Abstract
Concern exists about accepting live kidney donation from “medically complex donors” -those with risk factors for future kidney disease. This study’s aim was to examine variation in complex kidney donor use across United States (US) transplant centers. We conducted a retrospective cohort study of live kidney donors using Organ Procurement and Transplantation Network data. Donors with hypertension, obesity, or estimated glomerular filtration rate (eGFR) <60 ml/minute/1.73m2 were considered medically complex. Among 9319 donors, 2254 (24.2%) were complex: 1194 (12.8%) were obese, 956 (10.3%) hypertensive, and 392 (4.2%) had low eGFR. The mean proportion of medically complex donors at a center was 24% (range 0 – 65%.) In multivariate analysis, donor characteristics associated with medical complexity included spousal relationship to the recipient (OR 1.29, CI 1.06-1.56, p<0.01), low education (OR 1.19, CI 1.04-1.37, p=0.01), older age (OR 1.01 per year, CI 1.01-1.02, p<0.01), and non-US citizenship (OR 0.70, CI 0.51-0.97, p=0.01). Renal transplant centers with the highest transplant volume (OR 1.26, CI 1.02-1.57, p=0.03), and with a higher proportion of (living donation)/(all kidney transplants) (OR 1.97, CI 1.23-3.16, p<0.01) were more likely to use medically complex donors. Though controversial, the use of medically complex donors is widespread and varies widely across centers.
Introduction
The burgeoning wait-list for deceased donor kidney transplantation has driven a steady increase in live donor transplantation in the United States (US).(1, 2) Substantial concerns persist, however, about the acceptability of live donors with medical risk factors for future kidney disease. Examples of such risk factors include hypertension, obesity, and low glomerular filtration rate.(3-10) We refer to this group as “medically complex donors.”(11) We favor this term because it lacks stigma and reflects the challenging decision-making involved in counseling and accepting these donors in the absence of data about long-term risk of kidney disease.
Epidemiological studies of live donors suggest that their average risk of developing end-stage renal disease is less than 1%.(12-18) These studies, however, have not examined outcomes for the subset of complex live donors. In July 2007, the Organ Procurement Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) released a policy proposal regarding live donors that highlights the challenging clinical and ethical issues at stake. This proposal outlines policies to protect donors from coercion and identifies relative contraindications to live kidney donation such as elevated blood pressure and obesity.(19)
Appropriate live donor kidney transplantation requires transplant centers to consider simultaneously the interests of donors and recipients.(20, 21) These ethical duties are typically discharged through careful medical and psychological evaluation of the live donor and the processes of informed consent.(11, 22) However, transplant centers and staff may have strong and understandable motivations to accept medically complex live donors. The staff may feel an ethical responsibility toward recipients to accept medically complex donors. Additionally, centers must maintain adequate surgical volume because volume is a quality benchmark with which centers are compared and because volume helps pay for expensive infrastructure.(23, 24) Acceptance of medically complex live donors enables centers to increase transplant volume and generate needed revenue.(8, 11, 24, 25)
Live donor transplantation provides superior outcomes for recipients and does not require time on the wait-list.(6) Recipients and donors themselves, therefore, may put pressure on transplant staff to accept complex live donors. For donors, the choice to donate may stem from a sense of duty toward the recipient. Many donors report high satisfaction with the experience of organ donation.(26) In some cases, however, donors may feel coerced. The OPTN has Bylaw provisions that require transplant centers to protect donors from such coercion, such as through the use of a donor advocate.(19)
The aims of this study were to determine the prevalence of medically complex donor use for kidney transplantation in the United States and to identify donor, recipient, and center-level attributes associated with the acceptance of complex live donors. We hypothesized that parental and spousal donors would be more likely to be medically complex compared to other donors. We also hypothesized that medically complex live donors would be more likely to be accepted at centers with the highest volume of kidney transplants and at centers in Donor Service Areas (DSAs) with higher levels of market competitiveness. Each of these hypotheses reflects our view that the use of medically complex donors responds to demand perceived by donors, recipients, and center staff.(23, 27-29)
Materials and Methods
We used data from the OPTN to perform a retrospective cohort study of all live kidney donors in the US during an 18-month period from July 2004 through December 2005. These dates were selected because OPTN first began collecting data about donor weight and height from renal transplant centers in July 2004. Data obtained from the OPTN included clinical and demographic characteristics of donors and recipients, and the center where the transplant took place. The final dataset also included number of kidney transplants (live and deceased) at each center during this time period, and the median days on the wait-list for a deceased donor kidney transplant in each center’s DSA.
Outcome
We defined medically complex donors as those with any one of the following binary attributes, all of which were measured prior to donation: hypertension, obesity, or low GFR. Hypertension is a well-established risk factor for chronic kidney disease. The dataset reported whether the donor had a history of hypertension, as well as the donor’s pre-donation blood-pressure, but not use of blood pressure medications. Consistent with the JNC-VII report on hypertension, we defined donors as hypertensive if a history of hypertension was reported, if pre-donation systolic blood pressure was ≥140mm Hg, or if diastolic blood pressure was ≥90 mm Hg.(30) Obesity in the general population is associated with proteinuria, chronic kidney disease, and end-stage renal disease.(3, 31-33) Obesity has also been associated with renal insufficiency after nephrectomy.(34) We defined donors as obese if their body mass index (BMI) was ≥30.(35) In our study, low GFR was defined as estimated GFR (eGFR) < 60 ml/min/1.73m2. eGFR was calculated with the 4-variable Modification of Diet in Renal Disease (MDRD) equation. The 4-variable equation uses serum creatinine, age, race and gender.(36) GFR <60 ml/min/1.73m2 corresponds to chronic kidney disease stage III as established by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative.(37) Although eGFR may underestimate the measured or “actual” GFR, a study of live donors demonstrated that a pre-nephrectomy eGFR<69ml/minute/1.73m2 predicted a post-nephrectomy GFR<60ml/minute/1.73m2 as measured by iothalamate clearance.(38) Therefore, our study’s criterion for medical complexity of pre-nephrectomy eGFR<60ml/minute/1.73m2 is likely to identify individuals with reduced kidney function after donation.
