SUMMARY
The increasing availability of genomic and genetic tools to study olfaction—the sense of smell—has brought important new insights into how this chemosensory modality functions in different species. Newly sequenced mammalian genomes—from platypus to dog—have made it possible to infer how smell has evolved to suit the needs of a given species and how variation within a species may affect individual olfactory perception. This review will focus on recent advances in the genetics and genomics of mammalian smell, with a primary focus on rodents and humans.
Introduction
The ability to sense small molecules in the environment is an adaptation found in all living things from plants to humans. In animals, the chemical senses of smell and taste differ from the physical senses of vision, touch, and hearing in the diversity of possible stimuli that can be perceived to have a distinct taste or smell. Both small organic molecules and small proteins induce taste sensations. Volatile small molecule odorants as well as non-volatile proteins and non-volatile hydrocarbons all can induce olfactory sensations, depending on the species. Some animal species even perceive carbon dioxide [1].
Biomedical research in olfaction, which lagged behind investigation into vision in the last century, has experienced two recent phases of growth since the early 1990s. These growth phases correlate approximately with the discovery of the genes encoding the odorant receptors (ORs) by Buck and Axel in 1991 [2] and the subsequent completion of the sequencing of the human [3,4] and other mammalian genomes. The search for the receptors that mediate the detection of a large number of odorants was grounded in biochemical experiments in the mid-1980s that showed that odors stimulate the production of cyclic AMP (cAMP) via an olfactory-enriched adenylate cyclase [5,6]. Buck and Axel reasoned that odorants would be detected by a large family of G protein-coupled receptors (GPCRs), selectively expressed in the olfactory epithelium that would couple odor binding to the production of cAMP. These assumptions proved to be true and yielded not only a Nobel Prize to Buck and Axel for their discovery and subsequent characterization of the ORs but also a veritable boom of interest in tackling additional unsolved problems in the field of chemosensory perception. This review highlights recent discoveries concerning the olfactory organs, receptors, and ligands that mediate specific olfactory behaviors as well as detailing what comparative genomics has taught us about the sense of smell in diverse mammals.
Evolution of chemosensory receptor gene families in diverse mammalian species
The three most prominent genetic features of GPCRs that bind odors are the dramatic variation in the size of chemosensory receptor gene repertoires between species, the large number of pseudogenes in many species and especially humans, and the unparalleled genetic variability within species.
ORs were first identified in the rat [2], but subsequently annotated in a large number of mammalian species following genome sequencing and annotation. The size of the OR gene repertoire has been reported for the following mammals: opossum (1,518) [7], platypus (~700) [8], mouse (~1,500), human (~960), dog (~1,100), and rat (~1,500). The difference in the number of functional ORs between species is amplified by the differences in the ratio of pseudogenes, genes that have accumulated small deletions, point mutations, or frame shifts that prevent the expression of a functional OR protein. Among land mammals, humans have the highest pseudogene fraction with 51% (even excluding the large pseudogene family 7E), compared to 41% in chimpanzee, 30% in Old World monkeys, 15-20% in New World monkeys and lemurs, and around 20% in cattle, dog, rat, and mouse [9,10] (Table 1). The semi-aquatic platypus has a pseudogene fraction of 52% [8]. Marine mammals also have a high percentage of OR pseudogenes: Dall’s porpoises (78%), dwarf sperm whales (77%), minke whales (58%), and Steller’s sea lions (37%) [10]. The extremely high rate of OR pseudogenization in aquatic mammals may reflect the relative paucity of odor ligands encountered in such environments. The accumulation of OR pseudogenes in humans is presumed to be a consequence of relaxed purifying selection on many ORs. On the other hand it also has been shown that some ORs are adapting to human-specific odor perception requirements, as seen in signs of positive selection like human-specific subfamily extensions [11,12]. The ligands of the ORs that acquired a species-specific function in humans are not known but would be of interest to discover.
TABLE 1.
