Abstract
Previous studies have supported the hypothesis that macromolecular synthesis occurs in the brain during sleep as a response to prior waking activities and that prostaglandin D2 (PGD2) is an endogenous sleep substance whose effects are dependent on adenosine A2a receptor-mediated signaling. We compared gene expression in the cerebral cortex, basal forebrain and hypothalamus during PGD2-induced and adenosinergically-induced sleep to results from our previously-published study of recovery sleep (RS) after sleep deprivation (SD). Immediate early gene (IEG) expression in the cortex during sleep induced by PGD2- or by the selective adenosine A2a agonist CGS21680 showed limited similarity to that observed during RS while, in the basal forebrain and hypothalamus, widespread activation of IEGs not seen during RS occurred. In all three brain regions, PGD2 and CGS21680 reduced the expression of arc, a transcript whose expression is elevated during SD. Using GeneChips®, the majority of genes induced by either PGD2 or CGS21680 were induced by both, suggesting activation of the same pathways. However, gene expression induced in the brain after PGD2 or CGS21680 treatment was distinct from that described during RS after SD and apparently involves glial cell gene activation and signaling pathways in neural-immune interactions.
Keywords: Taqman® analysis, qPCR, Gene Chip, adenosine, cytokines, neural-immune
A number of chemical agents are known to induce sleep or a sleep-like state, but questions regarding the similarity of this state to physiologically normal sleep invariably arise. For example, benzodiazepines are soporific in both humans and rats but also have distinctive effects on the electroencephalogram (EEG) during sleep, including dose-dependent increases in non-rapid eye movement sleep (NREMS) and, paradoxically, reductions in slow wave (0.5–4 Hz) activity (SWA) and increases in higher frequency EEG oscillations (Achermann and Borbely 1987). In contrast to synthetic compounds, a variety of studies have demonstrated that prostaglandin D2 (PGD2) is an endogenous sleep-promoting substance. Intra-cerebral administration of PGD2 induces sleep, especially slow wave sleep (SWS), in rats and monkeys (Onoe et al. 1988). Inhibition of the enzyme responsible for PGD2 synthesis, PGD synthase (PGDS), with selenium compounds administered either intracerebroventricularly (Matsumura et al. 1991) or intravenously (Takahata et al. 1993) markedly suppresses sleep and blockade of PGD2 receptors inhibits physiological sleep (Qu et al. 2006). The level of PGD2 in the cerebrospinal fluid (CSF) of rats undergoes significant modulation by time of day, with a daytime peak and a nighttime trough (Pandey et al. 1995). CSF levels of PGD2 in rats increase during sleep deprivation (SD) and tend to increase along with an increasing propensity toward sleep under normal conditions (Ram et al. 1997).
The site of action for PGD2 has been identified as a sleep promoting zone (PGD2-SZ) located on ventral surface of the rostral basal forebrain (BF) outside the brain parenchyma (Matsumura et al. 1994; Gerashchenko et al. 1998). A number of lines of evidence implicate signaling by the A2a-adenosine receptor within the brain in the sleep-promoting mechanism of PGD2 (Satoh et al. 1996). Administration of a selective A2a-adenosine agonist (CGS21680), but not the selective A1-adenosine agonist cyclohexyladenosine, markedly induces sleep when administered to the PGD2-SZ (Satoh et al. 1996). The SWS-promoting effect of PGD2 is inhibited by pretreatment with KF17837, a highly selective A2a-adenosine antagonist (Satoh et al. 1996) and is blunted in adenosine A2a receptor-deficient mice (Urade et al. 2003). It is therefore hypothesized that PGD2 is coupled to A2a-adenosinergic signaling via the brain parenchyma and that the PGD2-SZ plays an important role as an interface between these two systems.
Widespread changes in gene expression have been documented within the brain in conjunction with changes in behavioral states (Cirelli 2002; Cirelli et al. 2004). The expression of a number of genes is upregulated during recovery sleep (RS) after SD (Terao et al. 2003b; Terao et al. 2006). Pharmacological manipulations that induce sleep might also be expected to induce changes in gene expression and, indeed, both PGD2- (Scammell et al. 1998) and adenosinergically-induced sleep (Scammell et al. 2001) are associated with changes in the expression of the immediate early gene (IEG) c-fos. If PGD2 and adenosinergic signaling induce physiological sleep, administration of these compounds should have transcriptional effects similar to those of RS. Identification of the molecular responses common to RS and pharmacologically-induced sleep might provide insights into the biochemical and functional consequences of sleep. Therefore, in the current study, we tested the hypothesis that some transcriptional effects are common to natural and chemically-induced sleep using the quantitative real-time polymerase chain reaction (qPCR) to assess the degree to which PGD2- and CGS21680-induced changes in gene expression parallel those known to occur during RS (Terao et al. 2003b; Terao et al. 2006). To identify additional genes regulated by PGD2 and CGS21680, we used high density oligonucleotide arrays. We find that gene expression in the cerebral cortex (Cx) during PGD2- and CGS21680-induced sleep shows surprisingly limited similarity to that observed during RS, and that BF and hypothalamus (Hy) gene expression is similar to that observed during SD, probably due to activation of adenosine A2a receptors by the PGD2 and adenosine signaling pathways in these regions.
Materials and methods
Animal Surgery
The methods used in these experiments were similar to those used in previous studies (Matsumura et al. 1994; Satoh et al. 1996; Satoh et al. 1999; Mizoguchi et al. 2001). Under pentobarbital anesthesia (50 mg/kg), 18 male Wistar rats were instrumented for standard recording of the EEG and electromyogram (EMG) and implanted with a stainless steel cannula (0.35 mm OD) for drug infusions in a midline position, 1.1 mm anterior to bregma, to a depth of 7.8 mm below the dura (Scammell et al. 1998). The cannulae thus were directed at the subarachnoid space under the rostral BF, the area defined as a PGD2-sensitive sleep-promoting zone.
Sleep Recording
After surgery, each rat was allowed at least a 7-day recovery period before being placed in the experimental chamber where EEG/EMG recording and continuous infusion of sterile physiological saline into the brain through the cannula at a rate of 0.2 μl/min occurred. Animals were maintained under an LD12:12 light cycle. After an acclimation period of 4 days, 24-h baseline recordings were collected for animal beginning at light offset (20:00 h). On the following day, the saline was replaced by infusion of one of the test solutions and animals were infused with either saline, PGD2 (200 pmol/min) or CGS21680 (20 pmol/min; n=5–7 animals/treatment) at a rate of 0.2 kl/min for a 2 h period beginning at light offset and the effects on sleep and wakefulness were assessed. Behavioral states were classified in 10 s epochs as either waking (W), non-rapid eye movement sleep (NREMS) or REMS as described previously (Matsumura et al. 1994; Satoh et al. 1999; Mizoguchi et al. 2001).
At the end of the infusion period (ZT14), rats were euthanized by injection of a lethal dose of pentobarbital. The brains were rapidly removed and the Cx, BF, and Hy were dissected, frozen on dry ice and stored at −70°C. These brain regions were chosen because of the putative involvement of BF and Hy structures in sleep regulation and the role of the Cx in generation of EEG rhythms. Dissection of the brain regions, total RNA isolation from the BF, Cx and Hy of each of the 18 experimental animals, and cDNA synthesis from these samples occurred as described previously (Terao et al. 2006).
Assessment of the Expression of Candidate Genes by qPCR
The primer and probe sequences for the genes listed in Table 1 along with that of glyceraldehyde-3-phosphate dehydrogenase (g3pdh). The methods used to measure mRNA levels using real-time amplification kinetics have been described previously (Terao et al. 2003a; Terao et al. 2003b; Terao et al. 2006).
Table 1.
Expression of members of the IEG family in the Cx, BF and Hy.
Candidate gene expression during recovery sleep (RS)* and PGD2- and CGS21680-induced sleep.
| Cerebral Cortex | Basal Forebrain | Hypothalamus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | RS | PGD2 | CGS | RS | PGD2 | CGS | RS | PGD2 | CGS |
| arc | – | ↓↓ | ↓↓ | – | ↓↓ | ↓↓ | – | ↓↓ | ↓↓ |
| c-fos | – | – | – | – | ↑↑↑↑ | ↑↑↑↑↑↑ | – | ↑↑↑ | ↑↑↑↑ |
| cjun | – | – | ↑↑ | – | ↑↑ | ↑↑ | – | ↑↑ | ↑ |
| egr-3 | ↑↑ | – | – | – | – | – | – | – | – |
| fra-1 | – | – | – | – | – | ↑↑ | – | – | ↑↑ |
| fra-2 | ↑ | – | – | ↑↑ | ↑↑↑↑ | ↑↑↑↑↑ | ↑↑ | ↑↑↑ | ↑↑↑↑ |
| grp78 | ↑ | – | – | – | – | – | – | – | – |
| grp94 | ↑ | – | ↑↑ | – | – | – | – | – | – |
| jun-B | – | – | – | – | ↑↑↑ | ↑↑↑↑ | – | ↑↑↑↑ | ↑↑↑↑↑↑ |
| jun-D | – | – | – | – | – | – | – | – | – |
| ngfi-a | – | – | – | – | – | – | – | – | ↑↑ |
| ngfi-b | – | – | – | – | – | ↑↑↑ | – | – | ↑↑↑↑↑ |
| nr4a3 | ↑ | – | – | ↑↑ | – | – | – | – | ↑↑↑ |
Number of arrows indicates x-fold change as determined by real-time RT-PCR analysis:
indicates gene is significantly upregulated but the magnitude is 1.5-fold or less.
indicates gene is significantly upregulated and the magnitude is 1.5- to 2.5-fold.
indicates gene is significantly upregulated and the magnitude is 2.5- to 3.5-fold.
indicates gene is significantly upregulated and the magnitude is 3.5- to 4.5-fold.
indicates gene is significantly upregulated and the magnitude is 4.5- to 5.5-fold.
indicates gene is significantly upregulated and the magnitude is 5.5- to 6.5-fold.
indicates gene is significantly downregulated but the magnitude is 1.5-fold or less.
indicates gene is significantly downregulated and the magnitude is 1.5- to 2.5-fold.
indicates gene is significantly downregulated and the magnitude is 2.5- to 3.5-fold.
No change relative to time-matched control.
Data from RS have been reported previously in Terao et al. (2006) and are included here for comparison.
Affymetrix GeneChip® Hybridization and GeneChip® Data Analysis
For cDNA synthesis, 5 kg aliquots of total RNA isolated from the 5–7 animals in each experimental condition were combined to create 9 RNA pools (3 brain regions - BF, Cx and Hy -from the 3 experimental conditions). The cDNA pools were used to synthesize cRNA probes which were then hybridized to replicate Affymetrix Rat Neurobiology U34 (RNU34) GeneChips®, resulting in a total of 18 GeneChip® hybridizations.
Data were analyzed with Microarray Suite 4.0 (Affymetrix, Santa Clara, CA) and GeneSpring (version 4.1.3, Silicon Genetics, Redwood City, CA). Hybridization signal was measured for the 1,322 gene elements on the RNU34 GeneChip®. Hybridization signal for each gene element on a GeneChip® is reported as an average difference (Avg Diff) value. The Avg Diff value was calculated by subtracting the background signal and the non-specific hybridization signal from the specific hybridization signal for each gene element. Some genes are represented by more than one gene element on the array, hence the use of the term gene element rather than gene in the Results section. The Affymetrix software automatically labels each gene element as being “present”, “marginal” or “absent” based on the Avg Diff value.
Two methods were used to identify gene elements exhibiting sleep state-dependent changes in expression. To evaluate qualitative (“all or none”) changes in expression between conditions within a brain region, gene elements were identified that met one of four criteria: 1) scored “present” in both GeneChips® hybridized with cRNA synthesized from the saline-infused animals (“control GeneChips®“) and absent in both “PGD2 GeneChips®”; 2) scored “present” in both control GeneChips® and absent in both “CGS21680 GeneChips®”; 3) scored “absent” in both control GeneChips® and present in both PGD2 GeneChips®; 4) scored “absent” in both control GeneChips® and present in both CGS21680 GeneChips®. To evaluate quantitative changes in expression between conditions, the Cross Gene Error Model (CGEM; http://www.silicongenetics.com/Support/GeneSpring/GSnotes/analysis_guides/error_model.pdf) of GeneSpring was used to identify those gene elements that were expressed at a level of intensity sufficient to be considered reliable in all six GeneChips® from a given brain region. Subsequent analyses were limited to the subset of gene elements identified by either of these two procedures.
