Abstract
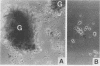
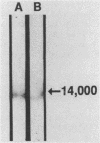
Nerve growth factor (NGF) is a neurotrophic factor essential for the development and maintenance of specific neuronal cell populations. In addition, NGF has biological effects on inflammatory cells. The aim of this study was to determine whether NGF is present in chronic inflammation, using isolated hepatic granulomas from mice infected with Schistosoma mansoni as the model. The schistosome granuloma is a complex T-cell-mediated immune response to the egg. Intact granulomas were isolated from the livers of infected mice and examined for the presence of NGF. In homogenized granuloma samples, radioimmunoassay and immunoblotting analyses detected an immunoreactive NGF that had the same molecular mass as that of purified murine NGF (13 kDa). Isolated granulomas cultured in vitro released soluble factor(s) with NGF-like neurite-promoting activity in a rat pheochromocytoma (PC12) bioassay. This activity was partially inhibited by a blocking anti-NGF antibody. There were two potential sources of this NGF-like neurite-promoting activity, either the schistosome egg or the host inflammatory response. Since neither isolated eggs nor soluble egg antigen had neurite-promoting activity, the inflammation was the source of this activity. The inability of the anti-NGF antibody to inhibit completely the granuloma-induced neurite outgrowth in the bioassay signifies that NGF is not the only neurotrophic factor present in these granulomas. The presence of NGF within the granulomas may indicate that NGF has a role in the granulomatous response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assouline J. G., Bosch P., Lim R., Kim I. S., Jensen R., Pantazis N. J. Rat astrocytes and Schwann cells in culture synthesize nerve growth factor-like neurite-promoting factors. Brain Res. 1987 Jan;428(1):103–118. doi: 10.1016/0165-3806(87)90087-3. [DOI] [PubMed] [Google Scholar]
- BRENER Z., PELLEGRINO J. Method for isolating schistosome granulomas from mouse liver. J Parasitol. 1956 Dec;42(6):564–564. [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Bryant M. G., Bloom S. R., Hamilton S. Abnormalities of vasoactive intestinal polypeptide-containing nerves in Crohn's disease. Gastroenterology. 1980 Nov;79(5 Pt 1):853–860. [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni A., Bigon E., Boarato E., Mietto L., Leon A., Toffano G. Interaction between nerve growth factor and lysophosphatidylserine on rat peritoneal mast cells. FEBS Lett. 1982 Feb 22;138(2):190–192. doi: 10.1016/0014-5793(82)80438-9. [DOI] [PubMed] [Google Scholar]
- DAVIS D. R., DOCKERTY M. B., MAYO C. W. The myenteric plexus in regional enteritis: a study of the number of ganglion cells in the ileum in 24 cases. Surg Gynecol Obstet. 1955 Aug;101(2):208–216. [PubMed] [Google Scholar]
- Davies A. M., Bandtlow C., Heumann R., Korsching S., Rohrer H., Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. 1987 Mar 26-Apr 1Nature. 326(6111):353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Heinrich S. P., Lee M. R., Yin H. S. Molecular cloning and expression of neuroleukin, a neurotrophic factor for spinal and sensory neurons. Science. 1986 Oct 31;234(4776):566–574. doi: 10.1126/science.3764429. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Mallat M., Houlgatte R., Brachet P., Prochiantz A. Lipopolysaccharide-stimulated rat brain macrophages release NGF in vitro. Dev Biol. 1989 May;133(1):309–311. doi: 10.1016/0012-1606(89)90322-9. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Switzer J., Coughlin M. D., Bienenstock J., Denburg J. A. Human basophilic cell differentiation promoted by 2.5S nerve growth factor. Int Arch Allergy Appl Immunol. 1988;86(4):453–457. doi: 10.1159/000234634. [DOI] [PubMed] [Google Scholar]
- Miyazawa Y., Fukuda Y., Imoto M., Koyama Y., Nagura H. Immunohistochemical studies on the distribution of nerve fibers in chronic liver diseases. Am J Gastroenterol. 1988 Oct;83(10):1108–1114. [PubMed] [Google Scholar]
