Abstract
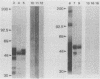
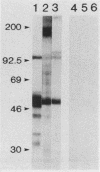
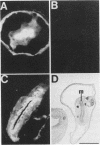
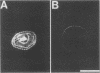
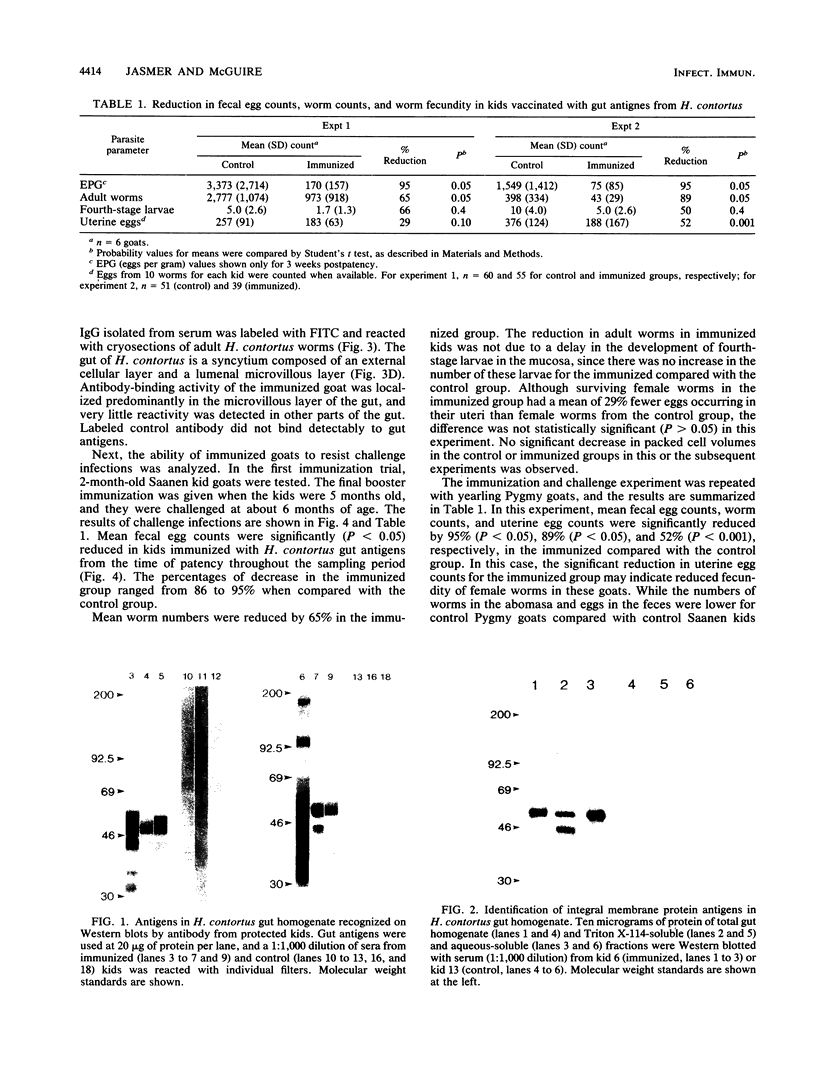
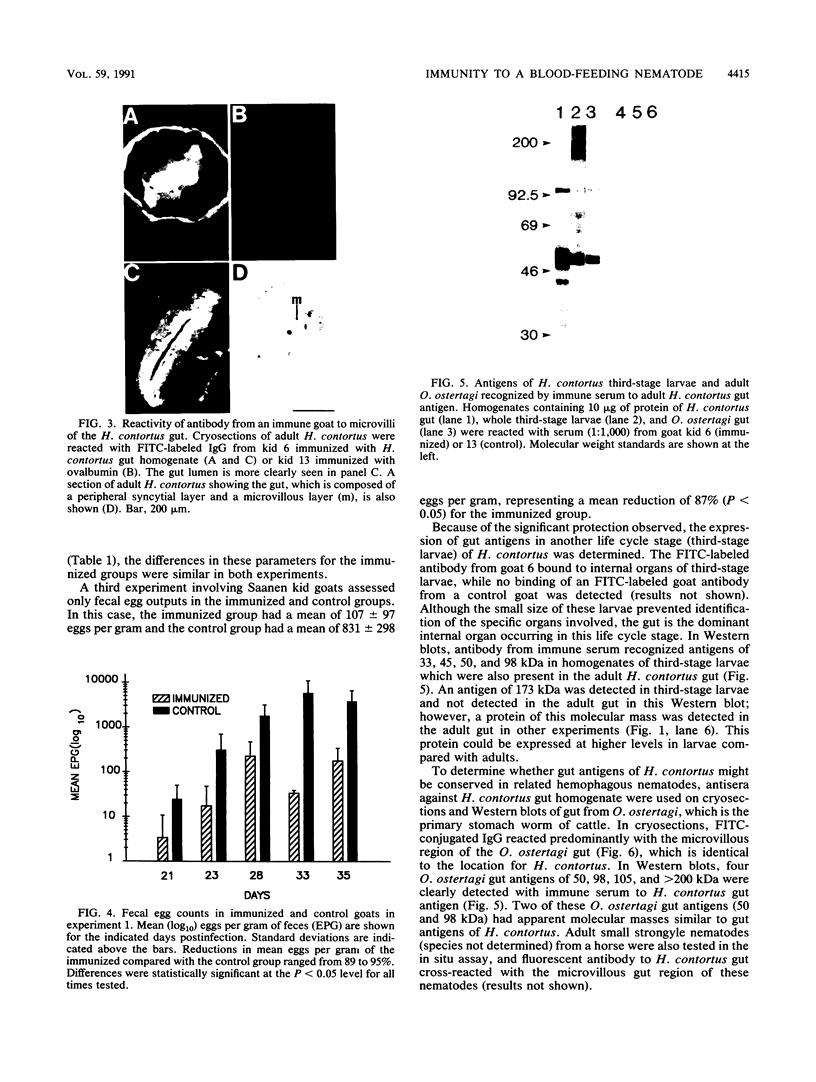
To determine the ability of gut antigens to induce a protective immune response against blood-feeding nematodes, isolated gut antigens were used to immunize goats against Haemonchus contortus. Immunization-induced antibody responses recognized parasite gut antigens which were associated predominantly with the microvillous membrane region of the parasite gut. Antibody from immune serum also recognized seven predominant gut proteins on a Western blot (immunoblot). Several of these proteins appeared to be integral membrane proteins on the basis of their solubility in the detergent Triton X-114, indicating that the presentation protocol stimulated an antibody response to microvillous membrane antigens. Three different age groups of goats ranging from less than 6 months to greater than 1 year were immunized for challenge experiments. After infection with 10(4) larvae, an 87 to 95% reduction in fecal egg counts for all age groups of goats was achieved in the immunized compared with the control group. The reduction of worms in immunized goats ranged from 65% (kids) to 89% (yearlings) compared with controls. These results indicate that gut antigens can induce significant protection against blood-feeding nematodes. Antibody to H. contortus gut antigens also cross-reacted with microvilli of other blood-feeding nematodes including Ostertagia ostertagi and small strongyles of horses, which indicates that epitopes associated with the gut are phylogenetically conserved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- CHRISTIE M. G., BRAMBELL M. R., CHARLESTON W. A. RESISTANCE TO A CHALLENGE INFECTION WITH HAEMONCHUS CONTORTUS CONFERRED BY PREVIOUS EXPERIENCE OF IMMATURE STAGES ONLY. J Comp Pathol. 1964 Oct;74:427–434. doi: 10.1016/s0368-1742(64)80049-7. [DOI] [PubMed] [Google Scholar]