Data values less than 0.01 were assigned a value of 0.01. The expression level for each gene element was normalized to the 50th percentile of the expression level of all gene elements on the same GeneChip® and, subsequently, to the median percentile of expression for that gene element across all GeneChips® from the same brain region (per GeneSpring’s recommended normalization procedures). Avg Diff values for the two control GeneChips® for each brain region and the two replicate GeneChips® hybridized in each experimental condition/brain region group were then averaged to provide expression level values for each gene element in each condition for each brain region. These Avg Diff values were used to calculate the percent change value between the pooled experimental and the pooled control group (drug infusion vs. saline control infusion). We report here all genes that show qualitative (“all or none”) changes across experimental conditions and those that are up- or down-regulated by at least 50% during either PGD2 or CGS21680 infusion when compared to the pooled saline-infusion control group in any of the three brain regions studied. The 50% threshold was empirically determined to be the minimum value required to identify genes commonly expressed in response to the experimental manipulations across all three brain regions examined. Fold difference data presented in the supplementary tables represent the fold difference between the means of each replicate pair in the control and treatment conditions.
Those genes that were upregulated or downregulated in response to PGD2 or CGS21680 were subsequently categorized according to function in a modified version of the GeneSpring ontological structure for gene categorization (Ashburner et al. 2000). The analyses presented herein are based on hybridizations to the probesets on the Affymetrix Rat Neurobiology U34 GeneChip®, each of which was designed to correspond to a particular Genbank accession number. In some cases, the Genbank accession numbers represent expressed sequence tags (ESTs). To identify the genes to which each of the ESTs actually correspond, we utilized the GeneSpider function of GeneSpring, which integrates the UniGene, Genbank and Locus Link databases. Since these databases have not been fully curated to eliminate redundancies, our analyses based on probe sets may identify two or more genetic elements as unique entities that, in fact, correspond to the same gene. Based on our knowledge of the literature, we have made every effort to eliminate such redundancies. For example, egr-1 (Genbank #M18416) is recognized as being synonymous with krox-24, but it is possible that there may still be other synonyms in our gene lists.
Confirmation of Results from GeneChip® Studies
On the basis of the GeneChip® results, several genes were selected for further analysis using the real-time fluorescence detection method as described above. The primer and probe sequences were chosen from the coding regions of the following genes using Primer Express v.1.0 Software (Perkin-Elmer Applied Biosystems, Foster City, CA): (1) complement component 3 (c3; GenBank accession #M29866): forward primer, GGTAAGGGTGGAACTGTTGCA; TaqMan® probe, 6FAM5′-CCAGCCTTCTGCAGCATGGCC-3′TAMRA; reverse primer, TGGTCTGGTAGTACCGCTTCTTG (amplicon size=74 bp); (2) the alpha 5 subunit of the GABAA receptor (gabra5; X51992): forward primer, CAGCTAGGACAGTTTTTGGAGTGA; TaqMan® probe, 6FAM5′-CACAGTGCTGACCATGACAACCCTCA-3′TAMRA; reverse primer, CGAATTCCGGGCACTGAT (amplicon size=71 bp); (3) glial fibrillary acidic protein (gfap; NM_017009): forward primer, CCTTGACCTGCGACCTTGAG; TaqMan® probe, 6FAM5′-TTGCGCGGCACGAACGAGTC-3′TAMRA; reverse primer, GCGCATTTGCCTCTCCAA (amplicon size=62 bp); (4) heat shock 27kDa protein (hsp27; AA998683; AI176658): forward primer, ATCAGCGGAGATCACCATTCC; TaqMan® probe, 6FAM5′-TCACTTTCGAGGCCCGTGCCC-3′TAMRA; reverse primer, CGACTCTGGGCCTCCAATT (amplicon size=64 bp); (5) insulin-like growth factor II (igf-II; X51992): forward primer, CGGCAGCTCAGATTTTGGAA; TaqMan® probe, 6FAM5′-AGTGTGTGTGCCCCAAACACGCAC-3′TAMRA; reverse primer, CCGCCGCGCAGTGT (amplicon size=62 bp); (6) metallothionein-1 and -2 (mt-1; M11794; AI102562): forward primer, CGTGCTGTGCCTGAAGTGA; TaqMan® probe, 6FAM5′-AACAGTGCTGCTGCCCTCAGGTGTAAA-3′TAMRA; reverse primer, GACTCTGAGTTGGTCCGGAAAT (amplicon size=72 bp); and (7) peripheral benzodiazepine receptor (pbr; NM_012515): forward primer, TTTGGTGCCCGGCAGAT; TaqMan® probe, 6FAM5′-CTGGGCTTTGGTGGACCTCAT-3′TAMRA; reverse primer, TTGCCACCCCACTGACAAG (amplicon size=61 bp). To confirm the specificity of the nucleotide sequences chosen for the primers and probes and the absence of DNA polymorphisms, BLASTN searches were conducted against the dbEST and nonredundant set of Genbank, EMBL, and DDBJ databases.
Statistical Analyses
Sleep state and real-time quantitative PCR measurements were analyzed using Statview 5.0 (Abacus Concepts, Berkeley, CA). Data were initially analyzed by ANOVA with alpha set at 0.05 to determine whether significant effects occurred in any of the parameters. Significant main effects determined by ANOVA were followed by the Tukey-Kramer post hoc test to determine whether experimental values differed from saline control values.
Results
Sleep Physiology
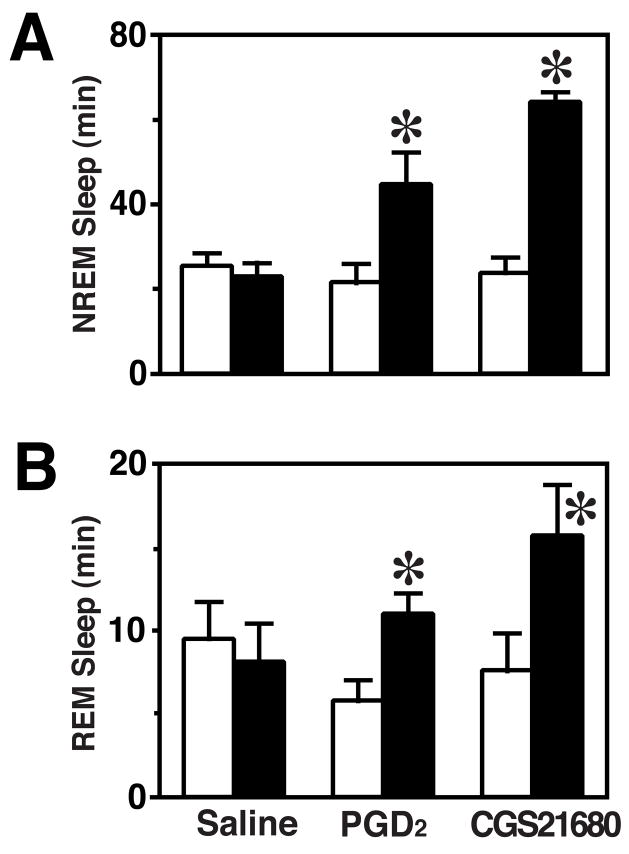
On the pre-treatment baseline day (P > 0.4; Fig. 1), the amount of time spent in wake, NREMS or REMS did not differ among treatment groups during 2 h corresponding to the infusion period on the experimental day. On the day of treatment, rats treated with PGD2 or CGS21680 spent less time awake (F(2,15)=24.66, P < 0.001) and more time in NREMS (F(2,15)=24.49, P < 0.001) during the infusion period (ZT12-ZT14) relative to the saline control group (Fig. 1). NREMS time was increased by roughly two-fold in PGD2-treated rats and threefold in CGS21680-treated rats (Fig. 1A). Similarly, REMS relative to baseline was dependent on treatment (F(2,15)=7.46, P = 0.006). Infusion of CGS21680 or PGD2 increased REMS by approximately two-fold relative to baseline (Fig. 1B), while saline infusion did not significantly affect REMS.
Fig. 1.
Amounts of NREM (A) and REM (B) sleep during the 2-h infusion period in each of the three groups relative to the baseline day. Open bars: baseline; shaded bars: experimental recording (2 h). *P < 0.05; by paired t test.
Taqman® Analyses of Candidate Genes
Since the effects of PGD2 and CGS21680 on sleep states from ZT12-ZT14 were roughly equivalent in magnitude to those observed during RS from ZT6-ZT8 after SD (Terao et al. 2006), we determined whether these pharmacological treatments had effects on gene expression in the Cx similar to those previously reported during RS (Terao et al. 2006). From our previous studies of RS (Terao et al. 2003a; Terao et al. 2003b; Terao et al. 2006), 13 genes were selected for qPCR analysis including several transcription factors (cfos, c-jun, egr-3, fra-1, fra-2, jun-B, jun-D, ngfi-a, ngfi-b, and nr4a3), heat shock protein (HSP) family members (grp78, and grp94), a gene associated with synaptic plasticity (arc), and glyceraldehyde-3-phosphate dehydrogenase (g3pdh) which was used as a denominator in the calculation of relative expression levels for these genes. As indicated in Table 1, five of these genes (egr-3, fra-2, grp78, grp94 and nr4a3) had increased expression and 8 genes had unchanged expression in the Cx during RS (Terao et al. 2006). However, none of these five genes showed increased expression in the Cx during PGD2-induced sleep and only grp94 showed increased expression during CGS21680-induced sleep (Fig. 2A). In contrast, both PGD2 and CGS21680 reduced the expression of arc in the Cx (Fig. 2B), a gene whose expression increased during SD (Terao et al. 2006). Increased expression of c-jun occurred in the Cx during CGS21680-induced sleep (Fig. 2C) without any significant change during RS or PGD2-induced sleep (Table 1).
Fig. 2.
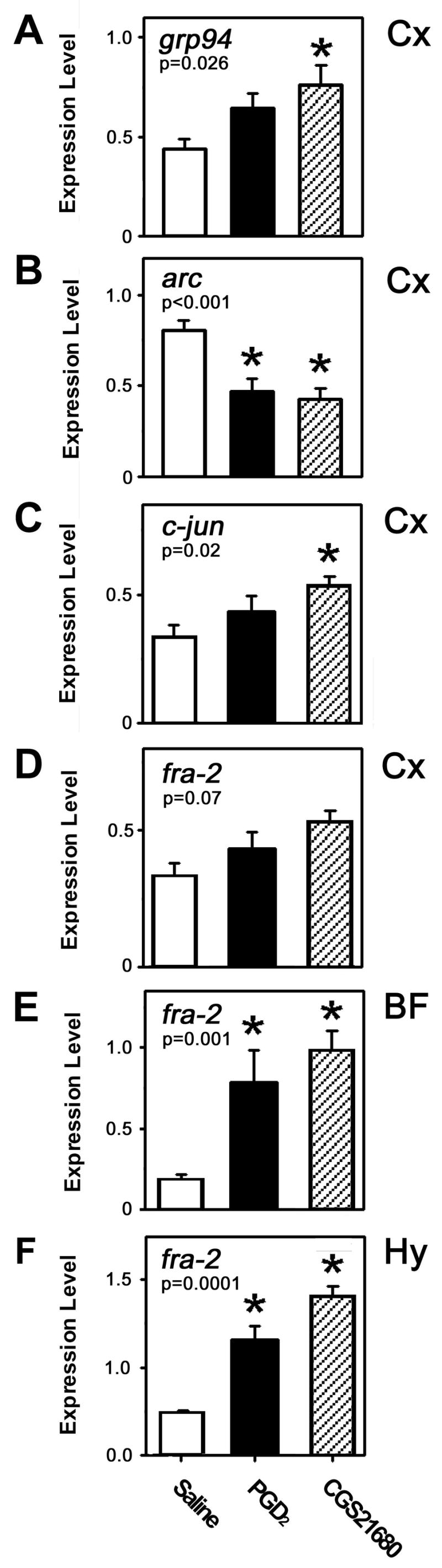
Expression of (A) grp94, (B) arc, and (C) c-jun in the cerebral cortex (Cx) during RS, and PGD2- or CGS21680-induced sleep. (D–F) Comparison of fra-2 expression in the Cx (E), basal forebrain (BF) (E) and hypothalamus (Hy) (F) across the three conditions. Numbers within each panel refer to the P value calculated based on ANOVA; * indicates P < 0.05 by Tukey-Kramer post hoc test.