Primary and secondary analyses
Our primary analysis was limited to donors for whom no data related to complexity were missing (these data include past history of hypertension, blood pressure, weight, height, serum creatinine, race, gender and age.) Patients without these missing data are referred to as the ‘primary cohort’.
For completeness, a secondary analysis was implemented among all donors, including those with missing data on medical complexity. Patients included in this secondary analysis are referred to as the ‘complete cohort’.
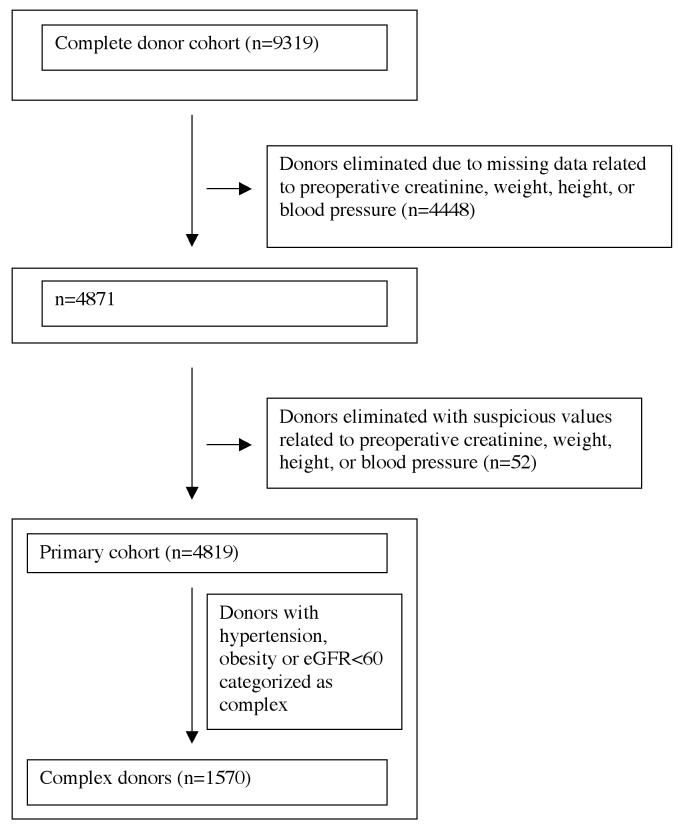
Generation of the primary cohort is depicted in Figure 1.40
Figure 1.
Generation of primary cohort
Elimination of clinically suspect data related to complexity
To eliminate clinically suspicious values, we coded as “missing” those values that seemed implausible for a patient accepted as a donor. We assumed that these data were incorrectly entered into the database.
The following pre-nephrectomy values were coded as missing: systolic blood pressure >170mm Hg or <80mmg Hg; diastolic blood pressure >105 or <40mm Hg; serum creatinine <0.5mg/dL or >2mg/dL; eGFR < 30 ml/min/1.73m2; weight <35kg; height<122cm; body mass index >45. Less than 1% of patients had data outside these thresholds.
Missing data on covariates unrelated to donor complexity
For binary characteristics (such as recipient history of prior dialysis), if an individual had a missing value in the OPTN dataset, we analyzed that individual as if he or she did not have the characteristic.
For interval scale variables (peak panel reactive antigen and mean days on the transplant wait-list), we imputed mean values for missing data. Unadjusted comparisons of medically complex and non-complex donors were similar before and after imputation for these variables.
Patient level characteristics
We explored for associations between medical complexity and the following donor characteristics: age, gender, ethnicity, relationship to recipient, US citizenship, and education level.
We similarly examined associations between donor medical complexity and characteristics of live donor kidney recipients that could have influenced whether a complex donor was used, including: age, gender, ethnicity, education level, blood type, end-stage renal disease caused by diabetes, peak panel reactive antigen (PRA), duration on the wait list, and prior renal transplantation.
Center level characteristics
Center volume
Center volume of live and deceased donor transplants had a non-Gaussian distribution with a rightward skew. Therefore, similar to prior studies, we categorized centers as having low, intermediate, or high volume of kidney transplants (live and deceased donor combined) by dividing centers into tertiles.(23) Centers in the lowest volume category performed a maximum of 54 kidney transplants during the study period. Centers in the intermediate volume category performed 55 - 123 kidney transplants while centers in the highest volume category performed >123 kidney transplants.
Proportion of (living donation)/(all kidney transplants)
This linear variable was calculated from center volume data.
Median wait-list time in the center’s donor service area
We obtained from UNOS the median wait-time for a deceased donor kidney in each DSA, using a cohort of patients wait-listed in 1999 (the most recent year for which this data was available for every DSA).
Market competitiveness
We defined the market to be the area covered by a DSA. We measured market competitiveness for each DSA by calculating the Herfindahl-Hirschman index (HHI), a measure of market concentration. The Department of Justice has used the HHI in the investigation of monopoly.(39, 40) The HHI has also been used by academic investigators, including those researching transplant centers, to examine the effects of market competitiveness on clinical practice and outcomes.(28, 41) The HHI is calculated as the sum of the squared market shares of each of the centers in a DSA. For example, a DSA with two transplant centers, one of which has 25% of the market and the other 75%, has a HHI of 0.252 + 0.752= 0.625. A DSA with a single center that has the entire market share would have a HHI of 1. The HHI varies between zero and one - an index of one indicates no competition and decreases in the index reflect higher competition.(39, 40)
The HHI had a U-shaped, non-Gaussian distribution. Therefore, a priori, we divided centers into three tertiles based on HHI values.
