Abstract
Limb movement-related neurons in the cerebellar nuclei (CN) typically produce bursts of discharge in association with movement. Consequently, given the inhibitory nature of the Purkinje cell (PC) projection to CN, it is puzzling that only a minority of movement-related PCs pause; the majority burst. Some of the movement-related CN activity may be the result of excitation from collaterals of mossy and climbing fiber projections to the cerebellar cortex. The only other input to CN is diffuse and neuromodulatory, from locus ceruleus and raphe nuclei. To investigate the role of the excitatory mossy fiber input, single units in CN were recorded in macaque monkeys during the performance of reaching and manipulation tasks, before and after blocking the PC input with local microinjections of GABAA antagonists (bicuculline or SR95531). After these injections, the movement-related modulation of CN discharge was greater and began earlier, compared with the modulation in the preinjection group of neurons. These observations indicate that an important excitatory drive is provided by extracerebellar inputs to CN, most likely from collaterals of mossy fibers. PCs may serve primarily to regulate this activity, by either pausing or bursting as necessary.
INTRODUCTION
Each of the large, excitatory projection neurons in the cerebellar nucleus (CN) receives GABAergic input from on the order of a hundred Purkinje cells (PCs) in the cerebellar cortex. Despite the inhibitory nature of this prominent projection, movement-related CN neurons predominantly increase their discharge during voluntary reaching and grasping movements (Fortier et al. 1989; Harvey et al. 1979; van Kan et al. 1993). One mechanism by which this increased discharge might be explained is if Purkinje cells were to decrease their firing with movement, thus disinhibiting their CN targets. This would seem to require that the majority of PCs show corresponding movement-related pauses in discharge, and that these pauses precede the excitatory discharge observed in CN. However, movement-related increases of Purkinje cell discharge are more commonly observed than decreases (Fortier et al. 1989; Harvey et al. 1977; Miller et al. 2002b). Furthermore, consistent differences in timing between PCs and CN neurons have not been demonstrated (Thach 1970).
The primary extracerebellar source of input to the cerebellar nuclei is by collaterals of mossy and climbing fibers projecting to the cerebellar cortex, which arise from the pontine nuclei, the nucleus reticularis tegmenti pontis, and the lateral reticular nucleus. There are also diffuse, neuromodulatory inputs from the locus ceruleus and raphe nuclei, mediated by norepinephrine, and serotonin, but these do not provide a significant source of movement-related modulation (Siggins et al. 1971). However, the excitatory mossy fiber (MF) projections do offer a potential explanation for the concurrent increases in CN and PC discharge (Houk et al. 1993; Shinoda et al. 1997; Thach 1970). The pontine and medullary nuclei giving rise to these fibers are known to receive movement-related signals from motor cortex and red nucleus (Robinson et al. 1987; Shinoda et al. 1992). Yet, although neurons in the pontine nuclei typically burst in association with movement (Matsunami 1987), their terminals are generally relatively simple in structure and widely ramifying (Shinoda et al. 1992, 1997), and might not provide a very strong input.
It has been suggested that the excitatory, recurrent connections between the cerebral cortex and cerebellar nuclei may be reciprocal even at the level of single cells (Tsukahara et al. 1983). There is some recent computational (Hua and Houk 1997) and electrophysiological evidence in support of this tight reciprocity (Holdefer et al. 2000). Such an architecture could give rise to positive feedback that would enhance the excitatory input to CN (Houk et al. 1993). Furthermore, a variety of intrinsic cellular mechanisms might further strengthen these MF collateral inputs. Among these are N-methyl-d-aspartate (NMDA) currents, postinhibitory rebound mediated by t-type Ca2+ channels, and regulation of Ca2+-dependent K+ channels (Aizenman and Linden 1999; Anchisi et al. 2001; Smith et al. 2002).
If the movement-related discharge of CN neurons results from PC pauses, then blocking the PC input with GABAA antagonists should decrease or eliminate the movement-related bursts. Instead, we report here an increase in movement-related discharge after GABAA antagonists. This is consistent with extracerebellar sources providing a strong movement-related drive to CN, which is then restrained and refined by inhibitory PC input.