When the candidate gene analyses were expanded to the BF and Hy, one gene, fra-2, showed consistent upregulation during RS, PGD2-, and CGS21680-induced sleep (Table 1 and Figs. 2D–F). As in the Cx, arc expression was significantly reduced during both PGD2- and CGS21680-induced sleep in the BF and Hy without any corresponding change during RS (Table 1). Three genes, c-fos, c-jun, and jun-B, were significantly upregulated in BF and Hy during both PGD2- and CGS21680-induced sleep without any corresponding change during RS (Table 1). Two genes, fra-1 and ngfi-b, were upregulated in both brain regions in CGS21680-induced sleep without any corresponding change in PGD2-induced sleep or RS (Table 1). Lastly, the expression of two genes, ngfi-a and nr4a3, was increased during CGS21680-induced sleep only in the Hy (Table 1).
Affymetrix GeneChip® Hybridizations
Comparisons of PGD2- and CGS21680-induced sleep with saline-treated animals
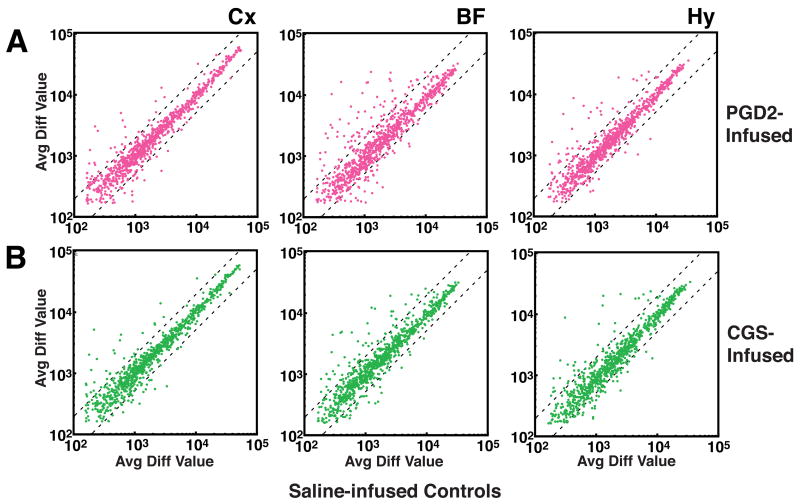
Affymetrix GeneChips® were used to assess the expression of 1,322 gene elements in the BF, Cx and Hy of PGD2- versus saline-treated rats (Fig. 3A) and in CGS21680- versus saline-treated rats (Fig. 3B). To identify candidate genes for further studies, we defined those gene elements that exhibited at least 1.5-fold greater signal in a treatment group (PGD2 or CGS21680) relative to saline as being upregulated, and those that exhibited at least 1.5-fold greater signal in the saline group relative to a treatment group (PGD2 or CGS21680) as being downregulated in that treatment group. By these criteria, considerably more gene elements were upregulated than downregulated in the two treatment groups relative to saline controls in the BF and Hy: 143 gene elements were upregulated in BF and/or Hy by PGD2 (Fig. 4A, Supplementary Table 1) and 98 were downregulated (Fig. 4B, Supplementary Table 2); 144 gene elements were upregulated in BF and/or Hy by CGS21680 (Fig. 4A, Supplementary Table 3) and 109 were downregulated (Fig. 4B, Supplementary Table 4). In contrast, the numbers of genes upregulated in the Cx were less than (PGD2) or equal to (CGS21680) those downregulated by the infusions (Fig. 4A vs. 4B).
Fig. 3.
(A) Comparison of gene expression values obtained in PGD2-treated vs. saline control groups in the cerebral cortex (Cx), basal forebrain (BF), and hypothalamus (Hy). (B) Comparison of gene expression values obtained in CGS21680-treated vs. saline control groups in three brain regions. Control groups are on the abscissa. Points that lie above the leftmost dotted line in each graph were expressed at 1.5-fold higher levels in the experimental condition relative to the control condition. Points that lie below the rightmost dotted line were expressed at 1.5-fold higher levels in the control condition relative to the experimental condition.
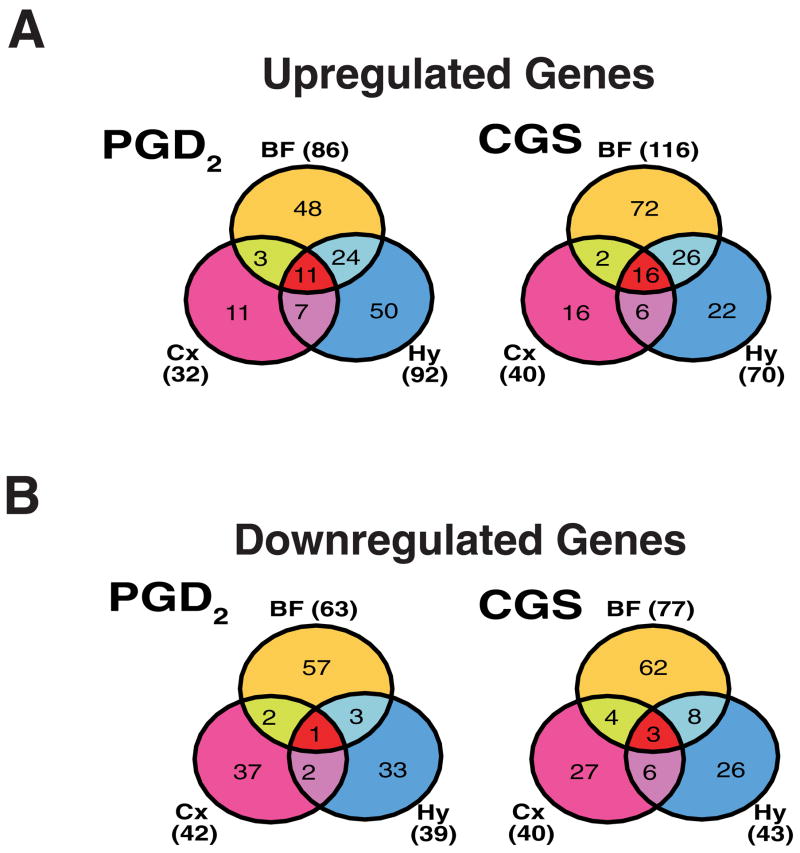
Fig. 4.
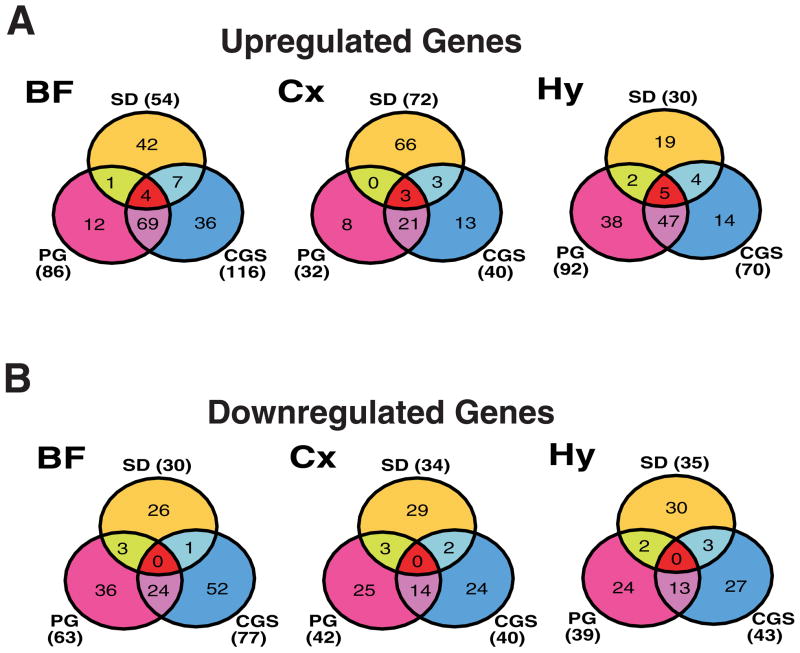
Venn diagrams illustrating the number of genes upregulated (A) or downregulated (B) by at least 50% in each of the three brain regions by PGD2 treatment (left) and CGS21680 treatment (right) during ZT12-14. Abbreviations: BF, basal forebrain; Cx, cortex; Hy, hypothalamus.
In PGD2-treated rats, gene elements classified as upregulated were more common by roughly 3-fold in BF (N=86) and Hy (N=92) than in Cx (N=32). In CGS21680-treated rats, the “responsiveness” of brain regions, as determined by the numbers of mRNAs upregulated subsequent to infusion, were comparable to PGD2 infusion, with the Cx being the least responsive (N=40) and the BF exhibiting the largest number of increased mRNAs (N=116), followed by the Hy (N=70).
A number of gene elements were upregulated in more than one brain region in PGD2- or CGS21680-treated rats (Fig. 4A, Table 2). Thirty-four gene elements were upregulated in two of three brain regions by PGD2 (Table 2A), and a partially overlapping set of 34 gene elements was upregulated in two of three brain regions by CGS21680 (Fig. 4A; Table 2B). Eleven gene elements representing 9 distinct genes were commonly upregulated across all three brain regions by PGD2 (Table 2A and Fig. 4A); these included complement component 3 (c3; GenBank accession #M29866), heat shock 27kDa protein (hsp27; AA998683; AI176658), and metallothionein-1 and -2 (mt-1; M11794; AI102562). Sixteen gene elements representing 14 distinct genes were commonly upregulated across all three brain regions by CGS21680 (Table 2B and Fig. 4A); c3, hsp27, and mt-1 were also among these genes. Nine transcripts were upregulated by both treatments in all three brain regions. Due to redundancy among gene elements on the GeneChip®, these nine elements reduced to six independent genes: glial fibrillary acidic proteins alpha and delta (gfap; AF028784), small inducible cytokine subfamily A20 (AF053312), peripheral-type benzodiazepine receptor (J05122), mt-1 (M11794; AI102562), c3 (M29866), and hsp27. In addition, other elements corresponding to hsp27 (M86389) and a metallothionein-2-related transcript (AI176456) were upregulated in two of three regions by both treatments, as were several other genes (see entries in bold in Tables 3A and 3B).
Table 2.
