Abstract
Rationale
This study examined the relationship between voluntary ethanol consumption and ethanol concentrations measured in the nucleus accumbens of ethanol dependent and non-dependent C57BL/6J mice.
Method
Mice were offered ethanol in a 2-bottle choice, limited access paradigm and consummatory behavior was monitored with lickometers. After baseline intake stabilized, mice received chronic intermittent ethanol (EtOH group) or air (CTL group) exposure by inhalation (16 hr/day for 4 days) and then resumed drinking. Brain ethanol levels during voluntary drinking were measured by microdialysis procedures and compared to brain ethanol concentrations produced during chronic intermittent ethanol vapor exposure.
Results
Voluntary ethanol consumption progressively increased over repeated cycles of chronic intermittent ethanol exposure but remained unchanged in CTL mice. Analysis of lick patterns indicated EtOH mice consumed ethanol at a faster rate compared to CTL mice. The greater and faster rate of ethanol intake in EtOH mice produced higher peak brain ethanol concentrations compared to CTL mice and these levels were similar to levels produced during chronic intermittent ethanol exposure.
Conclusions
These results show that in this model of dependence and relapse drinking, dependent mice exhibit enhanced voluntary ethanol consumption relative to non-dependent controls, which consequently produces blood and brain ethanol concentrations similar to those experienced during chronic intermittent ethanol exposure.
Keywords: Ethanol, Dependence, Withdrawal, Self, administration, Mice, Relapse, Microdialysis, Brain Ethanol Levels
Introduction
As a chronic relapsing disease, alcoholism constitutes a major health problem in the general population. Continued excessive ethanol consumption can lead to the development of dependence, which has been characterized as an allostatic state fueled by progressive dysregulation of brain reward and stress circuits (Koob 2003; Koob and Le Moal 2001). Such neuroadaptive changes have been suggested to play a prominent role in fostering transition from regulated ethanol use to more excessive and uncontrollable drinking. Additionally, the ability of ethanol to alleviate withdrawal-related dysphoria may serve as a powerful motivational force - enhancing vulnerability to relapse as well as favoring escalation of drinking to higher and more sustained levels (Becker 2000; Koob and Le Moal 2005). By the very nature of this relapsing disease, many ethanol dependent individuals make numerous attempts at curtailing their alcohol use, only to find themselves reverting back to patterns of excessive consumption again. Thus, it is common for many alcoholics to experience multiple episodes of heavy drinking interspersed by periods of attempted abstinence.
A number of animal models have been used to study the role of dependence in promoting enhanced relapse vulnerability as well as perpetuating ethanol use that escalates to excessive levels. Early studies produced mixed results, most likely due to the use of procedures that did not optimize establishing the positive reinforcing effects of ethanol as a context to then examine the development of the drug’s negative reinforcing capacity (Meisch 1983; Meisch 1984). More recently, studies in rats and mice have been successful in demonstrating enhanced propensity for ethanol self-administration in dependent animals (Finn et al. 2007; O’Dell et al. 2004; Roberts et al. 2000).
We have developed a mouse model of ethanol dependence and relapse that links procedures for ethanol self-administration with exposure to repeated cycles of chronic intermittent ethanol treatment by the inhalation route. We previously demonstrated enhanced voluntary ethanol consumption in dependent mice, which produced more than a 2-fold increase in blood ethanol levels compared to control mice (Becker and Lopez 2004). Additionally, elevated ethanol consumption in dependent mice occurred well beyond acute withdrawal and, with increased number of chronic ethanol exposure/withdrawal cycles, enhanced ethanol intake was further augmented and sustained for several weeks following final withdrawal compared to intake of a separate group of non-dependent mice (Lopez and Becker 2005).
In our previous work, amount of ethanol consumed was determined at the end of each limited access (2 hour) session, and total ethanol intake (g/kg) positively correlated with resultant blood ethanol levels (Becker and Lopez 2004). The present studies examined whether the temporal pattern of volitional ethanol consumption during the limited access drinking sessions is influenced by repeated cycles of chronic intermittent ethanol exposure. Additionally, using in vivo microdialysis procedures, we examined the temporal relationship between drinking and brain ethanol concentrations and whether enhanced ethanol intake in dependent animals results in higher brain ethanol concentrations. Finally, we examined blood and brain ethanol concentrations achieved after voluntary drinking in the context of levels produced during the chronic intermittent ethanol exposure regimen in dependent mice.
Methods
Subjects
Male C57BL/6J mice (10 weeks of age) obtained from Jackson Laboratories (Bar Harbor, ME) were individually housed and maintained in an AAALAC accredited animal facility under a 12 hr light cycle (lights on 0200 hr). Mice had free access to food and water at all times. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina and were consistent with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996). Mice were acclimated to the vivarium for two weeks prior to the experiments.
General Experimental Design
Mice were given access to ethanol using a 2-bottle choice, limited access procedure and standard lickometer circuits (described below). After establishing stable ethanol intake, mice were separated into chronic ethanol exposure/withdrawal (EtOH) and control (CTL) groups, counterbalanced based on intake during the final week of baseline drinking. The EtOH group received chronic intermittent exposure to ethanol vapor in inhalation chambers (16 hr/day for four days) while CTL mice were similarly handled, but placed in control chambers (see below). Limited access drinking sessions were suspended during inhalation exposure. After inhalation treatment, mice entered a 72 hr abstinence period and then were tested for ethanol intake for five consecutive days using limited access conditions as before. This pattern of chronic intermittent ethanol (or air) exposure followed by a 72 hr forced abstinence period and then five days of testing voluntary ethanol drinking was repeated for several Test cycles (detailed below).
Experiment 1
This study examined the temporal pattern of voluntary ethanol consumption at baseline and then following repeated cycles of chronic intermittent ethanol or air exposure. A sucrose fading procedure was employed to establish stable baseline voluntary intake of 15% (v/v) ethanol in the home cage. EtOH (n= 10) and CTL (n= 12) mice were tested for voluntary ethanol consumption over four Test cycles.