Comparative genomics of mammalian chemosensory receptor genes
| RECEPTOR TYPE | Where expressed (in mice) | # genes in humans | % pseudogenes in humans | # genes in mammals (range) | % pseudogenes in mammals (range) | Ligands |
|---|---|---|---|---|---|---|
| OR | OE (most OSNs)
SO (most OSNs) VNO (few OSNs) |
960 | 51 | 700-1,518 | 15-78 | Diverse volatile odorants
|
| V1R | VNO (apical)
OE (few OSNs in humans) |
117 | 98 | 65-849 | 50-100 | Diverse volatile odorants
|
| V2R | VNO (basal)
GG (V2r83, most OSNs) |
20 | 100 | 10-283 | 48-100 | Diverse non-volatile pheromones
|
| TAAR | OE (few OSNs)
GG (few OSNs) |
6 | 0 | 3-22 | low | Volatile amines |
Recently, a second family of olfactory GPCRs known as trace amine-associate receptors (TAARs) were described and shown to respond to volatile amines present in urine [13]. Humans have six TAAR genes [13], but different tetrapod species have between three and 22 members of the TAAR gene family and some fish species have up to 109 TAAR genes [14]. The behavioral relevance of the TAARs as odorant receptors is not yet known, but based on the pharmacology of mouse TAARs they may be important for receiving social cues related to sexuality and fear.
Two additional and distinct families of GPCRs, unrelated to ORs and TAARs, were found to be expressed in the vomeronasal organ (VNO) of some mammals and are called V1Rs and V2Rs, for class 1 and class 2 vomeronasal receptors. Both V1R and V2R gene families are exceptionally variable in gene number among mammals [15-17]. The V1R gene family has no functional member in the chimpanzee genome, whereas there are 270 in the platypus genome. Humans have two functional V1Rs but 115 pseudogenes. Dogs have only eight intact V1R genes and 54 pseudogenes, whereas the platypus has 579 V1R pseudogenes in addition to the 270 intact V1Rs [15,16]. The differences in size of the V1R repertoire are considered to be the largest variation in gene family size in all mammalian gene families [18]. V2R genes have completely degenerated in humans, chimpanzee, macaque, cow, and dog. In these species only V2R pseudogenes are found. In contrast, the opossum, the mouse, the rat, and the platypus have between 79 and 121 intact V2R genes and between 79 and 158 pseudogenes [16,17] (Table 1). The details of what ligands activate V1Rs and V2Rs have not yet been fully worked out, but the limited information available suggests that V1Rs detect volatile odorants and V2Rs are specialized for non-volatile protein ligands [19]. The underlying variability in VR number across species may reflect the relative importance of pheromone communication for a given mammal.
Genetic variability in humans and impact on the sense of smell
Beyond these differences in chemosensory GPCRs between species, large genetic differences between individuals of the same species have been found. Most work on intra-species variability has been performed in humans. However, it has been suggested that the considerable variability found for canine OR genes correlates with differences in olfactory abilities between individual dogs [20]. In mice, strain-specific sensitivity to isovaleric acid, the odor of sweaty socks, was mapped to a small genetic region [21], which was subsequently found to harbor a cluster of ORs [22]. It remains to be formally proven that some or all of these ORs are deleted or mutated in the isovaleric acid insensitive strains.
In humans, large genetic variability in OR genes has been shown on the sequence level, resulting in co-segregating pseudogenes [23], as well as on the structural level, resulting in copy-number variation that alters the number of copies of a given OR [24-26]. This discovery of the genetic variability in odor receptors suggested that inter-individual differences in sensitivity to odors [27] are at least partially caused by genetic variability. This hypothesis was recently confirmed for two odors, androstenone and isovaleric acid. Sensitivity to both odors was previously suggested to be genetically determined [28,29]. Genetic association studies coupled to receptor-ligand analysis showed that polymorphisms in OR7D4 [30] and OR11H7P [31] influence the sensitivity to androstenone and isovaleric acid, respectively. In parallel with targeted phenotype-genotype associations two groups have reported unbiased whole genome approaches that assigned genomic regions to specific aspects of odor perception [32,33]. These screens turned up genome regions devoid of OR genes [33] that presumably harbor genes affecting other aspects of peripheral olfactory function or more central aspects of odor perception.