METHODS
Behavior
Data for this study were obtained from 2 monkeys trained to perform either a button-pressing or grip task. Monkeys were required to initiate a trial by placing their left hand on a waist-level touch pad for 1 s and then to reach to and actuate a device located approximately at arm’s length in front of the shoulder. If successful, a tone sounded and a juice reward was delivered. Monkey T was required on alternate trials (as indicated by light-emitting diodes [LEDs] in each device) to reach to either a small button (7.5 mm diameter) or a key-grip device (14 × 26 × 22 mm), with a hold period of 1 s. The grip device was instrumented with force-sensitive resistors indicating thumb and index finger grip force. Monkey G was required to press a large square button (32 mm) at the end of a long cylinder (40 cm, 3.2 cm diameter) with a hold period of 0.25 s. The right arm of both monkeys was loosely restrained.
Surgical procedures
After training, aseptic surgery was performed with the animal under isoflurane anesthesia. A halo-type head restraint and a 20-mm i.d. stainless steel recording chamber were implanted over the arm and hand representations in the interpositus (IP) and dentate (DN) nuclei of the cerebellum (left hemisphere). The chamber was angled 15° lateral to medial in a coronal plane, and positioned stereotaxically (monkey T, AP: −8.0, ML: 13.0; monkey G, AP: −8.0, ML: 10.0). The dorsal dentate, at intermediate rostrocaudal levels, targets the arm and hand representation of the primary motor cortex (Hoover and Strick 1999). Interpositus also projects to arm M1, but its output is more strongly directed to magnocellular red nucleus (Flumerfelt et al. 1973). These limb-related areas of CN were the primary focus of our study. All procedures were approved by the Institutional Animal Care and Use Committee of Northwestern University.
Recording and local injection of GABAA antagonists
At the outset of these experiments recordings were made with single tungsten microelectrodes in CN to locate IP and DN, including regions that were well modulated by limb movement. Single-unit signals were recorded using standard techniques. Microelectrode signals were amplified, band-pass filtered (300–10,000 Hz), and discriminated using a DSP-based discrimination system (Plexon, Dallas, TX). Multiple single-unit activity included all waveforms that crossed an amplitude threshold set to twice the peak noise level. These recordings typically contained 2– 4 recognizable action potential waveforms.
Subsequent recordings were performed using a pressure injection and recording assembly modified from that described by Dias and Segraves (1997). A beveled, 30-gauge stainless steel tube was used for injections, and a 115-μm-diameter microelectrode inside this tube allowed both single-unit and multiple-unit activity to be discriminated at the site of the injection. The separation between the injection cannula and the tip of the microelectrode was typically 0.2 to 0.3 mm. The pressure pulse (supplied by a tank of nitrogen gas) applied to the assembly was precisely regulated with a pneumatic picopump (World Precision Instruments, PV830), which allowed gradual injections in small steps (<100 nl). Monitoring a visible meniscus during injection eliminated compliance of the connecting tubing as a source of error when determining the volume injected.
After preinjection recordings in CN, either the GABAA receptor antagonist bicuculline or SR95531 (GABA zine) (1 μg in a total of 1 μl) was injected over a 5- to 15-min period. For the next 30 – 45 min, additional data files were recorded from cells within 750 microns of the injection site. On rare occasions, it was possible to record the same cell before and after injection. Direct drug effects over a radius of 1 mm or less have been estimated for 1-μl injections of muscimol (Martin and Ghez 1999). Because the receptor affinity for bicuculline is significantly higher than that for muscimol, one would expect its spread, if anything, to be slightly less. Forty minutes is within the recovery time for comparable local injections of bicuculline (Schroeder et al. 1997). Indirect effects mediated by the locally ramifying axons of CN neurons were probably minimal, based on the specific behavioral deficits found for local injections of muscimol comparable sizes (Martin et al. 2000; Mason et al. 1998). The dosage of GABAA antagonist in this study was chosen to cause minimal behavioral effects, yet allow an adequate spread of the drug for subsequent sampling of neurons along a penetration. The effectiveness of the injection was also verified by a general increase in resting discharge rate and, on many occasions, by an exaggerated, periodic bursting of the resting discharge, which was never observed preinjection.