Experiment 2
This study examined voluntary ethanol consumption and resultant ethanol concentrations in brain using in vivo microdialysis procedures. After implantation of a guide cannula, mice were given daily limited access to 15% (v/v) ethanol + 5% (w/v) sucrose in drinking cages modified to accommodate dialysis (see below). Mice were transferred to the drinking cage daily at 0900 hr and returned to their home cage after the drinking session. Although C57BL/6J mice readily consume substantial amounts of ethanol without added sweeteners, sucrose was maintained in the ethanol solution to facilitate greater intake during microdialysis. We have previously shown enhanced ethanol intake in our model with or without sucrose and, importantly, repeated cycles of chronic intermittent ethanol exposure does not alter intake of 5% sucrose alone (Becker and Lopez 2004). Ethanol intake in EtOH (n= 17) and CTL (n= 10) groups of mice was assessed over three Test cycles. After completing Test 3, mice experienced another 72 hr abstinence period and then limited access sessions resumed for 1–2 days prior to microdialysis.
Experiment 3
This study examined brain and blood ethanol concentrations in mice immediately upon removal from the ethanol vapor chamber for comparison with concentrations measured after voluntary drinking. After establishing stable baseline ethanol (15% v/v) intake using the sucrose fading procedure, EtOH (n= 6) and CTL (n= 7) mice were tested for voluntary ethanol consumption under limited access conditions over four Test cycles. At the end of the fourth cycle, blood samples were collected from the retro-orbital sinus immediately following the final 2 hr limited access session to measure ethanol concentration resulting from voluntary ethanol drinking. A separate group of similarly treated EtOH mice (n= 14) were returned for a fifth cycle of chronic intermittent ethanol exposure and sacrificed immediately upon removal from the chamber at the end of treatment. Trunk blood and brain tissue (cortex) were collected to measure ethanol concentrations resulting from the chronic intermittent ethanol vapor exposure.
Limited Access Procedures
All mice in these studies were given limited access to ethanol solutions with tap water as the alternate choice (Becker and Lopez 2004; Lopez and Becker 2005). The 2 hr drinking sessions started at 1330 hr. The amount consumed was recorded daily (± 0.1 ml) and body weights were recorded weekly. Further, the ethanol and water bottles were attached to standard lickometer circuits as previously described (Griffin et al. 2007) to monitor the number of licks at each tube (lickometers were not used in Experiment 3). The position of the water and ethanol bottles was alternated daily to avoid side preferences.
Chronic Ethanol Inhalation Procedures
Chronic ethanol (or air) vapor exposure was delivered in Plexiglas inhalation chambers as previously described (Becker and Hale 1993; Becker and Lopez 2004; Lopez and Becker 2005). Chamber ethanol concentrations were monitored daily and air flow was adjusted to maintain ethanol concentrations within a range that yielded stable blood ethanol levels (150–200 mg/dl) throughout exposure. Prior to entry into the ethanol chambers, EtOH mice were administered ethanol (1.6 g/kg; 8% w/v) and the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg) by intraperitoneal injection in a volume of 20 ml/kg body weight. CTL mice were handled similarly, but received injections of saline and pyrazole before being placed in control chambers. The housing conditions were identical to those in the colony room.
Stereotaxic Surgery Procedures
Mice were anesthetized using a ketamine (120 mg/kg) and xylazine (6 mg/kg) cocktail and placed in a stereotaxic instrument, as previously described (Griffin and Middaugh 2006; Griffin et al. 2007). Unilateral guide cannulae (CMA/7; CMA Microdialysis, Sweden) were aimed at the nucleus accumbens [relative to Bregma: AP +1.5, ML +0.8 and DV −3.5] and secured directly to the skull using a light-cured resin system (Groseclose et al. 1998).
Microdialysis Procedures
The general procedure for probe placement and dialysate collection was similar to that previously described, with some modifications (Griffin et al. 2007). The day before the planned dialysis collection, probes were implanted without anesthesia 3 hr prior to the drinking session and mice were given their usual 2 hr access to ethanol, but dialysates were not collected. After this session, mice were placed in their home cage and the probes continued to be perfused overnight (0.2 μL/min). The next day, mice were placed back into their drinking cage on the usual schedule. Probes were perfused with filtered (0.22 microns) artificial cerebral spinal fluid (aCSF: NaCl 140 mM; Glucose 7.4 mM; KCl 3 mM; MgCl2 0.5 mM; CaCl2 1.2 mM; Na2HPO4 1.2 mM; NaH2PO4 0.3 mM; pH= 7.4) at 1.5 μL/min using a BeeHive syringe pump and 1 mL gas tight syringes (BAS, Inc). The active membrane portion of the microdialysis probes (CMA/7) extended 1 mm beyond the guide cannulae. FEP tubing was used for transfer lines (SciPro, Sanborn, NY) and dual channel swivels (Instech 375/d22QM) were used to route samples to the outside of the chamber to minimize disturbance of the mice. The outlet side of the probe had a total volume of 9.5 μL and a transit time of 6.3 min to reach the sample vial. To estimate probe recovery efficiency, we conducted an in vitro recovery experiment (at room temperature) with seven probes and calculated ethanol recovery to be 10.2 ± 0.9%.
Collection occurred in 20 min intervals into vials containing 6 μL of 0.75 M perchloric acid that was diluted to a final concentration of 0.15 M by the sample. Immediately after each collection interval, 2 μL of dialysate was removed for ethanol determination. The remainder of the sample was used for pilot studies establishing neurotransmitter assays for future studies using independent subjects.
Dialysate Ethanol Assay
All samples were analyzed for ethanol concentration within 24 hr of collection using gas chromatography with flame ionization detection (Griffin et al. 2007). Standards were prepared each day of the dialysis collection and sample concentrations were determined by interpolation from the standard curve. The limit of detection for this system is 0.05 mM ethanol.
Probe Placement Verification
After the collection procedure, mice were sacrificed and brains preserved in 10% formalin (Griffin and Middaugh 2006; Griffin et al. 2007). The brains were sectioned on a crytostat and stained with Cresyl violet. Mice were excluded from analysis if the probe was determined to be <60% in the nucleus accumbens (Paxinos and Franklin 2001).
