Abstract
Purpose
Treatment of metastatic colorectal carcinoma represents a major clinical challenge. We investigated the hypothesis that the desmoplastic reaction within liver elicited by metastatic adenocarcinoma, characterised by collagen I deposition and altered collagen IV distribution, promotes growth and survival of hepatic colorectal carcinoma metastases.
Experimental design
Partial hepatectomy specimens for metastatic colorectal adenocarcinoma were examined immunohistochemically for differential integrin expression. Human colorectal adenocarcinoma cell lines HT-29, KM12SM and KM12c were grown on wild type collagen I or IV, or cleavage resistant r/r collagen I, and assessed for their growth, survival and resistance to 5-fluorouracil. The effect of αvβ3 and αvβ5 integrin blockade by neutralising antibodies was examined.
Results
Collagen I, in contrast to collagen IV, significantly enhanced the growth, survival and chemoresistance of colorectal carcinoma cells. αvβ3 and αvβ5 integrin blockade significantly reduced colorectal carcinoma cell proliferation on collagen, especially in the cell line with most metastatic potential. These in vitro findings correlated with the pattern of integrin expression identified within resected hepatic colorectal carcinoma metastases. Using matrix metalloproteinase-resistant r/r collagen I as a dominant negative ligand for αv integrins we demonstrated a key role for this integrin-ligand interaction in mediating the survival and proliferation of colorectal carcinoma cells.
Conclusions
Desmoplasia has an important role in the development of hepatic colorectal carcinoma metastasis. The interaction between integrin and collagen I is identified as a potential therapeutic target.
Introduction
Colorectal carcinoma is the second commonest neoplasm condition in Western society, affecting 1 in 20 adults [2]. Despite improvements in diagnosis and treatment, approximately 50% of those who undergo a potentially curative resection die within 5 years, the majority due to metastatic disease [3, 4]. Even patients with early stage cancers may also succumb to the effects of metastatic disease [2].
The extracellular matrix (ECM) is produced and regulated by stromal cells and influences the growth of normal and neoplastic cells [1]. The desmoplastic reaction (DR) associated with invasive malignancy is characterised by dysregulated matrix turnover, with degradation of normal type IV collagen-rich basement membrane and accumulation of fibrillar collagens (predominantly type I) [5, 6]. In breast [7], pancreatic [8] and lung cancer [9] it has been suggested that changes in the tumour microenvironment due to the DR may benefit the tumour by enhancing proliferation, inducing a more invasive malignant phenotype, and increasing chemoresistance [6, 8-11]. Whilst there is increasing evidence for a tumour DR in primary malignancy far less is known about this axis in metastatic disease. Determining the role of the DR in metastatic disease is important because malignant tumours are able to seed a normal organ and elicit a desmoplastic wound healing response that may, in turn, influence the behaviour of the metastatic population. In contrast many primary cancers develop in a pre-existing inflammatory environment. Understanding the role of the DR in supporting metastatic cancer may inform therapeutic strategies.
In primary lung cancers the effects of DR on tumour are integrin mediated [9]. Integrins are cell surface receptors which, upon ligation with specific epitopes in the ECM, activate intracellular signalling pathways that regulate genes crucial for growth, differentiation [12] and apoptosis [13]. Intact and denatured collagens present a range of integrin ligands, including those for β1 [14] and αv [15, 16] integrins. Over-expression of these integrin sub-types might be important in the development of many epithelial cancers [17, 18]. A pronounced DR is known to be an independent poor prognostic factor in patients with colorectal carcinoma [19]. To date the limited numbers of in vitro studies examining the effect of matrix components on colorectal carcinoma cell growth have produced contradictory results [20, 21] and no unifying molecular mechanism has been defined to explain the poor prognosis associated with the development of a florid DR in primary or metastatic cancer.
In this study we have characterised the DR and integrin expression in partial hepatectomy specimens performed for hepatic colorectal carcinoma metastases and demonstrated how specific components of the desmoplastic matrix mediate a growth and survival advantage to colorectal carcinoma cells via integrins. We also demonstrate the importance of differential integrin expression by the cancer in vivo in hepatic metastases and describe a differential pattern of integrin expression for colorectal carcinomas with different metastatic phenotypes. Finally we demonstrate, mechanistically, the importance of collagen I degradation in regulating colorectal carcinoma cell growth, in particular those carcinoma cells with an aggressive metastatic phenotype. The key role played by the DR in colorectal carcinoma growth and survival identifies this process as a potential therapeutic target in the treatment of metastatic colorectal carcinoma.
Materials and Methods
Cell culture
Cells were cultured under standard conditions at 37°C and 5% CO2. HT-29 cells were obtained from the European Cell and Culture Collective (Porton Down, Wiltshire, UK) at passage number 140. HT-29 is a human colorectal carcinoma cell line derived from a primary grade 2 adenocarcinoma in 1964 and extensively studied since. The baseline parental cell line with the lowest metastatic potential has been used although variants with more metastatic potential have been established [22]. The KM12 cell line series (A kind gift of Professor IJ Fidler MD Anderson centre, Houston, TX, USA) consisting of progressively more aggressive liver metastasising variants derived from the original human KM12c cell line by intrasplenically injecting KM12c cells into nude mice and extracting cells from the resultant liver metastases, repeated several times to establish each metastatic line [23], were obtained at passage 12 (KM12c) and passage 21 (KM12SM). HT-29 and KM12c cell lines are poorly metastatic, and the KM12SM cell line is highly metastatic.
Collagen preparation
Collagens were dissolved in sterile 0.1 mM acetic acid to a final concentration of 1 mg/ml and plated at 15 μg/cm2. In each experiment a control of untreated tissue culture plastic was included. The matrix layers were blocked with 0.1% (w/v) bovine serum albumin. The Col 1a1r/r mice [24] produce type I collagen highly resistant to matrix metalloproteinase (MMP) digestion (A kind gift of Professor S Krane, Harvard medical school, MA, USA). Type I r/r collagen and its wild type control were extracted from mouse tails using the method of Cawston and Barrett [25].
Tinctorial and immunohistochemical staining
Samples were obtained from 10 separate patients after appropriate consent (LREC No: 215/99), who had undergone partial hepatectomy for metastatic colorectal carcinoma, and fixed in formalin. Sections were stained using a 0.1% picrosirius red solution (w/v) [26] or immunostained as described [27]. Negative controls using non-immune IgG instead of primary antibodies showed no staining. The primary antibodies and pre-treatments used were; Collagen I (Microwave, pH 6, Clone Col-1, Abcam, Cambridge, UK); Collagen IV (Pronase, Clone CIV 22, Dako Ely, Cambridgeshire, UK); α-smooth muscle actin (Microwave, pH 6, Clone 1A4, Sigma, Poole, Dorset, UK); β1 integrin (Pronase, Clone 4B7R, Santa Cruz, CA, USA); αvβ3 integrin (Microwave, pH 8, ab7167, Abcam, Cambridge, UK) and αvβ5 integrin (Microwave, pH 8, Clone 2Q1009, United States Biological, Swampscott, MA, USA). Analysis of the sections was undertaken by two pathologists who assessed the pattern and intensity of staining.