Data analysis
Perievent time histograms (PETHs) and rasters were constructed from the unit activity based on several salient behavioral events, which included the device LED onset, onset of reach, button press or grip, and delivery of the juice reward. The averaged firing rates in 10-ms bins were then smoothed using a Gaussian filter. The difference between the peak and trough firing rates was calculated for each histogram, and the largest difference was defined as the depth of modulation (DOM) for each neuron. Units were considered significantly modulated if the peak or trough in firing exceeded ±4 SD of the resting period discharge, when the monkey had his hand on the touch pad. Onset latencies for units with significant responses during reach were determined by following the smoothed modulation envelope in the PETH back in time from the peak or trough to the point where it fell within DOM/10 of the resting discharge.
Histology
After all recordings were completed, monkey T received a lethal dose of sodium pentobarbital and was perfused with heparinized PBS followed by 4% paraformaldehyde and 2% potassium ferrocyanide in 0.1 M phosphate buffer. Electrode penetrations in the IP and DN were reconstructed from 50-μm-thick histological sections after identifying the Prussian blue reaction products of small electrolytic lesions. Monkey G is still being used in subsequent experiments.
All the recording and injection sites for monkey T are shown in Fig.1. Penetration numbers at the top of each section may be used to identify the location of neurons shown in subsequent figures. Preinjection recording sites are indicated by the open circles, postinjection sites by the filled circles. The estimated area of effectiveness for these 1-μl injections (Martin and Ghez 1999) is shown by the large circle on each histological section. All the recording and injection sites were within the zone of dorsal, M1-projecting portions of the DN (Hoover and Strick 1999).
FIG. 1.

Reconstruction of recording sites for one (T) of the 2 monkeys. Small, open circles are preinjection recordings; filled circles show the location of single-neuron and multiple single-neuron recordings after the injection of the GABAA antagonists bicuculline or GABAzine (SR95531). Carets indicate the site of injection, and the large circles are estimates of the area of effectiveness for 1-μl injections. Stereotaxic coordinates in millimeters are shown below the sections.
RESULTS
The results of blocking Purkinje cell (PC) input to discrete regions in the cerebellar nucleus (CN) with local injections of GABAA antagonists in 2 monkeys are reported here. Figure 2 shows examples of movement-related discharge in the absence of drug delivery. Figure 2, A and B show cells recorded from the dentate nucleus (DN) displaying reach-related depths of modulation (DOMs) >80 spikes/s. Extremely high intrinsic firing rates would be required for these neurons if these responses had been mediated only by PC disinhibition. In B, the reach-related increase in discharge appears to have been preceded by a slight pause. If this pause represented PC input, it could have deinactivated t-type Ca2+ channels, and yielded a burst of action potentials when subsequently activated by even a small depolarizing input (Aizenman and Linden 1999). The cell in Fig. 2C shows a more modest DOM (33 spikes/s) that might more reasonably be explained by a simple disinhibitory mechanism. Figure 2D shows the typical bursting PC response during reaching (with a DOM of 114 spikes/s). Although many PCs pause during movement, a burst is more common.
FIG. 2.

Movement-related bursts of 3 cerebellar nucleus (CN) neurons (A, B, C) and a Purkinje cell (PC) (D) before drug injection. During voluntary reaching behavior, both inhibitory PCs and their CN neuronal targets typically increase their discharge. Perievent time histograms (PETHs) in this and subsequent figures are aligned with the onset of reach from the touch pad to the button or grip device.
CN discharge after local injection of GABAA antagonists
Figures 3-6 illustrate the effects of GABAA antagonists on the movement-related discharge in cerebellar nucleus. The cell illustrated in Fig. 3 (P25U2) was recorded from the most anterior penetration (Fig. 1), in an area of the DN that has projections to M1. It was unusual in 2 respects. We were able to hold the neuron throughout the entire interval before, during, and after injection of 1 μl of bicuculline. In general, it proved very difficult to do so, probably because of the mechanical disturbance caused by the injection. It was also unusual because its preinjection response included an initial pause in firing. Although atypical, this pause helps to make the effect of the bicuculline even clearer. Before injection (Fig. 3A), a reach-related pause in firing was followed by a brief return to, or slightly above, the resting level of discharge, after which the firing rate decreased again during the button press. A final, tonic increase in firing occurred at about 1.2 s, with the return of the hand from the button to the touch pad. Although relatively uncommon, such decreases in firing associated with reach were observed for 3 additional (out of 31) CN neurons.
