Abstract
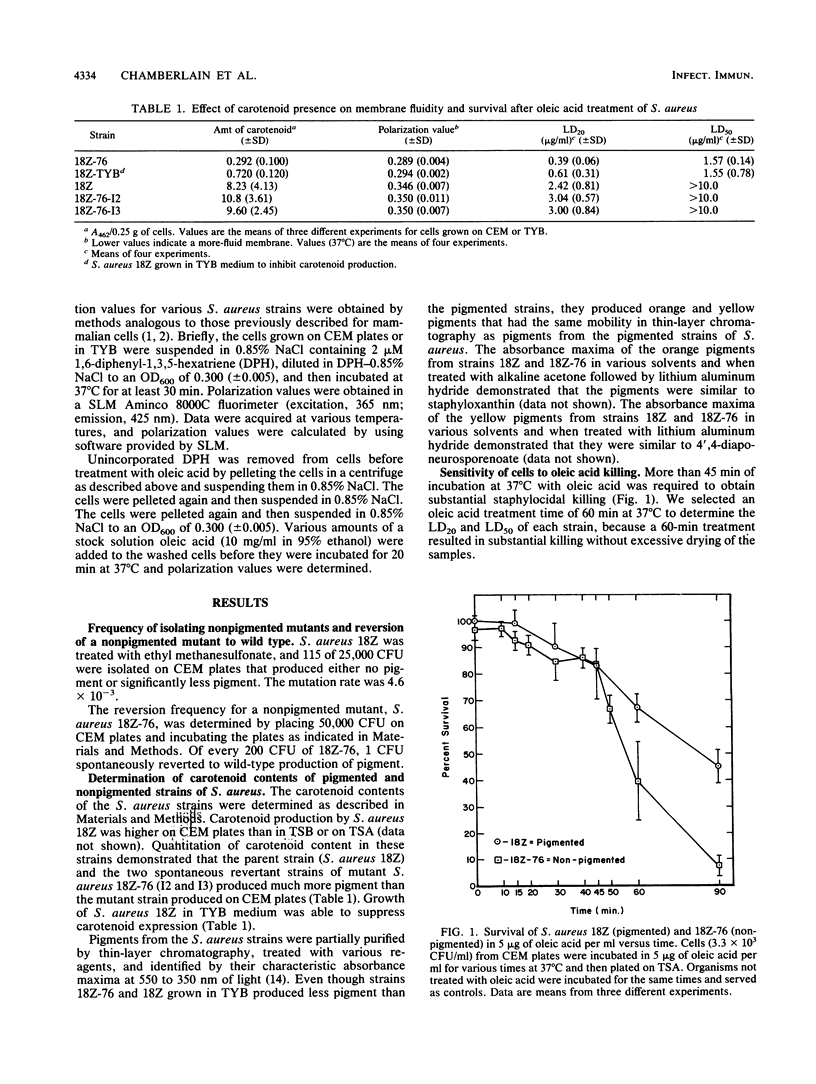
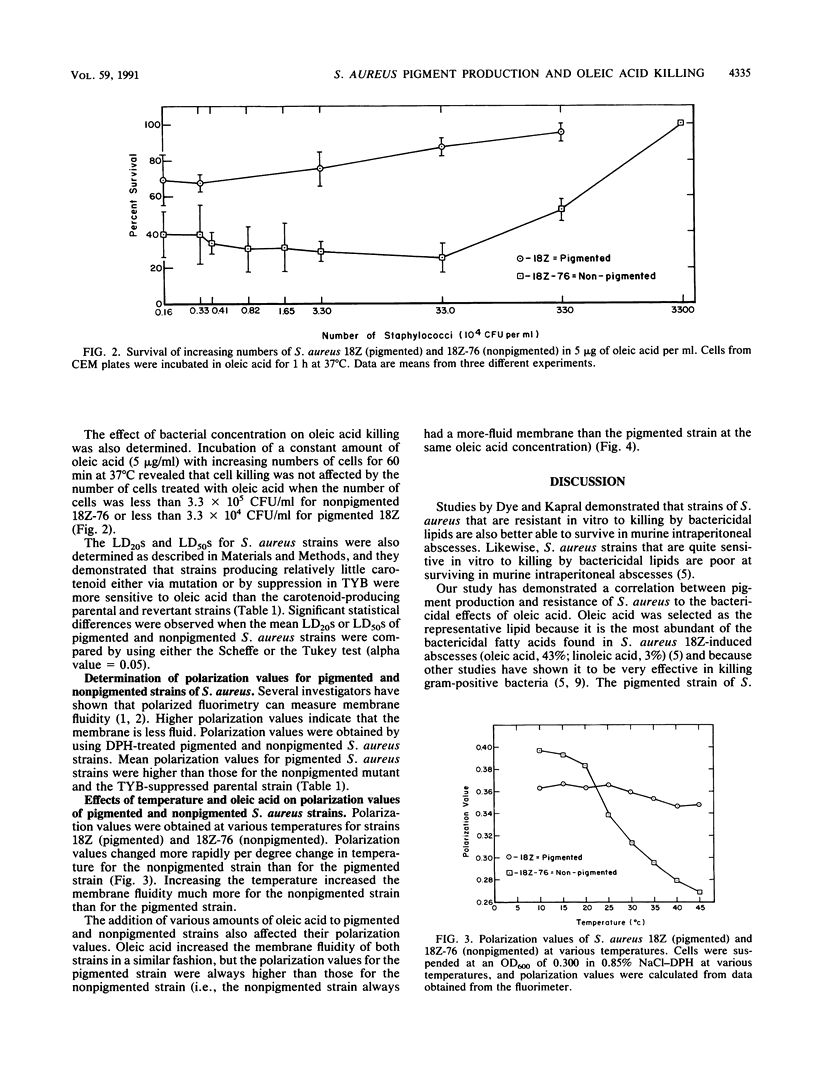
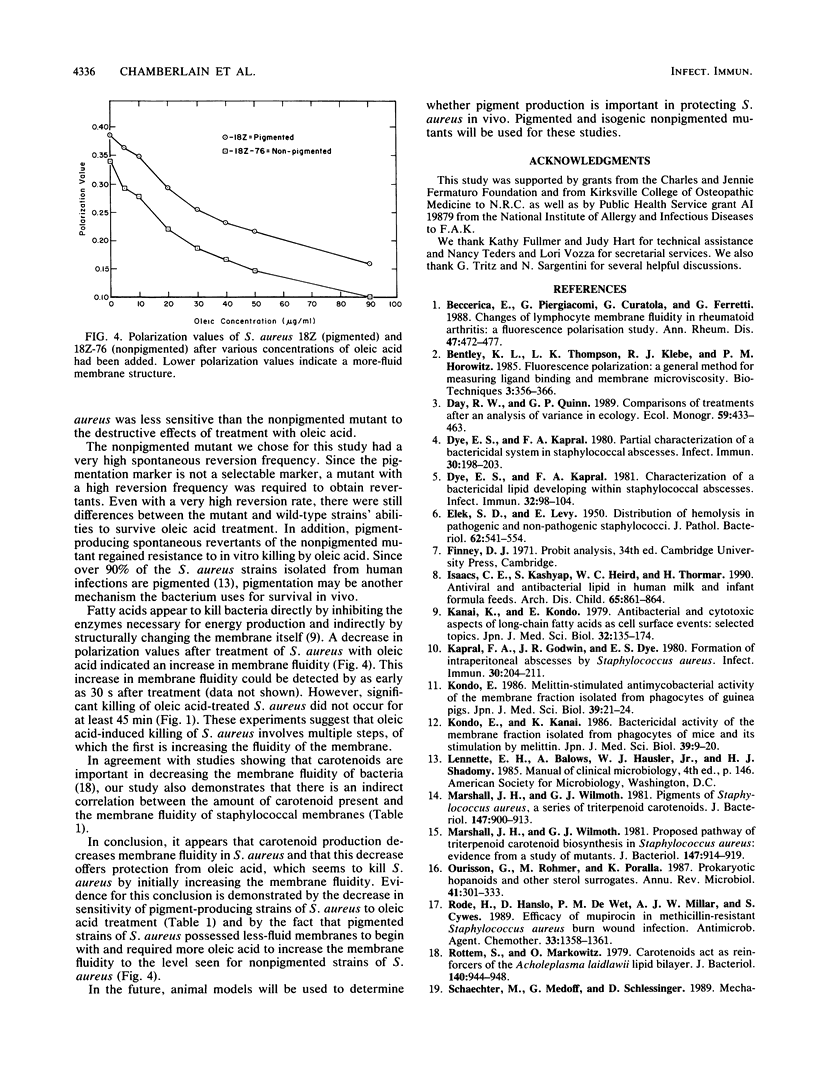
Staphylococcus aureus is susceptible to killing by host-derived fatty acids. Studies were performed to test for a correlation between carotenoid production by S. aureus and protection against oleic acid. Oleic acid killing of cells grown in carotenoid expression medium was determined as the dosage of oleic acid in 2 M NaCl-2 mM EDTA that would kill 20% of the cells in 60 min at 37 degrees C (i.e., the 20% lethal dose). Compared with the wild-type strain (18Z), a carotenoid-deficient mutant strain (18Z-76) and strain 18Z grown in a medium that suppressed carotenoid production both showed increased sensitivity to oleic acid. Spontaneous revertants of strain 18Z-76 that regained the ability to produce carotenoids were as resistant to oleic acid as the wild-type strain. Oleic acid was shown by fluorescence polarization to decrease polarization values. Lower polarization values indicate a more-fluid membrane. To determine whether protection against oleic acid killing might depend on carotenoid stabilization of membranes, fluorescence polarization values were determined for strains showing different levels of carotenoid production. An indirect correlation was found between membrane fluidity and carotenoid production. We were able to conclude that there is a direct correlation between carotenoid production (i.e., cell pigmentation), cell membrane stability, and resistance to oleic acid-induced cell killing.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beccerica E., Piergiacomi G., Curatola G., Ferretti G. Changes of lymphocyte membrane fluidity in rheumatoid arthritis: a fluorescence polarisation study. Ann Rheum Dis. 1988 Jun;47(6):472–477. doi: 10.1136/ard.47.