Data management and statistical analysis
Data were obtained from UNOS and transferred to STATA for analysis (Stata 9.0, Stata Corporation, College Station, TX.)
Association with outcome
The means of continuous variables for complex and non-complex donors were compared using the t-test. Categorical attributes were compared across these 2 groups using chi-square. All analyses and associations were considered significant when p<0.05.
A logistic regression model for the binary outcome of donor complexity was fit using individual-level (such as parental relationship to the recipient) and center-level variables (such as the volume of transplants at a center). All donor, recipient and center characteristics examined in univariate analysis were entered into the multivariate model (the complete list of these characteristics may be found in Table 5).
Table 5.
Univariate analysis of the primary cohort - donor, recipient and transplant center characteristics associated with donor medical complexity
| Primary cohort n=4819 |
Complete cohort n=9319 |
|||||
|---|---|---|---|---|---|---|
| Donor attribute (binary) |
OR | C.I. | p | OR | C.I. | p |
| Parent | 1.25 | 1.00-1.55 | 0.049 | 1.21 | 1.03-1.43 | 0.02 |
| Spouse | 1.33 | 1.11-1.58 | <0.01 | 1.23 | 1.07-1.41 | <0.01 |
| Female | 0.92 | 0.81-1.03 | 0.16 | 0.90 | 0.91-0.98 | 0.02 |
| Hispanic | 1.05 | 0.88-1.25 | 0.60 | 0.94 | 0.82-1.09 | 0.43 |
| Black | 1.26 | 1.07-1.49 | <0.01 | 1.12 | 0.98-1.28 | 0.09 |
| Low education * | 1.21 | 1.06-1.38 | <0.01 | 1.18 | 1.07-1.32 | <0.01 |
| Non US citizen | 0.68 | 0.50-0.91 | <0.01 | 0.74 | 0.59-0.94 | 0.01 |
|
Donor attribute (continuous) |
Complex |
Non- complex |
p | Complex |
Non- complex |
p |
| Age | 41.1 | 39.4 | <0.01 | 41.8 | 39.9 | <0.01 |
|
Recipient attribute (binary) |
OR | C.I. | p | OR | C.I. | p |
| Female | 1.10 | 0.97-1.24 | 0.13 | 1.09 | 0.99-1.20 | 0.08 |
| Hispanic | 1.05 | 0.88-1.25 | 0.57 | 0.96 | 0.83-1.11 | 0.55 |
| Black | 1.25 | 1.06-1.46 | <0.01 | 1.11 | 0.98-1.26 | 0.11 |
| Low education * | 1.08 | 0.95-1.22 | 0.23 | 1.08 | 0.98-1.19 | 0.13 |
| Cause ESRD: Diabetes |
1.12 | 0.97-1.29 | 0.14 | 1.12 | 1.00-1.26 | 0.045 |
| Dialysis | 1.11 | 0.98-1.26 | 0.12 | 1.09 | 0.99-1.20 | 0.09 |
| Prior kidney transplant |
0.99 | 0.81-1.21 | 0.94 | 0.93 | 0.79-1.09 | 0.38 |
| Blood type O | 0.92 | 0.82-1.04 | 0.21 | 0.95 | 0.87-1.05 | 0.32 |
|
Recipient attribute (continuous) |
Complex |
Non- complex |
P | Complex |
Non- complex |
p |
| Age (years) | 47.3 | 46.4 | 0.03 | 47.2 | 46.4 | 0.02 |
| Mean duration wait-list (days) ** |
313.0 | 333.6 | 0.055 | 312.2 | 324.0 | 0.14 |
| Peak PRA** | 10.1 | 9.7 | 0.54 | 9.3 | 9.8 | 0.27 |
|
Center attribute (Ordinal) |
||||||
|
% Complex donors |
p |
% Complex donors |
p | |||
| Category of center volume |
||||||
| Low | 28.7 | Reference | 24.3 | Reference | ||
| Intermediate | 31.9 | 0.19 | 24.6 | 0.88 | ||
| High | 33.5 | 0.04 | 24.0 | 0.88 | ||
| Category of market competitiveness *** |
||||||
| Low | 34.8 | 0.01 | 25.3 | 0.11 | ||
| Intermediate | 32.4 | 0.22 | 23.7 | 0.81 | ||
| High | 30.4 | Reference | 23.5 | Reference | ||
|
Center attribute (continuous) |
Complex |
Non- Complex |
p | complex |
Non- complex |
p |
| Proportion of live donors/total donors |
43.7% | 42.7% | 0.01 | 43.7% | 42.8% | <0.01 |
| Median days of wait-list time for deceased donor kidney in the center’s DSA |
1409.3 | 1464.8 | <0.01 | 1404.9 | 1462.6 | <0.01 |
Abbreviations ESRD: End-stage renal disease; PRA=panel reactive antigen; DSA=donor service area
Defined as no years of college education
Mean values were imputed for missing data on duration of waiting list time and peak PRA
Market competitiveness as measured by the Herfindahl-Hirschmann index; the “market” here is centers performing renal transplants within a single donor service area
Results
The complete cohort included 9319 live donors, of which 5550 (59.6%) were female. 6395 (68.6%) of these donors were white, 1325 (14.2%) were black, and 1165 (12.5%) were Hispanic. The majority (62.9%) of live donors were biologically related to the recipients. 1174 (12.6%) were spouses and 770 (8.3%) percent were parents. At least 2423 (26.0%) donors had no years of college education. The individual characteristics of the donors are presented in Table 1.
Table 1.