Clonogenic assays
Cells were cultured on uncoated plastic or type I or IV collagen in low serum media, or low serum media containing FU, for 48-56 hours. Cells were seeded in quadruplicate at 200 cells/well and left for 8 to 14 days until colonies had formed. Colonies were counted after Giemsa staining. The number of colonies (>16 cells) formed by cells exposed to FU was divided by the number of colonies in the untreated pair, giving an index of cell viability for each substratum.
Measurement of [3H]-thymidine incorporation
The effect cell-matrix interactions on proliferation was determined by measuring [3H]-thymidine incorporation. Experiments were conducted in triplicate in 24-well plates. Incorporation of [3H]-thymidine into cell DNA was determined as described [8]. When comparing proliferation of cancer cells on uncoated plastic vs. collagens, differences in cell adhesion were controlled by quantifying total DNA using PicoGreen Reagent (Invitrogen Ltd, Paisley, UK) in parallel triplicate wells. The results were expressed as counts.min−1.ng−1 DNA.
Neutralising antibody experiments
Neutralising antibodies used were: β1 integrin (Clone 4B4-azide free, Beckmann Coulter, High Wycombe, Buckinghamshire, UK); αvβ3 and αvβ5 integrins (Clones LM609 and P1F6-azide free, Chemicon, Chandlers Ford, Hampshire, UK).
Cell adhesion assay
Cells were pre-incubated with appropriate antibody (5-20 μg/ml), IgG control (Ancell antibodies, USA) or no antibody for 15 minutes before plating into uncoated or collagen coated wells in 10% fetal calf serum. After culturing under standard conditions for 2 hours, supernatants were removed and the wells washed to remove non-adherent cells. Adherent cells were fixed with formaldehyde, and stained with 1% methylene blue. Methylene blue was released from cells with 0.5 M HCl and absorbance measured at 620nm.
Effect of neutralising antibodies on cell proliferation
5 μg/ml β1 integrin neutralising antibody or control IgG was pre-incubated with cells for 15 minutes before plating, and [3H]-thymidine incorporation determined as described. For αvβ3 and αvβ5 integrin neutralising antibodies (5-10 μg/ml) it was possible that cell-mediated degradation of matrix was necessary to reveal epitopes so cells were plated out as described then switched to low serum media for 8 hours. The neutralizing antibody and controls were added in low serum media, left for a further 8 hours and [3H]-thymidine incorporation determined. DNA was measured in parallel wells to correct for potential differing adherent cell numbers.
Immunoblot analysis
1×106 cells were added to flasks coated with different matrices, and cultured for 16 hours. The media was changed to low serum media after washing and the cells cultured for 36 hours before processing.
To determine the amount of apoptosis as a result of FU treatment, Poly-ADP Ribose Polymerase (PARP) cleavage was assessed. After 8 hours of culture in low serum conditions FU (0-50 μg/ml) was added for 48-56 hours.
Cell monolayers were harvested into RIPA buffer containing mammalian protease inhibitors (Sigma, Poole, Dorset, UK). Protein concentrations were determined using the bicinchoninic acid protein assay kit (BCA-1, Sigma, Poole, Dorset, UK). Samples were prepared according to the Invitrogen Nupage protocol. 10-50 μg of protein was separated on Nupage 4-12% gels with MOPS running buffer. Samples were unreduced except those assessed for PARP cleavage. Proteins were transferred onto a PVDF membrane and the following antibodies applied; αv integrin, 1:1,000 (Clone AV1, Chemicon, Chandlers Ford, Hampshire, UK); β1 integrin, 1:1,000 (Clone JB1A, Chemicon, Chandlers Ford, Hampshire, UK); β3 integrin, 1:1,000 (Clone BB10, Chemicon, Chandlers Ford, Hampshire, UK); β5 integrin, 1:500 (Clone 343.11D1, Calbiochem, Beeston, Nottingham, UK); PARP, 1:1,500 (Clone C2-10, R&D systems, Abingdon, Oxon, UK). Equal sample loading was determined by immunoblotting for β-actin, 1:10,000 (Clone AC15, Sigma, Poole, Dorset, UK). Blots were processed using Western Breeze anti-mouse chemiluminescent detection system (Invitrogen Ltd, Paisley, UK), and the images captured using a Biorad FluorSmax imager (Biorad life sciences, Hemel Hempstead, Hertfordshire, UK).
Detection of apoptosis using flow cytometry
Apoptosis was quantified using the Annexin V Apoptosis detection kit (Calbiochem, PF032) utilizing appropriate controls for Annexin V and propidium iodide (PI) staining as directed by the manufacturer's protocol. A positive control was provided by colorectal carcinoma cells treated with a 3% formaldehyde solution. Samples were prepared using the same protocol as that used for determining the amount of PARP cleavage, and were analysed using the FACSCalibur system (Beckton Dickinson, UK).
Cell proliferation on Type I r/r collagen
The [3H]-thymidine and Pico green proliferation assay, as previously described, were performed for colorectal carcinoma cells cultured on proprietary collagen I (Sigma, Poole, Dorset, UK), r/r and wild type collagen I. The αvβ3 and αvβ5 integrin neutralising antibody experiments were also performed, as described, with KM12SM cells grown on these matrices.
Statistical Analysis
All results are expressed as means ± 95% confidence intervals and all experiments were repeated a minimum of three times. Statistical significance was assessed using Student's t-test.
Results
Staining pattern of collagens and integrins in resected liver
Picrosirius red staining demonstrated absence of fibrillar collagen within normal liver except for walls of vessels within portal tracts (Figure 1a). A matrix-rich stromal reaction was present within metastatic colorectal carcinoma (Figure 1b). Fibrillar collagen-rich desmoplasia was most dense in poorly differentiated areas of tumour, often enveloping individual tumour cells. The deposited matrix was populated by increased numbers of α-smooth muscle actin (α-sma) positive hepatic myofibroblasts (Figure 1c). The fibrotic neomatrix consisted predominantly of collagens I and III (Figure 1d). Immunostaining for collagen IV demonstrated disruption of the normal pattern within the metastasis compared to adjacent normal liver. Most residual collagen IV within the metastases was associated with fibrillar collagen bands (Figure 1e).
Figure 1.
