Abstract
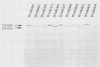
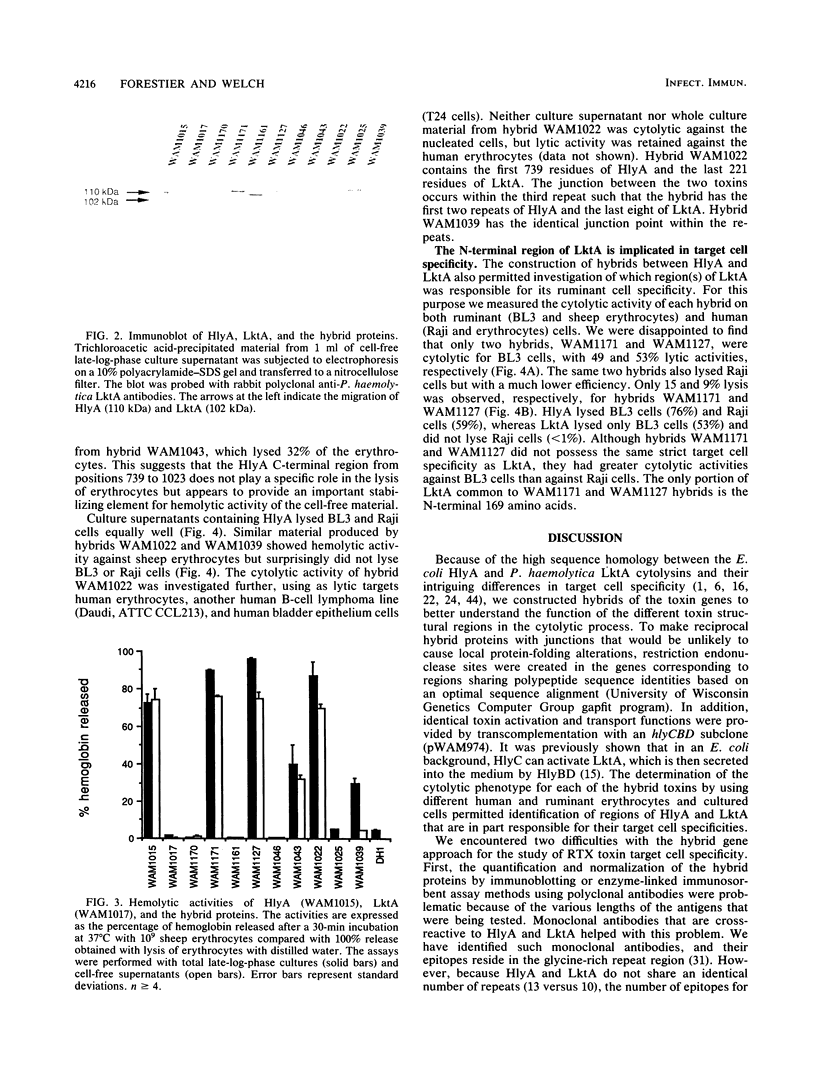
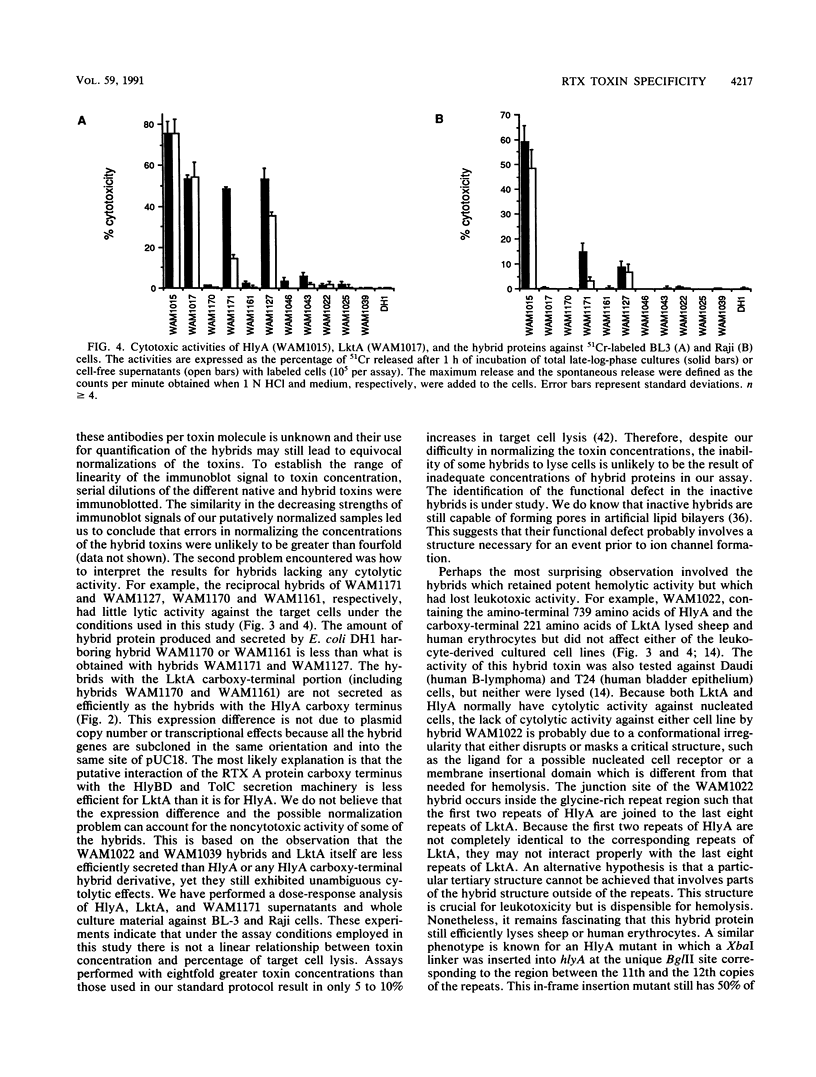
The Escherichia coli hemolysin (HlyA) and Pasteurella haemolytica leukotoxin (LktA) are cytolytic toxins encoded by genes belonging to the recently described RTX gene family. These cytotoxins are, respectively, 1,023 and 953 amino acids in length and are encoded by genes within identically organized operons. They share 45% amino acid sequence identities but differ in their target cell specificities. In vitro-derived recombinant hybrid genes between hlyA and lktA were constructed by using restriction endonuclease sites created by oligonucleotide site-directed mutagenesis. The cytolytic activity of hybrid proteins was investigated using as targets sheep erythrocytes and two cultured cell lines from different species (BL3, bovine leukemia-derived B lymphocytes; and Raji, human B-cell lymphoma cells). HlyA is cytolytic to all three cell types. LktA lyses only BL3 cells. Among the hybrid proteins displaying cytolytic activity, the striking finding is that the hemolytic activity of several LktA-HlyA hybrids was independent of any cytolytic activity against either cultured cell species. The hemolytic activity was associated with the HlyA region between amino acids 564 and 739. Structures that are critical for HlyA cytolytic activity against BL3 or Raji cells were destroyed when LktA-HlyA and HlyA-LktA hybrids were made, respectively, at amino acid positions 564 and 739 of HlyA. In contrast to HlyA, which lysed the two different cultured cell lines with equal efficiency, Lkt-HlyA hybrids possessing the amino-terminal 169 residues of LktA lysed BL3 cells more efficiently than Raji cells. This suggests that a significant but not exclusive element of the LktA ruminant cell specificity resides in the amino-terminal one-fifth of the protein. A molecular model of the functional domains of HlyA and LktA is presented.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluyut C. S., Simonson R. R., Bemrick W. J., Maheswaran S. K. Interaction of Pasteurella haemolytica with bovine neutrophils: identification and partial characterization of a cytotoxin. Am J Vet Res. 1981 Nov;42(11):1920–1926. [PubMed] [Google Scholar]
- Barry E. M., Weiss A. A., Ehrmann I. E., Gray M. C., Hewlett E. L., Goodwin M. S. Bordetella pertussis adenylate cyclase toxin and hemolytic activities require a second gene, cyaC, for activation. J Bacteriol. 1991 Jan;173(2):720–726. doi: 10.1128/jb.173.2.720-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Cytotoxic activity of partially purified Escherichia coli alpha haemolysin. J Med Microbiol. 1982 Feb;15(1):11–21. doi: 10.1099/00222615-15-1-11. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte viability in vitro. Infect Immun. 