Abstract
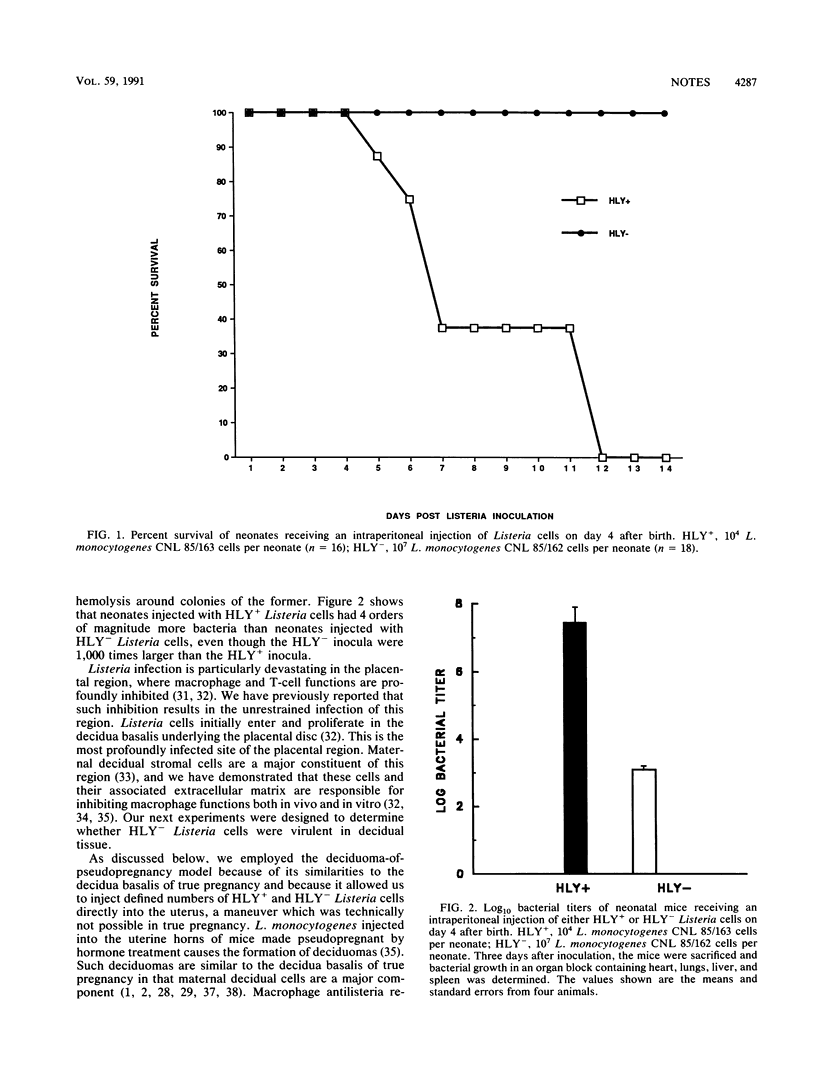
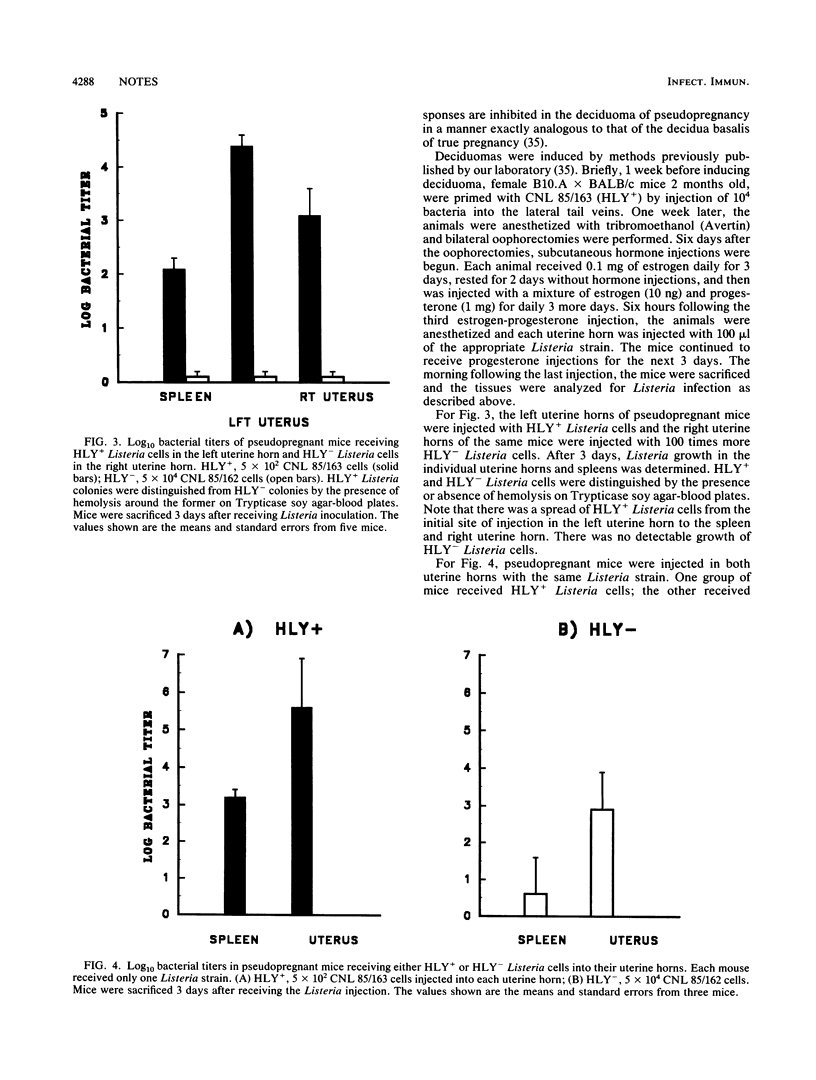
Listeriolysin is a 60-kDa protein which allows the growth of Listeria monocytogenes in macrophages and other cells and has been shown to be a virulence factor in Listeria infections of adult mice. However, the neonate and fetoplacental unit are major populations susceptible to listeriosis. Recent data indicate that macrophage and T-cell functions are markedly inhibited in these young mice, and the virulence of listeriolysin-negative (HLY-) and listeriolysin-positive (HLY+) Listeria cells in the setting of such inhibited macrophage and T-cell functions has not previously been examined. We now compare CNL 85/162, a transposon-induced, HLY- Listeria strain, and CNL 85/163, a spontaneous HLY+ revertant. We found that all 18 neonates injected with CNL 85/163 (HLY+) died within 12 days after an injection of 10(4) Listeria cells per mouse. In contrast, all 16 neonates injected with 1,000 times more CNL 85/162 (HLY-) cells survived more than 14 days. Three days after injection, growth of CNL 85/163 (HLY+) in the internal organs was more than 5 log greater than that of CNL 85/162 (HLY-). We also found that CNL 85/162 (HLY-) did not proliferate well in decidual tissue, which is a major component of the placental region. Our studies indicate that HLY- bacteria are not virulent in the neonate and the fetoplacental unit despite the inhibited immune functions at these sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berche P., Gaillard J. L., Sansonetti P. J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987 Apr 1;138(7):2266–2271. [PubMed] [Google Scholar]
- Bielecki J., Youngman P., Connelly P., Portnoy D. A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990 May 10;345(6271):175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- Camilli A., Goldfine H., Portnoy D. A. Listeria monocytogenes mutants lacking phosphatidylinositol-specific phospholipase C are avirulent. J Exp Med. 1991 Mar 1;173(3):751–754. doi: 10.1084/jem.173.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P. The listeriolysin O gene: a chromosomal locus crucial for the virulence of Listeria monocytogenes. Infection. 1988;16 (Suppl 2):S157–S159. doi: 10.1007/BF01639740. [DOI] [PubMed] [Google Scholar]
- Crainie M., Semeluk A., Lee K. C., Wegmann T. Regulation of constitutive and lymphokine-induced Ia expression by murine alpha-fetoprotein. Cell Immunol. 1989 Jan;118(1):41–52. doi: 10.1016/0008-8749(89)90356-0. [DOI] [PubMed] [Google Scholar]