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye E. S., Kapral F. A. Characterization of a bactericidal lipid developing within staphylococcal abscesses. Infect Immun. 1981 Apr;32(1):98–104. doi: 10.1128/iai.32.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye E. S., Kapral F. A. Partial characterization of a bactericidal system in staphylococcal abscesses. Infect Immun. 1980 Oct;30(1):198–203. doi: 10.1128/iai.30.1.198-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELEK S. D., LEVY E. Distribution of haemolysins in pathogenic and non-pathogenic staphylococci. J Pathol Bacteriol. 1950 Oct;62(4):541–554. doi: 10.1002/path.1700620405. [DOI] [PubMed] [Google Scholar]
- Isaacs C. E., Kashyap S., Heird W. C., Thormar H. Antiviral and antibacterial lipids in human milk and infant formula feeds. Arch Dis Child. 1990 Aug;65(8):861–864. doi: 10.1136/adc.65.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai K., Kondo E. Antibacterial and cytotoxic aspects of long-chain fatty acids as cell surface events: selected topics. Jpn J Med Sci Biol. 1979 Jun;32(3):135–174. doi: 10.7883/yoken1952.32.135. [DOI] [PubMed] [Google Scholar]
- Kapral F. A., Godwin J. R., Dye E. S. Formation of intraperitoneal abscesses by Staphylococcus aureus. Infect Immun. 1980 Oct;30(1):204–211. doi: 10.1128/iai.30.1.204-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E., Kanai K. Bactericidal activity of the membrane fraction isolated from phagocytes of mice and its stimulation by melittin. Jpn J Med Sci Biol. 1986 Feb;39(1):9–20. doi: 10.7883/yoken1952.39.9. [DOI] [PubMed] [Google Scholar]
- Kondo E. Melittin-stimulated antimycobacterial activity of the membrane fraction isolated from phagocytes of guinea pigs. Jpn J Med Sci Biol. 1986 Feb;39(1):21–24. doi: 10.7883/yoken1952.39.21. [DOI] [PubMed] [Google Scholar]