Demographic and clinical characteristics of live kidney donors (n=9319) at the time of transplantation in the complete cohort
| Donor attribute | Number |
|---|---|
| Age (s.d.) | 40.3 years (11.0) |
| Female (%) | 5550 (59.6) |
| Race (%) | |
| White | 6395 (68.6) |
| Black | 1325 (14.2) |
| Hispanic | 1165 (12.5) |
| Asian | 310 (3.3) |
| Other | 124 (1.3) |
| US Citizen (%) | 8859 (95.1) |
| Education (%) | |
| Grade school or less | 160 (1.7) |
| High school | 2263 (24.3) |
| Attended college/technical school | 1883 (20.2) |
| Associate/Bachelor degree | 1549 (16.6) |
| Graduate degree | 615 (6.6) |
| Unknown | 2849 (30.6) |
| Relation to recipient (%) | |
| Full sibling | 2521 (27.1) |
| Child | 1751 (18.8) |
| Spouse | 1174 (12.6) |
| Other biological | 692 (7.4) |
| Parent | 770 (8.3) |
| Non-biological, unrelated: Anonymous Donation | 123 (1.3) |
| Half-sibling | 107 (1.2) |
| Life partner | 61 (0.7) |
| Non-biological, unrelated: Paired Exchange | 45 (0.5) |
| Twin | 21 (0.3) |
| Non-biological, live/Deceased Donor Exchange | 13 (0.1) |
| Unrelated, directed donation | 2039 (21.9) |
Table 2 depicts the 2254 donors (24.2%) in the complete cohort who met criteria for medical complexity. This proportion assumes that donors with missing data, who did not otherwise meet criteria for medical complexity, were not complex. 1194 donors (12.8%) were obese, 956 (10.3%) were hypertensive, and 392 (4.2%) had an eGFR<60ml/min/1.73m2. 249 donors (2.7%) met more than one criterion for complexity; among these, 243 donors met 2 criteria for complexity, and 6 met 3 criteria.
Table 2.
Donors meeting criteria for medical complexity in the complete cohort
| Criteria for medical complexity | Number (%) |
|---|---|
| Any criterion | 2254 (24.2) |
| Obese (BMI ≥ 30) | 1194 (12.8) |
| Hypertensive (history of HTN, systolic BP ≥140, and/or diastolic BP ≥90mmHg) |
956 (10.3) |
| Low eGFR (< 60ml/min/m2) | 392 (4.2) |
| More than 1 criterion | 249 (2.7) |
Table 3 shows recipient characteristics. The mean age of recipients was 46.6 years. 3799 (40.8%) were female. 6210 (66.6%) were white, 1460 (15.6%) were black and 1146 (12.3%) were Hispanic.
Table 3.
Demographic and clinical characteristics of recipients of live donor kidney transplants in the complete cohort (n=9319)
| Recipient Attribute | |
|---|---|
| Age (s.d.) | 46.6 (13.7) |
| Female (%) | 3799 (40.8) |
| Race (%) | |
| White | 6210 (66.6) |
| Black | 1460 (15.6) |
| Hispanic | 1146 (12.3) |
| Asian | 350 (3.8) |
| Other | 153 (1.6) |
| Cause ESRD: Diabetes (%) | 1993 (21.4) |
| Chronic dialysis (%) | 5626 (60.4) |
| Peak panel reactive antigen (s.d) | 9.68 (22.5) |
| Prior kidney transplant (%) | 938 (10.1) |
| Blood type | |
| A | 3375 (36.2) |
| B | 1246 (13.4) |
| AB | 328 (3.5) |
| O | 4199 (45.1) |
| Other | 171 (0.2) |
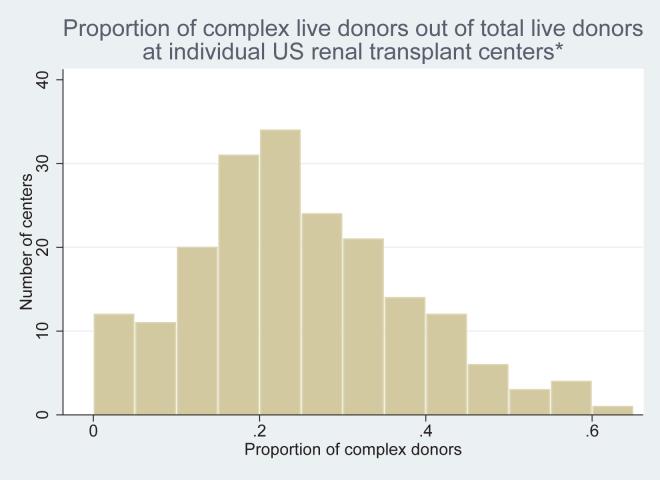
Center attributes are shown in Table 4. Live donors were distributed over 224 renal transplant centers. For centers performing > 5 live donor transplants in the period studied, the mean proportion of medically complex donors at an individual center was 24% (standard deviation 13%), with a range from 0 – 65% (see Figure 2).
Table 4.
Characteristics of transplant centers (n=224)
| Center attribute | Number |
|---|---|
| Mean center-specific number of kidney transplants (live and deceased donor) |
109 (1 - 474) |
| Mean center-specific number of live donors (range) | 88 (1 - 263) |
| Mean center-specific proportion of complex live donors +/- s.d. (range) * | 0.24 +/-0.13 (0 - 0.65) |
| Donor service areas (DSAs) | 57 |
| Mean DSA-specific Herfindahl-Hirschman index +/- s.d. (range) | 0.33 +/- 0.21 (0.1 - 1) |
Calculated for centers performing > 5 live donor transplants during study period
Figure 2.
Proportion of complex live donors out of total live donors at individual US renal transplant centers*
*Calculated for centers performing > 5 live donor transplants during study period
Missing data related to medical complexity
A large proportion of donors had incomplete data relating to medical complexity. Overall, 4448 donors (47.7% of complete cohort) had at least 1 missing data element. 3384 (36.3%) donors were missing weight, 2408 (25.8%) were missing height, 1237 (13.3%) were missing blood pressure, and 365 (3.9%) donors were missing serum creatinine.