- Crandall B. F. Alpha-fetoprotein: a review. Crit Rev Clin Lab Sci. 1981;15(2):127–185. doi: 10.3109/10408368109105870. [DOI] [PubMed] [Google Scholar]
- Delorme J., Benassayag C., Christeff N., Vallette G., Savu L., Nunez E. Age-dependent responses of the serum non-esterified fatty acids to adrenalectomy and ovariectomy in developing rats. Biochim Biophys Acta. 1984 Jan 17;792(1):6–10. doi: 10.1016/0005-2760(84)90275-3. [DOI] [PubMed] [Google Scholar]
- Domann E., Leimeister-Wächter M., Goebel W., Chakraborty T. Molecular cloning, sequencing, and identification of a metalloprotease gene from Listeria monocytogenes that is species specific and physically linked to the listeriolysin gene. Infect Immun. 1991 Jan;59(1):65–72. doi: 10.1128/iai.59.1.65-72.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin L. B., Shea C. M., Soberman R. J., Lu C. Y. Docosahexaenoic acid, a constituent of rodent fetal serum and fish oil diets, inhibits acquisition of macrophage tumoricidal function. J Immunol. 1990 Jun 15;144(12):4888–4897. [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Raveneau J., Beretti J. L., Lecroisey A., Vazquez-Boland J. A., Alouf J. E., Berche P. Purification and characterization of an extracellular 29-kilodalton phospholipase C from Listeria monocytogenes. Infect Immun. 1991 Jul;59(7):2382–2388. doi: 10.1128/iai.59.7.2382-2388.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hunziker R. D., Wegmann T. G. Placental immunoregulation. Crit Rev Immunol. 1986;6(3):245–285. [PubMed] [Google Scholar]
- Kathariou S., Rocourt J., Hof H., Goebel W. Levels of Listeria monocytogenes hemolysin are not directly proportional to virulence in experimental infections of mice. Infect Immun. 1988 Feb;56(2):534–536. doi: 10.1128/iai.56.2.534-536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Domann E., Chakraborty T. Detection of a gene encoding a phosphatidylinositol-specific phospholipase C that is co-ordinately expressed with listeriolysin in Listeria monocytogenes. Mol Microbiol. 1991 Feb;5(2):361–366. doi: 10.1111/j.1365-2958.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Lu C. Y., Calamai E. G., Unanue E. R. A defect in the antigen-presenting function of macrophages from neonatal mice. Nature. 1979 Nov 15;282(5736):327–329. doi: 10.1038/282327a0. [DOI] [PubMed] [Google Scholar]
- Lu C. Y., Changelian P. S., Unanue E. R. Alpha-fetoprotein inhibits macrophage expression of Ia antigens. J Immunol. 1984 Apr;132(4):1722–1727. [PubMed] [Google Scholar]