Species-specific olfactory organs detecting different odorant stimuli
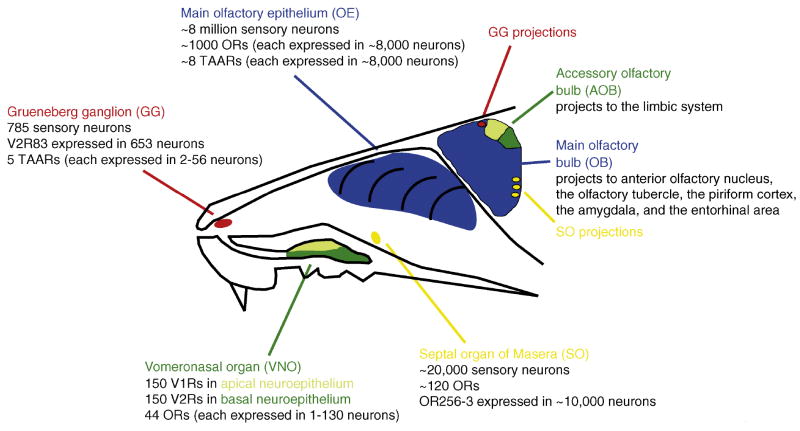
Mammals posses multiple organs for detecting odors: the olfactory epithelium (OE), the vomeronasal organ (VNO), the Grueneberg ganglion (GG), and the septal organ of Masera (SO) (reviewed in [34,35]). While rodents possess all four of these organs (Figure 1), some are missing in other mammals. There is increasingly strong data to support the idea that each organ has a specialized biological function, expresses specific chemosensory receptors and signal transduction components, and responds to chemically distinct stimuli (Figure 1; Table 1). The Grueneberg ganglion, which was only discovered in 1973, contains a small number of sensory neurons that were recently shown to mediate alarm pheromone detection in mice [36]. The septal organ of Masera, first described in 1943, is a patch of olfactory epithelium at the ventral base of the nasal septum that is found in a variety of mammals [37]. Recent work suggests that the septal organ is both broadly tuned to various volatile odors but also shows a unique sensitivity to mechanical stimulation [38]. The olfactory epithelium is largely devoted to detecting general odors but also detects volatile pheromones, while the opposite is true for the VNO.
Figure 1.
Schematic of the rodent olfactory system, indicating all four olfactory sensory organs, the classes of chemosensory receptor they express, and a summary of gene expression in each. Neurons in the VNO that express ORs project to the very rostral tip of the AOB (not indicated on the figure).
Just as there is some blurring of the chemical specificity of each of these organs, there is not strict segregation of a specific type of chemosensory receptor in each olfactory organ. The receptors that bind odors in most OSNs in the olfactory epithelium and in the septal organ are ORs. In the VNO, V1Rs and V2Rs are the principal receptors. The Grueneberg ganglion expresses V2R82 in the majority of sensory neurons, but TAARs are expressed in a subset of the neurons in the olfactory epithelium and the Grueneberg ganglion. Some neurons in the mouse VNO express ORs [39] and in humans, who lack a functional VNO, a V1R gene is expressed in the olfactory epithelium [40].
Not all sensory neurons in the olfactory epithelium appear to express olfactory GPCRs. Classic anatomical experiments indicated that the rodent olfactory epithelium has a specialized set of neurons with a characteristic projection pattern to olfactory bulb glomeruli known as the “necklace glomeruli,” so named for their appearance of linear beads on a string. Recent work from two groups suggests that these neurons express GC-D, a receptor guanylate cyclase [1,41], as well as carbonic anhydrase [1]. Behavior genetic and physiological experiments demonstrated that these GC-D neurons are extremely sensitive to carbon dioxide [1] but also required for responses to peptide hormones and urine components [41].
Genetic analysis of the mouse VNO confirms the behavioral importance of this organ in the social and reproductive biology of rodents. Targeted deletion of a large cluster of V1Rs produced mice with deficits in maternal and sexual behavior [42]. Mice lacking the TRPC2 ion channel, the transduction channel necessary for signaling of both V1Rs and V2Rs, show severe defects in male [43,44] and female sexual behavior [45]. In contrast, humans and other primates possess only a vestigial VNO by anatomical criteria [46]. The VNO is also vestigial by genetic criteria, as TRPC2 is nonfunctional in humans and other Old World primates [47]. Not only is the VNO vestigial in humans, but humans also lack other innovations in chemosensory signaling that mice retain. For instance, small secreted proteins in rodent urine called MUPs and in tear secretions from the extraorbital lacrimal gland called ESPs [48] are likely to act as pheromone signals in mice [49]. Humans entirely lack both the MUP and ESP gene families. Whether humans have largely lost the ability to communicate social cues with chemical signals typically received by the VNO or whether we use our olfactory epithelium for that purpose remains an open question.