FIG. 3.

PETHs aligned to reach for a CN neuron recorded before and after local injection of bicuculline. A: before injection, a reach-related pause in firing was observed. B: 12 min after the end of the injection, the pause was eliminated, and an excitation associated with reach was unmasked. C: some recovery of the reach-related pause was seen after 39 min. D: behavior was stable during the recording of this neuron. Median time for movement of hand from the touch pad to button press is plotted for each of the 2-min files recorded from this neuron. Bicuculline (1.0 μl at 1 μg/1 μl) was injected over a 15-min period, as shown by the hatched bar. Times at which the PETHs in A, B, and C were recorded are indicated by the corresponding letters. Error bars show the 25th and 75th percentiles for each point.
FIG. 6.
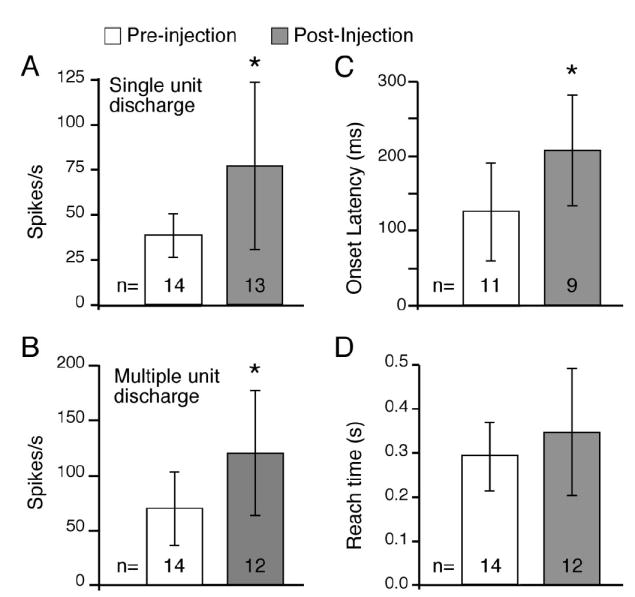
Summary measures. A: for both monkeys G and T the largest depth of modulation (DOM) was chosen from PETHs after alignment to different task-related events (see METHODS). DOMs were significantly greater after injection of GABAA antagonists both for single-unit and multiunit (B) recordings. C: onset of activity associated with reach began earlier after GABAA antagonists as compared with preinjection (P < 0.05, means −209 vs. −126 ms). D: latencies for hand movement from the touch pad was a simple measure of the monkey’s ability to perform the task. Unlike the neuronal discharge, there was no significant difference in this behavioral measure before and after injection. Error bars: SD.
By 12 min after the bicuculline injection, the resting discharge of this neuron increased dramatically, from about 50 to a bit over 100 spikes/s. More important, the initial pause disappeared, and was replaced by a burst of 80 spikes/s above the new resting discharge. This burst began 120 ms before reach onset and was followed by a further, gradual increase during the hold period (Fig. 3B). By 39 min after the injection, some recovery from the GABAA antagonist was evident (Fig.3C). The reach-related increase in firing reached only 61 spike/s and began 20 ms after the beginning of the reach. In addition, there was a decrease in firing preceding reach, reminiscent of the pause seen before injection. Despite the dramatic changes in this cell’s discharge, and presumably other neighboring cells, the monkey’s behavior was largely unaffected. This is demonstrated in Fig. 3D, in which the median time required to reach from the touch pad to the button is plotted as a function of time. Time points corresponding to the histograms in Fig. 3,A–C are indicated. There was no significant change in the reach time associated with the injection (unpaired t-test, P = 0.6). The dramatic changes in the discharge of this neuron after injection of bicuculline were not the indirect result of changes in the monkey’s motor behavior.
Because it was very difficult to hold the same neuron before and after injection, comparisons were made between groups of neurons recorded before and after injection during a given penetration. Several examples are shown in Fig. 4 for 2 penetrations (P34 and P42, Fig. 1) in the DN of monkey T. These between-group comparisons show effects similar to those of Fig. 3: elevated spontaneous discharge rate, a reach-related burst in discharge as large as or larger than that before injection, and a shift of the response to earlier onsets relative to reach.
