Abstract
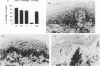
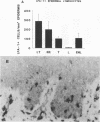
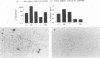
Leprosy presents as a clinical spectrum that is precisely paralleled by a spectrum of immunological reactivity. The disease provides a useful and accessible model, in this case in the skin, in which to study the dynamics of cellular immune responses to an infectious pathogen, including the role of adhesion molecules in those responses. In lesions characterized by strong delayed-type hypersensitivity against Mycobacterium leprae (tuberculoid, reversal reaction, and Mitsuda reaction), the overlying epidermis exhibited pronounced keratinocyte intracellular adhesion molecule 1 (ICAM-1) expression and contained lymphocytes expressing the ICAM-1 ligand, LFA-1. Conversely, in lesions in which delayed-type hypersensitivity was lacking (lepromatous), keratinocyte ICAM-1 expression was low and LFA-1+ lymphocytes were rare. Expression of these adhesion molecules on the cells within the dermal granulomas was equivalent throughout the spectrum of leprosy. The percentage of lymphocytes in these granulomas containing mRNA coding for gamma interferon and tumor necrosis factor alpha, synergistic regulators of ICAM-1 expression, paralleled epidermal ICAM-1 expression. In lesions of erythema nodosum leprosum, a reactional state of lepromatous leprosy thought to be due to immune complex deposition, keratinocyte ICAM-1 expression and gamma interferon mRNA+ cells were both prominent. Antibodies to LFA-1 and ICAM-1 blocked the response of both alpha beta and gamma delta T-cell clones in vitro to mycobacteria. Overall, the expression of adhesion molecules by immunocompetent epidermal cells, as well as the cytokines which regulate such expression, correlates with the outcome of the host response to infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. N., Sarma V., Mitra R. S., Dixit V. M., Nickoloff B. J. Marked synergism between tumor necrosis factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J Clin Invest. 1990 Feb;85(2):605–608. doi: 10.1172/JCI114481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnetson R. S., Bjune G., Pearson J. M., Kronvall G. Cell mediated and humoral immunity in "reversal reactions". Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):267–274. [PubMed] [Google Scholar]
- Bierer B. E., Burakoff S. J. T cell adhesion molecules. FASEB J. 1988 Jul;2(10):2584–2590. doi: 10.1096/fasebj.2.10.2838364. [DOI] [PubMed] [Google Scholar]