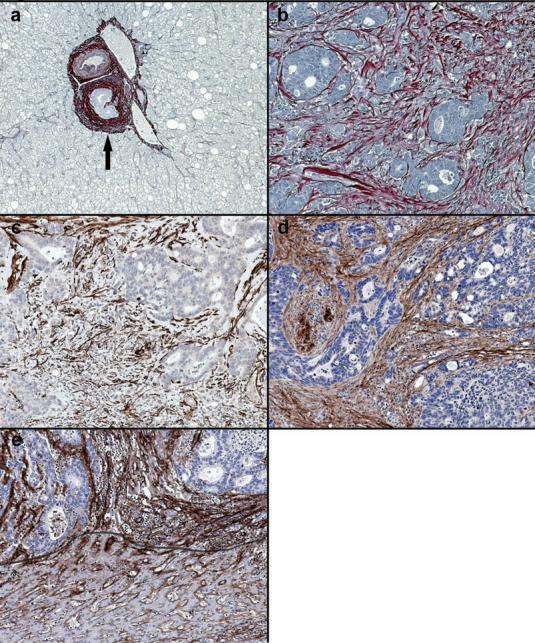
Expression of collagen within desmoplastic reaction. (a) In normal liver picrosirius red positive fibrillar collagen is only present within portal tracts (arrowed). (b) In areas of tumour desmoplasia picrosirius red positive fibrillar collagen is abundant. This is most dense in association with the most poorly differentiated areas. (c) Activated hepatic myofibroblasts demonstrated by α-smooth muscle actin immunopositivity are plentiful within the deposited desmoplastic matrix. (d) Collagen I is the major component of desmoplastic neomatrix. (e) In normal liver collagen IV is found in the space of Disse (below line) but within areas of tumour desmoplasia deposition of collagen IV is denser and more widespread. Images representative of staining patterns identified in livers of 10 different patients. 100x magnification.
β1 integrin expression was demonstrated in normal liver tissue and throughout the colorectal carcinoma metastases (Figure 2a). Within metastases β1 integrins were expressed by both tumour and stromal cells at higher levels compared to normal liver. However, the pattern of β1 integrin expression within the metastases was irregular, being most dense and uniformly distributed in well differentiated areas of the tumour, and often less strongly expressed or absent in poorly differentiated areas (Figure 2b).
Figure 2.
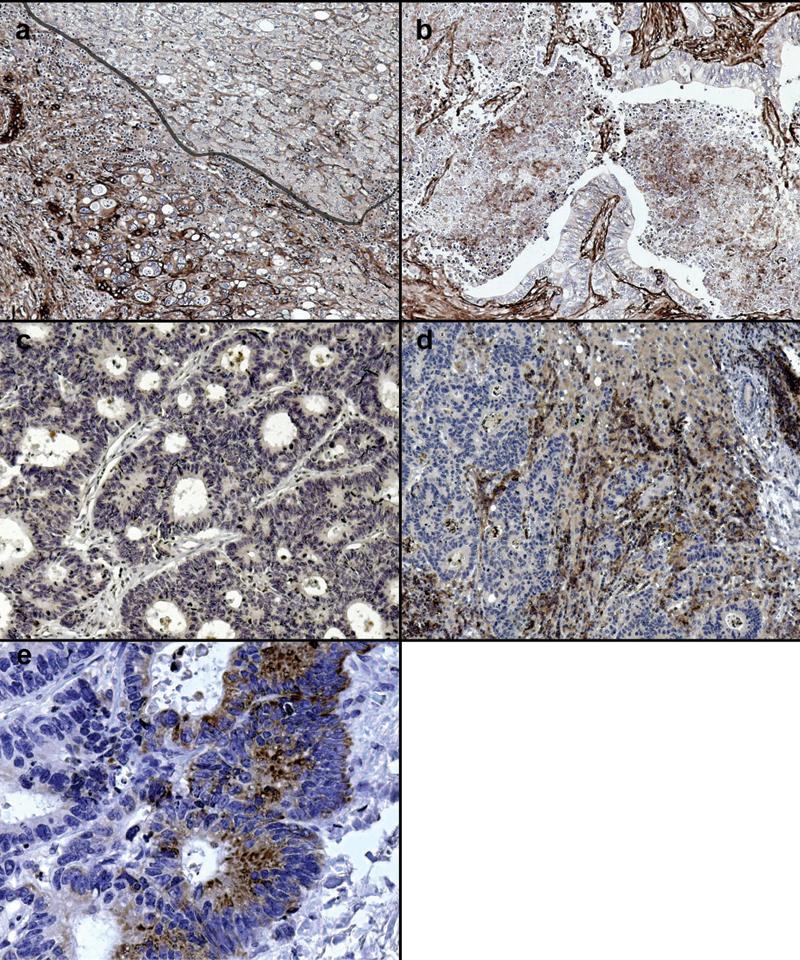
Expression of β1 and αv integrins within hepatic colorectal carcinoma metastases. (a) Normal liver has a uniform pattern of β1 integrin expression on hepatocyte basement membranes (upper right) whilst expression was increased and disorganised in areas of tumour desmoplasia (lower left). (b) Less β1 integrin expression is observed in poorly differentiated areas of tumour. (c) Well differentiated tumour expresses minimal αvβ5 whilst there is marked expression by epithelial and stromal cells in poorly differentiated (d) areas. (e). αvβ5 integrins were most highly expressed at the invading margin of the tumour. Images representative of staining patterns identified in livers of 10 different patients. 100x magnification (e, 400x magnification).
In well differentiated areas of the metastases αvβ5 integrin was expressed at very low levels (Figure 2c) in contrast to stronger expression within poorly differentiated areas (Figure 2d). Expression was highest along the invading margin of the metastases (Figures 2e).
αvβ3 integrin expression has been previously described in primary human adenocarcinoma [28] and was strong throughout the metastases. Although expression appeared to be highest in poorly differentiated areas, the differences were not as marked as those identified for αvβ5 integrin expression (data not shown).
Collagens regulate colorectal carcinoma cell proliferation
Having demonstrated abundant collagen I in hepatic colorectal carcinoma metastases in vivo we examined the effect of collagen I on tumour cell growth and chemoresistance in vitro. Culture on collagen I significantly increased proliferation of all colorectal carcinoma cell lines compared to tissue culture plastic and collagen IV. Collagen IV significantly inhibited proliferation of HT-29 and KM12c cells compared to tissue culture plastic and collagen I. The anti-proliferative effect of type IV collagen was less marked in KM12SM cells (Figure 3a).
Figure 3.