Blood and Brain Tissue Ethanol Assays
Blood ethanol concentrations were determined using the Analox© method, as previously described (Lopez and Becker 2005). Brain tissue (~20 mg cortex) was collected following rapid decapitation. Samples were homogenized in ice-cold deionized water and analyzed using an enzymatic spectrophotometric assay previously described (Becker and Hale 1993).
Data Analysis
Measures of ethanol intake (g/kg) and licks at the ethanol tube were averaged over the 5-day limited access sessions during baseline and each of the test cycles for each subject. These data were analyzed by 2-way ANOVA, with Group (EtOH vs. CTL) as a between-subjects factor and Test Cycle as a repeated measure. Post hoc comparisons used Newman-Keuls test, when appropriate. Ethanol concentration in dialysate samples also was analyzed by 2-way ANOVA. Peak ethanol concentrations and area under the curve (from last baseline sample to last experimental dialysate sample) were analyzed by Students t-tests. Correlation analyses were conducted using Pearson’s Product Moment analysis. For all analyses, significance levels were set at p< 0.05.
Results
Experiment 1
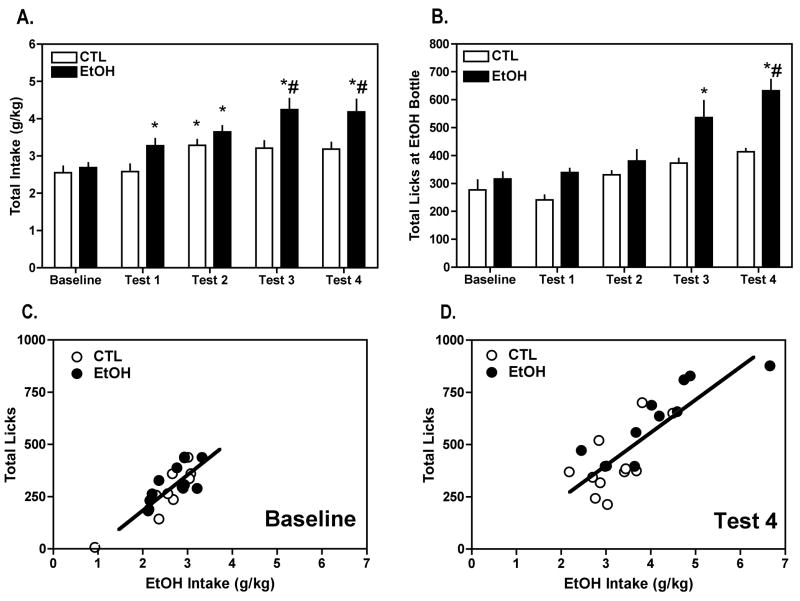
In agreement with previous findings from our laboratory (Becker and Lopez 2004; Lopez and Becker 2005), repeated cycles of chronic intermittent ethanol exposure significantly increased voluntary ethanol intake. That is, while baseline intake (~2.5 g/kg) was similar in both groups, and drinking in CTL mice remained relatively unchanged, EtOH mice exhibited a progressive increase in ethanol consumption, with average intake reaching ~4.1 g/kg by Test 4 (Figure 1A). This impression was supported by ANOVA, which revealed a significant Group × Test Cycle interaction [F(4,80)= 3.07, p= 0.0209]. Post hoc analysis indicated that ethanol consumption in the EtOH mice was significantly greater during Test 1, Test 2, Test 3, and Test 4 relative to their own baseline while in the CTL group intake during only Test 2 was significantly different from baseline levels (all ps< 0.05). In addition, ethanol intake was significantly greater in EtOH mice compared to CTL mice during Test 3 and Test 4 (ps< 0.05).
Figure 1.
Repeated cycles of chronic intermittent ethanol exposure increased voluntary (A) ethanol intake (g/kg) and (B) consummatory behavior (i.e., total cumulative licks at the ethanol tube) in C57BL/6J mice drinking ethanol (15% v/v). Data are presented as mean ± s.e.m. weekly values for baseline and the four test cycles for EtOH (n= 10) and CTL (n= 12) groups of mice (see procedural details for Experiment 1). Total cumulative licks positively correlated with total ethanol intake during (C) baseline and (D) following the fourth cycle of chronic intermittent ethanol exposure. The positive correlations indicate that licks at the ethanol tube accurately reflect amount of ethanol consumed during the limited access sessions.
* significantly differs from respective baseline level (p< 0.05); # significantly differs from CTL group (p< 0.05).
Consistent with increased overall ethanol drinking, total licks registered at the ethanol tube also increased in the EtOH group from about 300 total licks at baseline to almost 700 total licks during Test 4. In contrast, total licks at the ethanol tube remained relatively stable during baseline and test sessions for the CTL group (Figure 1B). ANOVA indicated a significant Group × Test Cycle interaction [F(4,80)= 3.38, p= 0.0132], as well as significant main effects of Group [F(1,20)= 7.48, p= 0.0127] and Test Cycle [F(1,4)= 19.26, p < 0.0001]. Post hoc analysis indicated that total licks were significantly greater in the EtOH mice during Test 3 and Test 4 compared to their baseline level and greater than the CTL mice during Test 4 (ps< 0.05). Additionally, ethanol consumption (g/kg) was positively correlated with consummatory behavior (total licks) under baseline conditions (r2= 0.6888 p< 0.0001) (Figure 1C), as well as for Test 4 (r2= 0.6829, p< 0.0001) (Figure 1D). This outcome indicates that licking behavior accurately reflected ethanol intake.