Genes exhibiting increased expression among two or more brain regions examined in the PGD2-treated rats (A) and in the CGS21680-treated rats (B).
| Table 2A. 33 distinct genes are commonly upregulated by at least 50% in two or more brain regions in response to PGD2.
| |||
|---|---|---|---|
| Brain Regions | Probeset Name | Gene Name | Genbank # |
| BF and Cx (n=3) | M54987_at | Rat corticotropin releasing hormone (CRH) gene, complete cds. | M54987 |
| X60769mRNA_at | CCAAT/enhancer binding protein (C/EBP), beta (Cebpb) | X60769 | |
| X87157_at | Neurolysin (metallopeptidase M3 family) (Nln) | X87157 | |
| BF and Hy (n=24) | AF030091UTR#1_at | Cyclin L1 (Ccnl1) | M54987 |
| AJ222813_s_at | Interleukin 18 (Il18) | X60769 | |
| D00913_at | ICAM-1; Rattus sp. mRNA for intercellular adhesion molecule-1, complete cds. | X87157 | |
| D00913_g_at | ICAM-1; Rattus sp. mRNA for intercellular adhesion molecule-1, complete cds. | M54987 | |
| J02722cds_at | heme oxygenase; Rat heme oxygenase gene, complete cds. | X60769 | |
| J04563_at | putative; Rat cAMP phosphodiesterase mRNA, 3′ end (Phosphodiesterase 4B (Pde4b)) | X87157 | |
| M34253_at | Interferon regulatory factor 1 (Irf1) | M54987 | |
| M34253_g_at | Interferon regulatory factor 1 (Irf1) | X60769 | |
| M63122_at | Tumor necrosis factor receptor superfamily, member 1a (Tnfrsf1a) | X87157 | |
| M86389cds_s_at | Heat shock 27kDa protein 1 (Hspb1) | M54987 | |
| rc_AA945583_at | Hydroxysteroid (17-beta) dehydrogenase 10 (Hsd17b10) | X60769 | |
| rc_AI071965_s_at | Rattus norvegicus cDNA clone UI-R-C2-nl-e-03-0-UI 3′, mRNA sequence (Heat shock 70kD protein 1A (Hspa1a)) | X87157 | |
| rc_AI179610_at | Heme oxygenase 1 (Hmox1) | M54987 | |
| rc_AI236828_s_at | Signal transducer and activator of transcription 3 (Stat3) | X60769 | |
| U14647_at | Caspase 1 (Casp1) | X87157 | |
| U14647_g_at | Caspase 1(Casp1) | M54987 | |
| U42719_at | Complement component 4a (C4a) | X60769 | |
| U77777_s_at | Interleukin 18 (Il18) | X87157 | |
| X03347cds_g_at | FBR murine osteosarcoma, complete proviral sequence integrated in Rattus genome. | M54987 | |
| X17053cds_s_at | Rat immediate-early serum-responsive JE gene. | X60769 | |
| X17053mRNA_s_at | Rat immediate-early serum-responsive JE gene. | X87157 | |
| X54686cds_at | R.norvegicus pJunB gene. | M54987 | |
| X91810_at | Signal transducer and activator of transcription 3 (Stat3) | X60769 | |
| Z75029_s_at | Heat shock 70kD protein 1A (Hspa1a) | X87157 | |
| Cx and Hy (n=7) | M58587_at | Interleukin 6 receptor (Il6r) | M58587 |
| rc_AI146018_s_at | Transcribed locus, highly similar to NP_004792.1 neurexin 1 [Homo sapiens] | AI146018 | |
| rc_AI170268_at | Beta-2 microglobulin (B2m) | AI170268 | |
| rc_AI176456_at | Transcribed locus, highly similar to NP_032656.1 metallothionein 2 [Mus musculus] | AI176456 | |
| U31866_g_at | Transcribed locus, moderately similar to NP_598738.1 transferrin [Mus musculus] | U31866 | |
| U78090_s_at | Potassium channel regulator 1 (LOC245960) | U78090 | |
| X62952_at | Vimentin (Vim) | X62952 | |
| BF, Cx and Hy (n=11) | AF028784cds#1_s_at | Rattus norvegicus glial fibrillary acidic proteins alpha and delta (GFAP) gene. | AF028784 |
| AF053312_s_at | Small inducible cytokine subfamily A20 (Chemokine (C-C motif) ligand 20 (Ccl20)) | AF053312 | |
| J05122_at | Benzodiazepin receptor, Peripheral-type (Bzrp) | J05122 | |
| L16764_s_at | Heat shock 70kD protein 1A (Hspa1a) | L16764 | |
| M11794cds#2_f_at | Rat metallothionein-2 and metallothionein-1 genes, complete cds. | M11794 | |
| M29866_s_at | Complement component 3 (C3) | M29866 | |
| rc_AA998683_g_at | Heat shock 27kDa protein 1 (Hspb1) | AA998683 | |
| rc_AI102562_at | Metallothionein | AI102562 | |
| rc_AI176658_s_at | Heat shock 27kDa protein 1 (Hspb1) | AI176658 | |
| U48596_at | Mitogen activated protein kinase kinase kinase 1 (Map3k1) | U48596 | |
| X52477_at | Complement component 3 (C3) | X52477 | |
| Table 2B. 38 distinct genes are commonly upregulated by at least 50% in two or more brain regions in response to CGS21680.
| |||
| Brain Regions | Probeset Name | Gene Name | Genbank # |
|
| |||
| BF and Cx (n=2) | L16764_s_at | Heat shock 70kD protein 1A (Hspa1a) | L16764 |
| Y09507_at | Hypoxia inducible factor 1, alpha subunit (Hif1a) | Y09507 | |
| BF and Hy (n=26) | AF030091UTR#1_at | Cyclin L1 (Ccnl1) | AF030091 |
| AF030091UTR#1_g_at | Cyclin L1 (Ccnl1) | AF030091 | |
| AJ222813_s_at | Interleukin 18 (Il18) | AJ222813 | |
| D00913_at | ICAM-1; Rattus sp. mRNA for intercellular adhesion molecule-1, complete cds. | D00913 | |
| D00913_g_at | ICAM-1; Rattus sp. mRNA for intercellular adhesion molecule-1, complete cds. | D00913 | |
| M17960_at | Insulin-like growth factor 2 (Igf2) | M17960 | |
| M26744_at | Interleukin 6 (Il6) | M26744 | |
| M63122_at | Tumor necrosis factor receptor superfamily, member 1a (Tnfrsf1a) | M63122 | |
| M86389cds_s_at | Heat shock 27kDa protein 1 (Hspb1) | M86389 | |
| rc_AI071965_s_at | Rattus norvegicus cDNA clone UI-R-C2-nl-e-03-0-UI 3′, mRNA sequence (Heat shock 70kD protein 1A (Hspa1a)) | Hspa1a)) AI071965 | |
| rc_AI137246_s_at | Ig VH193020=anti-insulin 193020 monoclonal antibody heavy chain variable region | S65980 | |
| rc_AI176710_at | Nuclear receptor subfamily 4, group A, member 3 (Nr4a3) | AI176710 | |
| S77528cds_s_at | NFIL-6; Rattus sp. C/EBP-related transcription factor (Nfil6) mRNA, complete cds. | S77528 | |
| U14647_g_at | Caspase 1 (Casp1) | U14647 | |
| U17254_at | Immediate early gene transcription factor NGFI-B (Nuclear receptor subfamily 4, group A, member 1 (Nr4a1)) | U17254 | |
| U17254_g_at | Immediate early gene transcription factor NGFI-B (Nuclear receptor subfamily 4, group A, member 1 (Nr4a1)) | U17254 | |
| U42719_at | Complement component 4a (C4a) | U42719 | |
| U48596_at | Mitogen activated protein kinase kinase kinase 1 (Map3k1) | U48596 | |
| U77777_s_at | Interleukin 18 (Il18) | U77777 | |
| X03347cds_g_at | FBR murine osteosarcoma, complete proviral sequence integrated in Rattus genome. | X03347 | |
| X06769cds_at | unnamed protein product; c-fos protein (AA 1-380); Rat c-fos mRNA. | X06769 | |
| X06769cds_g_at | unnamed protein product; c-fos protein (AA 1-380); Rat c-fos mRNA. | X06769 | |
| X17053mRNA_s_at | Rat immediate-early serum-responsive JE gene. | X17053 | |
| X54686cds_at | R.norvegicus pJunB gene. | X54686 | |
| X91810_at | Signal transducer and activator of transcription 3 (Stat3) | X91810 | |
| Z75029_s_at | Heat shock 70kD protein 1A (Hspa1a) | Z75029 | |
| Cx and Hy (n=6) | M54987_at | Rat corticotropin releasing hormone (CRH) gene, complete cds. | M54987 |
| rc_AA964003_s_at | MHC class II RT1-D beta1 chain haplotype a (Arrestin, beta 2 (Arrb2)) | AA964003 | |
| rc_AI146018_s_at | Transcribed locus, highly similar to NP_004792.1 neurexin 1 [Homo sapiens] | AI146018 | |
| rc_AI176456_at | Transcribed locus, highly similar to NP_032656.1 metallothionein 2 [Mus musculus] | AI176456 | |
| U31866_g_at | Transcribed locus, moderately similar to NP_598738.1 transferrin [Mus musculus] | U31866 | |
| X62952_at | Vimentin (Vim) | X62952 | |
| BF, Cx and Hy (n=16) | AF028784cds#1_s_at | Rattus norvegicus glial fibrillary acidic proteins alpha and delta (GFAP) gene. | AF028784 |
| AF053312_s_at | Small inducible cytokine subfamily A20 (Chemokine (C-C motif) ligand 20 (Ccl20)) | AF053312 | |
| J05122_at | Benzodiazepin receptor, Peripheral-type (Bzrp) | J05122 | |
| M11794cds#2_f_at | Rat metallothionein-2 and metallothionein-1 genes, complete cds. | M11794 | |
| M23697_at | Plasminogen activator, tissue (Plat) | M23697 | |
| M29866_s_at | Complement component 3 (C3) | M29866 | |
| M34253_g_at | Interferon regulatory factor 1 | M34253 | |
| M58587_at | Interleukin 6 receptor (Il6r) | M58587 | |
| rc_AA945583_at | Hydroxysteroid (17-beta) dehydrogenase 10 | AA945583 | |
| rc_AA998683_g_at | Heat shock 27kDa protein 1 | AA998683 | |
| rc_AI102562_at | Metallothionein | AI102562 | |
| rc_AI176658_s_at | Heat shock 27kDa protein 1 | AI176658 | |
| U14647_at | Caspase 1 (Casp1) | U14647 | |
| X17053cds_s_at | Rat immediate-early serum-responsive JE gene. | X17053 | |
| X52477_at | Complement component 3 (C3) | X52477 | |
| X60769mRNA_at | CCAAT/enhancer binding protein (C/EBP), beta (Cebpb) | X60769 | |
Table 3.
Genes exhibiting decreased expression among two or more brain regions examined in the PGD2-treated rats (A) and in the CGS21680-treated rats (B).
| Table 3A. 8 genes are commonly downregulated by at least 50% in two or more brain regions in response to PGD2.
| |||
|---|---|---|---|
| Brain Regions | Probeset Name | Gene Name | Genbank # |
| BF and Cx | AF042714_at | Neurexophilin 4 (Nxph4) | AF042714 |
| U88036_at | Solute carrier family 21 (organic anion transporter), member 5 (Slc21a5) | U88036 | |
| BF and Hy | AJ001029_at | SRY-box containing gene 10 (Sox10) | AJ001029 |
| M59786_at | Calcium channel, voltage-dependent, alpha 1C subunit (Cacna1c) | M59786 | |
| X17012mRNA_ | Rat IGFII gene for insulin-like growth factor II. | X17012 | |
| Cx and Hy | AF081365_s_at | Potassium inwardly-rectifying channel, subfamily J, member 1 (Kcnj1) | AF081365 |
| AFFX-DapX-M_at | 26.7% identity to the Escherichia coli bifunctional biotin operon repressor | L38424 | |
| BF, Cx and Hy | X51992_at | Gamma-aminobutyric acid A receptor, alpha 5 (Gabra5) | X51992 |
| Table 3B. 20 distinct genes are commonly downregulated by at least 50% in two or more brain regions in response to CGS21680.
| |||
| Brain Regions | Probeset Name | Gene Name | Genbank # |
|
| |||
| BF and Cx | U16845_at | Neurotrimin (RNU16845) | U16845 |
| U73142_at | Mitogen activated protein kinase 14 (Mapk14) | U73142 | |
| X15468cds_at | Rat mRNA for GABA(A) receptor beta-3 subunit. | X15468 | |
| Z11548_at | Glutamate receptor, ionotropic, kainate 2 (Grik2) | Z11548 | |
| BF and Hy | AB016160_g_at | Gamma-aminobutyric acid (GABA) B receptor, 1 (Gabbr1) | AB016160 |
| M31076_at | Transforming growth factor alpha (Tgfa) | M31076 | |
| M32867_at | Potassium voltage gated channel, shaker related subfamily, member 4 (Kcna4) | M32867 | |
| rc_AA893870_g_at | EST197673 Normalized rat placenta; cDNA clone RPLAM86 3′ end, mRNA sequence. | AA893870 | |
| rc_AI235758_s_at | Protein kinase, cAMP dependent regulatory, type II beta (Prkar2b) | AI235758 | |
| S94371_g_at | GluR-4c; glutamate receptor subunit 4c | S94371 | |
| X12589cds_s_at | potassium channel protein (AA 1-495), voltage-dependent. | X12589 | |
| X14788_s_at | CAMP responsive element binding protein 1 (Creb1) | X14788 | |
| Cx and Hy | AF021935_at | Ser-Thr protein kinase related to the myotonic dystrophy protein kinase (Pk428) | AF021935 |
| M58040_at | Transferrin receptor (Tfrc) | M58040 | |
| M59980_s_at | Potassium voltage gated channel, Shal-related family, member 2 (Kcnd2) | M59980 | |
| rc_AA925246_at | Cathepsin K (Ctsk) | AA925246 | |
| S55933_i_at | GABAA receptor alpha 4 subunit [rats, mRNA, 1843 nt]. | S55933 | |
| S94371_at | GluR-4c; glutamate receptor subunit 4c {alternatively spliced}. | S94371 | |
| BF, Cx and Hy | X17012mRNA_s_at | Rat IGFII gene for insulin-like growth factor II. | X17012 |
| U88036_at | Solute carrier family 21 (organic anion transporter), member 5 (Slc21a5) | U88036 | |
| X51992_at | Gamma-aminobutyric acid A receptor, alpha 5 (Gabra5) | X51992 | |
Entries in bold type are commonly downregulated by both PGD2 and CGS21680 in at least two of the same brain regions.