- Mascola L., Sorvillo F., Neal J., Iwakoshi K., Weaver R. Surveillance of listeriosis in Los Angeles County, 1985-1986. A first year's report. Arch Intern Med. 1989 Jul;149(7):1569–1572. [PubMed] [Google Scholar]
- Mengaud J., Braun-Breton C., Cossart P. Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol Microbiol. 1991 Feb;5(2):367–372. doi: 10.1111/j.1365-2958.1991.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Geoffroy C., Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991 Mar;59(3):1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder G. L., Macon G. R. Uterine and oviducal protein secretion during early pregnancy in the mouse. J Reprod Fertil. 1987 Sep;81(1):287–294. doi: 10.1530/jrf.0.0810287. [DOI] [PubMed] [Google Scholar]
- O'Shea J. D., Kleinfeld R. G., Morrow H. A. Ultrastructure of decidualization in the pseudopregnant rat. Am J Anat. 1983 Mar;166(3):271–298. doi: 10.1002/aja.1001660304. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab Invest. 1989 Jul;61(1):27–36. [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Role of local immunosuppression in murine fetoplacental listeriosis. J Clin Invest. 1987 Apr;79(4):1234–1241. doi: 10.1172/JCI112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988 Jun 1;140(11):3947–3955. [PubMed] [Google Scholar]
- Redline R. W., McKay D. B., Vazquez M. A., Papaioannou V. E., Lu C. Y. Macrophage functions are regulated by the substratum of murine decidual stromal cells. J Clin Invest. 1990 Jun;85(6):1951–1958. doi: 10.1172/JCI114658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R. W., Shea C. M., Papaioannou V. E., Lu C. Y. Defective anti-listerial responses in deciduoma of pseudopregnant mice. Am J Pathol. 1988 Dec;133(3):485–497. [PMC free article] [PubMed] [Google Scholar]
- Storb R., Prentice R. L., Buckner C. D., Clift R. A., Appelbaum F., Deeg J., Doney K., Hansen J. A., Mason M., Sanders J. E. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983 Feb 10;308(6):302–307. doi: 10.1056/NEJM198302103080602. [DOI] [PubMed] [Google Scholar]
- Weitlauf H. M., Suda-Hartman M. Changes in secreted uterine proteins associated with embryo implantation in the mouse. J Reprod Fertil. 1988 Nov;84(2):539–549. doi: 10.1530/jrf.0.0840539. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Damjanov A., Weiss J., Liotta L. A., Damjanov I. Mouse endometrial stromal cells produce basement-membrane components. Differentiation. 1986;32(1):49–58. doi: 10.1111/j.1432-0436.1986.tb00555.x. [DOI] [PubMed] [Google Scholar]