- Clemetson K. J., Bienz D., Zahno M. L., Lüscher E. F. Distribution of platelet glycoproteins and phosphoproteins in hydrophobic and hydrophilic phases in Triton X-114 phase partition. Biochim Biophys Acta. 1984 Dec 19;778(3):463–469. doi: 10.1016/0005-2736(84)90395-x. [DOI] [PubMed] [Google Scholar]
- Crawford T. B., McGuire T. C., Henson J. B. Detection of equine infectious anemia virus in vitro by immunofluorescence. Arch Gesamte Virusforsch. 1971;34(4):332–339. doi: 10.1007/BF01242979. [DOI] [PubMed] [Google Scholar]
- Egerton J. R., Suhayda D., Eary C. H. Laboratory selection of Haemonchus contortus for resistance to ivermectin. J Parasitol. 1988 Aug;74(4):614–617. [PubMed] [Google Scholar]
- Gamble H. R., Purcell J. P., Fetterer R. H. Purification of a 44 kilodalton protease which mediates the ecdysis of infective Haemonchus contortus larvae. Mol Biochem Parasitol. 1989 Feb;33(1):49–58. doi: 10.1016/0166-6851(89)90041-8. [DOI] [PubMed] [Google Scholar]
- Klei T. R. Immunity and potential of vaccination. Vet Clin North Am Equine Pract. 1986 Aug;2(2):395–402. doi: 10.1016/s0749-0739(17)30724-1. [DOI] [PubMed] [Google Scholar]
- Maher P. A., Singer S. J. Anomalous interaction of the acetylcholine receptor protein with the nonionic detergent Triton X-114. Proc Natl Acad Sci U S A. 1985 Feb;82(4):958–962. doi: 10.1073/pnas.82.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. A. Hookworm infection in man. Adv Parasitol. 1979;17:315–384. doi: 10.1016/s0065-308x(08)60552-7. [DOI] [PubMed] [Google Scholar]
- Miller T. A. Industrial development and field use of the canine hookworm vaccine. Adv Parasitol. 1978;16:333–342. doi: 10.1016/s0065-308x(08)60577-1. [DOI] [PubMed] [Google Scholar]
- Miller T. A. Vaccination against the canine hookworm diseases. Adv Parasitol. 1971;9:153–183. doi: 10.1016/s0065-308x(08)60161-x. [DOI] [PubMed] [Google Scholar]
- Munn E. A., Greenwood C. A., Coadwell W. J. Vaccination of young lambs by means of a protein fraction extracted from adult Haemonchus contortus. Parasitology. 1987 Apr;94(Pt 2):385–397. doi: 10.1017/s0031182000054032. [DOI] [PubMed] [Google Scholar]