We performed sensitivity analyses to estimate the maximum impact of missing data on our estimates of donor hypertension, obesity and low eGFR. If all donors with missing data on blood pressure were classified as hypertensive, the number of hypertensive donors increased from 956 to 2193 (23.5%). If all donors with missing data on weight were classified as obese, the number of obese donors increased from 1194 to 4578 (49.1%). If all donors with missing data on serum creatinine were classified as having a low eGFR, the number of donors with low eGFR increased from 392 to 757 (8.1%).
Missing data were associated with higher center volume. Fifty-one percent of donors at the high volume centers, 43% of donors at the intermediate volume centers and 35% of donors in the lowest volume centers were missing data related to medical complexity (p<0.01.)
Characteristics associated with donor medical complexity
Univariate analyses are shown in Table 5 and results of multivariable logistic regression are shown in Table 6. In the following text, odds ratios and p values correspond to analysis of the primary cohort using multivariate regression.
Table 6.
Multivariate analysis of the primary cohort - donor, recipient and transplant center characteristics associated with donor medical complexity
| Primary cohort n=4819 |
Complete cohort n=9319 |
|||||
|---|---|---|---|---|---|---|
| Donor attribute | OR | C.I. | p | OR | C.I. | p |
| Parent | 1.21 | 0.94-1.56 | 0.13 | 1.14 | 0.93-1.37 | 0.20 |
| Spouse | 1.29 | 1.06-1.56 | <0.01 | 1.18 | 1.02-1.36 | 0.03 |
| Low education** | 1.19 | 1.04-1.37 | 0.01 | 1.18 | 1.06-1.32 | <0.01 |
| Female | 0.87 | 0.77-0.99 | 0.03 | 0.85 | 0.77-0.94 | <0.01 |
| Non US citizen | 0.70 | 0.51-0.97 | 0.03 | 0.79 | 0.62-1.02 | 0.07 |
| Age | 1.01 | 1.01-1.02 | <0.01 | 1.02 | 1.01-1.02 | <0.01 |
| Center attribute | OR | C.I. | p | OR | C.I. | p |
| Category of center volume |
||||||
| Low | Reference | Reference | ||||
| Intermediate | 1.20 | 0.95-1.52 | 0.13 | 1.04 | 0.86-1.26 | 0.69 |
| High | 1.26 | 1.02-1.57 | 0.03 | 1.01 | 0.84-1.21 | 0.92 |
| Category of market competitiveness *** |
||||||
| Low | 1.14 | 0.96-1.35 | 0.14 | 1.00 | 0.88-1.14 | 0.96 |
| Intermediate | 1.06 | 0.91-1.23 | 0.48 | 0.95 | 0.84-1.07 | 0.39 |
| High | Reference | Reference | ||||
| Proportion of live donors/total donors |
1.97 | 1.23-3.16 | <0.01 | 2.12 | 1.44-3.11 | <0.01 |
| 6 month interval of wait-list time for deceased donor kidney in the center’s DSA |
0.97 | 0.95-0.99 | <0.01 | 0.97 | 0.95-0.99 | <0.01 |
Results from multivariable logistic regression. Only hypothesized variables and those significant in the primary analysis are shown.
Defined as no years of college education
Market competitiveness as measured by the Herfindahl-Hirschmann index; the “market” here is centers performing renal transplants within a single donor service area. A high score reflects low competition; therefore, the high category is used as reference in the regression
Donor characteristics
Spousal relationship to the recipient (OR 1.29, CI 1.06-1.56, p<0.01), low education (OR 1.19, CI 1.04-1.37, p=0.01), older age (OR 1.01 per year, CI 1.01-1.02, p<0.01), female gender (OR 0.87, CI 0.77 – 0.99, p=0.03), and non-US citizenship (OR 0.70, CI 0.51-0.97, p=0.03) were each associated with donor medical complexity.
Recipient characteristics and medically complex donors
No recipient characteristics were associated with donor complexity.
Center characteristics and medically complex donors
Highest center volume was associated with use of medically complex donors. Compared to lowest volume centers, the centers in the highest volume category had a higher odds of using medically complex donors (OR 1.26, CI 1.02-1.57, p=0.03). An increasing proportion of (living donation)/(all kidney transplants) at a center was also associated with use of medically complex donors (OR 1.97, CI 1.23-3.16, p<0.01). Median wait-list time in a DSA had a small inverse association (OR 0.97, CI 0.95-0.99, p<0.01 per 6 months of wait-time) with complex donor use.
Use of medically complex donors was not associated with market competition.
Discussion
This study has three main findings. First, despite growing concern about the safety and appropriateness of accepting kidney donation from medically complex live donors, nearly a quarter of live kidney donors have risk factors for future kidney disease at the time of their nephrectomies.(10, 19) Second, wide variation in the proportion of donors who are medically complex is evident among US renal transplant centers. Third, center-specific factors such as kidney transplant volume are associated with the use of complex live donors.