- Bjorvatn B., Barnetson R. S., Kronvall G., Zubler R. H., Lambert P. H. Immune complexes and complement hypercatabolism in patients with leprosy. Clin Exp Immunol. 1976 Dec;26(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Bjune G., Barnetson R. S., Ridley D. S., Kronvall G. Lymphocyte transformation test in leprosy; correlation of the response with inflammation of lesions. Clin Exp Immunol. 1976 Jul;25(1):85–94. [PMC free article] [PubMed] [Google Scholar]
- Ciccone E., Viale O., Pende D., Malnati M., Battista Ferrara G., Barocci S., Moretta A., Moretta L. Specificity of human T lymphocytes expressing a gamma/delta T cell antigen receptor. Recognition of a polymorphic determinant of HLA class I molecules by a gamma/delta clone. Eur J Immunol. 1989 Jul;19(7):1267–1271. doi: 10.1002/eji.1830190718. [DOI] [PubMed] [Google Scholar]
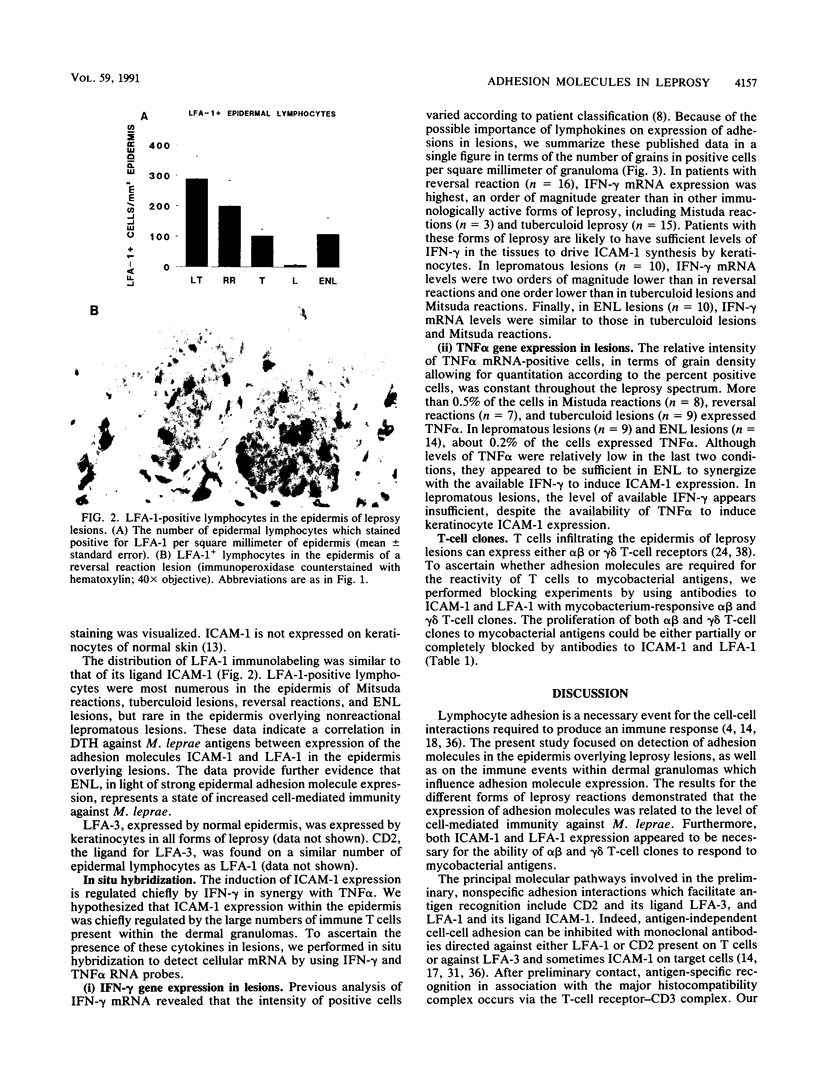
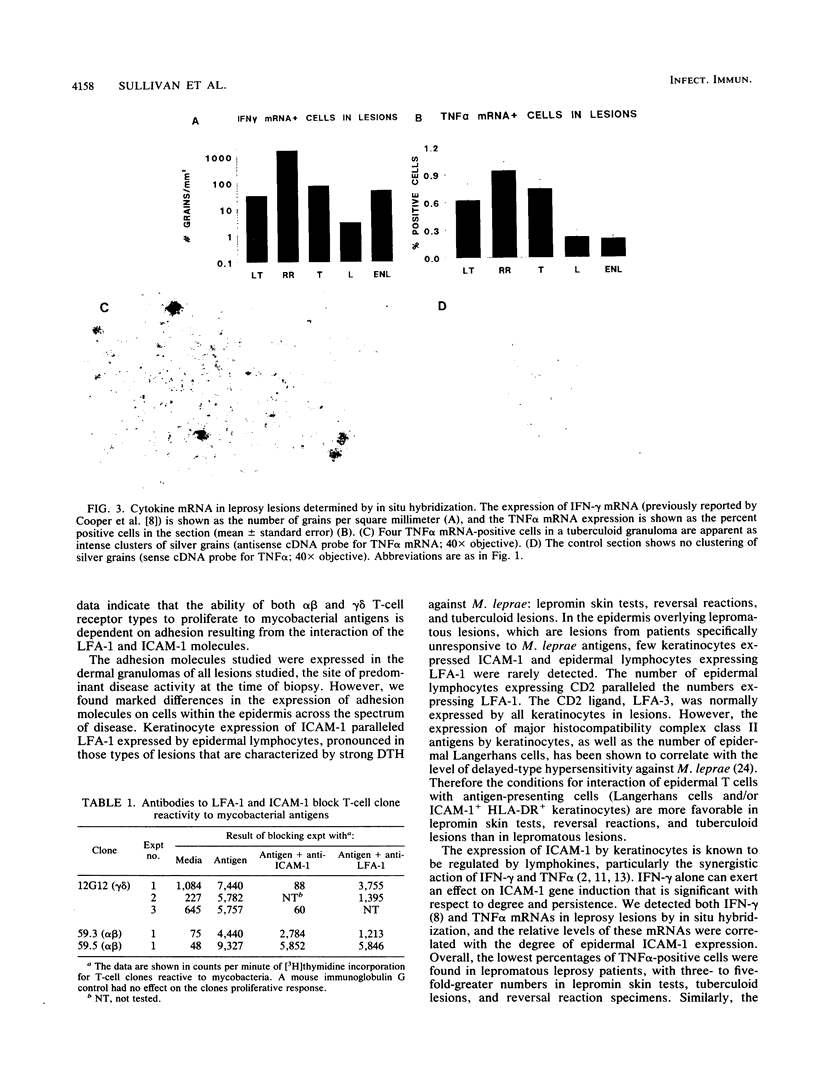
- Cooper C. L., Mueller C., Sinchaisri T. A., Pirmez C., Chan J., Kaplan G., Young S. M., Weissman I. L., Bloom B. R., Rea T. H. Analysis of naturally occurring delayed-type hypersensitivity reactions in leprosy by in situ hybridization. J Exp Med. 1989 May 1;169(5):1565–1581. doi: 10.1084/jem.169.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan E., Modlin R. L., Rea T. H. An in situ immunohistological study of Mitsuda reactions. Int J Lepr Other Mycobact Dis. 1985 Sep;53(3):404–409. [PubMed] [Google Scholar]
- Dustin M. L., Singer K. H., Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. 1988 Apr 1;167(4):1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Myrvang B., Samuel D. R., Ross W. F., Lofgren M. Mechanism of "reactions" in borderline tuberculoid (BT) leprosy. A preliminary report. Acta Pathol Microbiol Scand Suppl. 1973;236(0):45–53. [PubMed] [Google Scholar]
- Griffiths C. E., Voorhees J. J., Nickoloff B. J. Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989 Apr;20(4):617–629. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Kupper T. S. Mechanisms of cutaneous inflammation. Interactions between epidermal cytokines, adhesion molecules, and leukocytes. Arch Dermatol. 1989 Oct;125(10):1406–1412. doi: 10.1001/archderm.125.10.1406. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Johnson A. H., Hartzman R. J., Woody J. N. Antigen-specific human T lymphocyte clones: induction, antigen specificity, and MHC restriction of influenza virus-immune clones. J Immunol. 