To evaluate the temporal pattern of ethanol intake during limited access drinking sessions, lick data were expressed as cumulative licks in 5-min bins across baseline and subsequent Test cycles of the experiment (Figure 2A). Baseline lick rates for EtOH and CTL groups did not statistically differ and for clarity, these values were collapsed across groups. Likewise, lick rates for the CTL group were collapsed across test cycles, since analysis did not indicate a statistical difference in the pattern of lick responses for CTL mice over Test cycles. As shown in Figure 2A, most consummatory behavior occurred in the first 40 minutes of ethanol access (first eight 5-min bins) in both EtOH and CTL groups. However, EtOH mice not only displayed greater total number of licks with each test cycle, but they also exhibited a progressively faster rate of intake (steeper rise) over repeated test sessions. This steeper rise in cumulative licks in EtOH mice compared to CTL mice was most apparent during the initial period (first 40 min) of the 2 hr drinking sessions (Figure 2B). Analysis of cumulative licks over the first 40 min of the drinking sessions (Figure 2C) revealed a significant Group × Test Cycle interaction [F(4,80)= 3.50, p= 0.0110]. Post hoc analysis indicated that licking responses for EtOH mice during Test 2, Test 3, and Test 4 were significantly greater than baseline (ps< 0.05). In contrast, licks at the ethanol tube during this initial period of access did not significantly change over test cycles for the CTL group. Additionally, total licks during the first 40-min period was significantly greater for EtOH compared to CTL mice during Test 3 and Test 4 (ps< 0.05).
Figure 2.
Cumulative licking responses registered at the ethanol tube progressively increase over repeated cycles of chronic intermittent ethanol exposure in EtOH mice compared to the CTL group in Experiment 1. Data are presented as weekly mean ± s.e.m. (A) total cumulative licks in 5-min bins for the entire 2-hr limited access drinking sessions (for clarity, CTL data are averaged over the four test cycles), (B) cumulative licks in 5-min bins during the first 40 min of the limited access sessions, and (C) total cumulative licks for the first 40 min of the limited access sessions across baseline and test cycles. * significantly differs from respective baseline level (p< 0.05);
# significantly differs from CTL group (p< 0.05).
Finally, water intake and licks at the water tube were measured in tandem with ethanol throughout the experiment. Although there was occasional sampling from the water tube by mice in both groups, water intake (ml) and the number of licks measured at the water tube were essentially neglible (data not shown).
Experiment 2
Ethanol consumption was similar for EtOH and CLT groups during the baseline phase of the study (~2.8 g/kg). However, while ethanol intake remained relatively stable in the CTL group, ethanol intake in EtOH mice progressively increased over the three test cycles (Figure 3A). ANOVA revealed a significant main effect of Group [F(1,25)= 10.47, p= 0.0034]. Although the Group × Test Cycle interaction term did not achieve statistical significance, separate analysis of baseline data indicated no significant difference between EtOH and CTL groups [t(25)=1.319, p=0.1992]. Thus, the overall greater ethanol intake in EtOH mice compared to the CTL group was primarily attributed to increased intake during the repeated test cycles.
Figure 3.
Mean ± s.e.m. (A) ethanol intake (g/kg) and (B) total cumulative licks at the ethanol tube during baseline and the three test cycles for EtOH (n= 17) and CTL (n= 10) groups of mice in Experiment 2 (prior to microdialysis testing).
Consistent with the increased ethanol intake by EtOH mice, their total licks at the ethanol tube increased from ~490 during baseline to ~710 total licks during the third test period (Figure 3B). Total licks registered at the ethanol tube in CTL mice remained relatively stable throughout the experiment. ANOVA indicated a significant main effect of Group [F(1,25)= 10.58, p= 0.0033]. Again, while the Group × Test Cycle interaction term did not achieve statistical significance, data shown in Figure 3B suggest that greater licking behavior directed at the ethanol tube in EtOH mice compared to CTL mice is primarily accounted for by larger group differences during the test periods, especially during the final test cycle.
Consistent with results from the previous study, most ethanol consumption occurred during the initial 40 minutes of the drinking sessions (Figure 4A) and the difference in lick rates between EtOH and CTL groups progressively increased over test cycles during this time frame (Figure 4B). Analysis of cumulative licks over the first 40 min of the drinking sessions (Figure 4C) revealed a significant Group × Test Cycle interaction [F(3,75)= 3.71, p= 0.0151]. Post hoc analyses indicated no difference in lick rates for the CTL group during different phases of the experiment. In contrast, ethanol licks during this time frame (first 40 min of the drinking sessions) for the EtOH group were significantly greater at Test 3 and Test 4 than at baseline, as well as being greater than the CTL group (ps< 0.05). As in Experiment 1, although there was occasional sampling from the water bottle by mice during the limited access sessions, water intake and licks at the water tube were very low in both EtOH and CTL groups (data not shown).
Figure 4.
Increased cumulative licking responses registered at the ethanol tube over repeated cycles of chronic intermittent ethanol exposure in EtOH mice compared to the CTL group in Experiment 2. Data are presented as weekly mean ± s.e.m. (A) total cumulative licks in 5-min bins for the entire 2-hr limited access drinking sessions (for clarity, CTL data are averaged over the three test cycles), (B) cumulative licks in 5-min bins during the first 40 min of the limited access sessions, and (C) total cumulative licks for the first 40 min of the limited access sessions across baseline and test cycles.
* significantly differs from respective baseline level (p< 0.05); # significantly differs from CTL group (p< 0.05).