Downregulated gene elements were more common in the BF in both PGD2-treated (n=63) and CGS21680-treated rats (n=77) than in either the Cx or Hy (Fig. 4B, Supplementary Tables 2 and 4). Table 3A presents the 8 gene elements downregulated by at least 50% in more than one brain region in PGD2-treated rats. Only the alpha 5 subunit of the GABAA receptor (gabra5; X51992) was found to be downregulated across all three brain regions in PGD2-sleep (Fig. 4B, Table 3A). Table 3B presents the 21 transcripts downregulated by at least 50% in more than one brain region in CGS21680-treated rats; gabra5 was also downregulated in all three brain regions during CGS21680-induced sleep, as was insulin-like growth factor II (igf-II; X51992) and the organic anion transporter, slc21a5 (Fig. 4B, Table 3B). In addition, seven transcripts were downregulated in two of three regions by PGD2 (Table 3A) and 18 were downregulated in two of three regions by CGS21680 (Table 3B).
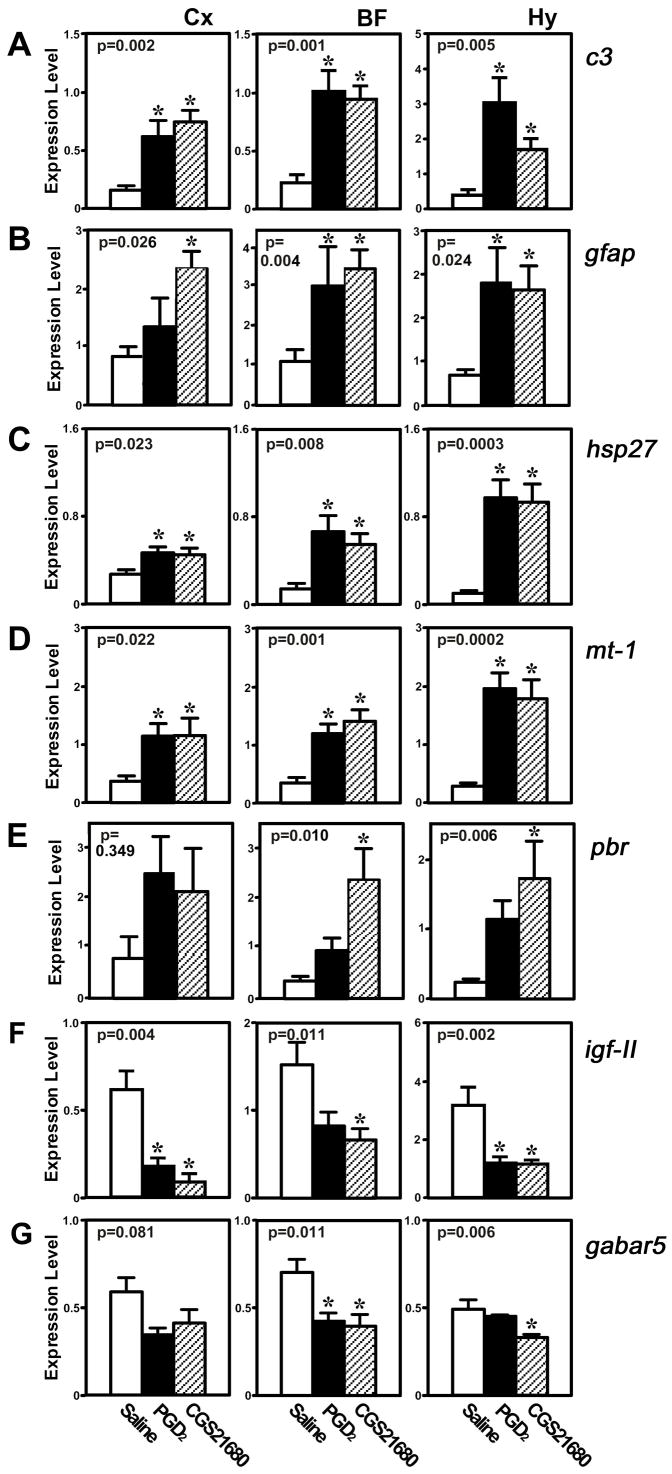
Since the GeneChip® analyses were based on pooled samples, the expression of seven of the above transcripts (c3, gabra5, gfap, hsp27, igf-II, mt-1, and pbr) was measured by Taqman® analysis to confirm that their levels were significantly changed in all three brain areas by both treatments (Fig. 5). Taqman® confirmed that the expression of the c3, gfap, hsp27 and mt-1 transcripts was significantly elevated in Cx, BF and Hy of PGD2- and CGS21680-treated rats in comparison to saline controls (Fig. 5A–D). In contrast, pbr expression was significantly upregulated only by CGS21680 in the BF and Hy (Fig. 5E). Igf-II expression was significantly downregulated in all three regions by both treatments (Fig. 5F), but Taqman® only confirmed downregulation of gabra5 in the BF and Hy in both treatments (Fig. 5G). Since 5 of the 30 gene × brain region × condition comparisons suggested as upregulated by the Affymetrix analyses were not confirmed as significant by Taqman analyses (Fig. 5A–E), we estimate the False Discovery Rate to be at least 17% for upregulated genes. Similarly, since 4 of the 12 genes suggested as downregulated by the Affymetrix analyses were not confirmed as significant by Taqman analyses (Fig. 5F–G), we estimate the False Discovery Rate to be at least 33% for downregulated genes.
Fig. 5.
Taqman® analysis of 5 genes identified as being significantly changed in three brain regions by PGD2 and CGS21680 infusion. (A) Complement component 3 (c3). (B) Heat shock 27kDa protein (hsp27). (C) Metallothionein-1 (mt-1) and -2 (mt-2). (D) Insulin-like growth factor II (igf-II). (E) The alpha 5 subunit of the GABAA receptor (gabra5). (F) Glial fibrillary acidic protein (gfap), (G) Peripheral benzodiazepine receptor (pbr). Abbreviations: BF=basal forebrain; Hy=hypothalamus; Cx=cerebral cortex. Numbers within each panel refer to the P value calculated based on ANOVA; * indicates P < 0.05 by Tukey-Kramer post hoc test.
Comparison of current results to prior study of recovery sleep
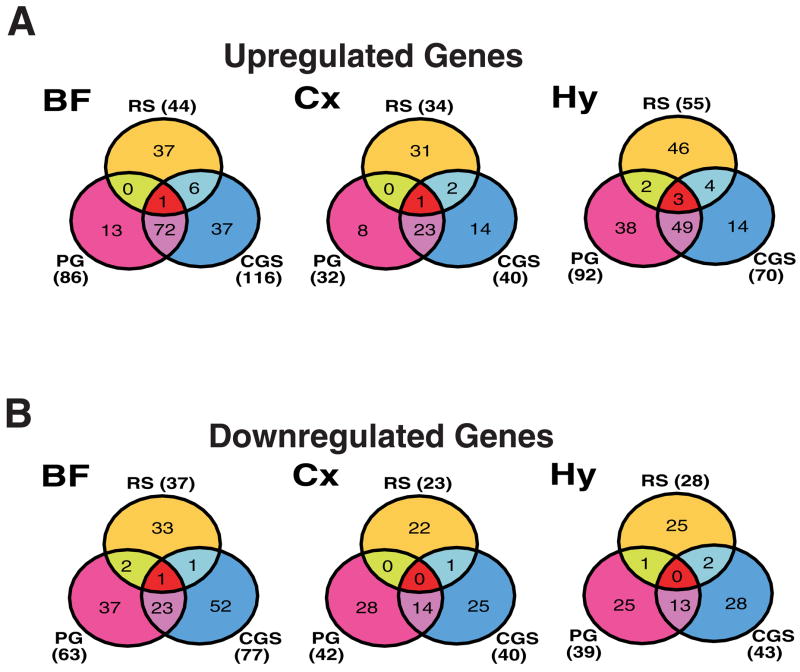
We compared the results of these GeneChip® studies to those of our previous report (Terao et al. 2006) to determine whether pharmacologically-induced sleep induced changes in gene expression similar to those that occur during SD and/or RS. Of the genes that were upregulated in one or more brain regions by both PGD2 and CGS21680, 10 were also upregulated during SD (Table 4A; Fig. 6A) and 5 during RS (albeit in a region-specific manner Table 4B; Fig. 7A). Both caspase 1 (U14647) and CCAAT/enhancer binding protein (C/EBP), beta (X60769) were upregulated by SD, PGD2 and CGS21680 in more than one brain region (Table 4A). Although several gene elements were upregulated region-specifically in common between RS and CGS21680 or between RS and PGD2 (Fig. 7A, Table 4B), none were upregulated in common in more than one brain region in all three treatment groups. However, several gene elements were upregulated in common in one brain region between SD and PGD2 (Supplementary Table 5A), RS and PGD2 (Supplementary Table 4C), SD and CGS21680 (Supplementary Table 6A), or RS and CGS21680 (Supplementary Table 5C).
Table 4.
