Abstract
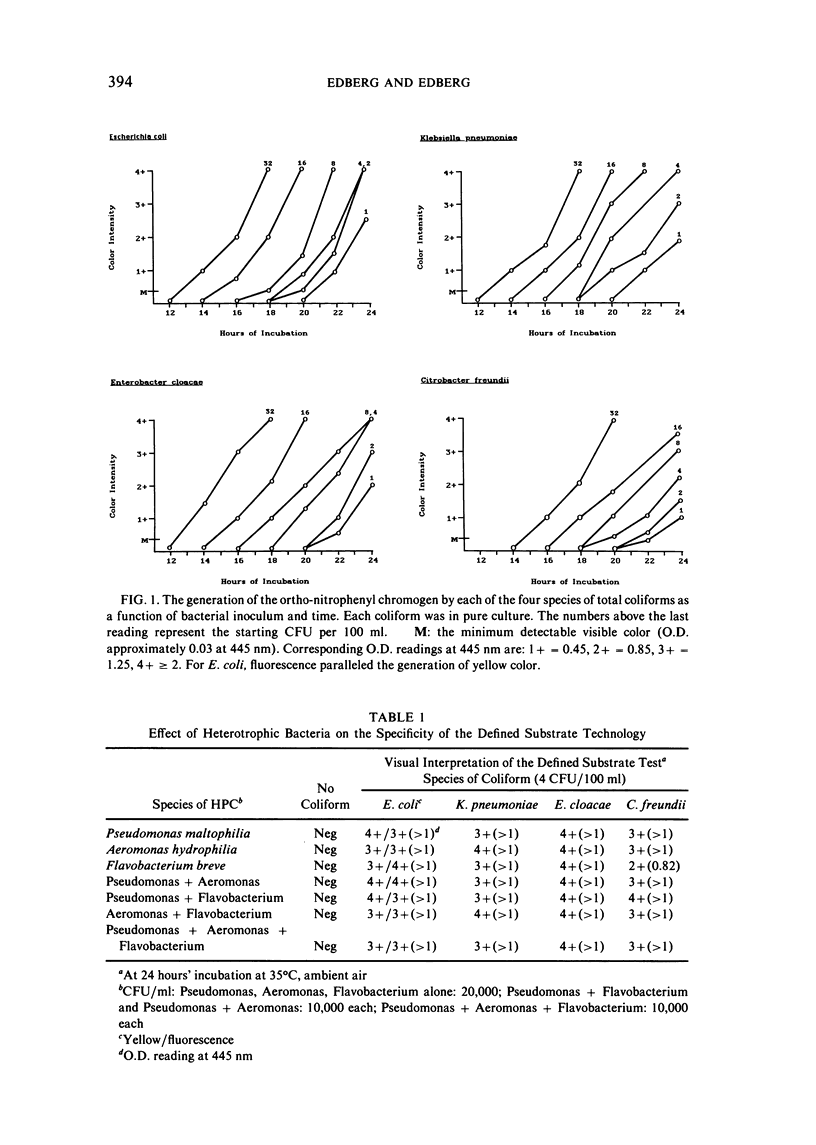
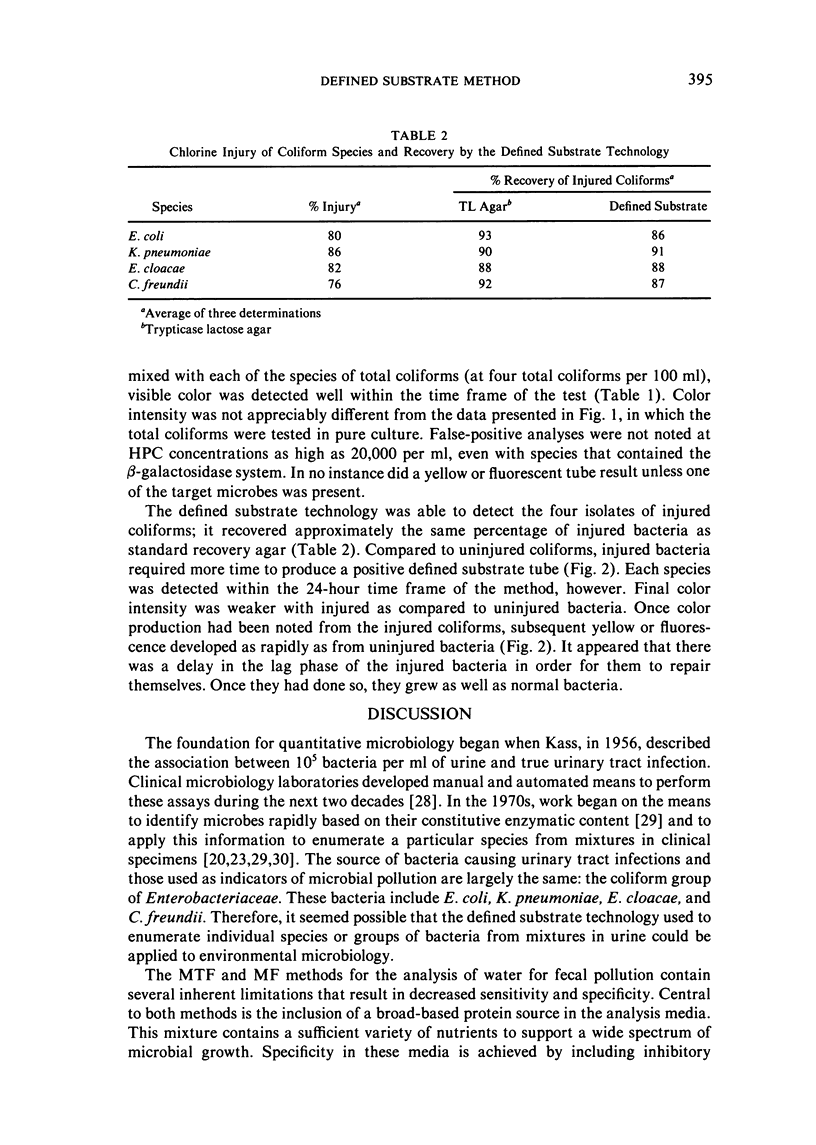
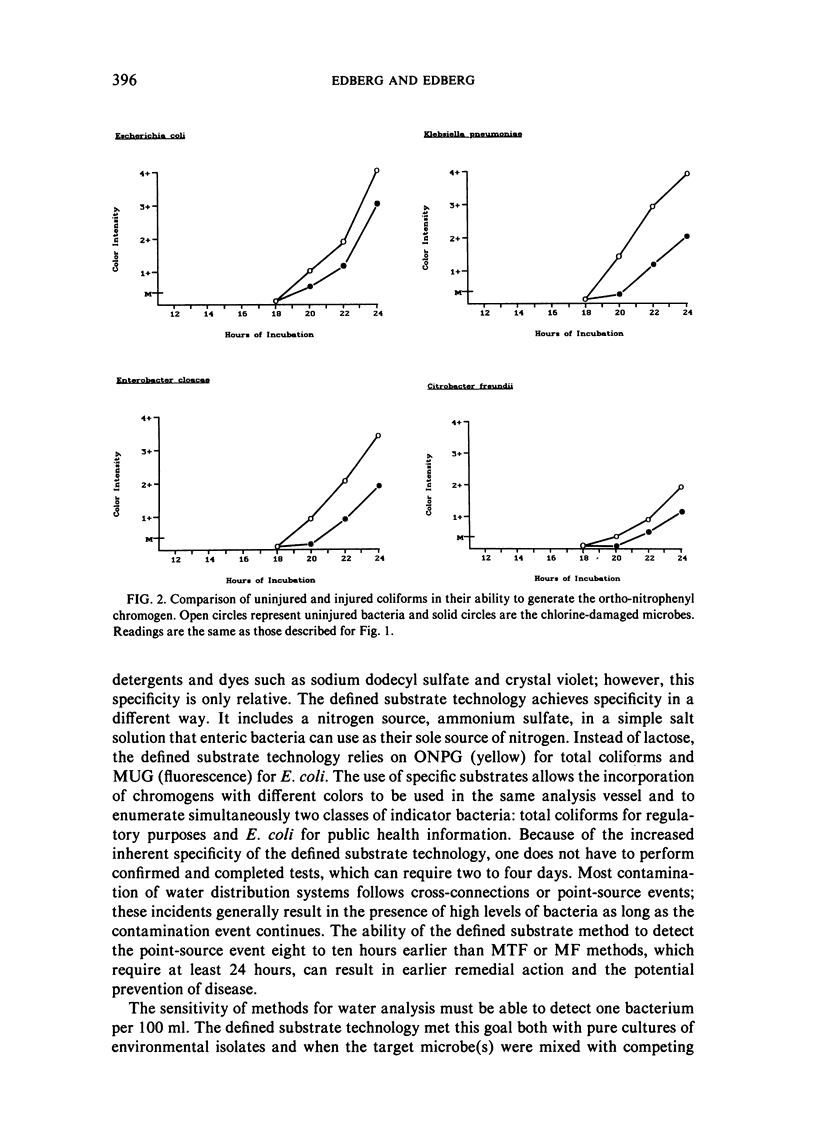
The examination of water and other environmental sources for microbial pollution is a major public health undertaking. Currently, there are two accepted methods in use: the multiple-tube fermentation (MTF) and the membrane filtration (MF) tests. Both methods are designed to enumerate the secondary indicator group, total coliforms. Both tests suffer several inherent limitations, including a time delay of three to seven days to obtain a definitive result, the subjective nature of the test interpretation, and the inability to provide directly useful public health information. A defined substrate technology, originally used to enumerate specific bacterial species from mixtures in clinical urine specimens, was applied to water testing; the technology was constituted to enumerate simultaneously both total coliforms and the primary indicator bacterium E. coli. Examination of environmental isolates of these two classes of target microbes showed sensitivity equal to available methods, with potentially greater specificity. It was not subject to inhibition by bacteria other than the targets, grew injured coliforms, did not require confirmatory tests, and the maximum time to a positive was 24 hours. The defined substrate technology provides both regulatory and directly useful public health information.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Evaluation of recovery methods to detect coliforms in water. Appl Environ Microbiol. 1977 Mar;33(3):590–595. doi: 10.1128/aem.33.3.590-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry W. B., Hanks J. B., Thomason B. M., Murlin A. M., Biddle J. W., Croom J. M. Salmonellae as an index of pollution of surface waters. Appl Microbiol. 1972 Sep;24(3):334–340. doi: 10.1128/am.24.3.334-340.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Atkinson B., Chambers C., Moore M. H., Palumbo L., Zorzon C. F., Singer J. M. Clinical evaluation of the MICRO-ID, API 20E, and conventional media systems for identification of Enterobacteriacea. J Clin Microbiol. 1979 Aug;10(2):161–167. doi: 10.1128/jcm.10.2.161-167.