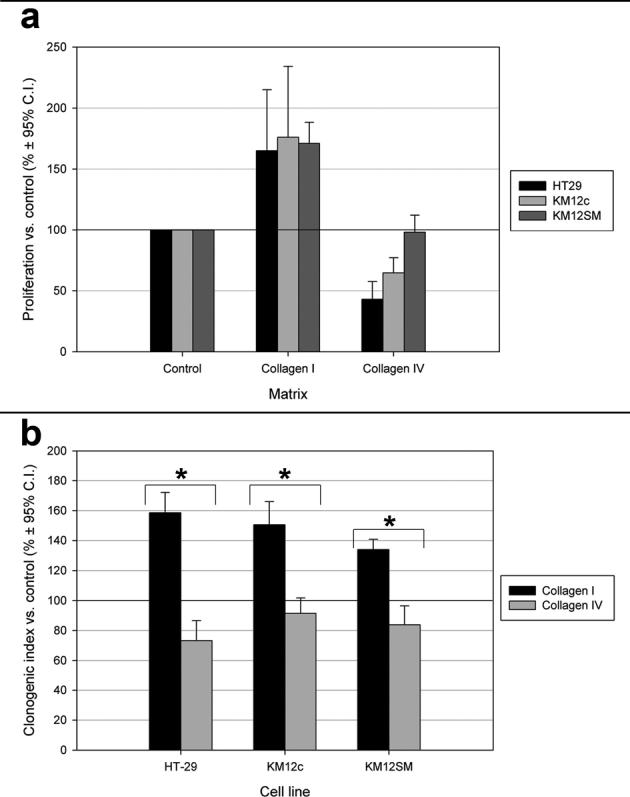
Influence of different matrices on colorectal adenocarcinoma cell proliferation and survival. (a) Collagen I was significantly growth promoting compared to collagen IV and tissue culture plastic (control) in all cell lines. The inhibitory effects of collagen IV on cell growth were not observed in KM12SM cells. (b) Colorectal carcinoma cell lines gain a significant survival advantage after FU exposure when cultured on collagen I compared to tissue culture plastic and collagen IV. *, p<0.05.
Collagens regulate colorectal carcinoma cell response to chemotherapeutic agents
Clonogenic assays were utilised to determine colorectal carcinoma cell survival after exposure to the chemotherapeutic agent FU. The clonogenic indices for the HT-29, KM12c and KM12SM cell lines grown on tissue culture plastic without treatment were 0.433, 0.50 and 0.52 respectively. A dose response curve to FU was determined for each cell line. The IC-50 used for subsequent experiments for each cell line were: HT-29, 1 μg/ml for 56 hours; KM12c, 0.125 μg/ml for 48 hours; KM12SM, 0.25 μg/ml for 48 hours.
Clonogenic assays demonstrated a significant survival advantage for HT-29, KM12c and KM12SM cells when grown on collagen I compared to collagen IV following exposure to FU (Figure 3b). In the absence of FU the different matrix substrata had no significant effect on cell survival, with similar colony numbers and sizes observed. The survival benefits of collagen I to KM12SM cells, although still significant, were reduced in magnitude in comparison to HT-29 and KM12c cells.
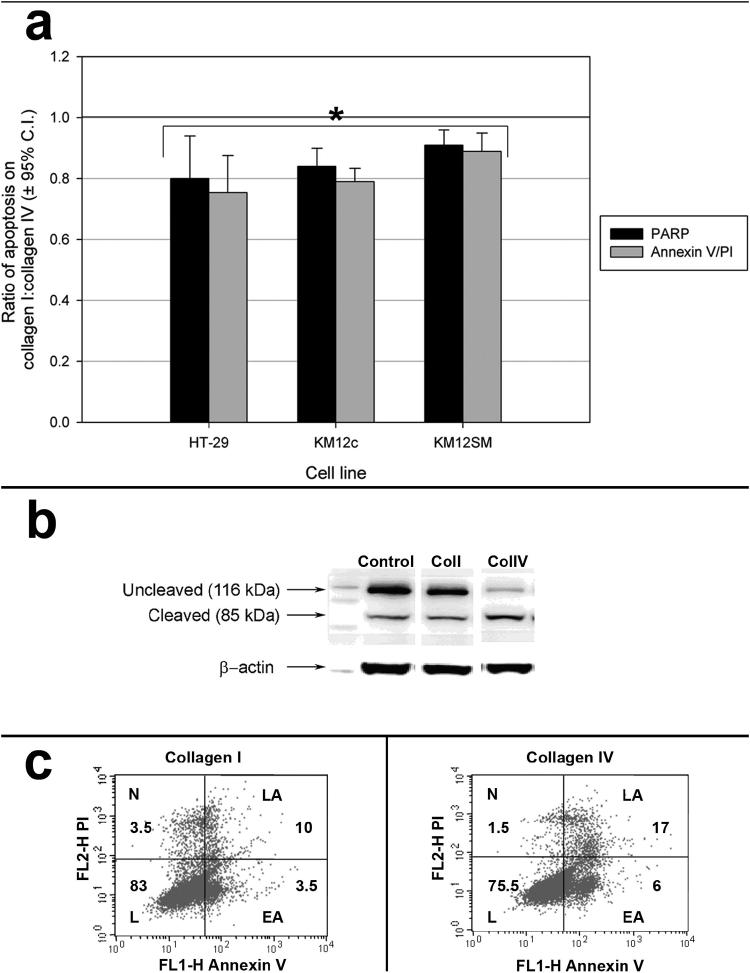
Collagen I enhances chemoresistance
Enhanced survival is the product of both changes in proliferation and apoptosis. The apoptotic response of colorectal carcinoma cell lines grown on different matrices to FU was assessed by analysing PARP cleavage. A dose response curve for each cell line was determined. For cells grown on collagen I and IV and exposed to FU, the percentage of uncleaved and cleaved PARP was then determined by densitometric analysis of immunoblots, and the ratio of cleaved/uncleaved PARP was compared.
There was a significant reduction in PARP cleavage in response to FU in each cell line when cultured on collagen I, compared to collagen IV (Figure 4a and b). The chemoprotective effect of type I collagen was greatest for HT-29 & KM12c cells where apoptosis was reduced by approximately 20% (p<0.05). Collagen I reduced the amount of PARP cleavage in the KM12SM line by approximately 10%.
Figure 4.
Reduction in apoptosis in response to FU with culture on collagen I compared to collagen IV. (a) Colorectal adenocarcinoma cell line apoptosis after exposure to chemotherapy (FU) is reduced by culture on collagen I compared with collagen IV, measured by both PARP cleavage and Annexin V/PI staining and FACS analysis. Results expressed as means ± 95% confidence intervals. *, p<0.05. (b) Representative immunoblot from which PARP cleavage was determined after densitometry. (c) Representative plot of FACS analysis for Annexin V (FL1-H, x-axis) and PI (FL2-H, y-axis) staining of FU treated HT-29 cells cultured on either collagen I (left) or collagen IV (right) with percentages indicated (L, live; N, necrotic; LA, late apoptotic; EA, early apoptotic).
Apoptosis was also determined after identical treatment regimens by FACS analysis following Annexin V/PI staining. This confirmed the results obtained using the clonogenic and PARP assays (Figures 4a and c).