1982 May;36(2):455–461. doi: 10.1128/iai.36.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Moulds T. L., Struck D. K. Secretion of the Pasteurella leukotoxin by Escherichia coli. FEMS Microbiol Lett. 1989 Jul 15;51(1):169–173. doi: 10.1016/0378-1097(89)90502-8. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Post D., Struck D. K. Identification and characterization of the Pasteurella haemolytica leukotoxin. Infect Immun. 1987 Oct;55(10):2348–2354. doi: 10.1128/iai.55.10.2348-2354.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Welch R. A. Alterations of amino acid repeats in the Escherichia coli hemolysin affect cytolytic activity and secretion. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5269–5273. doi: 10.1073/pnas.85.14.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier C., Welch R. A. Nonreciprocal complementation of the hlyC and lktC genes of the Escherichia coli hemolysin and Pasteurella haemolytica leukotoxin determinants. Infect Immun. 1990 Mar;58(3):828–832. doi: 10.1128/iai.58.3.828-832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeberg O. V., Orskov I. In vitro cytotoxic effect of alpha-hemolytic Escherichia coli on human blood granulocytes. Infect Immun. 1984 Jul;45(1):255–260. doi: 10.1128/iai.45.1.255-260.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Gray L., Mackman N., Nicaud J. M., Holland I. B. The carboxy-terminal region of haemolysin 2001 is required for secretion of the toxin from Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):127–133. doi: 10.1007/BF02428042. [DOI] [PubMed] [Google Scholar]
- Gygi D., Nicolet J., Frey J., Cross M., Koronakis V., Hughes C. Isolation of the Actinobacillus pleuropneumoniae haemolysin gene and the activation and secretion of the prohaemolysin by the HlyC, HlyB and HlyD proteins of Escherichia coli. Mol Microbiol. 1990 Jan;4(1):123–128. doi: 10.1111/j.1365-2958.1990.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Highlander S. K., Chidambaram M., Engler M. J., Weinstock G. M. DNA sequence of the Pasteurella haemolytica leukotoxin gene cluster. DNA. 1989 Jan-Feb;8(1):15–28. doi: 10.1089/dna.1.1989.8.15. [DOI] [PubMed] [Google Scholar]
- Highlander S. K., Engler M. J., Weinstock G. M. Secretion and expression of the Pasteurella haemolytica Leukotoxin. J Bacteriol. 1990 May;172(5):2343–2350. doi: 10.1128/jb.172.5.2343-2350.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmel M. E., Yates M. D., Lauerman L. H., Squire P. G. Purification and partial characterization of a macrophage cytotoxin from Pasteurella haemolytica. Am J Vet Res. 1982 May;43(5):764–767. [PubMed] [Google Scholar]