The finding that 24.2% of donors met criteria for medical complexity bears comparison to the recent nationwide study of US transplant center policies for live donors by Mandelbrot et al.(42) Our analysis found that 12.8% of donors were obese. This proportion of obese donors is supported by Mandelbrot’s report that only 10% of centers exclude donors with BMI>=30, as well as the rising prevalence of obesity in the United States. Our finding that 10.3% of live donors were hypertensive is consistent with the fact that substantial diversity exists as far as center policy about accepting hypertensive donors. In Mandelbrot’s study, for instance, only 49% of centers reported excluding donors with “any borderline blood pressures” or “persistent borderline blood pressures.” On the other hand, the single-center study by Textor et al. on outcomes with hypertensive donors provoked debate about the acceptability of using such donors.(4, 5) Our finding that 4.2% of donors had eGFR<60ml/min/1.73m2 is not easily compared to existing data on center policies, because most centers use renal function cut-offs related to creatinine clearance. Although transplant staff may consider eGFR in their donor assessment, this information was not assessed by Mandelbrot et al.(42)
As we hypothesized, a number of donor attributes were associated with increased likelihood of donor complexity, including a spousal relationship to the recipient. Parents were more likely to be medically complex donors in univariate analysis, although not in our multivariate model (p=0.13.) The fact that spouses were more likely to be medically complex donors suggests that transplant centers take into account a close relationship between donor and recipient in deciding whether to proceed with nephrectomy.(43, 44) These findings could also be consistent with the view that spouses and parents are more likely to accept greater risk due to a sense of obligation. Alternatively, these groups might be more susceptible to social pressure or coercion to donate.
The association of low education (defined as no years of college) with donor medical complexity raises the troubling possibility that some medically complex donors were less likely to understand that they had risk factors for kidney disease. We acknowledge the possibility that limited education of a donor could be associated with or confounded by other important donor characteristics not measured in our study, such as socio-economic status. In any case, transplant staff who evaluate donors should consider whether a donor’s education level might impede comprehension of potential risks of donation.
The association of donor age to complexity may be explained by the fact that the prevalence of hypertension and decreased GFR rise with age. Additionally, centers may be more likely to accept complex older donors because they are perceived to have fewer years of life during which the negative influence of medical complexity could adversely affect their health.
We also demonstrated a relationship between non-US citizenship and complex donors. These non-US citizen donors may represent a mix of those who have traveled from other countries for donation and US residents who do not have citizenship. The finding that non-US citizenship is associated with a lower odds of donor complexity may reflect caution on behalf of transplant professionals, who may not be assured of the long-term care of these patients. Alternatively, in the case of donors from abroad, it may be that the barriers to coming to the US impede arrival of all but the most committed and healthy.
Our analysis did not demonstrate any significant associations between recipient characteristics and use of medically complex live donors in multivariate analysis. This finding suggests that the decision by a transplant center to accept a medically complex donor is most influenced by donor characteristics and the relationship of the donor to the recipient.
We also examined associations between center-level attributes and donor medical complexity, using a logistic modeling strategy that adjusted for differences in donor and recipient populations across centers. Multivariate regression revealed an association between highest center volume and use of medically complex donors. Additionally, an increasing proportion of live donor transplants as a fraction of the total center kidney transplant volume was associated with acceptance of medically complex donors. It is plausible that the greater clinical experience within higher volume centers leads to greater comfort evaluating donors with risk factors for future kidney disease.(23) For instance, obesity is associated with chronic kidney disease and may also increase risk for operative complications;(9) higher volume centers and those that perform a higher proportion of living donor transplantation may be more willing to accept obese donors on the basis of surgical judgment.
We also found that use of complex donors is associated with a small but significant decrease in median wait-list time in a DSA. Interestingly, in a recent analysis of wait-listed kidney transplant candidates between 1995 and 2002, Segev et al. found that recipients at centers with the longest deceased donor wait-times were 2.3-fold more likely to undergo live donor transplantation. The authors proposed that renal transplant candidates may be more likely to seek live donors and that live donors may be more likely to come forward when it is known that wait-times for organs are prolonged.(45) Given our results, it may be that centers are more likely to perform live donor transplants when wait-lists are long, but that use of medically complex donors leads to a small but significant decrease in average days on the wait-list in a DSA.
We hypothesized that DSAs with a greater level of market competition - where centers may face financial pressure to increase the number of transplants - would be more likely to accept medically complex donors. This hypothesis was supported only in univariate analysis and use of complex donors was actually associated with centers in DSAs with lower market competition. The lack of significance in multivariate regression suggests that other center attributes, such as transplant volume or average wait-list time, are more important factors affecting center practice.
This study is the first to examine variation in the acceptance of medically complex live kidney donors across transplant centers and to identify donor and center level attributes associated with donor complexity. The study, however, is limited by missing data, primarily on pre-donation weight, height, and blood pressure of donors. To address this problem, we performed a primary analysis restricted to donors with complete data and a secondary analysis using all donors.(46) We did not pursue a strategy of imputing missing values on medical complexity, given that complexity was our main outcome. Notably, we also found that high and intermediate volume centers were more likely to have missing data on medical complexity than small volume centers. This greater proportion of missing data in high and intermediate volume centers may provide a reason why donor complexity was not associated with volume or market competition in our secondary analysis of the complete cohort. The fact that important data were missing for a large proportion of donors underscores the need for better compliance by transplant staff with reporting clinical information on donors. Improved compliance will increase costs and logistical demands for transplant centers. Such compliance will, however, be vital to proposed future cohort studies of donor outcomes.(8)
Another limitation of our study is that the criteria for donor medical complexity that we used are not exhaustive and some may disagree with our classification.(11) Our criteria for donor complexity, however, are supported by large epidemiological studies on risk factors for chronic kidney disease.(31, 32, 47, 48) We were also limited to characterizing donors using existing OPTN data fields. For instance, results of ambulatory blood pressure monitoring are not reported to the OPTN. Additionally, we calculated eGFR from serum creatinine and demographic data; programs do not report results of other methods of GFR measurement (such as iothalamate clearance) to the OPTN. Published literature on donor evaluation suggests that different programs have various approaches to defining the lower limit of acceptable renal function. Some authors, for instance, have advocated for a cutoff of 80ml/minute/1.73 m2 whereas others have proposed age-specific guidelines for adequate renal function in a donor.(6, 8, 38, 49, 50) Given this variety in proposed cut-offs for adequate renal function in donors, we felt that 60 ml/min/1.73m2 was a conservative threshold for which the MDRD study equation has been shown to have reasonable specificity for detecting low renal function.(38) Our cut-offs for obesity and hypertension followed conventional definitions.(30, 35)
CONCLUSIONS
This analysis of a comprehensive OPTN dataset on live kidney donors demonstrates that twenty-four percent of donors had risk factors for future kidney disease. Since this proportion assumes that donors with missing data did not otherwise meet criteria for medical complexity, the true proportion of complex donors may be higher. The wide variation in donor complexity across centers suggests that, in the absence of consensus guidelines establishing medical contraindications to donation, the use of complex donors will be strongly associated with issues such as the donor’s relation to the recipient, donor education level or center volume.(19) Studies of long-term outcomes for medically complex donors are needed to guide policy about acceptance of these donors. In the meantime, however, transplant professionals who evaluate medically complex donors should highlight the lack of definitive data about outcomes in order to ensure the integrity of informed consent.