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Gam K., Bottenbley C. J., Singer J. M. Rapid spot test for the determination of esculin hydrolysis. J Clin Microbiol. 1976 Aug;4(2):180–184. doi: 10.1128/jcm.4.2.180-184.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Novak M., Slater H., Singer J. M. Direct inoculation procedure for the rapid classification of bacteria from blood culture. J Clin Microbiol. 1975 Dec;2(6):469–473. doi: 10.1128/jcm.2.6.469-473.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Piscitelli V., Cartter M. Phenotypic characteristics of coliform and noncoliform bacteria from a public water supply compared with regional and national clinical species. Appl Environ Microbiol. 1986 Sep;52(3):474–478. doi: 10.1128/aem.52.3.474-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Pittman S., Singer J. M. Esculin hydrolysis by Enterobacteriaceae. J Clin Microbiol. 1977 Aug;6(2):111–116. doi: 10.1128/jcm.6.2.111-116.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Samuels S. Rapid, colorimetric test for the determination of hippurate hydrolysis by group B Streptococcus. J Clin Microbiol. 1976 Jan;3(1):49–50. doi: 10.1128/jcm.3.1.49-50.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. M., LeChevallier M. W., Waarvick C. E., Seidler R. J. Coliform species recovered from untreated surface water and drinking water by the membrane filter, standard, and modified most-probable-number techniques. Appl Environ Microbiol. 1981 Mar;41(3):657–663. doi: 10.1128/aem.41.3.657-663.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. M., Seidler R. J., LeChevallier M. W. Impact of verification media and resuscitation on accuracy of the membrane filter total coliform enumeration technique. Appl Environ Microbiol. 1981 May;41(5):1144–1151. doi: 10.1128/aem.41.5.1144-1151.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. M., Waarvick C. E., Seidler R. J., LeChevallier M. W. Failure of the most-probable-number technique to detect coliforms in drinking water and raw water supplies. Appl Environ Microbiol. 1981 Jan;41(1):130–138. doi: 10.1128/aem.41.1.130-138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong D., Damaré J. M., Weiner R. M., Colwell R. R. Bacteria associated with false-positive most-probable-number coliform test results for shellfish and estuaries. Appl Environ Microbiol. 