Integrin neutralisation inhibits colorectal carcinoma cell adhesion and proliferation
After demonstrating that collagen I regulates colorectal carcinoma cell growth and survival, and that colorectal carcinoma cells express β1 and αv integrins in vivo, we examined the role of these integrins in mediating cell growth and survival. Immunoblotting demonstrated that KM12 cell lines differed in integrin expression depending on their metastatic potential (Data not shown). The highly metastatic KM12SM line expressed αv, β3 and β5 integrins at higher levels, especially when cultured on collagens, compared with the poorly metastatic KM12c line.
Neutralising antibodies were used to examine the contribution of integrin sub-units to colorectal carcinoma cell adhesion. β1 integrin neutralising antibody exerts a significant concentration dependent antagonism of cellular adhesion of between 40-60% for each cell line on both collagen I and IV (Data not shown). In contrast, αvβ3 and αvβ5 neutralising antibodies had minimal effect on adhesion.
β1 integrin neutralisation reduced proliferation for all cell lines in a concentration dependent manner, indicating that β1 integrin has an important role in regulating both adhesion and proliferation (Figure 5a). Although neutralisation of αvβ3 integrin had no effect on cell adhesion, it did significantly reduce proliferation of KM12SM cells on collagens I and IV (but not tissue culture plastic) in a concentration dependent manner. Previous work has shown reduced survival of colorectal carcinoma cell lines after treatment with S247 (highly potent and selective antagonist that inhibits binding of purified αvβ3 to vitronectin) [29]. There was no effect on proliferation of HT-29 and KM12c cells on any substratum (Figure 5b). A similar anti-proliferative effect was demonstrated on collagens with αvβ5 integrin neutralising antibody in both metastatic and poorly-metastatic cell lines (Figure 5c). The magnitude of this effect was greatest for KM12SM cell line.
Figure 5.
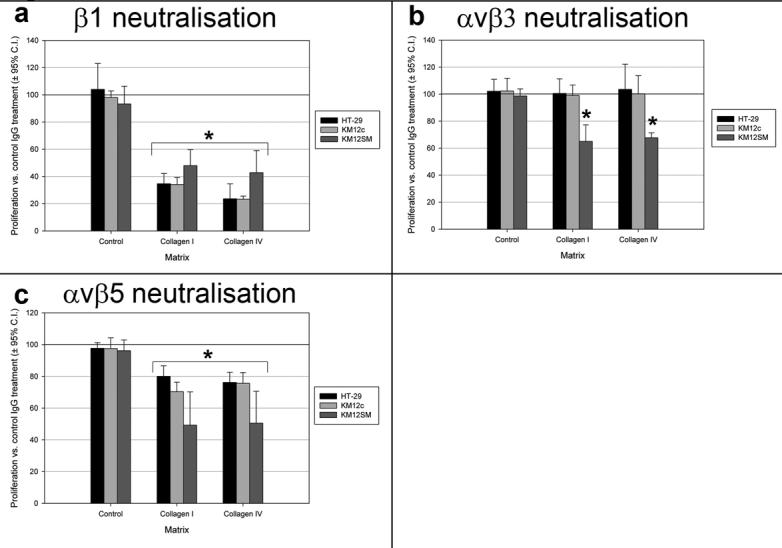
Effect of integrin neutralization on proliferation of colorectal adenocarcinoma cell lines on different sub-cellular matrices. (a) β1 integrin neutralizing antibody significantly reduced proliferation on collagen I and IV. (b) αvβ3 integrin neutralisation significantly reduced proliferation of KM12SM cells on collagen I and IV. (c) αvβ5 integrin neutralisation significantly reduced proliferation on collagen I and IV. Expressed as means ± 95% confidence intervals, compared with IgG isotype control. *, p<0.05 vs. control antibody.
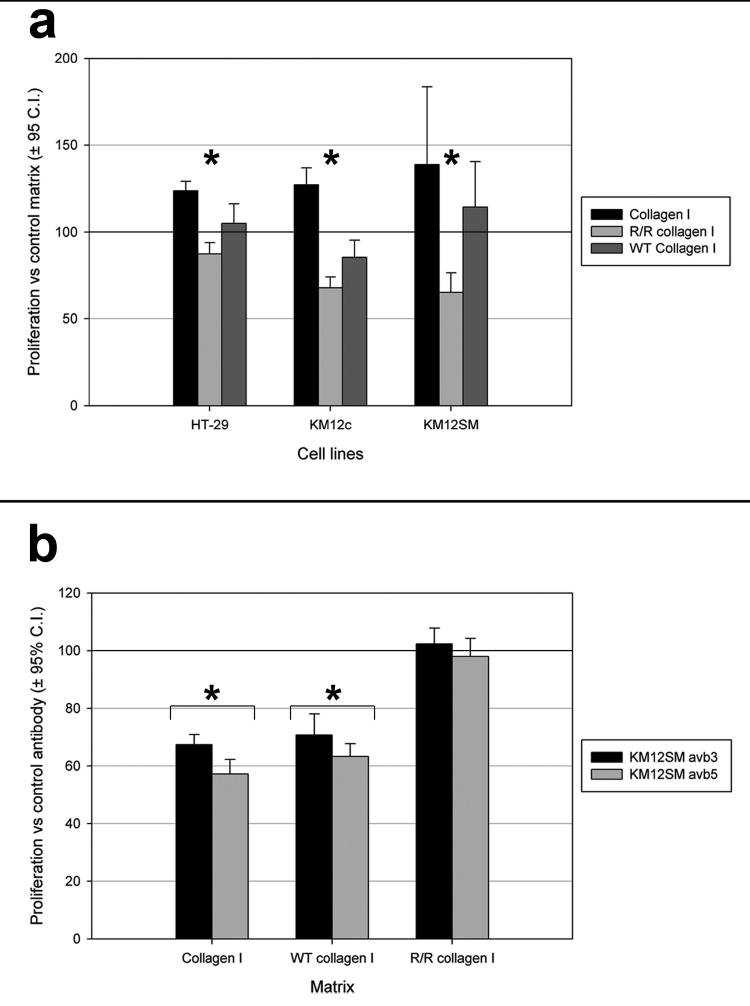
Prevention of collagen I breakdown attenuates the proliferative effect on colorectal carcinoma cells
The inhibition of collagen-dependent colorectal carcinoma cell proliferation by αvβ3 and αvβ5 blockade suggested that these integrins encountered stimulating ligands within the collagen. It has been reported that ligands for these integrins are hidden in intact collagen but revealed by collagen degradation [15, 16].
We used MMP resistant type I r/r collagen as a dominant negative ligand to determine whether exposure of cryptic αv binding epitopes mediates the growth promoting effect of collagen I. Cell lines cultured on type I r/r collagen proliferated less compared with cells cultured on wild-type and proprietary collagen type I (Figure 6a). Proliferation of KM12SM cells was most inhibited by culture on type I r/r collagen.