FIG. 4.
Enhancement of movement-related discharge after GABAA antagonists. A: small, steplike increase in discharge of a CN neuron (P34U6) recorded before injection. B: after 1 μl bicuculline, another neuron (P34U8) increased its firing rate by more than 140 spikes/s. This cell was recorded within 250 μm of the neuron shown above. C: 40 spikes/s DOM before injection (P42U1). D: after the GABAA antagonist GABAzine (SR95531), the neuron P42U2 showed an increase in firing of 46 spikes/s, which began 220 ms before the onset of reach. For this and subsequent figures, the horizontal dashed line indicates spontaneous firing rate, and the vertical dashed line marks the onset of modulation. Median times at which the reach and button (or grip) phases of the task ended are indicated by the heavy vertical lines within the box under the reach-aligned PETH. Shaded regions show the 25th and 75th percentiles of the variation in these times.
The preinjection neuron in the left column of Fig. 4 showed an increase in firing rate associated with a reach of 30 spikes/s above the spontaneous rate of 25 (onset latency: −150 ms). After recording this neuron, 1 μl of bicuculline was injected, and 22 min later, another neuron was recorded 250 μm ventral to this site (Fig. 4B). Despite having blocked its PC inputs, the neuron’s discharge increased by more than 140 spikes/s during reach (onset latency −170 ms) from a spontaneous rate of 60 spikes/s. Periodic bursting appeared in the resting discharge while the monkey’s hand was on the touch pad. Many CN neurons showed this bursting behavior after bicuculline. Because it was never seen before injection, such bursting was considered to be good evidence that the drug was actually ejected from the cannula in sufficient quantity to affect the recorded cell. Multiunit activity during penetration 34 increased from 96 to 110 spikes/s after injection.
A neuron recorded in the DN after the more selective GABAA antagonist GABAzine (SR95531) is shown in Fig. 4D. GABAzine, unlike bicuculline, does not block an apaminsensitive, Ca2+-dependent K+ conductance. The preinjection neuron recorded along this penetration increased its firing by 43 spikes/s, beginning 70 ms before the onset of reach (Fig. 4C). By 26 min after injection of 1 μl of GABAzine, a neuron was isolated 550 μm dorsal to the injection site (Fig. 4D). Modulation during reach persisted (46 spikes/s) and began earlier than for the preinjection neuron (−220 ms). Activity during the touch pad period also showed periodic bursting, although not as dramatically as that seen after bicuculline (Fig. 4B). However, the spontaneous rate of this neuron was about 25 spikes/s higher than that of the previous neuron recorded before injection. In addition to this single-neuron recording, multiple-unit activity was recorded after GABAzine from an additional 3 sites along this penetration, all of which were well modulated during behavior (mean DOM = 158 spikes/s). Multiunit activity was not recorded before the injection during penetration 42.
A similar set of neurons recorded from 2 penetrations in monkey G are shown in Fig. 5. Those recorded along the same penetration (P20 and P14) are aligned in a column. The preinjection neurons (Fig. 5, A and D) showed modest increases in firing associated with reach (DOM: 30 and 22 spikes/s, onsets at −110 and −120 ms, respectively). Bicuculline (1 μl) injections were then made at the same depth as these baseline recordings. Subsequently, neurons along P20 were recorded 50 and 300 μm from the site of injection (Fig. 5, B and C). Phasic, increased firing was observed for both units (56 and 43 spikes/s) at relatively early latencies, and from an elevated baseline rate. Highly periodic bursting was also present both during the touch pad period and during and subsequent to the button press. This burst pattern occasionally made onset time difficult to determine with certainty. The neuron shown in Fig. 5E was phasically modulated by reach after bicuculline (67 spikes/s; onset at −140 ms), with periodic bursting discharge both before and after reach. A second neuron, recorded simultaneously from the same electrode, showed little behavioral modulation and no periodic bursting (Fig. 5F). It is possible, though unlikely, that for some reason bicuculline was not able to gain access to its receptors. Multiple-unit activity before and after the injection in the 2 experiments shown in this figure averaged 71 and 164 spikes/s (left column) and 47 and 85 spikes/s (right column).
