Abstract
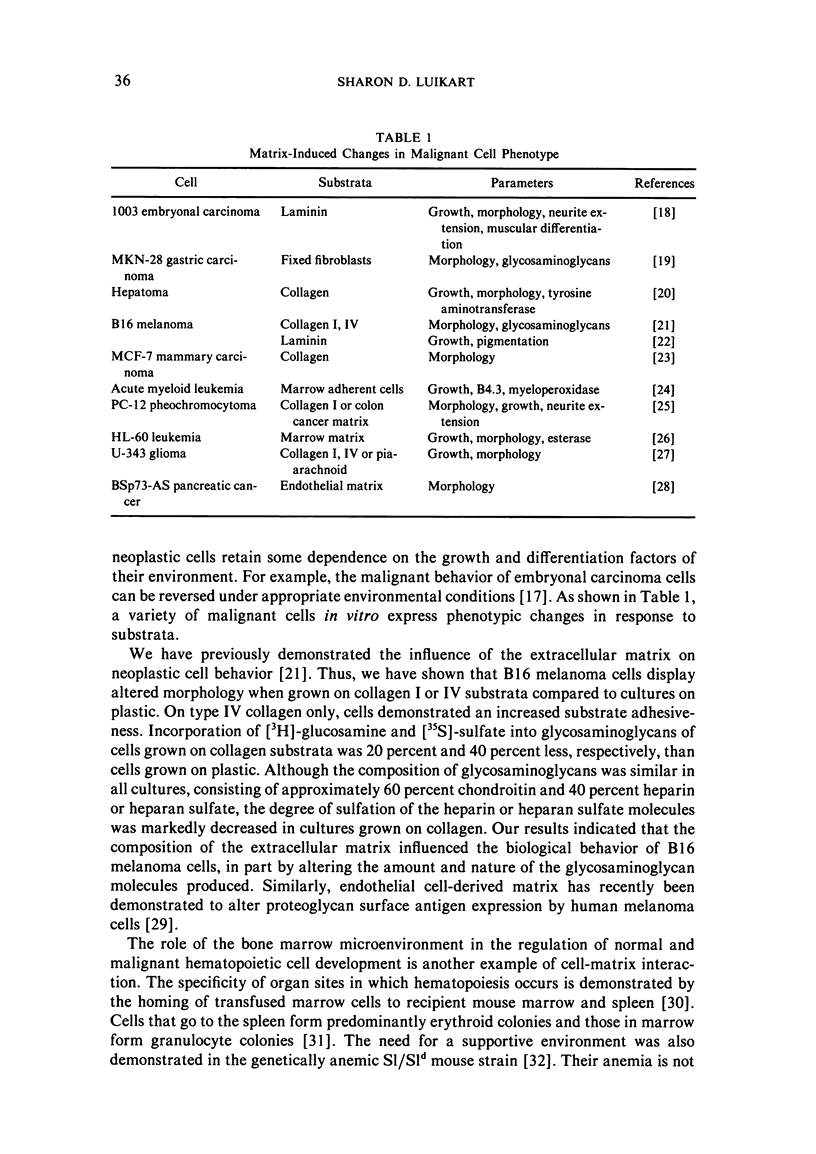
Extracellular matrix molecules, including collagen, glycosaminoglycans (usually linked to a protein core as proteoglycan), elastin, and glycoproteins, influence the initiation and maintenance of differentiation of a variety of cell types. These molecules bind to the cell surface at specific sites and nonspecifically by electrostatic forces. Such interactions may alter the cell's response to growth and differentiation factors. After neoplastic transformation, most cells retain some dependence on these factors. This paper reviews the influence of matrix components on the phenotype of a variety of malignant cells and concludes that in vitro studies of malignant cell behavior require the utilization of an appropriate microenvironment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellot F., Luis J., el Battari A., Secchi J., Cau P., Marvaldi J., Pichon J. Extracellular material secreted by human colonic adenocarcinoma cell lines promotes spreading in serum-free medium and induces neurite outgrowth of PC-12 cells. Int J Cancer. 1985 Nov 15;36(5):609–615. doi: 10.1002/ijc.2910360515. [DOI] [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Darmon M. Y. Laminin provides a better substrate than fibronectin for attachment, growth, and differentiation of 1003 embryonal carcinoma cells. In Vitro. 1982 Dec;18(12):997–1003. doi: 10.1007/BF02796374. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Saxén L., Timpl R. The extracellular matrix and kidney differentiation. Prog Clin Biol Res. 1982;91:429–442. [PubMed] [Google Scholar]
- Fulton A. B., Wan K. M., Penman S. The spatial distribution of polyribosomes in 3T3 cells and the associated assembly of proteins into the skeletal framework. Cell. 1980 Jul;20(3):849–857. doi: 10.1016/0092-8674(80)90331-1. [DOI] [PubMed] [Google Scholar]
- Gatmaitan Z., Jefferson D. M., Ruiz-Opazo N., Biempica L., Arias I. M., Dudas G., Leinwand L. A., Reid L. M. Regulation of growth and differentiation of a rat hepatoma cell line by the synergistic interactions of hormones and collagenous substrata. J Cell Biol. 1983 Oct;97(4):1179–1190. doi: 10.1083/jcb.97.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. J. Extracellular matrices and the control of cell proliferation and differentiation in vitro. Prog Clin Biol Res. 1984;145:103–128. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Gospodarowicz D., Vlodavsky I., Savion N. The role of fibroblast growth factor and the extracellular matrix in the control of proliferation and differentiation of corneal endothelial cells. Vision Res. 1981;21(1):87–103. doi: 10.1016/0042-6989(81)90141-3. [DOI] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Illmensee K., Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci U S A. 1976 Feb;73(2):549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Cannon F. B., Laurie G. W., Hassell J. R., Aumailley M., Terranova V. P., Martin G. R., DuBois-Dalcq M. Biological activities of laminin. J Cell Biochem. 1985;27(4):317–325. doi: 10.1002/jcb.240270402. [DOI] [PubMed] [Google Scholar]
- Kujawa M. J., Tepperman K. Culturing chick muscle cells on glycosaminoglycan substrates: attachment and differentiation. Dev Biol. 1983 Oct;99(2):277–286. doi: 10.1016/0012-1606(83)90277-4. [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Bing J. T., Kleinman H. K., Hassell J. R., Aumailley M., Martin G. R., Feldmann R. J. Localization of binding sites for laminin, heparan sulfate proteoglycan and fibronectin on basement membrane (type IV) collagen. J Mol Biol. 1986 May 5;189(1):205–216. doi: 10.1016/0022-2836(86)90391-8. [DOI] [PubMed] [Google Scholar]