Figure 6.
Influence of αv integrin neutralization on colorectal adenocarcinoma cell line proliferation on wild type and r/r type I collagens. (a) Proliferation of colorectal adenocarcinoma cell lines was reduced when grown on r/r collagen I compared to proprietary (Collagen I) and wild-type (WT) collagen I. Expressed as means ± 95% confidence intervals, compared with culture on tissue culture plastic. *, p<0.05 r/r collagen I vs. WT and propriety collagen I. (b) αvβ3 and αvβ5 neutralisation significantly reduced KM12SM proliferation on proprietary and wild type collagen I but not when cultured on r/r collagen I. Expressed as means ± 95% confidence intervals, compared with IgG control. *, p<0.05.
This suggested that colorectal carcinoma cells degrade matrix to reveal αv integrin ligands as they adopt a more aggressive metastatic phenotype, in turn promoting their own growth. To examine this possibility proliferation assays were performed using integrin neutralisation on the different types of collagen I. Antibodies to the αv subunit had no influence on KM12SM proliferation on type I r/r collagen. In contrast this antibody significantly reduced proliferation of KM12SM cells on both the wild type control and proprietary collagen I (Figure 6b).
Discussion
These studies provide clear support for the importance of a desmoplastic reaction in the development, growth and chemoresistance of hepatic colorectal carcinoma metastases. Activated liver myofibroblasts present in these areas are a likely source of these collagens. Colorectal carcinoma cell lines derive a growth and survival advantage, and increased chemoresistance, as a result of contact with collagen I, a major component of this desmoplastic reaction. There is clear evidence that colorectal carcinoma integrin expression (specifically αv) and turnover of collagen I enhance growth.
Resected hepatic colorectal carcinoma metastases showed a desmoplastic reaction, consisting predominantly of collagen I forming discrete fibrils throughout the stroma. Interestingly this was often at its most dense in poorly differentiated areas. Colorectal carcinoma cells may provoke an inflammatory reaction within the liver [30], which initiates the hepatic wound healing response, with transdifferentiation of hepatic stellate cells into fibrogenic hepatic myofibroblasts. In vitro studies have suggested that interactions between colorectal carcinoma cells and stromal cells contribute to the development of primary colorectal cancers [31], and studies using animal models suggest that redistribution and expansion of myofibroblasts play an important role in the formation of established hepatic colorectal carcinoma metastases [32]. A desmoplastic response in these circumstances can be viewed as a fibrogenic response to inflammation. Successful colorectal carcinoma cell clones may have been selected for their ability to respond to components of the desmoplastic reaction with enhanced proliferation and survival. Indeed, studies in several different primary cancers [7, 33] suggest that this response may be a feature of aggressive neoplasms.
Previous studies investigating the apoptotic and proliferative responses of colorectal carcinoma cells to different matrix components have produced highly variable results [20, 21]. Our studies demonstrate that interstitial collagens consistently enhance colorectal carcinoma cell growth. Within the desmoplastic area in vivo the alteration in collagen IV distribution may also be important to the colorectal carcinoma phenotype. Our immunohistochemical staining showing dysregulated deposition was specific for the α1 & 2 chains of collagen IV. Type IV collagen possess many sequences on the α2, 3 and 6 chains that inhibit proliferation, migration, and MMP production in other cancer cells [34, 35]. It is of interest that the inhibitory effects of collagen IV on colorectal carcinoma cell proliferation in vitro depended on the metastatic potential of the clone. Collagen I, in contrast, was always growth promoting. Poorly metastatic clones show a reduction in proliferation when cultured on collagen IV but aggressively metastatic clones do not. Thus the ability to establish growth, overcoming inhibitory influences, within a virgin collagen IV-rich environment is a feature of aggressive malignancy.
Antagonisation of β1 integrin reduced both adhesion and proliferation for all colorectal carcinoma cell clones. However, β1 integrin blockade had a more marked effect on proliferation of the poorly metastatic colorectal carcinoma cells. In contrast, highly metastatic colorectal carcinoma cells express αv integrins at higher levels, and the inhibition of proliferation by αvβ3 and αvβ5 blockade demonstrated their key growth regulatory role. Integrin blockade of this nature is consistent with integrin gene-deletion in other systems, supporting true integrin antagonism [36]. Despite some debate existing about the agonist effects of integrin blocking agents with respect to angiogenesis, our data is consistent with true antagonism. The in vivo data demonstrating the differential expression of different integrin sub-types within hepatic colorectal carcinoma metastases supports our in vitro data, showing different patterns of integrin expression dependent on the malignant phenotype of colorectal carcinoma. Through enhanced expression of αv integrins the highly metastatic colorectal carcinoma cell clones may respond to a wider repertoire of matrix components and proliferate not only on collagen I but also collagen IV. The antibody used for staining of, and blockade of, β1 complexes does not differentiate between specific complexes, this data does demonstrate the importance of these complexes.
Degradation of both collagens IV and I reveals key growth regulatory RGD sequences that can be ligated by αv integrin sub-types. RGD sequences are expressed by degraded collagens, fibronectin and vitronectin [15, 16], and are important in the development of other malignancies [17, 37]. The effect of αv integrin blockade on colorectal carcinoma cells grown on collagens suggests that cells encountered these growth promoting ligands after collagen degradation, substantiated by the results obtained for cells grown on r/r collagen I combined with αv integrin blockade.
Exposure of colorectal carcinoma cells to collagen I is associated with a more invasive phenotype [38] and up-regulation of αvβ5 integrins may be associated with invasion [39]. There may, therefore, be complex interplay between matrix components and colorectal carcinoma, with integrins acting as intermediates. Alterations in integrin expression, conformation and adhesiveness are under the control of ras signalling pathways (inside-out signalling) [12, 40], but can also be influenced by the ECM (outside-in signalling) [1, 12]. Components of the desmoplastic matrix could modify the properties of integrins expressed by colorectal carcinoma cells. Indeed our immunoblotting studies support this by showing increased αv, β3 and β5 expression in more highly metastatic clones.
Candidate MMPs involved in the development of hepatic colorectal carcinoma metastases include MMPs-2, 7, 9 and 14 [41-43]. Our own studies using gelatin zymography (Data not shown) have shown that colorectal carcinoma cells produce active MMP-2 and 9, whilst hepatic myofibroblasts express higher amounts of MMP-2, 9 and 14 [44]. The importance of matrix remodelling by colorectal carcinoma-derived MMPs in generating matrix-derived proliferative signals to carcinoma cells is illustrated by our studies showing that MMP-resistant r/r collagen I provides a poorer proliferative stimulus than normal type I collagen. In r/r collagen I, collagenase-resistance prevents the initial cleavage [24] and unwinding that reveals αv binding epitopes. By demonstrating a reduction in proliferation in response to r/r collagen I we have further supported our αv integrin neutralising antibody experiments. This experimental approach is particularly robust as it obviates the need to identify or inhibit specific MMPs involved in the process of matrix remodelling.