FIG. 5.
Bicuculline effects on movement-related discharge recorded from 2 penetrations in monkey G. Left column: neuron recorded before injection, and 2 neurons recorded after 1 μl of bicuculline within 300 μm of the preinjection neuron. Postinjection neurons were well modulated by reach, and showed a pronounced bursting pattern in their spontaneous activity. Right column: 2 neurons are shown that were recorded at the same site after bicuculline and within 500 μm of the preinjection neuron. Although CN neurons were typically well modulated by the task, there were occasional exceptions, even for neurons recorded from the same electrode (P14U3 and P14U4).
To summarize the population data recorded before and after injection, the DOM was calculated for all the neurons recorded along any penetration where a successful injection was made. As shown in Fig. 6A, discriminated, single neurons in the GABAA antagonist group had significantly greater DOMs compared with neurons recorded before injection during the same experiments (77 vs. 39 spikes/s, respectively; P < 0.05). Similarly, multiple-unit activity increased significantly from 60 to 120 spikes/s (Fig. 6B).
The onset of reach-related single-unit activity postinjection also occurred earlier than that recorded preinjection (Fig. 6B, P < 0.005, means −209 vs. −126 ms). Onset latencies were compared for the pre- and postinjection cells that were significantly modulated during reach (> ±4SD of resting discharge), which included 11 and 9 neurons, respectively. These differences were probably not simply that the larger modulations after GABAA antagonists were more easily detected because the DOM and onset latencies for the pre- and postinjection neurons were not correlated (r = 0.11).
The significant differences in firing rate and onset latency shown in Fig. 6 occurred despite the lack of detectable behavioral deficits. Figure 6D shows the mean latency for hand movement from the touch pad to the button or grip device for all the files from which the neuronal data for this figure were taken. There were no significant differences in the mean reach times before and after injection (means 0.29 vs. 0.35 s; P = 0.26). Two additional neurons were recorded after an injection that caused a behavioral deficit. In both cases, the DOM of the cells recorded after the injection was significantly larger than that of the preinjection cells. However, because such changes in behavior would have complicated the analysis of movement-related discharge, these neurons were not included in the statistics or summary graphs of Fig. 6.
Having demonstrated that blocking PC input to the nuclei serves generally to increase the magnitude of the movement-related discharge, in a very limited series of 3 experiments, we also attempted to cause the opposite effect. We injected the NMDA receptor antagonist, AP5 (30 mM, 1 μl), to block the excitatory MF input to CN. In each of these sessions, we held a single cell before, during, and after the injection of AP5. Two of these experiments were consistent with our hypothesis that significant movement-related excitation is conveyed to CN by mossy fiber collaterals. Figure 7 shows the results for the most representative of these cases. The initial movement-related burst was subtly modulated by a slight pause occurring at 0 s, and there was a second, small decrease in activity beginning at about 0.9 s. By 18 min after the injection, the bursts disappeared, whereas both of the underlying decreases became more prominent. The spontaneous rate actually increased somewhat after the injection, an effect that may be attributable to disinhibition of CN through the block of excitatory input to inhibitory interneurons. Such an effect has been reported in cerebral cortex (Li et al. 2002). In the second experiment, a large movement-related burst was virtually eliminated after injection, but without the increase in spontaneous rate.
FIG. 7.

Effect of injection of the NMDA receptor antagonist AP5. A: modulation of a CN neuron before injection included a prominent burst that was modified by a small inhibitory pause. B: excitatory components of the movement-related discharge modulation are lost as a result of blocking the mossy fiber (MF) input to CN. PC-related pauses remain.
The third experiment, however, was inconclusive. Shortly after the injection, both the spontaneous rate and the movement-related discharge increased. However, by 30 min, there had been a further increase in overall discharge such that the movement-related depth of modulation was somewhat obscured. Although unexpected, this result could have occurred if the neuron received significant inhibitory input from interneurons that were themselves activated by mossy fibers or by collaterals of CN output neurons.
Although these numbers are small, this result provides further evidence that most of the movement-related bursts seen in CN are the result of mossy fiber inputs, not disinhibition from PCs. The role of PC input to CN appears to be largely one of restraint, both of the overall discharge, and of the fine details of CN modulation.