Future prospects
The increasing scale and rapidly decreasing cost of genome sequencing opens up many new opportunities in olfaction. For instance, the advent of personal genome sequencing may make it feasible to correlate a given human’s olfactory capacities with variation in her or his genome. Future sequenced genomes of mammals with interesting ecology and lifestyles—naked mole rats, bats, elephants, and others would be of interest—will provide further insights into selective pressures that shape the olfactory subgenome. As approaches to express odorant receptors in heterologous cells continue to match specific ORs with their ligands [50], it will become possible to do large-scale analysis relating the structure of an odorant receptor to its functional properties. Examining smell at angstrom resolution by solving crystal structures of ORs in various liganded states seems plausible now that several related non-olfactory GPCR structures have been solved [51]. Aside from the curiosities of this sensory modality, all of this ferment in the field of smell may ultimately provide translationally important advances in disease biomarkers, including early diagnosis of neurodegenerative and psychiatric disease [52] and the detection of specific disease states by changes in body odor [53].
Acknowledgments
Work in the authors’ laboratory is supported by the National Institutes of Health (DC006711, DC009600), the Howard Hughes Medical Institute, and funded in part by a grant to R. Axel and L.B.V. from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative. A.K. is supported by a 2008 Branco Weiss Society in Science Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND ANNOTATIONS
- 1.Hu J, Zhong C, Ding C, Chi Q, W A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 2.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 3.Zozulya S, Echeverri F, Nguyen T. The human olfactory receptor repertoire. Genome Biol. 2001;2:RESEARCH0018. doi: 10.1186/gb-2001-2-6-research0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glusman G, Yanai I, Rubin I, Lancet D. The complete human olfactory subgenome. Genome Res. 2001;11:685–702. doi: 10.1101/gr.171001. [DOI] [PubMed] [Google Scholar]
- 5.Pace U, Hanski E, Salomon Y, Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985;316:255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- 6.Sklar PB, Anholt RR, Snyder SH. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986;261:15538–15543. [PubMed] [Google Scholar]
- 7.Aloni R, Olender T, Lancet D. Ancient genomic architecture for mammalian olfactory receptor clusters. Genome Biol. 2006;7:R88. doi: 10.1186/gb-2006-7-10-r88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, Grutzner F, Belov K, Miller W, Clarke L, Chinwalla AT, et al. Genome analysis of the platypus reveals unique signatures of evolution. Nature. 2008;453:175–183. doi: 10.1038/nature06936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouquier S, Giorgi D. Olfactory receptor gene repertoires in mammals. Mutat Res. 2007;616:95–102. doi: 10.1016/j.mrfmmm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Kishida T, Kubota S, Shirayama Y, Fukami H. The olfactory receptor gene repertoires in secondary-adapted marine vertebrates: evidence for reduction of the functional proportions in cetaceans. Biol Lett. 2007;3:428–430. doi: 10.1098/rsbl.2007.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilad Y, Man O, Glusman G. A comparison of the human and chimpanzee olfactory receptor gene repertoires. Genome Res. 2005;15:224–230. doi: 10.1101/gr.2846405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Go Y, Niimura Y. Similar numbers but different repertoires of olfactory receptor genes in humans and chimpanzees. Mol Biol Evol. 2008;25:1897–1907. doi: 10.1093/molbev/msn135. [DOI] [PubMed] [Google Scholar]
- ••13.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. The authors’ search for GPCRs other than ORs that are expressed in mouse OSNs resulted in the discovery of a second class of candidate odorant receptors, the trace amine-associated receptors (TAARs). The TAAR gene family has 15 members in mice and 6 in humans. In the mouse, individual TAAR genes are expressed in non-overlapping subsets of neurons in the olfactory epithelium, like ORs. Some TAARs were shown to be activated by volatile amines found in mouse urine.