Genes upregulated or downregulated in a region-specific manner by PGD2, CGS21680 and sleep deprivation or recovery sleep.
| Table 4A. Genes commonly upregulated at least 50% in response to CGS21680 and PGD2 and during sleep deprivation (Terao et al., 2005).
| |||
|---|---|---|---|
| Brain Regions | Probeset Name | Gene Name | Genbank # |
| BF (n=4) | rc_AA998683_g_at | Heat shock 27kDa protein 1 (Hspb1) | AA998683 |
| U14647_at | Caspase 1 (Casp1) | U14647 | |
| U42719_at | Complement component 4a (C4a) | U42719 | |
| X60769mRNA_at | CCAAT/enhancer binding protein (C/EBP), beta (Cebpb) | X60769 | |
| Cx (n=3) | M11794cds#2_f_at | metallothionein 2; metallothionein 1; Rat metallothionein-2 and metallothionein-1 genes | M11794 |
| rc_AI229237_at | Opioid receptor-like (Oprl) | AI229237 | |
| X60769mRNA_at | CCAAT/enhancer binding protein (C/EBP), beta (Cebpb) | X60769 | |
| Hy (n=5) | J05122_at | Benzodiazepin receptor, Peripheral-type (Bzrp) | J05122 |
| rc_AI176456_at | Transcribed locus, highly similar to NP_032656.1 metallothionein 2 | AI176456 | |
| U14647_at | Caspase 1 (Casp1) | U14647 | |
| U47031_at | Purinergic receptor P2X, ligand-gated ion channel, 4 (P2rx4) | U47031 | |
| X91810_at | Signal transducer and activator of transcription 3 (Stat3) | X91810 | |
| Table 4B. Genes are commonly upregulated at least 50% in response to CGS21680 and PGD2 and during recovery sleep (Terao et al., 2005).
| |||
| Brain Regions | Probeset Name | Gene Name | Genbank # |
|
| |||
| BF | U23056_at | CEA-related cell adhesion molecule 10 (Ceacam10) | U23056 |
| Cx | M11794cds#2_f_at | metallothionein 2; metallothionein 1; Rat metallothionein-2 and metallothionein-1 genes | M11794 |
| Hy (n=3) | AF027954_at | Bcl-2-related ovarian killer protein (Bok) | AF027954 |
| J05122_at | Benzodiazepin receptor, Peripheral-type (Bzrp) | J05122 | |
| rc_AI176456_at | Transcribed locus, highly similar to NP_032656.1 metallothionein 2 | AI176456 | |
| Table 4C. Genes commonly downregulated at least 50% in response to CGS21680 and PGD2 and during recovery sleep (Terao et al., 2005).
| |||
| Brain Regions | Probeset Name | Gene Name | Genbank # |
|
| |||
| BF | AF005720mRNA#3_s_at | ClC-2Sa; Rattus norvegicus chloride channel (ClC-2) gene, alternatively spliced | AF005720 |
Entries in bold type were upregulated by both PGD2 and CGS21680 and during both sleep deprivation in more than one brain region.
Fig. 6.
Venn diagrams illustrating the number of genes upregulated (A) or downregulated (B) in each of the three brain regions by PGD2 treatment, CGS21680 treatment, and sleep deprivation (Terao et al. 2006). Abbreviations: BF, basal forebrain; Cx, cortex; Hy, hypothalamus.
Fig. 7.
Venn diagrams illustrating the number of genes upregulated (A) or downregulated (B) in each of the three brain regions by PGD2 treatment, CGS21680 treatment, and recovery sleep (RS) (Terao et al. 2006). Abbreviations: BF, basal forebrain; Cx, cortex; Hy, hypothalamus.
Of the genes that were downregulated in one or more regions of the brain by both PGD2 and CGS21680, only one was also found to be downregulated during RS (albeit in a region-specific manner) in our previously reported study (Terao et al. 2006): in the BF (Fig. 7B, Table 4C), the clc-2sa transcript (AF005720), encoding a chloride channel, was downregulated during RS and after both PGD2 and CGS21680 infusions. In neither the Cx nor the Hy were any gene elements uniformly downregulated across the SD, PGD2 and CGS21680 GeneChips® (Fig. 6B) or across RS, PGD2 and CGS21680 GeneChips® (Fig. 7B). Several gene elements were, however, downregulated in one region in common between SD and PGD2 (Supplementary Table 5B), RS and PGD2 (Supplementary Table 5D), SD and CGS21680 (Supplementary Table 6B), or RS and CGS21680 (Supplementary Table 6D).
Discussion
Methodological Considerations and Candidate Gene Studies
In recent years, two strategies have been used to compare the brain gene expression profile between sleep and wakefulness (Cirelli 2002; Terao et al. 2003a; Terao et al. 2003b; Cirelli et al. 2004; Terao et al. 2006). One approach has been to sacrifice groups of animals 12 h out of phase from one another when each group can be expected to have a different distribution of sleep/wake states (“sleep history”) in the hours prior to sacrifice. The circadian confound in such comparisons of “spontaneous wake” vs. “spontaneous sleep” is eliminated by comparison to a group of animals subjected to SD during the “spontaneous sleep” period (Cirelli 2002; Cirelli et al. 2004). The second approach has been to impose SD during the first half of the light phase when sleep is normally at its maximal occurrence followed by an opportunity for RS during the second half of the light phase (Terao et al. 2003a; Terao et al. 2003b; Terao et al. 2006). The expectation is that SD will exacerbate the drive to sleep that is normally high in the first half of the lights on period and that the subsequent RS will be characterized by increased sleep consolidation and intensity which will result in enhancement of the molecular concomitants of sleep, particularly those associated with the restorative aspects of sleep. Both of these approaches suffer from a reliance on SD (prolonged wakefulness) which is thought to be stressful to experimental subjects at both the system (Gip et al. 2004) and cellular (Terao et al. 2003b) levels.
In contrast to the above experimental paradigms, induction of sleep by pharmacological means is likely less stressful to experimental subjects. In the case of induction of sleep by infusion of PGD2 and CGS21680 in particular, sleep induction occurs by changing the perfusion from saline to one of the above drugs (dissolved in saline) from a remote location, without handling the animal (Matsumura et al. 1995). Thus, this experimental paradigm is conceivably a powerful tool for the analysis of gene expression that accompanies a change in arousal state. However, sleep is induced by chemical means rather than occurring spontaneously. Thus, the present study sought to address two questions: (1) what changes in gene expression occur after activation of the PGD2 and adenosine A2a signaling pathways? and (2) how similar are these changes to those that occur during “normal” sleep (specifically, RS after SD)?
To determine whether activation of the PGD2 and adenosine A2a signaling pathways results in molecular changes similar to those incurred during RS after SD, we compared the expression of 13 genes, primarily from the IEG and HSP families, that had been characterized during RS in both mouse (Terao et al. 2003a; Terao et al. 2003b) and rat (Terao et al. 2006) brain. As indicated in Table 1, although 5 of these genes were upregulated during RS in the rat Cx (Terao et al. 2006), none of these 5 genes changed its expression during PGD2-induced sleep and only one gene, grp94, was upregulated in the Cx during CGS21680-induced sleep (Fig. 2A). Thus, on the basis of this admittedly small panel of biomarkers, we conclude that either (1) the molecular pathways that underlie PGD2- and CGS21680-induced sleep have little similarity to RS; (2) that the gene expression profiles induced by PGD2- and CGS21680 are more similar to prolonged wakefulness; or (3) that the elevated expression of these 5 biomarkers during RS are an artifact related to the SD which precedes the RS period. On the other hand, reduced levels of arc and IEGs have been reported in the cortex during spontaneous sleep (Cirelli et al., 2004). With respect to the third possibility, we (Wisor et al. 2006) have recently compared the expression of these same five genes in the Cx during RS to sleep induced by three systemically administered pharmacological agents known to affect different aspects of GABAergic neurotransmission: a GABAA agonist (zolpidem), a GABAA modulator (triazolam), and a GABAB agonist (gamma-hydroxybutyrate). Although SD and RS occurred in this study during the lights off period -- 12 h out of phase from the previous gene expression study (Terao et al. 2006) -- three of the five mRNAs (egr-3, grp78, and grp94) were still elevated during RS. Each of the drugs studied effectively induced sleep, but each drug induced only a subset of the molecular changes in the Cx associated with RS. We conclude that pharmacologically-induced sleep, whether induced by PGD2, CGS21680 or GABAergic agents, at most activates a subset of the molecular pathways that are activated during RS.
Table 1 provides other evidence that pharmacologically-induced sleep is distinct from RS. In the BF and Hy, although PGD2-induced sleep is accompanied by downregulation of the expression of arc and upregulation of c-fos, c-jun and jun-B and CGS21680-induced sleep is characterized by these same changes plus upregulation of ngfi-b in both regions and increased levels of ngfi-a and nr4a3 in the Hy, none of these changes occur in RS. It is likely that activation of these IEGs is due to the action of these compounds on adenosine A2a signaling pathways. On the other hand, one gene, fra-2, is upregulated in BF and Hy during PGD2- and CGS21680-induced sleep as well as RS (Fig. 2D and Table 1). The expression of fra-2 in the Cx has been shown to be related to the amplitude of EEG delta power (Wisor et al. 2006), although there is no evidence of elevation of this transcript in the Cx after PGD2 or CGS21680 treatment (Table 1).
PGD2 and A2a Signaling and GeneChip® Studies
As stated above, the other goal of this study was to identify changes in gene expression that occur after activation of the PGD2 and adenosine A2a signaling pathways that result in sleep induction. Table 1 provides an initial glimpse into these pathways and indicates that such activation is likely to be brain-region specific, since the BF and Hy appear to be more similar in their expression patterns than either region is to the Cx. For a broader assessment, we conducted GeneChip® studies in which we compared the expression of 1,322 gene elements during PGD2-and CGS21680-induced sleep across the three brain regions. Fig. 4A demonstrates that this general trend holds true for upregulated genes: the BF and Hy are more similar to each other than either area is to the Cx. However, this appears not to be the case for downregulated genes (Fig. 4B): about 50% more transcripts are downregulated in the BF than in either the Hy or Cx. Presumably, this reflects the proximity of the BF to the site of the infusion cannula that targeted the PGD2-SZ in the subarachnoid space.
As illustrated in Tables 2A and 2B, six genes were upregulated in all three brain regions in response to both PGD2 and CGS21680 treatment: peripheral-type benzodiazepine receptor, c3, gfap, hsp27, mt-1, and small inducible cytokine subfamily A20. Upregulation of three of these genes, c3, hsp27, and mt-1, was confirmed in all three regions by Taqman® analysis (Fig. 5). The peripheral benzodiazepine receptor (PBR) is a mitochondrial protein, involved in the regulation of cholesterol transport from the outer to the inner mitochondrial membrane, the rate-determining step in steroid hormone biosynthesis (Papadopoulos 2004). The PBR is highly conserved across the animal kingdom and a PBR-homologous sequence also exists in Arabidopsis. PBRs are involved in a functional structure designated as the mitochondrial permeability transition (MPT) pore which controls apoptosis (Jorda et al. 2005). The PBR has also been suggested as part of the mitochondrial membrane biogenesis process involved in increased cell proliferation (cancer, gliosis) and tissue repair (nerve damage and ischemia-reperfusion injury). Following various types of nerve injury, reactive gliosis is accompanied by high expression of the PBR, leading to the hypothesis that PBRs can be used as a sensitive marker for CNS injury (Gehlert et al. 1997). Complement component c3 is implicated in the pathogenesis of inflammatory disorders of the CNS such as multiple sclerosis, Alzheimer’s disease, and trauma (Boos et al. 2005). Complement c3 mRNA was upregulated 2–28 days post-optic nerve crush, indicating local synthesis of complement in the optic nerve (Ohlsson et al. 2003). GFAP is a well-known astrocytic marker and astrocytes proliferate at sites of neural injury. Hsp27 shows a distinctive widespread spatial and temporal pattern of induction in CNS glial and neuronal cells following kainate administration (Akbar et al. 2001). HSP27 mRNA level was increased 2.5-fold in the medial septum and 15-fold in the hippocampus 3 days after fimbria-fornix lesions; this increase persisted for 10 days (Anguelova and Smirnova 2000). Three and 10 days after lesion, HSP27 protein levels were increased in the septum (4.5 and 5-fold, respectively) and hippocampus (65 and 10-fold, respectively). Interestingly, the morphology of the HSP27-positive cells was indistinguishable from that of GFAP-immunoreactive cells (Anguelova and Smirnova 2000), suggesting that these cells are glia. Metallothionein-I and -II are anti-inflammatory, neuroprotective, antioxidant proteins expressed during experimental autoimmune encephalitis (EAE) and multiple sclerosis, in which they might play a protective role (Espejo et al. 2005). Several roles for MT action have been described in the CNS, including zinc metabolism, free radical scavenging, and protection and regeneration following neurological injury (Dittmann et al. 2005). According to SwissPROT database, Genbank accession #AF053312 described above as small inducible cytokine subfamily A20, has several other synonyms including CCL20, macrophage inflammatory protein 3 alpha (MIP-3-alpha), liver and activation-regulated chemokine (CC chemokine LARC), beta chemokine exodus-1 and CC chemokine ST38. CC chemokine ST38 was originally identified through its upregulation in ischemic brain tissue using a differential display technique (Utans-Schneitz et al. 1998). CCL20 is the only chemokine known to interact with receptor CCR6. Together, this ligand/receptor pair is responsible for the chemoattraction of immature dendritic cells, effector/memory T-cells and B-cells (Schutyser et al. 2003). MIP-3alpha expression is under the direct control of NF-kappaB, a key transcription factor of immune and inflammatory responses (Sugita et al. 2002). In relapsing EAE, the major intracerebral source of macrophage inflammatory protein-3alpha/CCL20 are astrocytes (Ambrosini et al. 2003). Taken together, these results suggest a pattern of gene expression induced in the brain after PGD2 or CGS21680 treatment that is distinct from that previously described during RS after SD (Terao et al. 2006) and may involve glial cell gene activation and the signaling pathways involved in neural-immune interactions. Interestingly, glial cell activation has been observed in previous studies of “spontaneous sleep” (Cirelli et al. 2004).