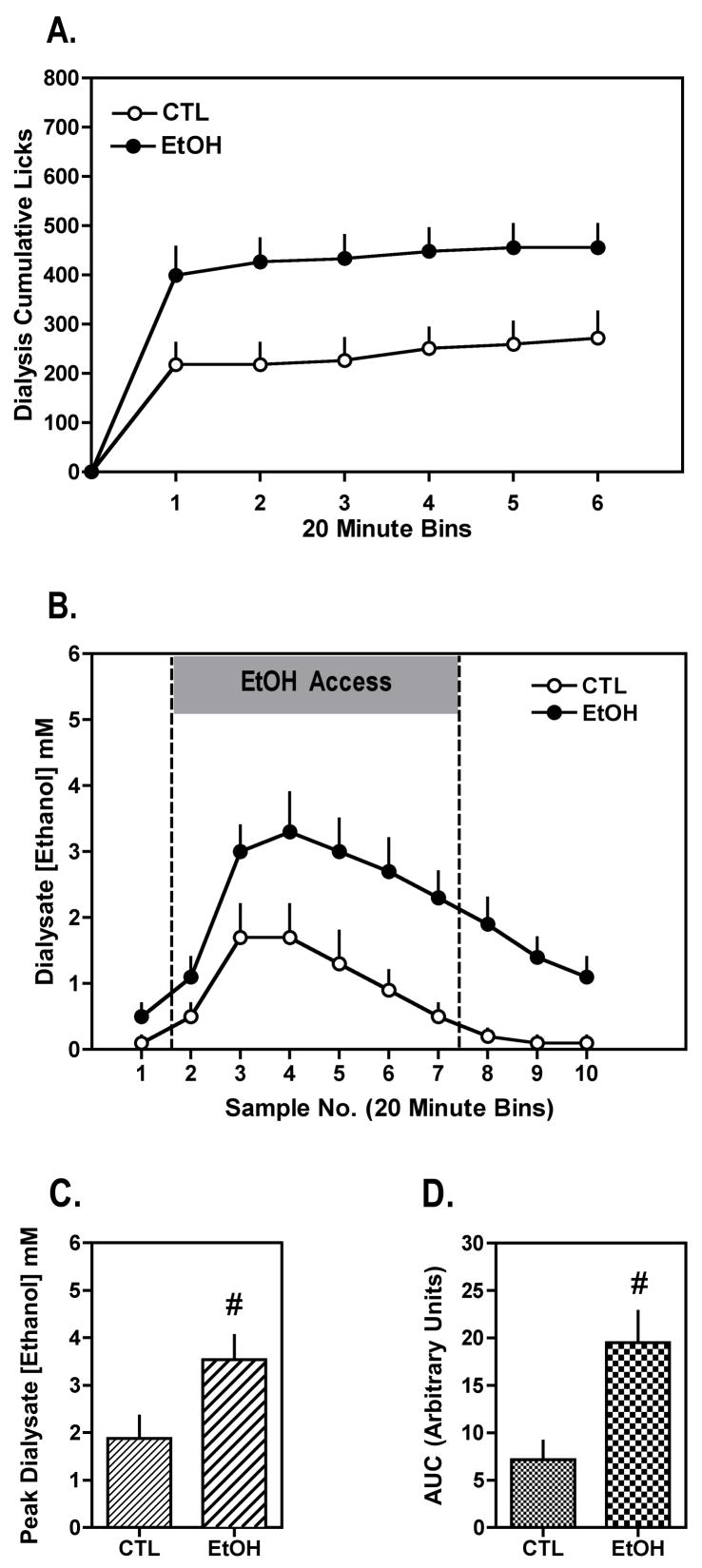
During the microdialysis session, EtOH mice continued to exhibit greater ethanol intake compared to the CTL group. This impression was supported by analysis of licks registered at the ethanol tube (expressed as cumulative licks in 20-min bins to correspond with the dialysate collection time), which indicated a significant main effect of Group [F(1,17)= 7.83, p= 0.0123]. As shown in Figure 5A, the greater licking responses at the ethanol tube in the EtOH group compared to the CTL group was apparent after the first 20 minutes of the drinking session. Further, this greater ethanol consumption (lick rate), particularly in the first sample period, produced significantly higher dialysate ethanol concentrations in the nucleus accumbens of EtOH mice in comparison to CTL mice during the limited access session, and this effect continued for an additional three sample time points following the drinking session (Figure 5B) [F(1,17)= 7.13, p= 0.0162]. Additionally, the greater voluntary ethanol intake by EtOH mice compared to the CTL group during the dialysis procedure resulted in significantly higher peak ethanol levels in dialysate (Figure 5C) [t(17)= 2.16, p= 0.0450]. Similar results were obtained when the data were expressed as area under the curve over the entire collection period (Figure 5D) [t(17)= 2.63, p= 0.0175]. Both peak ethanol concentrations and the area under the curve measures were positively correlated with total ethanol intake during the drinking session (r2= 0.2545, p= 0.0276 and r2= 0.2564, p= 0.0276, respectively). Taken together, these findings indicate that ethanol concentrations measured in the nucleus accumbens dialysate samples are a direct consequence of ethanol voluntarily consumed by the mice during the dialysis session.
Figure 5.
Increased ethanol intake results in increased brain ethanol concentrations in EtOH compared to CTL mice during the microdialysis experiment that followed the third test cycle in Experiment 2. Data are presented for EtOH (n= 12) and CTL (n= 7) groups as mean ± s.e.m. for (A) cumulative lick responses at the ethanol tube in 20-min bins, (B) ethanol concentration in 20-min dialysis samples prior to, during, and 1 hr after the 2-hr limited access drinking session, (C) peak ethanol concentration in dialysis samples, and (D) area under the curve for dialysate ethanol concentration over the entire collection period.
# significantly differs from CTL group (p< 0.05).
The possibility that metabolic tolerance contributes to increased ethanol intake exhibited by the EtOH group was addressed by comparing slopes of the elimination phase of brain ethanol concentrations. Linear regression analysis on dialysate samples 3–10 was performed to generate slopes for each subject. Analysis of these data revealed no significant difference in the rate of ethanol elimination for EtOH and CTL groups (slope for EtOH group= −0.37 ± 0.07 and CTL group= −0.29 ± 0.09) [t(17)= 0.80, p= 0.4331]. Thus, it does not appear that a faster rate of ethanol elimination in brain (metabolic tolerance) as a function of repeated chronic intermittent ethanol exposure cycles can simply account for enhanced voluntary ethanol consumption in the EtOH mice.
Microdialysis probe placements are depicted in Figure 6. Some mice were excluded from data analysis for the microdialysis portion of Experiment 2 (EtOH group: n= 5; CTL group: n= 3) due to poor probe placement, lickometer malfunction during dialysis, or damaged dialysis transfer lines. Additionally, Figure 6 shows representative photomicrographs of the histological preparation used to determine probe placements from this study in an EtOH and a CTL mouse.