- 14.Hashiguchi Y, Nishida M. Evolution of trace amine associated receptor (TAAR) gene family in vertebrates: lineage-specific expansions and degradations of a second class of vertebrate chemosensory receptors expressed in the olfactory epithelium. Mol Biol Evol. 2007;24:2099–2107. doi: 10.1093/molbev/msm140. [DOI] [PubMed] [Google Scholar]
- 15.Young JM, Kambere M, Trask BJ, Lane RP. Divergent V1R repertoires in five species: Amplification in rodents, decimation in primates, and a surprisingly small repertoire in dogs. Genome Res. 2005;15:231–240. doi: 10.1101/gr.3339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grus WE, Shi P, Zhang J. Largest vertebrate vomeronasal type 1 receptor gene repertoire in the semiaquatic platypus. Mol Biol Evol. 2007;24:2153–2157. doi: 10.1093/molbev/msm157. [DOI] [PubMed] [Google Scholar]
- 17.Young JM, Trask BJ. V2R gene families degenerated in primates, dog and cow, but expanded in opossum. Trends Genet. 2007;23:212–215. doi: 10.1016/j.tig.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Grus WE, Shi P, Zhang YP, Zhang J. Dramatic variation of the vomeronasal pheromone receptor gene repertoire among five orders of placental and marsupial mammals. Proc Natl Acad Sci U S A. 2005;102:5767–5772. doi: 10.1073/pnas.0501589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Touhara K. Molecular biology of peptide pheromone production and reception in mice. Adv Genet. 2007;59:147–171. doi: 10.1016/S0065-2660(07)59006-1. [DOI] [PubMed] [Google Scholar]
- 20.Lesniak A, Walczak M, Jezierski T, Sacharczuk M, Gawkowski M, Jaszczak K. Canine olfactory receptor gene polymorphism and its relation to odor detection performance by sniffer dogs. J Hered. 2008;99:518–527. doi: 10.1093/jhered/esn057. [DOI] [PubMed] [Google Scholar]
- 21.Griff IC, Reed RR. The genetic basis for specific anosmia to isovaleric acid in the mouse. Cell. 1995;83:407–414. doi: 10.1016/0092-8674(95)90118-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- 23.Menashe I, Man O, Lancet D, Gilad Y. Different noses for different people. Nat Genet. 2003;34:143–144. doi: 10.1038/ng1160. [DOI] [PubMed] [Google Scholar]
- ••24.Nozawa M, Kawahara Y, Nei M. Genomic drift and copy number variation of sensory receptor genes in humans. Proc Natl Acad Sci U S A. 2007;104:20421–20426. doi: 10.1073/pnas.0709956104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••25.Young JM, Endicott RM, Parghi SS, Walker M, Kidd JM, Trask BJ. Extensive copy-number variation of the human olfactory receptor gene family. Am J Hum Genet. 2008;83:228–242. doi: 10.1016/j.ajhg.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••26.Hasin Y, Olender T, Khen M, Gonzaga-Jauregui C, Kim PM, Eckehart Urban A, Snyder M, Gerstein MB, Lancet D, Korbel JO. High-resolution copy-number variation map reflects human olfactory receptor diversity and evolution. PLoS Genet. 2008 doi: 10.1371/journal.pgen.1000249. in press. These three detailed studies of copy-number variation (CNV) in the human OR repertoire collectively demonstrate that intact OR genes, OR pseudogenes, and V1R genes are all enriched in copy-number variable regions in the human genome. Furthermore, the authors identify fifteen OR genes that are deleted in some individuals and three OR genes that are duplicated in a subpopulation, as well as a novel hybrid OR that results from the joining of two ancestral OR genes.
- 27.Amoore JE. Specific anosmia and the concept of primary odors. D. Reidel Publishing Company. Chemical Senses and Flavor 2. 1977:267–281. [Google Scholar]
- 28.Whissell-Buechy D, Amoore JE. Odour-blindness to musk: simple recessive inheritance. Nature. 1973;242:271–273. doi: 10.1038/242271a0. [DOI] [PubMed] [Google Scholar]
- 29.Wysocki CJ, Beauchamp GK. Ability to smell androstenone is genetically determined. Proc Natl Acad Sci U S A. 1984;81:4899–4902. doi: 10.1073/pnas.81.15.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••30.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449:468–472. doi: 10.1038/nature06162. This study shows that naturally occurring variation in the OR7D4 gene correlates with differences in the perception of androstenone and androstadienone, the two known ligands of OR7D4. A common variant of OR7D4 contains two single nucleotide polymorphisms that result in two amino acid substitutions that severely impair function in vitro. Subjects with one or two non-functional alleles of OR7D4 are less sensitive to androstenone and androstadienone.
- ••31.Menashe I, Abaffy T, Hasin Y, Goshen S, Yahalom V, Luetje CW, Lancet D. Genetic elucidation of human hyperosmia to isovaleric acid. PLoS Biol. 2007;5:e284. doi: 10.1371/journal.pbio.0050284. The authors test the association between the segregating pseudogene genotypes of 43 ORs and odor detection thresholds to four odors. They find an association between single nucleotide polymorphisms in a receptor that is activated by isovaleric acid, OR11H7P, and the sensitivity to isovaleric acid.