1982 Jan;128(1):233–238. [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Shaw S. The CD2-LFA-3 and LFA-1-ICAM pathways: relevance to T-cell recognition. Immunol Today. 1989 Dec;10(12):417–422. doi: 10.1016/0167-5699(89)90039-X. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Bakke A. C., Vaccaro S. A., Horwitz D. A., Taylor C. R., Rea T. H. Tissue and blood T-lymphocyte subpopulations in erythema nodosum leprosum. Arch Dermatol. 1985 Feb;121(2):216–219. [PubMed] [Google Scholar]
- Modlin R. L., Mehra V., Jordan R., Bloom B. R., Rea T. H. In situ and in vitro characterization of the cellular immune response in erythema nodosum leprosum. J Immunol. 1986 Feb 1;136(3):883–886. [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Mueller C., Gershenfeld H. K., Lobe C. G., Okada C. Y., Bleackley R. C., Weissman I. L. A high proportion of T lymphocytes that infiltrate H-2-incompatible heart allografts in vivo express genes encoding cytotoxic cell-specific serine proteases, but do not express the MEL-14-defined lymph node homing receptor. J Exp Med. 1988 Mar 1;167(3):1124–1136. doi: 10.1084/jem.167.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea T. H., Levan N. E. Variations in dinitrochlorobenzene responsivity in untreated leprosy: evidence of a beneficial role for anergy. Int J Lepr Other Mycobact Dis. 1980 Jun;48(2):120–125. [PubMed] [Google Scholar]
- Rea T. H., Shen J. Y., Modlin R. L. Epidermal keratinocyte Ia expression, Langerhans cell hyperplasia and lymphocytic infiltration in skin lesions of leprosy. Clin Exp Immunol. 1986 Aug;65(2):253–259. [PMC free article] [PubMed] [Google Scholar]
- Rea T. H., Taylor C. R. Serum and tissue lysozyme in leprosy. Infect Immun. 1977 Dec;18(3):847–856. doi: 10.1128/iai.18.3.847-856.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51(5):451–465. [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Ridley D. S. Reactions in leprosy. Lepr Rev. 1969 Apr;40(2):77–81. doi: 10.5935/0305-7518.19690016. [DOI] [PubMed] [Google Scholar]
- Ridley D. S. The pathogenesis of the early skin lesion in leprosy. J Pathol. 1973 Nov;111(3):191–206. doi: 10.1002/path.1711110307. [DOI] [PubMed] [Google Scholar]