- Issartel J. P., Koronakis V., Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991 Jun 27;351(6329):759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- Keane W. F., Gekker G., Schlievert P. M., Peterson P. K. Enhancement of endotoxin-induced isolated renal tubular cell injury by toxic shock syndrome toxin 1. Am J Pathol. 1986 Jan;122(1):169–176. [PMC free article] [PubMed] [Google Scholar]
- Keane W. F., Welch R., Gekker G., Peterson P. K. Mechanism of Escherichia coli alpha-hemolysin-induced injury to isolated renal tubular cells. Am J Pathol. 1987 Feb;126(2):350–357. [PMC free article] [PubMed] [Google Scholar]
- Kolodrubetz D., Dailey T., Ebersole J., Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989 May;57(5):1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Koronakis E., Hughes C. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 1989 Feb;8(2):595–605. doi: 10.1002/j.1460-2075.1989.tb03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig E., Dailey T., Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: homology to the alpha-hemolysin/leukotoxin gene family. Infect Immun. 1990 Apr;58(4):920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo R. Y., Strathdee C. A., Shewen P. E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987 Sep;55(9):1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Mackman N., Baker K., Gray L., Haigh R., Nicaud J. M., Holland I. B. Release of a chimeric protein into the medium from Escherichia coli using the C-terminal secretion signal of haemolysin. EMBO J. 1987 Sep;6(9):2835–2841. doi: 10.1002/j.1460-2075.1987.tb02580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Identification of polypeptides required for the export of haemolysin 2001 from E. coli. Mol Gen Genet. 1985;201(3):529–536. doi: 10.1007/BF00331351. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Characterisation of HlyC and mechanism of activation and secretion of haemolysin from E. coli 2001. FEBS Lett. 1985 Aug 5;187(2):339–344. doi: 10.1016/0014-5793(85)81272-2. [DOI] [PubMed] [Google Scholar]
- Noegel A., Rdest U., Springer W., Goebel W. Plasmid cistrons controlling synthesis and excretion of the exotoxin alpha-haemolysin of Escherichia coli. Mol Gen Genet. 1979 Oct 1;175(3):343–350. doi: 10.1007/BF00397234. [DOI] [PubMed] [Google Scholar]
- Pellett S., Boehm D. F., Snyder I. S., Rowe G., Welch R. A. Characterization of monoclonal antibodies against the Escherichia coli hemolysin. Infect Immun. 1990 Mar;58(3):822–827. doi: 10.1128/iai.58.3.822-827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982 Jan;35(1):91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Regulation of expression of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Nov;171(11):5955–5962. doi: 10.1128/jb.171.11.5955-5962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991 Mar;5(3):521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]