- Murphy R. A., Singer R. H., Saide J. D., Pantazis N. J., Blanchard M. H., Byron K. S., Arnason B. G., Young M. Synthesis and secretion of a high molecular weight form of nerve growth factor by skeletal muscle cells in culture. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4496–4500. doi: 10.1073/pnas.74.10.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oger J., Arnason B. G., Pantazis N., Lehrich J., Young M. Synthesis of nerve growth factor by L and 3T3 cells in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1554–1558. doi: 10.1073/pnas.71.4.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis N. J., Jensen R. Nerve growth factor, not laminin, is the major neurite-promoting component in medium conditioned by mouse L929 fibroblast cells. Brain Res. 1988 May 1;468(1):123–137. doi: 10.1016/0165-3806(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Pearce F. L., Thompson H. L. Some characteristics of histamine secretion from rat peritoneal mast cells stimulated with nerve growth factor. J Physiol. 1986 Mar;372:379–393. doi: 10.1113/jphysiol.1986.sp016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D., Bridson W., Rayford P. L. Rapid calculation of radioimmunoassay results. J Lab Clin Med. 1969 Nov;74(5):770–781. [PubMed] [Google Scholar]
- Smith A. P., Varon S., Shooter E. M. Multiple forms of the nerve growth factor protein and its subunits. Biochemistry. 1968 Sep;7(9):3259–3268. doi: 10.1021/bi00849a032. [DOI] [PubMed] [Google Scholar]
- Snider W. D., Johnson E. M., Jr Neurotrophic molecules. Ann Neurol. 1989 Oct;26(4):489–506. doi: 10.1002/ana.410260402. [DOI] [PubMed] [Google Scholar]
- Stemple D. L., Mahanthappa N. K., Anderson D. J. Basic FGF induces neuronal differentiation, cell division, and NGF dependence in chromaffin cells: a sequence of events in sympathetic development. Neuron. 1988 Aug;1(6):517–525. doi: 10.1016/0896-6273(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Stenger R. J., Warren K. S., Johnson E. A. An ultrastructural study of hepatic granulomas and schistosome egg shells in murine hepatosplenic schistosomiasis mansoni. Exp Mol Pathol. 1967 Aug;7(1):116–132. doi: 10.1016/0014-4800(67)90041-x. [DOI] [PubMed] [Google Scholar]
- Thorpe L. W., Perez-Polo J. R. The influence of nerve growth factor on the in vitro proliferative response of rat spleen lymphocytes. J Neurosci Res. 1987;18(1):134–139. doi: 10.1002/jnr.490180120. [DOI] [PubMed] [Google Scholar]
- Thorpe L. W., Werrbach-Perez K., Perez-Polo J. R. Effects of nerve growth factor on the expression of interleukin-2 receptors on cultured human lymphocytes. Ann N Y Acad Sci. 1987;496:310–311. doi: 10.1111/j.1749-6632.1987.tb35781.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varilek G. W., Weinstock J. V., Williams T. H., Jew J. Alterations of the intestinal innervation in mice infected with Schistosoma mansoni. J Parasitol. 1991 Jun;77(3):472–478. [PubMed] [Google Scholar]
- Varon S., Nomura J., Shooter E. M. The isolation of the mouse nerve growth factor protein in a high molecular weight form. Biochemistry. 1967 Jul;6(7):2202–2209. doi: 10.1021/bi00859a043. [DOI] [PubMed] [Google Scholar]
- Walicke P. A. Basic and acidic fibroblast growth factors have trophic effects on neurons from multiple CNS regions. J Neurosci. 1988 Jul;8(7):2618–2627. doi: 10.1523/JNEUROSCI.08-07-02618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E., Nohr D., Millan M. J., Stein C., Müller S., Gramsch C., Herz A. Peptide neuroanatomy of adjuvant-induced arthritic inflammation in rat. Agents Actions. 1988 Dec;25(3-4):255–259. doi: 10.1007/BF01965027. [DOI] [PubMed] [Google Scholar]
- Weinstock J. V., Boros D. L. Organ-dependent differences in composition and function observed in hepatic and intestinal granulomas isolated from mice with Schistosomiasis mansoni. J Immunol. 1983 Jan;130(1):418–422. [PubMed] [Google Scholar]
- Weskamp G., Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and in peripheral tissues. J Neurochem. 1987 Jun;48(6):1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Wahl S. M., Cheever A. W., Wahl L. M. Fibroblast stimulation in schistosomiasis. I. Stimulation in vitro of fibroblasts by soluble products of egg granulomas. J Infect Dis. 1981 Sep;144(3):254–262. doi: 10.1093/infdis/144.3.254. [DOI] [PubMed] [Google Scholar]
- Young M., Oger J., Blanchard M. H., Asdourian H., Amos H., Arnason B. G. Secretion of a nerve growth factor by primary chick fibroblast cultures. Science. 1975 Jan 31;187(4174):361–362. doi: 10.1126/science.1167427. [DOI] [PubMed] [Google Scholar]