Figure 6.

Microdialysis probe placements for EtOH and CTL mice included in Experiment 2. (A) Bold lines, representing 1 mm of active dialysis membrane, indicate probe placements for each mouse included in the dialysis experiment. (B) Photomicrographs of coronally sectioned mouse brains show probe and guide tracts in formalin-fixed tissue used to determine placements. The brackets show the location of the active portion of the dialysis membrane below the guide cannula tract in a CTL and EtOH mouse. Adapted from Paxinos and Franklin, 2001 (Academic Press).
Experiment 3
As in previous experiments, ethanol intake progressively increased over successive Test cycles in EtOH mice compared to the CTL group (Table 1). ANOVA indicated a significant Group × Test Cycle interaction [F(4,44)= 4.2, p= 0.006]. Post-hoc analysis indicated that ethanol intake was significantly greater in EtOH mice compared to CTL mice during all of the Test cycles, but not during Baseline (ps< 0.05). Additionally, blood ethanol concentrations were determined in EtOH and CTL groups immediately following the last drinking session during Test cycle 4. As shown in Table 2, greater ethanol drinking in EtOH mice produced blood ethanol levels that were nearly 2-fold greater than that of CTL mice [t(11)= 2.60, p= 0.027], consistent with our previous data (Becker and Lopez 2004).
Table 1.
Ethanol Intake (g/kg) for Dependent (EtOH) and Non-dependent (CTL) Groups
| Weekly Ethanol Consumption (g/kg)
|
|||||
|---|---|---|---|---|---|
| Baseline | Test 1 | Test 2 | Test 3 | Test 4 | |
| CTL (n= 7) | 2.1 ± 0.19 | 2.0 ± 0.21 | 2.2 ± 0.15 | 2.4 ± 0.1 | 2.9 ± 0.15 |
| Weekly Ethanol Consumption (g/kg)
| |||||
| EtOH (n= 6) | 2.2 ± 0.16 | 2.9 ± 0.24*# | 3.8 ± 0.32*# | 4.0 ± 0.26*# | 3.7 ± 0.17*# |
Experiment 3 intake values are means ± s.e.m.
p< 0.05, differs from respective baseline level (within-group comparison)
p< 0.05, differs from CTL group at corresponding Test cycle (between-group comparison)
Table 2.
Blood and Brain Ethanol Concentrations Related to Voluntary Drinking and Chronic Intermittent Ethanol Vapor Exposure
| Blood EtOH Levels | Brain EtOH Levels | |||||
|---|---|---|---|---|---|---|
| Experiment | Group | N | (mg/dl) | (mM) | (mg/dl) | (mM) |
| 3 | CTL | 7 | 64.7 ± 13.8 | 13.9 ± 3 | ||
|
| ||||||
| EtOH | 6 | 128.6 ± 22§ | 27.7 ± 4.7§ | |||
|
| ||||||
| 3 | EtOH | 14 | 168.9 ± 13‡ | 36.4 ± 2.9 | 131.7 ± 17‡ | 28.4 ± 3.5 |
|
| ||||||
| 2 | CTL | 7 | 87.2 ± 22 | 18.79 ± 5† | ||
|
| ||||||
| EtOH | 12 | 164.3 ± 24 | 35.4 ± 5† | |||
Values for ethanol concentrations are means ± s.e.m.
Values estimated from Figure 5C using probe recovery determinations (see methods)
For Exp 3: p= 0.027, differs from respective CTL group
For Exp 3: p> 0.05 (n.s.), blood vs. brain ethanol levels
A separate group of similarly treated EtOH mice (n= 14) received an additional (5th) cycle of chronic intermittent ethanol exposure and were sacrificed immediately upon removal from the inhalation chambers. As depicted in Table 2, blood ethanol concentrations were slightly higher than brain ethanol concentrations, but this was not statistically significant [t(26)= 1.76, p= 0.091]. These data demonstrate that the chronic intermittent ethanol exposure regimen yielded blood ethanol concentrations in the targeted range of 150–200 mg/dl (see methods) and this exposure produced brain ethanol concentrations of similar magnitude in the same mice.
Finally, peak brain ethanol concentrations determined from the microdialysis study (Experiment 2) are presented in Table 2 for comparative purposes. These values, expressed as mg/dl and mM, were extrapolated from the data shown in Figure 5C and adjusted based on calculated (10%) probe recovery efficiency (see methods). Although conditions for collecting brain ethanol concentrations are quite distinct for Experiments 2 and 3, the data enable a number of relevant comparisons. For instance, estimated peak brain ethanol concentrations measured during dialysis in EtOH mice as a consequence of voluntary drinking (35.4 mM) are remarkably similar to ethanol concentrations measured in brain tissue immediately upon removal from the ethanol vapor chambers (28.4 mM). Additionally, blood ethanol concentrations achieved following voluntary ethanol consumption during an index limited access drinking session were not only significantly greater in EtOH mice (27.7 mM) compared to CTL mice (13.9 mM), but the greater ethanol intake exhibited by EtOH mice produced blood ethanol levels that approached blood ethanol concentrations registered immediately after chronic ethanol vapor exposure (36.4 mM).
Discussion
Results from these studies demonstrated that EtOH mice not only consumed a significantly greater total amount of ethanol compared to CTL mice as a result of dependence and withdrawal experience (induced through repeated chronic intermittent ethanol exposures), but EtOH mice also exhibited a faster rate of ethanol intake compared to CTL mice. This greater and faster rate of consumption produced significantly higher ethanol concentrations measured in the nucleus accumbens by conventional microdialysis procedures in EtOH mice relative to non-dependent controls. To our knowledge, this is the first report of tracking and contrasting changes in brain ethanol concentrations that relate to oral self-administration of ethanol in dependent and non-dependent mice. Moreover, blood and brain ethanol levels achieved as a result of up-regulated voluntary ethanol drinking in EtOH mice were similar to those measured immediately following chronic intermittent ethanol exposure. Taken together, these data suggest that ethanol dependent mice increase their voluntary drinking behavior to produce blood and brain ethanol levels in a range consistent with their prior experience of chronic intermittent ethanol exposure.