- Neilson J. T., Van de Walle M. J. Partial protection of lambs against Haemonchus contortus by vaccination with a fractionated preparation of the parasite. Vet Parasitol. 1987 Feb;23(3-4):211–221. doi: 10.1016/0304-4017(87)90007-0. [DOI] [PubMed] [Google Scholar]
- Opdebeeck J. P., Wong J. Y., Dobson C. Hereford cattle protected against Boophilus microplus with antigens purified by immunoaffinity chromatography from larval and adult ticks. Immunology. 1989 Jul;67(3):388–393. [PMC free article] [PubMed] [Google Scholar]
- Opdebeeck J. P., Wong J. Y., Jackson L. A., Dobson C. Hereford cattle immunized and protected against Boophilus microplus with soluble and membrane-associated antigens from the midgut of ticks. Parasite Immunol. 1988 Jul;10(4):405–410. doi: 10.1111/j.1365-3024.1988.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Preston J. M., Allonby E. W. The influence of breed on the susceptibility of sheep of Haemonchus contortus infection in Kenya. Res Vet Sci. 1979 Mar;26(2):134–139. [PubMed] [Google Scholar]
- Prichard R. K., Hall C. A., Kelly J. D., Martin I. C., Donald A. D. The problem of anthelmintic resistance in nematodes. Aust Vet J. 1980 May;56(5):239–251. doi: 10.1111/j.1751-0813.1980.tb15983.x. [DOI] [PubMed] [Google Scholar]
- Reduker D. W., Jasmer D. P., Goff W. L., Perryman L. E., Davis W. C., McGuire T. C. A recombinant surface protein of Babesia bovis elicits bovine antibodies that react with live merozoites. Mol Biochem Parasitol. 1989 Jul;35(3):239–247. doi: 10.1016/0166-6851(89)90210-7. [DOI] [PubMed] [Google Scholar]
- Smith W. D., Angus K. W. Haemonchus contortus: attempts to immunise lambs with irradiated larvae. Res Vet Sci. 1980 Jul;29(1):45–50. [PubMed] [Google Scholar]
- Wescott R. B. Anthelmintics and drug resistance. Vet Clin North Am Equine Pract. 1986 Aug;2(2):367–380. doi: 10.1016/s0749-0739(17)30722-8. [DOI] [PubMed] [Google Scholar]
- Willadsen P., Riding G. A., McKenna R. V., Kemp D. H., Tellam R. L., Nielsen J. N., Lahnstein J., Cobon G. S., Gough J. M. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J Immunol. 1989 Aug 15;143(4):1346–1351. [PubMed] [Google Scholar]