How do we integrate these results with our current understanding of the role of PGD2 and adenosine in sleep regulation? PGD2 and adenosine, a product of cellular metabolic activity, likely play key roles in the endogenous regulation of sleep, as demonstrated by the profound somnogenic effects evident in Fig. 1. The increases in NREMS and REMS that we observed after intracerebral PGD2 infusion replicate numerous reports that PGD2 induces physiologically normal sleep in a number of mammalian species (Hayaishi 2002; Huang et al. 2007). Sleep induction by intracerebral infusion of two other sleep-inducing factors, interleukin-1 and tumor necrosis factor-alpha (TNFα), may be dependent on the activation of prostaglandin synthesis (Terao et al. 1998a; Terao et al. 1998b; Krueger et al. 2001). TNFα activates the nuclear factor NFκB (Paik et al. 2002), which in turn can activate PGD2 gene expression (Ward et al. 2004). This cascade of events provides a possible mechanism for the induction of sleep by TNFα (Krueger et al. 2001). Since the expression of at least one of the genes upregulated in all three brain regions in response to both PGD2 and CGS21680 treatment, MIP-3alpha, is also under the direct control of NFκB (Sugita et al. 2002), this molecule may be a nexus of functional significance for cytokine-mediated sleep.
Activation of PGD2 receptors results in an increase in extracellular adenosine levels (Mizoguchi et al. 2001). Administration of a selective A2a-adenosine agonist (CGS21680), but not a selective A1-adenosine agonist (cyclohexyladenosine), results in increased sleep when administered to the PGD2-SZ (Satoh et al. 1996). The sleep-promoting effect of PGD2 was inhibited by pretreatment with KF17837, a highly selective A2a-adenosine antagonist. Adenosine receptor activation induces NFκB expression (Basheer et al. 2004), providing a means for crosstalk between the adenosinergic and prostaglandin signaling systems. The complex interactions of the adenosine and PGD2 signaling systems may allow for redundancy in sleep regulatory systems, and thus provides an explanation for the relatively modest effects of gene inactivation in either of these two systems on sleep (Mizoguchi et al. 2001; Hayaishi 2002; Hayaishi and Urade 2002; Stenberg et al. 2003; Urade et al. 2003). These interactions also provide a basis for the strong similarities in gene profiles that were observed between PGD2 and CGS21680 treatment groups. The considerable overlap in genes upregulated or downregulated by CGS21680 and PGD2 — the majority of genes induced by either agent were induced by both —further supports the contention that the effects of both of these agents ultimately converge on the same sleep regulatory pathways in the brain.
In contrast to the upregulated genes, there was a more limited set of genes that were downregulated by PGD2 and CGS21680 treatment (Table 3). Although gabra5 was the only transcript downregulated on the GeneChips® in all three regions by both drugs, this downregulation in the Cx was not confirmed by Taqman® analysis (p=0.08; Fig. 5G) although downregulation of igf-II was confirmed (Fig. 5F). The alpha5 subunit of the GABAA receptor appears to be necessary for the development of tolerance to the sedative action of diazepam in mice, in contrast to alpha1-GABAA receptors which mediate the acute sedative action of diazepam (van Rijnsoever et al. 2004). IGF-II expression is normally restricted to the mesenchymal support structures of the brain, including the choroid plexus and leptomeninges (DeChiara et al. 1991) where its expression is coincident with that of IGF binding protein-2. In the chronic phase after CNS injury (7–14 d), an increase in both IGF-II mRNA and protein was observed specifically and focally in the marginal astrocytes forming the limiting glial membrane of the wound (Walter et al. 1999).
Comparison to Previous GeneChip® Studies of RS
Collectively, the current report and a previous report from our laboratory (Terao et al. 2006) have identified genes that are upregulated in common among three conditions characterized by elevated levels of sleep and SWA: (1) RS after SD, (2) CGS21680 treatment, and (3) PGD2 treatment. Given the elevated levels of sleep after these treatments, parallel changes in gene expression might be expected. On the other hand, since CNS concentrations of PGD2 (Ram et al. 1997) and adenosine (Porkka-Heiskanen et al. 1997; Basheer et al. 2004) are elevated during sustained wake (not during sustained sleep), experimentally-induced increases in the levels of PGD2 or adenosinergic tone via CGS21680 infusion could induce changes in gene expression similar to those that occur during SD. Indeed, SD-upregulated genes were more in common with those that were induced by drug treatment (Fig. 6) than were RS-upregulated genes (Fig. 7), although the numbers are too small to provide firm conclusions. The upregulation of metallothionein genes encoding metal chelators implicated in ion homeostasis and detoxification (Burdette and Lippard 2003) in Cx by SD, PGD2 and CGS21680 (Table 4A) indicates an effect of all three treatments on the disposition of ionic species in the brain. Sleep may serve to counteract wake-associated changes in ion compartmentalization, as previous studies have postulated (Benington and Heller 1995; Cirelli et al. 2005) and perhaps serves a detoxification role (Inoue et al. 1995).
In our previous report (Terao et al. 2006), we found that BF and Cx were more similar to each other in response to SD than either region was to the Hy. In the current study, changes in gene expression were more similar in comparisons of BF to Hy than in comparisons of either region to Cx by several criteria. First, the number of upregulated genes was approximately two-to threefold greater in both Hy and BF than in Cx for both treatments (Fig. 4A). Second, upregulated genes were more common than downregulated genes in BF and Hy by at least 36%, whereas the numbers of genes upregulated in the Cx were equal to or fewer than downregulated genes for CGS21680 and PGD2 treatments, respectively (Fig. 4A vs. 4B). Third, while well over 20 gene elements were upregulated in common in BF and Hy by either treatment, fewer than eight were upregulated in common between Cx and either other region (Table 2). Finally, similarities in the functional categorization of genes affected by PGD2 or CGS21680, as delineated by GeneSpring’s ontological structure for gene categorization (Ashburner et al. 2000; Cirelli et al. 2004), are more common in comparisons between BF and Hy than in comparisons involving Cx (Table 5). Genes associated with induced cell growth (e.g., the immediate early serum-responsive JE gene, and signal transducer and activator of transcription 3- stat3) or cell death (e.g., tumor necrosis factor receptor superfamily, member 1a and caspase 1) make up more than one third of upregulated transcripts in both BF and Hy in both treatment groups but less than one quarter of such transcripts in Cx.
Table 5.
Functional classifications of genes upregulated or downregulated by PGD2 or CGS21680.
Percent of genes in each functional category whose mRNA expression levels were changed by at least 50% after PGD2 or CGS21680 infusion.
| Biological Process Category | # Genes on Chip | % Genes on Chip | % of mRNAs upregulated by PGD2 |
% of mRNAs upregulated by CGS21680
|
||||
|---|---|---|---|---|---|---|---|---|
| BF (n=86) | Cx (n=32) | Hy (n=92) | BF (n=116) | Cx (n=40) | Hy (n=70) | |||
| Cell Adhesion | 39 | 3 | 6 | 3 | 5 | 7 | 5 | 6 |
| Cell Maintenance, Growth and Death | 490 | 37 | 51 | 22 | 37 | 45 | 23 | 39 |
| Neurotransmission | 290 | 22 | 19 | 9 | 13 | 17 | 8 | 6 |
| Intracellular Signal | 132 | 10 | 7 | 6 | 1 | 11 | 0 | 1 |
| Physiology | 29 | 2 | 3 | 3 | 4 | 3 | 3 | 4 |
| Other | 342 | 26 | 14 | 56 | 39 | 16 | 63 | 44 |
| Biological Process Category | # Genes on Chip | # Genes on Chip | % of mRNAs downregulated by PGD2
|
% of mRNAs downregulated by CGS21680
|
||||
| BF (n=63) | Cx (n=42) | Hy (n=39) | BF (n=77) | Cx (n=40) | Hy (n=43) | |||
|
| ||||||||
| Cell Adhesion | 39 | 3 | 2 | 0 | 0 | 3 | 3 | 2 |
| Cell Maintenance, Growth and Death | 490 | 37 | 33 | 31 | 41 | 34 | 45 | 33 |
| Neurotransmission | 290 | 22 | 30 | 26 | 21 | 22 | 38 | 26 |
| Intracellular Signal | 132 | 10 | 8 | 10 | 13 | 10 | 5 | 12 |
| Physiology | 29 | 2 | 0 | 2 | 0 | 0 | 5 | 2 |
| Other | 342 | 26 | 27 | 31 | 26 | 31 | 5 | 26 |
The similarity of measurements from BF and Hy in the current study may be a consequence of the infusion site. Infusions were delivered into the subarachnoid space, which is bordered by the BF, ventral Hy and only a small portion of the Cx. Thus, absent any widespread diffusion of PGD2 or CGS21680 in the ventricular system, the bulk of the direct physiological response to infusions would be expected to occur in BF and Hy. In addition, the sleep promoting efficacy of adenosinergic agents (Satoh et al. 1999) and PGD2 (Matsumura et al. 1994) are greatest when they are targeted to the vicinity of BF and Hy. Therefore, the molecular response to PGD2 and CGS21680 might be expected to be most robust within BF and Hy. In contrast, any changes in gene expression in the Cx are likely to be secondary to the direct effects of these soporific agents on BF and Hy.
Limitations of the Present Study
Several limitations should be acknowledged in comparison of the present data with that from our previous study (Terao et al. 2006). First, the rats in the RS group (Terao et al. 2006) were instrumented only for EEG/EMG recording, whereas the rats in the PGD2- and CGS21680-treated groups were also prepared with intraparenchymal cannulae. Second, all animals (experimentals and controls) in the PGD2- and CGS21680-infusion experiment experienced an infusion during the experimental period, whereas the RS group did not. Third, the current study required no direct interactions between the experimenter and subjects during the experimental session (other than the moment when animals were euthanized) since infusions were performed remotely, whereas SD by gentle handling requires interaction between experimenter and subject. The differences between these two protocols in the amount of locomotor activity, stress and sensory input experienced by the subjects are therefore not trivial. Also, the RS group was sacrificed at ZT8 during the lights-on period, whereas the PGD2- and CGS21680-treated groups were sacrificed at ZT14 during the dark period. The RS began at a time of day (ZT6) when the propensity to sleep is high, but sleep need (as reflected in EEG delta power and sleep consolidation) is low and approaching its daily nadir. On the other hand, at the time when PGD2 and CGS21680-infused rats were killed (ZT14), sleep propensity is low due to the circadian waking signal but, in control rats, sleep need is increasing due to the fact that rats are mostly awake between ZT12 and ZT14. Lastly, the amount of sleep differed between these groups: in the RS group, sleep duration increased approximately 16% whereas sleep duration doubled during either pharmacological treatment in the present study.