Results from these studies confirm our previous findings indicating that repeated cycles of chronic intermittent ethanol exposure significantly increases voluntary ethanol consumption in C57BL/6J mice (Becker and Lopez 2004; Lopez and Becker 2005). A key feature of this dependence and relapse drinking model is that mice are given ample opportunity to self-administer ethanol prior to the dependence manipulation. Thus, once the positive reinforcing effects of ethanol are established during baseline drinking, voluntary ethanol intake progressively increases following repeated cycles of chronic intermittent ethanol exposure. We have found that the increased drinking results in significantly elevated blood ethanol levels, the effect is facilitated when chronic ethanol inhalation is delivered in an intermittent rather than continuous fashion, and increased drinking is evident whether ethanol is presented alone or with sucrose (Becker and Lopez 2004; Lopez and Becker 2005). Collectively, these results along with findings of others (Finn et al. 2007; O’Dell et al. 2004; Roberts et al. 2000) demonstrate a role for dependence and withdrawal experience in facilitating and perpetuating excessive ethanol drinking.
In the present study we assessed the temporal pattern of ethanol intake during the free-choice 2 hr limited access drinking sessions by using lickometer circuitry. The majority of licking responses directed at the ethanol tube occurred within the first 20–40 min of the drinking sessions in both CTL and EtOH groups. Because licking strongly correlated with ethanol intake as we (Griffin et al. 2007) and others have shown (Ford et al. 2005a; Ford et al. 2005b), this indicates that the majority of ethanol intake occurred during this time period. Additionally, not only did the EtOH group evidence greater total cumulative licks at the end of the drinking sessions in comparison to the CTL group, but the rate of ethanol drinking during the initial (20–40 min) period of the sessions was significantly greater in EtOH compared to CTL mice. Moreover, this difference progressively increased over the repeated test cycles. Consistent with greater rates of intake by EtOH mice, we also found blood ethanol levels were approximately 2-fold higher in EtOH mice compared to that registered by CTL mice, as previously reported (Becker and Lopez 2004). Further, the blood ethanol levels reached as a result of voluntary consumption in EtOH mice approached blood ethanol levels measured after exposure to the chronic intermittent ethanol treatment regimen. This is consistent with the idea that EtOH mice up-regulated their drinking behavior to achieve blood ethanol levels experienced during the chronic ethanol exposure procedure.
In the present study, using in vivo microdialysis procedures we found significantly higher peak ethanol concentrations in samples from the nucleus accumbens in EtOH compared to CTL mice. A substantial amount of ethanol was consumed within the first 20 min of the drinking session and dialysate ethanol levels peaked 40–60 min later, an effect consistent with oral absorption of ethanol (Desroches et al. 1995; Griffin et al. 2007). Consistent with the greater amount of ethanol intake during the microdialysis session, EtOH mice evidenced higher peak brain ethanol levels that were sustained (relative to the CTL group) during the later time points of the drinking session as well as at least one hour following termination of ethanol access. Importantly, ethanol levels in the dialysis samples positively correlated with ethanol intake during the dialysis/drinking session. Thus, a consequence of greater and faster ethanol drinking in the EtOH (dependent) group is significantly greater ethanol concentrations in brain compared to non-dependent controls.
The ethanol levels detected in dialysate from nucleus accumbens in the present study are consistent with our previous report in voluntarily drinking (non-dependent) C57BL/6J mice (Griffin et al. 2007) and another study involving limited access operant oral self-administration in rats (Doyon et al. 2003). Under artificial (in vitro) conditions, ethanol recovery by the microdialysis probes in the current study was calculated to be approximately 10%. This suggests that actual ethanol levels in the nucleus accumbens were about 10 times higher than concentrations measured in the dialysate samples. Although caution must be used when extrapolating values based on probe recovery (Hsiao et al. 1990; Robinson et al. 2000), it is remarkable that these estimated peak values were very similar to brain ethanol concentrations measured immediately upon removal from the ethanol vapor chamber (Table 2). Again, these results suggest that EtOH mice increased their consumption of ethanol to achieve brain ethanol levels in a range similar to that experienced during chronic intermittent ethanol exposure.
The biological mechanism driving the escalation in ethanol drinking in dependent mice is unknown. It does not appear that increased ethanol consumption can be attributed to a general need to simply hydrate. Although ethanol is always presented as a choice, with water as the alternative fluid, very little water is consumed during the limited access sessions. This is not surprising because an important feature of the model is that the mice are not water or food deprived at any time during the course of the experiment. Thus, mice target almost all of their drinking responses at the ethanol tube, and the greater amount consumed (especially in the initial period of the drinking session) by EtOH mice suggests greater avidity for ethanol as a function of dependence and withdrawal experience. Recent work in our laboratory (Lopez et al. 2008) as well as work by others with rats (Brown et al. 1998; Roberts et al. 2000) using operant procedures also support the idea that enhanced ethanol self-administration in dependent animals may be related to enhanced reinforcing effects of ethanol. Future studies will need to examine the relative contributions of enhanced sensitivity to the rewarding effects of ethanol and possible tolerance to the aversive effects of the drug as motivational factors that engender excessive drinking in dependent animals.