- Romani N., Koide S., Crowley M., Witmer-Pack M., Livingstone A. M., Fathman C. G., Inaba K., Steinman R. M. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989 Mar 1;169(3):1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Luce G. E., Quinones R., Gress R. E., Springer T. A., Sanders M. E. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986 Sep 18;323(6085):262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- Shiohara T., Moriya N., Saizawa K., Gotoh C., Yagita H., Nagashima M. Evidence for involvement of lymphocyte function-associated antigen 1 in T cell migration to epidermis. J Immunol. 1991 Feb 1;146(3):840–845. [PubMed] [Google Scholar]
- Simon J. C., Cruz P. D., Jr, Bergstresser P. R., Davis L. S., Tigelaar R. E. Phorbol myristate acetate-activated keratinocytes stimulate proliferation of resting peripheral blood mononuclear lymphocytes via a MHC-independent, but protein kinase C- and intercellular adhesion molecule-1-dependent, mechanism. J Immunol. 1991 Jan 15;146(2):476–484. [PubMed] [Google Scholar]
- Simon J. C., Cruz P. D., Jr, Bergstresser P. R., Tigelaar R. E. Low dose ultraviolet B-irradiated Langerhans cells preferentially activate CD4+ cells of the T helper 2 subset. J Immunol. 1990 Oct 1;145(7):2087–2091. [PubMed] [Google Scholar]
- Singer K. H., Denning S. M., Whichard L. P., Haynes B. F. Thymocyte LFA-1 and thymic epithelial cell ICAM-1 molecules mediate binding of activated human thymocytes to thymic epithelial cells. J Immunol. 1990 Apr 15;144(8):2931–2939. [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Stach J. L., Strobel M., Fumoux F., Bach J. F. Defect in the generation of cytotoxic T cells in lepromatous leprosy. Clin Exp Immunol. 1982 Jun;48(3):633–640. [PMC free article] [PubMed] [Google Scholar]
- Uyemura K., Deans R. J., Band H., Ohmen J., Panchamoorthy G., Morita C. T., Rea T. H., Modlin R. L. Evidence for clonal selection of gamma/delta T cells in response to a human pathogen. J Exp Med. 1991 Sep 1;174(3):683–692. doi: 10.1084/jem.174.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. F., Turk J. L., Wemambu S. N. Mechanisms of reactions in leprosy. Int J Lepr Other Mycobact Dis. 1971 Apr-Jun;39(2):417–428. [PubMed] [Google Scholar]
- Wemambu S. N., Turk J. L., Waters M. F., Rees R. J. Erythema nodosum leprosum: a clinical manifestation of the arthus phenomenon. Lancet. 1969 Nov 1;2(7627):933–935. doi: 10.1016/s0140-6736(69)90592-3. [DOI] [PubMed] [Google Scholar]
- Wong L., Salgame P., Torigian V. K., Fu T. H., Rea T. H., Modlin R. L. CD2 expression and function in lepromatous leprosy. Infect Immun. 1989 Sep;57(9):2815–2819. doi: 10.1128/iai.57.9.2815-2819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]