Much data also exists for the role of non-integrin mediated cell-matrix interactions. CD44 has been implicated in promoting tumour metastasis growth but, reflecting the complexity of the extracellular milieu, this may be closely related to both integrin expression and MMP activity [45]. There may also be a role for discoidin domain receptors (DDR) which are known to be up regulated in colorectal carcinoma and are integrin-independent [46]. Collagen I and III are ligands for both DDR1 and DDR2 although only when collagen has an intact structure since no signalling is observed with heat-denatured collagen. Our finding that collagenase-resistant r/r collagen I provides a poor proliferative stimulus is inconsistent with DDRs playing a fundamental role since the 3 dimensional structure of collagen I is preserved in r/r collagen, suggesting that DDRs should still be active [47].
We have demonstrated evidence in vivo of a pronounced desmoplastic reaction in hepatic colorectal carcinoma metastases, and that colorectal carcinoma cells express β1 and αv integrins. We propose that the desmoplastic reaction is a dynamic phenomenon, with degradation of normal collagen IV-rich matrix occurring simultaneously with deposition of fibrillar collagens. The desmoplastic reaction arises as a consequence of the provocation of a classical wound healing response with activation of hepatic myofibroblasts by metastatic tumour cells. The resultant fibrotic neomatrix accumulation may provide a rapidly replenishing resource of neo-epitopes that could function as ligands for growth regulatory integrins expressed by colorectal carcinoma cells. This environment might select for colorectal carcinoma cells expressing the collagen I binding integrins. As the collagen IV-rich matrix in the space of Disse and the desmoplastic neomatrix rich in collagen I are degraded by metastatic cells, possibly in association with hepatic myofibroblasts, key growth regulatory neo-epitopes are revealed that act as ligands for αv integrins. This process may perpetuate the fibrotic response as hepatic myofibroblast proliferation is enhanced by ligation of αvβ3 integrin [48]. The differential integrin expression characterises a more aggressive malignant phenotype in which the growth inhibitory effects of collagen IV are lost. These data suggest that inhibiting desmoplastic matrix synthesis or turnover could offer new therapeutic options in patients with metastatic colorectal carcinoma.
Acknowledgments
Professor IJ Fidler (Chairman of Cancer Biology, MD Anderson centre, Houston, Texas, USA) for providing the KM12 cell line series and his invaluable advice; Professor S Krane (Emeritus Professor Harvard Medical School, Boston, USA) for the r/r collagen mouse and controls.
Grant support: JAC received a clinical training fellowship from the Royal College of Surgeons of England/ Society of Academic and Research surgeons; TAA and TJK were Wellcome Trust Clinical Training Fellows; JPI was a MRC Senior Clinical Fellow, and received a Wessex Cancer Trust Research Grant, and gratefully acknowledges support from the estates of V Lyons and J Clarke.
Abbreviations
- α-sma
α-smooth muscle actin
- DR
desmoplastic reaction
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- PARP
poly-ADP ribose polymerase
- PI
propidium iodide
Footnotes
Financial disclosures: None
No conflicts of interest exist
Statement of Clinical Relevance
Colorectal carcinoma (CRC) affects 5% of adults in the West. 50% of those who undergo a potentially curative resection die within 5 years, the majority due to metastatic disease. Surgery is the only available curative treatment for hepatic CRC metastases but only 10-20% of patients are operable at presentation. 70% of patients develop a recurrence within the first year after hepatectomy, and the 5 year survival is 26-49%. The need for targeted strategies is clear, and an understanding of the biological behaviour of metastatic CRC in the liver is essential to inform their development.
The desmoplastic reaction associated with invasive malignancy is characterised by an accumulation of fibrillar collagens. In primary malignancy these changes benefit the tumour. To date, little is known about this in metastatic disease.
Our studies demonstrate the importance of a desmoplastic reaction to hepatic CRC metastases. Contact with collagen I, a major component of this desmoplastic reaction, gives CRC cells a growth and survival advantage, and increased chemoresistance. Expression of integrins by colorectal carcinoma and turnover of collagen I enhances growth. This suggests that targeting the integrin-matrix interactions in hepatic colorectal carcinoma metastases may present a specific therapeutic opportunity.
Reference List
- 1.Radisky D, Muschler J, Bissell MJ. Order and disorder: the role of extracellular matrix in epithelial cancer. Cancer Invest. 2002;20:139–53. doi: 10.1081/cnv-120000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodey B, Bodey B, Jr., Siegel SE, Kaiser HE. Prognostic significance of matrix metalloproteinase expression in colorectal carcinomas. In Vivo. 2000;14:659–66. [PubMed] [Google Scholar]
- 3.Myers RE, Balshem AM, Wolf TA, Ross EA, Millner L. Screening for colorectal neoplasia: physicians' adherence to complete diagnostic evaluation. Am J Public Health. 1993;83:1620–2. doi: 10.2105/ajph.83.11.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardingham JE, Kotasek D, Sage RE, Eaton MC, Pascoe VH, Dobrovic A. Detection of circulating tumor cells in colorectal cancer by immunobead-PCR is a sensitive prognostic marker for relapse of disease. Mol Med. 1995;1:789–94. [PMC free article] [PubMed] [Google Scholar]
- 5.Ohtani H. Stromal reaction in cancer tissue: pathophysiologic significance of the expression of matrix-degrading enzymes in relation to matrix turnover and immune/inflammatory reactions. Pathol Int. 1998;48:1–9. doi: 10.1111/j.1440-1827.1998.tb03820.x. [DOI] [PubMed] [Google Scholar]
- 6.De WO, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–47. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 7.Meng L, Zhou J, Sasano H, Suzuki T, Zeitoun KM, Bulun SE. Tumor necrosis factor alpha and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor gamma: mechanism of desmoplastic reaction. Cancer Res. 2001;61:2250–5. [PubMed] [Google Scholar]
- 8.Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–37. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 9.Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–8. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 10.Dingemans KP, Zeeman-Boeschoten IM, Keep RF, Das PK. Transplantation of colon carcinoma into granulation tissue induces an invasive morphotype. Int J Cancer. 1993;54:1010–6. doi: 10.1002/ijc.2910540625. [DOI] [PubMed] [Google Scholar]