- Marshall J. H., Wilmoth G. J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriol. 1981 Sep;147(3):900–913. doi: 10.1128/jb.147.3.900-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. H., Wilmoth G. J. Proposed pathway of triterpenoid carotenoid biosynthesis in Staphylococcus aureus: evidence from a study of mutants. J Bacteriol. 1981 Sep;147(3):914–919. doi: 10.1128/jb.147.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourisson G., Rohmer M., Poralla K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu Rev Microbiol. 1987;41:301–333. doi: 10.1146/annurev.mi.41.100187.001505. [DOI] [PubMed] [Google Scholar]
- Rode H., Hanslo D., de Wet P. M., Millar A. J., Cywes S. Efficacy of mupirocin in methicillin-resistant Staphylococcus aureus burn wound infection. Antimicrob Agents Chemother. 1989 Aug;33(8):1358–1361. doi: 10.1128/aac.33.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Markowitz O. Carotenoids acts as reinforcers of the Acholeplasma laidlawii lipid bilayer. J Bacteriol. 1979 Dec;140(3):944–948. doi: 10.1128/jb.140.3.944-948.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. F. Bacterial triterpenoids. Microbiol Rev. 1984 Sep;48(3):181–198. doi: 10.1128/mr.48.3.181-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormar H., Isaacs C. E., Brown H. R., Barshatzky M. R., Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987 Jan;31(1):27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche H., Ruysschaert J. M., Defrise-Quertain F., Willemsens G., Cornelissen F., Marichal P., Cools W., Van Cutsem J. The interaction of miconazole and ketoconazole with lipids. Biochem Pharmacol. 1982 Aug 15;31(16):2609–2617. doi: 10.1016/0006-2952(82)90707-9. [DOI] [PubMed] [Google Scholar]