1981 Jan;41(1):35–45. doi: 10.1128/aem.41.1.35-45.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg H. D., Gavan T. L., Smith P. B., Sonnenwirth A., Taylor W., Martin W. J., Rhoden D., Balows A. Collaborative investigation of the AutoMicrobic System Enterobacteriaceae biochemical card. J Clin Microbiol. 1980 Jun;11(6):694–702. doi: 10.1128/jcm.11.6.694-702.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N. J., Zeigler W. L., Reed F. C., Stukel T. A., Rice E. W. Comparison of membrane filter, multiple-fermentation-tube, and presence-absence techniques for detecting total coliforms in small community water systems. Appl Environ Microbiol. 1986 May;51(5):1007–1012. doi: 10.1128/aem.51.5.1007-1012.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Cameron S. C., McFeters G. A. New medium for improved recovery of coliform bacteria from drinking water. Appl Environ Microbiol. 1983 Feb;45(2):484–492. doi: 10.1128/aem.45.2.484-492.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Stuart D. G. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972 Nov;24(5):805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows P. S., Anderson J. G., Patel K., Mullins B. W. Variability in gas production by Escherichia coli in enrichment media and its relationship to pH. Appl Environ Microbiol. 1980 Aug;40(2):309–312. doi: 10.1128/aem.40.2.309-312.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G. B., Gubbins P., Morgan V. A critical appraisal of the membrane filter technic. Health Lab Sci. 1965 Oct;2(4):227–237. [PubMed] [Google Scholar]
- SHIPE E. L., Jr, CAMERON G. M. A comparison of the membrane filter with the most probable number method for coliform determinations from several waters. Appl Microbiol. 1954 Mar;2(2):85–88. doi: 10.1128/am.2.2.85-88.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff L. J., Morrison S. M., Mayeux J. V. Synergistic false-positive coliform reaction on M-Endo MF medium. Appl Microbiol. 1970 Nov;20(5):778–781. doi: 10.1128/am.20.5.778-781.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