- 32.Pinto JM, Thanaviratananich S, Hayes MG, Naclerio RM, Ober C. A genome-wide screen for hyposmia susceptibility Loci. Chem Senses. 2008;33:319–329. doi: 10.1093/chemse/bjm092. [DOI] [PubMed] [Google Scholar]
- ••33.Knaapila A, Keskitalo K, Kallela M, Wessman M, Sammalisto S, Hiekkalinna T, Palotie A, Peltonen L, Tuorila H, Perola M. Genetic component of identification, intensity and pleasantness of odours: a Finnish family study. Eur J Hum Genet. 2007 doi: 10.1038/sj.ejhg.5201804. In a whole-genome scan to identify loci affecting the perception of twelve familiar odors such as lemon, chocolate, rose or onion in 26 families, the authors found a heritable component of several perceptual traits. Linkage evidence for cinnamon pleasantness and the intensity of paint thinner odor is demonstrated. Interestingly, the identified loci do not contain OR genes, and may harbor other genes that affect the central processing of odor information.
- 34.Breer H, Fleischer J, Strotmann J. The sense of smell: multiple olfactory subsystems. Cell Mol Life Sci. 2006;63:1465–1475. doi: 10.1007/s00018-006-6108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma M. Encoding olfactory signals via multiple chemosensory systems. Crit Rev Biochem Mol Biol. 2007;42:463–480. doi: 10.1080/10409230701693359. [DOI] [PubMed] [Google Scholar]
- ••36.Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321:1092–1095. doi: 10.1126/science.1160770. This paper provides persuasive behavioral evidence that the Grueneberg ganglion functions to detect alarm pheromone produced by stressed mice. While intact mice freeze when they encounter alarm pheromone substances, mice whose Grueneberg ganglion has been surgically ablated are oblivious to the alarm substance.
- 37.Tian H, Ma M. Molecular organization of the olfactory septal organ. J Neurosci. 2004;24:8383–8390. doi: 10.1523/JNEUROSCI.2222-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci. 2007;10:348–354. doi: 10.1038/nn1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levai O, Feistel T, Breer H, Strotmann J. Cells in the vomeronasal organ express odorant receptors but project to the accessory olfactory bulb. J Comp Neurol. 2006;498:476–490. doi: 10.1002/cne.21067. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez I, Greer CA, Mok MY, Mombaerts P. A putative pheromone receptor gene expressed in human olfactory mucosa. Nat Genet. 2000;26:18–19. doi: 10.1038/79124. [DOI] [PubMed] [Google Scholar]
- 41.Leinders-Zufall T, Cockerham R, Michalakis S, Biel M, Garbers D, Reed R, Zufall F, Munger S. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci U S A. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- 43.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 44.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 46.Trotier D, Eloit C, Wassef M, Talmain G, Bensimon JL, Doving KB, Ferrand J. The vomeronasal cavity in adult humans. Chem Senses. 2000;25:369–380. doi: 10.1093/chemse/25.4.369. [DOI] [PubMed] [Google Scholar]
- 47.Liman ER, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci U S A. 2003;100:3328–3332. doi: 10.1073/pnas.0636123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••48.Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. This paper provides persuasive evidence that MUPs, small proteins produced in urine, directly mediate aggressive behavior in mice, in the absence of small molecule pheromones.
- ••49.Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. These authors use elegant biochemical purification to identify male-specific peptides secreted from tear glands that directly activate female VNO neurons.
- 50.Zhuang H, Matsunami H. Synergism of accessory factors in functional expression of mammalian odorant receptors. J Biol Chem. 2007;282:15284–15293. doi: 10.1074/jbc.M700386200. [DOI] [PubMed] [Google Scholar]
- 51.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 52.Pantelis C, Brewer W. Olfactory Impairment in Neuropsychiatric Disorders. In: Brewer W, Castle D, Pantelis C, editors. Olfaction and the Brain. Cambridge University Press; Cambridge: 2006. [Google Scholar]
- 53.Willis CM, Church SM, Guest CM, Cook WA, McCarthy N, Bransbury AJ, Church MR, Church JC. Olfactory detection of human bladder cancer by dogs: proof of principle study. Bmj. 2004;329:712. doi: 10.1136/bmj.329.7468.712. [DOI] [PMC free article] [PubMed] [Google Scholar]