It is also possible that increased drinking in dependent animals is related to metabolic tolerance, i.e. increased ethanol metabolism. Chronic ethanol exposure induces expression of the cytochrome P450 enzyme, CYP2E1 in liver and can increase ethanol metabolic capacity beyond that provided by alcohol dehydrogenase alone (Alderman et al. 1989; Lieber 1999). Thus, increased ethanol metabolism may reduce blood ethanol levels that leads to reduced ethanol exposure in brain, which may foster greater drinking. However, our previous work indicated that repeated cycles of chronic intermittent ethanol exposure does not significantly alter blood ethanol elimination rates compared to controls (Becker 1994). Furthermore, although catalase appears to be an important metabolic pathway for ethanol in brain, CYP2E1 also contributes significantly to brain ethanol metabolism (Zimatkin et al. 2006). Chronic ethanol exposure induces CYP2E1 expression in the brain (Yadav et al. 2006), suggesting the possibility of reduced brain ethanol levels in dependent subjects. However, results from the current microdialysis experiment indicate that brain ethanol metabolism (expressed as rate of decline in ethanol concentration) was not statistically different between EtOH and CTL groups. Similarly, another report indicated that after gastric intubation of ethanol, brain ethanol levels in microdialysis samples were similar between ethanol exposed and ethanol naïve rats (Quertemont et al. 2003). Thus, it does not appear that greater ethanol consumption in the EtOH group can simply be attributed to pharmacokinetic factors. Nevertheless, in the current study, EtOH and CTL groups consumed different amounts of ethanol (as expected) and this may complicate interpretations about the relationship between ethanol pharmacokinetics and drinking behavior. Future studies will be required to further address this issue of potential differences in metabolic tolerance by equating ethanol exposure in both EtOH and CTL groups through experimenter-administered ethanol.
In summary, results from this series of experiments demonstrate that escalation of voluntary ethanol intake over repeated cycles of chronic intermittent ethanol exposure was directly related to increased licking behavior during the early portion of the limited access drinking sessions. This apparent early ‘loading’ of ethanol through greater and faster consumption produced significantly higher peak concentrations of ethanol that were sustained over a longer period of time in the nucleus accumbens of EtOH mice in comparison to CTL mice. Moreover, greater voluntary ethanol consumption in dependent mice produced blood and brain ethanol levels that approximated those levels experienced during the chronic ethanol exposure regimen. The underlying mechanisms that drive this escalation of drinking, as well as the extent to which resulting elevated brain ethanol concentrations play a role in perpetuating enhanced ethanol drinking in dependent mice remains to be determined.
Acknowledgments
This work was supported by NIH grants AA10716, AA14095 and AA13885. The authors thank Judi S. Randall and Kay Fernandes for excellent technical assistance in conducting this work.
References
- Alderman J, Kato S, Lieber CS. The microsomal ethanol oxidizing system mediates metabolic tolerance to ethanol in deermice lacking alcohol dehydrogenase. Arch Biochem Biophys. 1989;271:33–9. doi: 10.1016/0003-9861(89)90252-x. [DOI] [PubMed] [Google Scholar]
- Becker HC. Positive relationship between the number of prior ethanol withdrawal episodes and the severity of subsequent withdrawal seizures. Psychopharmacology. 1994;116:26–32. doi: 10.1007/BF02244867. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–8. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Brown G, Jackson A, Stephens DN. Effects of repeated withdrawal from chronic ethanol on oral self-administration of ethanol on a progressive ratio schedule. Behav Pharm. 1998;9:149–61. [PubMed] [Google Scholar]
- Desroches D, Orevillo C, Verina D. Sex- and strain-related differences in first-pass alcohol metabolism in mice. Alcohol. 1995;12:221–6. doi: 10.1016/0741-8329(94)00098-x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–82. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased Drinking During Withdrawal From Intermittent Ethanol Exposure Is Blocked by the CRF Receptor Antagonist d-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alter ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol. 2005a;37:23–33. doi: 10.1016/j.alcohol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABA(A) receptors differentially modulate Ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005b;29:1630–40. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD. The influence of sex on extracellular dopamine and locomotor activity in C57BL/6J mice before and after acute cocaine challenge. Synapse. 2006;59:74–81. doi: 10.1002/syn.20218. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD, Becker HC. Voluntary ethanol drinking in mice and ethanol concentrations in the nucleus accumbens. Brain Res. 2007;1138:208–13. doi: 10.1016/j.brainres.2006.12.071. [DOI] [PubMed] [Google Scholar]
- Groseclose CH, Draughn RA, Tyor WR, Sallee FR, Middaugh LD. Long-term intracranial cannula stabilization in mice with light cured resin composites. J Neurosci Meth. 1998;79:31–36. doi: 10.1016/s0165-0270(97)00158-1. [DOI] [PubMed] [Google Scholar]
- Hsiao JK, Ball BA, Morrison PF, Mefford IN, Bungay PM. Effects of different semipermeable membranes on in vitro and in vivo performance of microdialysis probes. J Neurochem. 1990;54:1449–52. doi: 10.1111/j.1471-4159.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–4. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968–1998)--a review. Alcohol Clin Exp Res. 1999;23:991–1007. [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increase both self-administration and the reinforcing value of ethanol in C57BL/6J mice. Alcohol Clin & Exp Res. 2008;32:163A. [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Meisch R. Relationship between physical dependence on ethanol and reinforcing properties of ethanol in animals. NIAAA Research Monographs. 1983;13:27–32. [Google Scholar]
- Meisch RA. Alcohol self-administration by experimental animals. In: Smart RG, Cappell HD, Glaser FB, Israel Y, Kalant H, Popham RE, Schmidt W, Sellers EM, editors. Research Advances in Alcohol and Drug Problems. Plenum Press; New York: 1984. pp. 23–45. [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–82. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; Academic Press: 2001. [Google Scholar]
- Quertemont E, Green HL, Grant KA. Brain ethanol concentrations and ethanol discrimination in rats: effects of dose and time. Psychopharmacology (Berl) 2003;168:262–70. doi: 10.1007/s00213-003-1437-7. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–94. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Lara JA, Brunner LJ, Gonzales RA. Quantification of Ethanol Concentrations in the Extracellular Fluid of the Rat Brain: In Vivo Calibration of Microdialysis Probes. J Neurochem. 2000;75:1685–1693. doi: 10.1046/j.1471-4159.2000.0751685.x. [DOI] [PubMed] [Google Scholar]
- Yadav S, Dhawan A, Singh RL, Seth PK, Parmar D. Expression of constitutive and inducible cytochrome P450 2E1 in rat brain. Mol Cell Biochem. 2006;286:171–80. doi: 10.1007/s11010-005-9109-z. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res. 2006;30:1500–5. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]