Funding Sources
Dr. Reese is supported by NIH Career Development Award, K23 - DK078688-01
Disclaimer: “This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.”
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Danovitch GM, Cohen DJ, Weir MR, Stock PG, Bennett WM, Christensen LL, et al. Current status of kidney and pancreas transplantation in the United States, 1994-2003. Am J Transplant. 2005;5(4 Pt 2):904–15. doi: 10.1111/j.1600-6135.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–8. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 4.Textor SC, Taler SJ, Driscoll N, Larson TS, Gloor J, Griffin M, et al. Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation. 2004;78(2):276–82. doi: 10.1097/01.tp.0000128168.97735.b3. [DOI] [PubMed] [Google Scholar]
- 5.Herman ES, Rafey MA, Akalin E, Winston JA, Murphy B. Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation. 2005;79(12):1768–9. doi: 10.1097/01.tp.0000167702.03032.1f. [DOI] [PubMed] [Google Scholar]
- 6.Davis CL, Delmonico FL. Living-donor kidney transplantation: a review of the current practices for the live donor. J Am Soc Nephrol. 2005;16(7):2098–110. doi: 10.1681/ASN.2004100824. [DOI] [PubMed] [Google Scholar]
- 7.Rea DJ, Heimbach JK, Grande JP, Textor SC, Taler SJ, Prieto M, et al. Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney Int. 2006;70(9):1636–41. doi: 10.1038/sj.ki.5001799. [DOI] [PubMed] [Google Scholar]
- 8.Delmonico F. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79(6 Suppl):S53–66. [PubMed] [Google Scholar]
- 9.Heimbach JK, Taler SJ, Prieto M, Cosio FG, Textor SC, Kudva YC, et al. Obesity in living kidney donors: clinical characteristics and outcomes in the era of laparoscopic donor nephrectomy. Am J Transplant. 2005;5(5):1057–64. doi: 10.1111/j.1600-6143.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 10.Ommen E, Winston J, Murphy B. Medical risks for living donors: absence of proof is not proof of absence. Clinical Journal of the American Society of Nephrology. 2006;1:885–895. doi: 10.2215/CJN.00840306. [DOI] [PubMed] [Google Scholar]
- 11.Reese P, Caplan A, Kesselheim A, Bloom R. Creating a Medical, Ethical and Legal Framework for Complex Living Kidney Donors. Clinical Journal of the American Society of Nephrology. 2006;1(6):1148–53. doi: 10.2215/CJN.02180606. [DOI] [PubMed] [Google Scholar]
- 12.Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tyden G, Groth CG. Kidney donors live longer. Transplantation. 1997;64(7):976–8. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Fehrman-Ekholm I, Duner F, Brink B, Tyden G, Elinder CG. No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation. 2001;72(3):444–9. doi: 10.1097/00007890-200108150-00015. [DOI] [PubMed] [Google Scholar]
- 14.Gossmann J, Wilhelm A, Kachel HG, Jordan J, Sann U, Geiger H, et al. Long-term consequences of live kidney donation follow-up in 93% of living kidney donors in a single transplant center. Am J Transplant. 2005;5(10):2417–24. doi: 10.1111/j.1600-6143.2005.01037.x. [DOI] [PubMed] [Google Scholar]
- 15.Najarian JS, Chavers BM, McHugh LE, Matas AJ. 20 years or more of follow-up of living kidney donors. Lancet. 1992;340(8823):807–10. doi: 10.1016/0140-6736(92)92683-7. [DOI] [PubMed] [Google Scholar]
- 16.Narkun-Burgess DM, Nolan CR, Norman JE, Page WF, Miller PL, Meyer TW. Forty-five year follow-up after uninephrectomy. Kidney Int. 1993;43(5):1110–5. doi: 10.1038/ki.1993.156. [DOI] [PubMed] [Google Scholar]
- 17.Rizvi SA, Naqvi SA, Jawad F, Ahmed E, Asghar A, Zafar MN, et al. Living kidney donor follow-up in a dedicated clinic. Transplantation. 2005;79(9):1247–51. doi: 10.1097/01.tp.0000161666.05236.97. [DOI] [PubMed] [Google Scholar]
- 18.Ellison MD, McBride MA, Taranto SE, Delmonico FL, Kauffman HM. Living kidney donors in need of kidney transplants: a report from the organ procurement and transplantation network. Transplantation. 2002;74(9):1349–51. doi: 10.1097/00007890-200211150-00025. [DOI] [PubMed] [Google Scholar]
- 19.UNOS. Guidelines for the medical evaluation of living kidney donors (Living donor committee) Policy proposals issued for public comment URL http://www.unos.org/PublicComment/pubcommentPropSub_208.pdf Accessed: July 25 2007 [cited July 25 2007]; Available from: http://www.unos.org/PublicComment/pubcommentPropSub_208.pdf
- 20.Truog RD. The ethics of organ donation by living donors. N Engl J Med. 2005;353(5):444–6. doi: 10.1056/NEJMp058155. [DOI] [PubMed] [Google Scholar]
- 21.Organ Donation: Opportunities for Action. Institute of Medicine, National Academies Press; Washington, DC: 2006. [Google Scholar]
- 22.Abecassis M, Adams M, Adams P, Arnold RM, Atkins CR, Barr ML, et al. Consensus statement on the live organ donor. Jama. 2000;284(22):2919–26. doi: 10.1001/jama.284.22.2919. [DOI] [PubMed] [Google Scholar]