- Loring J., Glimelius B., Weston J. A. Extracellular matrix materials influence quail neural crest cell differentiation in vitro. Dev Biol. 1982 Mar;90(1):165–174. doi: 10.1016/0012-1606(82)90222-6. [DOI] [PubMed] [Google Scholar]
- Luikart S. D., Maniglia C. A., Sartorelli A. C. Influence of collagen substrata on glycosaminoglycan production by B16 melanoma cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3738–3742. doi: 10.1073/pnas.80.12.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch E. A., Siminovitch L., Till J. E., Russell E. S., Bernstein S. E. The cellular basis of the genetically determined hemopoietic defect in anemic mice of genotype Sl-Sld. Blood. 1965 Oct;26(4):399–410. [PubMed] [Google Scholar]
- Pourreau-Schneider N., Martin P. M., Charpin C., Jacquemier J., Saez S., Nandi S. How culture conditions modulate the morphofunctional differentiation of the human estradiol-sensitive mammary cell line (MCF-7). J Steroid Biochem. 1984 Jan;20(1):407–415. doi: 10.1016/0022-4731(84)90243-7. [DOI] [PubMed] [Google Scholar]
- Raz A., Zöller M., Ben-Ze'ev Cell configuration and adhesive properties of metastasizing and non-metastasizing BSp73 rat adenocarcinoma cells. Exp Cell Res. 1986 Jan;162(1):127–141. doi: 10.1016/0014-4827(86)90431-3. [DOI] [PubMed] [Google Scholar]
- Reimann J., Burger H. In vitro proliferation of haemopoietic cells in the presence of adherent cell layers. II. Differential effect of adherent cell layers derived from various organs. Exp Hematol. 1979 Jan;7(1):52–58. [PubMed] [Google Scholar]
- Rettig W. J., Real F. X., Spengler B. A., Biedler J. L., Old L. J. Human melanoma proteoglycan: expression in hybrids controlled by intrinsic and extrinsic signals. Science. 1986 Mar 14;231(4743):1281–1284. doi: 10.1126/science.3633135. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Gatmaitan Z., Mackensen S., Giambrone M. A., Ponce P., Reid L. M. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980 Oct;87(1):255–263. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutka J. T. The K.G. McKenzie award lecture--1986. Effects of extracellular matrix proteins on the growth and differentiation of an anaplastic glioma cell line. Can J Neurol Sci. 1986 Nov;13(4):301–306. doi: 10.1017/s0317167100036611. [DOI] [PubMed] [Google Scholar]
- Schölzel C., Löwenberg B. Stimulation of proliferation and differentiation of acute myeloid leukemia cells on a bone marrow stroma in culture. Exp Hematol. 1985 Aug;13(7):664–669. [PubMed] [Google Scholar]
- Sobue M., Takeuchi J., Tsukidate K., Toida M., Goto K., Nakashima N. Influence of fixed fibroblasts on glycosaminoglycan synthesis of human gastric carcinoma cells in vitro. Exp Cell Res. 1983 Dec;149(2):527–534. doi: 10.1016/0014-4827(83)90363-4. [DOI] [PubMed] [Google Scholar]
- Sénéchal H., Wahrmann J. P., Delain D., Macieira-Coelho A. Modulation of differentiation in vitro. II. Influence of cell spreading and surface events on myogenesis. In Vitro. 1984 Sep;20(9):692–698. doi: 10.1007/BF02618874. [DOI] [PubMed] [Google Scholar]
- Thomas D. B. The differentiation of transplanted haematopoietic cells derived from bone marrow, spleen and fetal liver. J Anat. 1971 Nov;110(Pt 2):297–306. [PMC free article] [PubMed] [Google Scholar]
- Wolf N. S. Dissecting the hematopoietic microenvironment. III. Evidence for a positive short range stimulus for cellular proliferation. Cell Tissue Kinet. 1978 Jul;11(4):335–345. [PubMed] [Google Scholar]