Other limitations apply to the molecular approaches used in the present study. First, the GeneChip® results reported here are based on replicate pooled samples from each brain region in each treatment condition and, thus, rigorous statistical analysis of the GeneChip® data was not possible. On the other hand, followup Taqman® analyses of individual brain samples (Fig. 5) confirmed significant variation due to experimental treatment in 19 of the 21 comparisons (7 genes × 3 brain regions). Secondly, our analyses are based on brain regions and, consequently, may not have revealed nucleus- or cell type-specific changes in gene expression. Thirdly, the 50% threshold for inclusion of a gene in further analyses was arbitrary with no particular biological significance; there is no guarantee that a 2- or 3-fold change has any biological meaning or that an expression change < 50% is not meaningful. The 50% criterion was chosen to be as inclusive as possible, although it is recognized that there are likely to be more false positives in the gene lists than there would be if the criterion were a 3-fold change. On the other hand, a more stringent criterion would increase the likelihood of false negatives, which would render screening by GeneChip® of limited utility. Fourth, most of our attention in the analyses has focused on genes that are commonly up- or down-regulated in all three brain regions. Our logic for this focus was based on the assumption that mRNAs that are particularly important in mediating the response to PGD2 or CGS21680 are likely to have altered expression throughout the brain. However, as discussed above, Table 1 and Fig. 4 indicate that the BF and Hy may be more similar than the Cx with respect to upregulated genes whereas the Cx and Hy may be more similar than the BF with respect to downregulated genes. Lastly, it should be noted that the present analyses are based on transcript levels whereas the great majority of important cellular activity is mediated at the protein level.
Supplementary Material
Table S1 gene elements upregulated by PGD2.
Table S2 gene elements downregulated by PGD2.
Table S3 gene elements upregulated by CGS21680.
Table S4 gene elements downregulated by CGS21680.
Table S5 gene elements upregulated or downregulated in common by PGD2 and SD or by PGD2 and RS.
Table S6 gene elements upregulated or downregulated in common by CGS21680 and SD or by CGS21680 and RS.
Acknowledgments
Research supported by NIH RO1 HL/MH59658 and by a grant from the Genome Network Project from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Achermann P, Borbely AA. Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Hum Neurobiol. 1987;6:203–210. [PubMed] [Google Scholar]
- Akbar MT, Wells DJ, Latchman DS, et al. Heat shock protein 27 shows a distinctive widespread spatial and temporal pattern of induction in CNS glial and neuronal cells compared to heat shock protein 70 and caspase 3 following kainate administration. Brain Res Mol Brain Res. 2001;93:148–163. doi: 10.1016/s0169-328x(01)00199-1. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Columba-Cabezas S, Serafini B, et al. Astrocytes are the major intracerebral source of macrophage inflammatory protein-3alpha/CCL20 in relapsing experimental autoimmune encephalomyelitis and in vitro. Glia. 2003;41:290–300. doi: 10.1002/glia.10193. [DOI] [PubMed] [Google Scholar]
- Anguelova E, Smirnova T. Differential expression of small heat shock protein 27 in the rat hippocampus and septum after fimbria-fornix lesion. Neurosci Lett. 2000;280:99–102. doi: 10.1016/s0304-3940(00)00762-x. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, et al. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Boos L, Szalai AJ, Barnum SR. C3a expressed in the central nervous system protects against LPS-induced shock. Neurosci Lett. 2005;387:68–71. doi: 10.1016/j.neulet.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Burdette SC, Lippard SJ. Meeting of the minds: metalloneurochemistry. Proc Natl Acad Sci U S A. 2003;100:3605–3610. doi: 10.1073/pnas.0637711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C. How sleep deprivation affects gene expression in the brain: a review of recent findings. J Appl Physiol. 2002;92:394–400. doi: 10.1152/jappl.2002.92.1.394. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, Hill S, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- Dittmann J, Fung SJ, Vickers JC, et al. Metallothionein biology in the ageing and neurodegenerative brain. Neurotox Res. 2005;7:87–93. doi: 10.1007/BF03033779. [DOI] [PubMed] [Google Scholar]
- Espejo C, Penkowa M, Demestre M, et al. Time-course expression of CNS inflammatory, neurodegenerative tissue repair markers and metallothioneins during experimental autoimmune encephalomyelitis. Neuroscience. 2005;132:1135–1149. doi: 10.1016/j.neuroscience.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Stephenson DT, Schober DA, et al. Increased expression of peripheral benzodiazepine receptors in the facial nucleus following motor neuron axotomy. Neurochem Int. 1997;31:705–713. doi: 10.1016/s0197-0186(97)00007-7. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Beuckmann CT, Kanaoka Y, et al. Dominant expression of rat prostanoid DP receptor mRNA in leptomeninges, inner segments of photoreceptor cells, iris epithelium, and ciliary processes. J Neurochem. 1998;71:937–945. doi: 10.1046/j.1471-4159.1998.71030937.x. [DOI] [PubMed] [Google Scholar]
- Gip P, Hagiwara G, Sapolsky RM, et al. Glucocorticoids influence brain glycogen levels during sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1057–1062. doi: 10.1152/ajpregu.00528.2003. [DOI] [PubMed] [Google Scholar]
- Hayaishi O. Molecular genetic studies on sleep-wake regulation, with special emphasis on the prostaglandin D(2) system. J Appl Physiol. 2002;92:863–868. doi: 10.1152/japplphysiol.00766.2001. [DOI] [PubMed] [Google Scholar]
- Hayaishi O, Urade Y. Prostaglandin D2 in sleep-wake regulation: recent progress and perspectives. Neuroscientist. 2002;8:12–15. doi: 10.1177/107385840200800105. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol. 2007;7:33–38. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Inoue S, Honda K, Komoda Y. Sleep as neuronal detoxification and restitution. Behav Brain Res. 1995;69:91–96. doi: 10.1016/0166-4328(95)00014-k. [DOI] [PubMed] [Google Scholar]
- Jorda EG, Jimenez A, Verdaguer E, et al. Evidence in favour of a role for peripheral-type benzodiazepine receptor ligands in amplification of neuronal apoptosis. Apoptosis. 2005;10:91–104. doi: 10.1007/s10495-005-6064-9. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obal FJ, Fang J, et al. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Takahata R, Hayaishi O. Inhibition of sleep in rats by inorganic selenium compounds, inhibitors of prostaglandin D synthase. Proc Natl Acad Sci U S A. 1991;88:9046–9050. doi: 10.1073/pnas.88.20.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Kinoshita G, Satoh S, et al. A novel apparatus that permits multiple routes for infusions and body-fluid collections in a freely-moving animal. J Neurosci Methods. 1995;57:145–149. doi: 10.1016/0165-0270(94)00107-r. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Nakajima T, Osaka T, et al. Prostaglandin D2-sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain. Proc Natl Acad Sci U S A. 1994;91:11998–12002. doi: 10.1073/pnas.91.25.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Eguchi N, Kimura K, et al. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2001;98:11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson M, Bellander BM, Langmoen IA, et al. Complement activation following optic nerve crush in the adult rat. J Neurotrauma. 2003;20:895–904. doi: 10.1089/089771503322385827. [DOI] [PubMed] [Google Scholar]
- Onoe H, Ueno R, Fujita I, et al. Prostaglandin D2, a cerebral sleep-inducing substance in monkeys. Proc Natl Acad Sci U S A. 1988;85:4082–4086. doi: 10.1073/pnas.85.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J, Lee JY, Hwang D. Signaling pathways for TNFa-induced COX-2 expression: mediation through MAP kinases and NFkB, and inhibition by certain nonsteroidal anti-inflammatory drugs. Adv Exp Med Biol. 2002;507:503–508. doi: 10.1007/978-1-4615-0193-0_77. [DOI] [PubMed] [Google Scholar]
- Pandey HP, Ram A, Matsumura H, et al. Concentration of prostaglandin D2 in cerebrospinal fluid exhibits a circadian alteration in conscious rats. Biochem Mol Biol Int. 1995;37:431–437. [PubMed] [Google Scholar]
- Papadopoulos V. In search of the function of the peripheral-type benzodiazepine receptor. Endocr Res. 2004;30:677–684. doi: 10.1081/erc-200043971. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, et al. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, et al. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci U S A. 2006;103:17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram A, Pandey HP, Matsumura H, et al. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Res. 1997;751:81–89. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Suzuki F, et al. Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc Natl Acad Sci U S A. 1996;93:5980–5984. doi: 10.1073/pnas.93.12.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Koike N, et al. Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur J Neurosci. 1999;11:1587–1597. doi: 10.1046/j.1460-9568.1999.00569.x. [DOI] [PubMed] [Google Scholar]
- Scammell T, Gerashchenko D, Urade Y, et al. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci U S A. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Stenberg D, Litonius E, Halldner L, et al. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res. 2003;12:283–290. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- Sugita S, Kohno T, Yamamoto K, et al. Induction of macrophage-inflammatory protein-3alpha gene expression by TNF-dependent NF-kappaB activation. J Immunol. 2002;168:5621–5628. doi: 10.4049/jimmunol.168.11.5621. [DOI] [PubMed] [Google Scholar]
- Takahata R, Matsumura H, Kantha SS, et al. Intravenous administration of inorganic selenium compounds, inhibitors of prostaglandin D synthase, inhibits sleep in freely moving rats. Brain Res. 1993;623:65–71. doi: 10.1016/0006-8993(93)90010-k. [DOI] [PubMed] [Google Scholar]
- Terao A, Matsumura H, Saito M. Interleukin-1 induces slow-wave sleep at the prostaglandin D2-sensitive sleep-promoting zone in the rat brain. J Neurosci. 1998a;18:6599–6607. doi: 10.1523/JNEUROSCI.18-16-06599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao A, Matsumura H, Yoneda H, et al. Enhancement of slow-wave sleep by tumor necrosis factor-alpha is mediated by cyclooxygenase-2 in rats. Neuroreport. 1998b;9:3791–3796. doi: 10.1097/00001756-199812010-00005. [DOI] [PubMed] [Google Scholar]
- Terao A, Greco MA, Davis RW, et al. Region-specific changes in immediate early gene expression in response to sleep deprivation and recovery sleep in the mouse brain. Neuroscience. 2003a;120:1115–1124. doi: 10.1016/s0306-4522(03)00395-6. [DOI] [PubMed] [Google Scholar]
- Terao A, Wisor JP, Peyron C, et al. Gene expression in the rat brain during sleep deprivation and recovery sleep: an Affymetrix GeneChip study. Neuroscience. 2006;137:593–605. doi: 10.1016/j.neuroscience.2005.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao A, Steininger TL, Hyder K, et al. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience. 2003b;116:187–200. doi: 10.1016/s0306-4522(02)00695-4. [DOI] [PubMed] [Google Scholar]
- Urade Y, Eguchi N, Qu WM, et al. Sleep regulation in adenosine Ay receptor-deficient mice. Neurology. 2003;61:S94–96. doi: 10.1212/01.wnl.0000095222.41066.5e. [DOI] [PubMed] [Google Scholar]
- Utans-Schneitz U, Lorez H, Klinkert WE, et al. A novel rat CC chemokine, identified by targeted differential display, is upregulated in brain inflammation. J Neuroimmunol. 1998;92:179–190. doi: 10.1016/s0165-5728(98)00204-5. [DOI] [PubMed] [Google Scholar]
- van Rijnsoever C, Tauber M, Choulli MK, et al. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004;24:6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter HJ, Berry M, Hill DJ, et al. Distinct sites of insulin-like growth factor (IGF)-II expression and localization in lesioned rat brain: possible roles of IGF binding proteins (IGFBPs) in the mediation of IGF-II activity. Endocrinology. 1999;140:520–532. doi: 10.1210/endo.140.1.6463. [DOI] [PubMed] [Google Scholar]
- Ward C, Walker A, Dransfield I, et al. Regulation of granulocyte apoptosis by NF-kappaB. Biochem Soc Trans. 2004;32:465–467. doi: 10.1042/BST0320465. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Morairty SR, Huynh NT, et al. Gene expression in the rat cerebral cortex: comparison of recovery sleep and hypnotic-induced sleep. Neuroscience. 2006;141:371–378. doi: 10.1016/j.neuroscience.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 gene elements upregulated by PGD2.
Table S2 gene elements downregulated by PGD2.
Table S3 gene elements upregulated by CGS21680.
Table S4 gene elements downregulated by CGS21680.
Table S5 gene elements upregulated or downregulated in common by PGD2 and SD or by PGD2 and RS.
Table S6 gene elements upregulated or downregulated in common by CGS21680 and SD or by CGS21680 and RS.