- 23.Axelrod DA, Guidinger MK, McCullough KP, Leichtman AB, Punch JD, Merion RM. Association of center volume with outcome after liver and kidney transplantation. Am J Transplant. 2004;4(6):920–7. doi: 10.1111/j.1600-6143.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 24.Howard RJ. The challenging triangle: balancing outcomes, transplant numbers and costs. Am J Transplant. 2007;7(11):2443–5. doi: 10.1111/j.1600-6143.2007.01961.x. [DOI] [PubMed] [Google Scholar]
- 25.Steiner R. How should we ethically select living kidney donors when they all are at risk? Am J Transplant. 2005;5(5):1172–3. doi: 10.1111/j.1600-6143.2005.00886.x. [DOI] [PubMed] [Google Scholar]
- 26.Clemens KK, Thiessen-Philbrook H, Parikh CR, Yang RC, Karley ML, Boudville N, et al. Psychosocial health of living kidney donors: a systematic review. Am J Transplant. 2006;6(12):2965–77. doi: 10.1111/j.1600-6143.2006.01567.x. [DOI] [PubMed] [Google Scholar]
- 27.Robinson JC, Luft HS. The impact of hospital market structure on patient volume, average length of stay, and the cost of care. J Health Econ. 1985;4(4):333–56. doi: 10.1016/0167-6296(85)90012-8. [DOI] [PubMed] [Google Scholar]
- 28.Scanlon DP, Hollenbeak CS, Lee W, Loh E, Ubel PA. Does competition for transplantable hearts encourage ‘gaming’ of the waiting list? Health Aff (Millwood) 2004;23(2):191–8. doi: 10.1377/hlthaff.23.2.191. [DOI] [PubMed] [Google Scholar]
- 29.Hartz AJ, Krakauer H, Kuhn EM, Young M, Jacobsen SJ, Gay G, et al. Hospital characteristics and mortality rates. N Engl J Med. 1989;321(25):1720–5. doi: 10.1056/NEJM198912213212506. [DOI] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 31.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. Jama. 2004;291(7):844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 32.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46(5):871–80. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Chertow GM, Hsu CY, Johansen KL. The enlarging body of evidence: obesity and chronic kidney disease. J Am Soc Nephrol. 2006;17(6):1501–2. doi: 10.1681/ASN.2006040327. [DOI] [PubMed] [Google Scholar]
- 34.Praga M, Hernandez E, Herrero JC, Morales E, Revilla Y, Diaz-Gonzalez R, et al. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int. 2000;58(5):2111–8. doi: 10.1111/j.1523-1755.2000.00384.x. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization Obesity. 2007 2007. [cited 9/29/2007]; Available from: http://www.who.int/topics/obesity/en/
- 36.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 37.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 38.Rook M, Hofker HS, van Son WJ, Homan van der Heide JJ, Ploeg RJ, Navis GJ. Predictive capacity of pre-donation GFR and renal reserve capacity for donor renal function after living kidney donation. Am J Transplant. 2006;6(7):1653–9. doi: 10.1111/j.1600-6143.2006.01359.x. [DOI] [PubMed] [Google Scholar]
- 39.Herfindahl O. Concentration in the US Steel Industry. New York: Columbia: 1950. [Google Scholar]
- 40.Hirschman A. The paternity of an index. American Economics Review. 1964;54:761–2. [Google Scholar]
- 41.Hyman D, Kovacic W. Monopoly, Monopsony, And Market Definition: An Antitrust Perspective On Market Concentration Among Health Insurers. Health Affairs. 2004;23(6):25–28. doi: 10.1377/hlthaff.23.6.25. [DOI] [PubMed] [Google Scholar]
- 42.Mandelbrot DA, Pavlakis M, Danovitch GM, Johnson SR, Karp SJ, Khwaja K, et al. The medical evaluation of living kidney donors: a survey of US transplant centers. Am J Transplant. 2007;7(10):2333–43. doi: 10.1111/j.1600-6143.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- 43.Popp FC, Eggert N, Hoy L, Lang SA, Obed A, Piso P, et al. Who is willing to take the risk? Assessing the readiness for living liver donation in the general German population. J Med Ethics. 2006;32(7):389–894. doi: 10.1136/jme.2005.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neuhaus TJ, Wartmann M, Weber M, Landolt MA, Laube GF, Kemper MJ. Psychosocial impact of living-related kidney transplantation on donors and partners. Pediatr Nephrol. 2005;20(2):205–9. doi: 10.1007/s00467-004-1749-9. [DOI] [PubMed] [Google Scholar]
- 45.Segev DL, Gentry SE, Montgomery RA. Association between waiting times for kidney transplantation and rates of live donation. Am J Transplant. 2007;7(10):2406–13. doi: 10.1111/j.1600-6143.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- 46.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd John Wiley and Sons; New York: 2002. [Google Scholar]
- 47.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17(5):1444–52. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 48.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–8. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 49.Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43(1):112–9. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 50.Gonwa TA, Atkins C, Zhang YA, Parker TF, Hunt JM, Lu CY, et al. Glomerular filtration rates in persons evaluated as living-related donors--are our standards too high? Transplantation. 1993;55(5):983–5. doi: 10.1097/00007890-199305000-00005. [DOI] [PubMed] [Google Scholar]