- 11.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 12.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339(Pt 3):481–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 14.Jokinen J, Dadu E, Nykvist P, et al. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem. 2004;279:31956–63. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- 15.Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–31. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Rodriguez D, Petitclerc E, et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–79. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petitclerc E, Stromblad S, von Schalscha TL, et al. Integrin alpha(v)beta3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res. 1999;59:2724–30. [PubMed] [Google Scholar]
- 18.Sawai H, Funahashi H, Yamamoto M, et al. Interleukin-1alpha enhances integrin alpha(6)beta(1) expression and metastatic capability of human pancreatic cancer. Oncology. 2003;65:167–73. doi: 10.1159/000072343. [DOI] [PubMed] [Google Scholar]
- 19.Sis B, Sarioglu S, Sokmen S, Sakar M, Kupelioglu A, Fuzun M. Desmoplasia measured by computer assisted image analysis: an independent prognostic marker in colorectal carcinoma. J Clin Pathol. 2005;58:32–8. doi: 10.1136/jcp.2004.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zvibel I, Halpern Z, Papa M. Extracellular matrix modulates expression of growth factors and growth-factor receptors in liver-colonizing colon-cancer cell lines. Int J Cancer. 1998;77:295–301. doi: 10.1002/(sici)1097-0215(19980717)77:2<295::aid-ijc20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Kouniavsky G, Khaikin M, Zvibel I, et al. Stromal extracellular matrix reduces chemotherapy-induced apoptosis in colon cancer cell lines. Clin Exp Metastasis. 2002;19:55–60. doi: 10.1023/a:1013880326925. [DOI] [PubMed] [Google Scholar]
- 22.Price JE, Daniels LM, Campbell DE, Giavazzi R. Organ distribution of experimental metastases of a human colorectal carcinoma injected in nude mice. Clin Exp Metastasis. 1989;7:55–68. doi: 10.1007/BF02057181. [DOI] [PubMed] [Google Scholar]
- 23.Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–71. [PubMed] [Google Scholar]
- 24.Wu H, Byrne MH, Stacey A, et al. Generation of collagenase-resistant collagen by site-directed mutagenesis of murine pro alpha 1(I) collagen gene. Proc Natl Acad Sci U S A. 1990;87:5888–92. doi: 10.1073/pnas.87.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cawston TE, Barrett AJ. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979;99:340–5. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- 26.Iredale JP, Benyon RC, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–49. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shek FW, Benyon RC, Walker FM, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–98. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karadag A, Fedarko NS, Fisher LW. Dentin matrix protein 1 enhances invasion potential of colon cancer cells by bridging matrix metalloproteinase-9 to integrins and CD44. Cancer Res. 2005;65:11545–52. doi: 10.1158/0008-5472.CAN-05-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinmuth N, Liu W, Ahmad SA, et al. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–87. [PubMed] [Google Scholar]
- 30.Yoong KF, Afford SC, Randhawa S, Hubscher SG, Adams DH. Fas/Fas ligand interaction in human colorectal hepatic metastases: A mechanism of hepatocyte destruction to facilitate local tumor invasion. Am J Pathol. 1999;154:693–703. doi: 10.1016/S0002-9440(10)65316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauptmann S, Siegert A, Berger S, et al. Regulation of cell growth and the expression of extracellular matrix proteins in colorectal adenocarcinoma: a fibroblast-tumor cell coculture model to study tumor-host interactions in vitro. Eur J Cell Biol. 2003;82:1–8. doi: 10.1078/0171-9335-00283. [DOI] [PubMed] [Google Scholar]
- 32.Higashi N, Ishii H, Fujiwara T, Morimoto-Tomita M, Irimura T. Redistribution of fibroblasts and macrophages as micrometastases develop into established liver metastases. Clin Exp Metastasis. 2002;19:631–8. doi: 10.1023/a:1020946300690. [DOI] [PubMed] [Google Scholar]
- 33.Buchholz M, Biebl A, Neesse A, et al. SERPINE2 (protease nexin I) promotes extracellular matrix production and local invasion of pancreatic tumors in vivo. Cancer Res. 2003;63:4945–51. [PubMed] [Google Scholar]
- 34.Petitclerc E, Boutaud A, Prestayko A, et al. New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem. 2000;275:8051–61. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 35.Maeshima Y, Sudhakar A, Lively JC, et al. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science. 2002;295:140–3. doi: 10.1126/science.1065298. [DOI] [PubMed] [Google Scholar]
- 36.McHugh KP, Hodivala-Dilke K, Zheng MH, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–40. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar CC, Malkowski M, Yin Z, et al. Inhibition of angiogenesis and tumor growth by SCH221153, a dual alpha(v)beta3 and alpha(v)beta5 integrin receptor antagonist. Cancer Res. 2001;61:2232–8. [PubMed] [Google Scholar]
- 38.Brabletz T, Spaderna S, Kolb J, et al. Down-regulation of the homeodomain factor Cdx2 in colorectal cancer by collagen type I: an active role for the tumor environment in malignant tumor progression. Cancer Res. 2004;64:6973–7. doi: 10.1158/0008-5472.CAN-04-1132. [DOI] [PubMed] [Google Scholar]
- 39.Schramm K, Krause K, Bittroff-Leben A, Goldin-Lang P, Thiel E, Kreuser ED. Activated K-ras is involved in regulation of integrin expression in human colon carcinoma cells. Int J Cancer. 2000;87:155–64. doi: 10.1002/1097-0215(20000715)87:2<155::aid-ijc1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 40.Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–42. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng ZS, Shu WP, Cohen AM, Guillem JG. Matrix metalloproteinase-7 expression in colorectal cancer liver metastases: evidence for involvement of MMP-7 activation in human cancer metastases. Clin Cancer Res. 2002;8:144–8. [PubMed] [Google Scholar]
- 42.Mook OR, Van OC, Ackema EG, Van MF, Frederiks WM. In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem. 2003;51:821–9. doi: 10.1177/002215540305100613. [DOI] [PubMed] [Google Scholar]
- 43.Bendardaf R, Lamlum H, Vihinen P, Ristamaki R, Laine J, Pyrhonen S. Low collagenase-1 (MMP-1) and MT1-MMP expression levels are favourable survival markers in advanced colorectal carcinoma. Oncology. 2003;65:337–46. doi: 10.1159/000074647. [DOI] [PubMed] [Google Scholar]
- 44.Benyon RC, Hovell CJ, Da GM, Jones EH, Iredale JP, Arthur MJ. Progelatinase A is produced and activated by rat hepatic stellate cells and promotes their proliferation. Hepatology. 1999;30:977–86. doi: 10.1002/hep.510300431. [DOI] [PubMed] [Google Scholar]
- 45.Samanna V, Wei H, Ego-Osuala D, Chellaiah MA. Alpha-V-dependent outside-in signaling is required for the regulation of CD44 surface expression, MMP-2 secretion, and cell migration by osteopontin in human melanoma cells. Exp Cell Res. 2006;312:2214–30. doi: 10.1016/j.yexcr.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Shrivastava A, Radziejewski C, Campbell E, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 47.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhou X, Murphy FR, Gehdu N, Zhang J, Iredale JP, Benyon RC. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem. 2004;279:23996–4006. doi: 10.1074/jbc.M311668200. [DOI] [PubMed] [Google Scholar]