DISCUSSION
The results of this study demonstrate that movement-related burst discharge in CN not only persists after PC inputs are blocked, but actually increases in magnitude. It also occurs at shorter latency before reach. These results support an important role for motor-related, excitatory inputs directly to CN, and are consistent with the regulation of this drive by inhibitory inputs from PCs.
Effectiveness of local injections of GABAA antagonists
Because the results of this study relied mainly on comparisons between separate groups of neurons recorded before and after injection of GABAA antagonists, we must address a potential sampling bias between groups. This is particularly important when interpreting differences in modulation and onset latency. We minimized this source of bias by only using penetrations yielding both pre- and postinjection data points, thus ensuring that comparable regions of CN were sampled for both groups.
All neurons in the GABAA antagonist group were recorded 10–40 min postinjection, which is within the time period for which bicuculline effects have been shown to persist after local injections (Schroeder et al. 1997). Also, all of the units in the antagonist group were recorded within 750 μm of the site of injection. This is well within the zone (1-mm radius) for which local injections of 1 μl of muscimol reduced glucose metabolism (Martin and Ghez 1999). If, as is possible, the spread of bicuculline were actually less than that of muscimol, it could lead to an underestimate of the effect that we reported, if some of the postinjection cells were actually outside the effective radius. For most cells, the effectiveness of the injection was verified by spontaneous bursting of the resting discharge. Those few neurons without rhythmic bursting after bicuculline came from penetrations where such bursting was clearly observed for other neurons, indicating that the drug had been successfully delivered (P34, Fig. 4; P14, Fig. 5). In any case, if some inactivations were less than complete, it follows that a complete block would likely have yielded even larger effects than those we report here.
Extracerebellar drive to CN
There are 2 main extracerebellar inputs to CN: climbing fibers and mossy fibers. In a study in the rat, 91% of the labeled climbing fibers from the inferior olive (IO) gave off thin collaterals that ramified within a single nucleus (Sugihara et al. 1999). However, the firing rates of IO neurons are almost certainly too slow to have mediated the robust response modulation observed in the present study, particularly because they lack the powerful synapse of the cerebellar cortex. Twenty percent of the mossy fibers projecting from pontine nuclei (PN) to the cerebellar cortex have been observed to emit sparse collaterals, ramifying in a single nucleus (Shinoda et al. 1992). Virtually all MF inputs from the lateral reticular nucleus (LRN) have axon collaterals to CN often innervating more than one nucleus (Wu et al. 1999). We recorded in regions of the dentate that would be expected to receive M1-related inputs from MF collaterals (Shinoda et al. 1997).
Both of these sources of mossy fibers receive projections that carry motor information, and could mediate the modulation we observed in CN that persisted after injection of GABAA antagonists. The PN receive widespread inputs from neocortical areas, including motor and premotor cortex (Brodal and Bjaalie 1997). LRN also receives a direct projection from motor cortex, although most of its motor input derives from collaterals of the rubrospinal pathway (Allen and Tsukahara 1974; Bruckmoser et al. 1970; Robinson et al. 1987). Microstimulation of motor or premotor areas has been shown to elicit short-latency excitatory effects in CN, which may be mediated by these MF collaterals (Allen et al. 1976, 1978; Miller et al. 2002a; Shinoda et al. 1997). However, these excitatory effects are relatively infrequent and weak compared with the accompanying inhibitory effects, which are almost certainly mediated mainly by mossy fiber input to the cerebellar cortex, and from that point, PC inhibition of the nucleus. In summary, the anatomical data and the infrequency of short-latency excitatory effects after neocortical stimulation have left the effectiveness of MF collaterals in CN an open question.
The major finding of this study is that extracerebellar input provides an excitatory drive to CN that is more effective than would be expected from these anatomical and microstimulation studies. Not only were movement-related increases in discharge no smaller after local injection of GABAA antagonists, they were actually greater. Both the spontaneous and the movement-related activity increased, but the latter increase was larger, resulting in greater depths of modulation. This is probably not attributed to a simple, overall increase in excitability. In the lateral geniculate nucleus, bicuculline increased the visually driven DOM of neurons, whereas micro-iontophoresis of glutamate, which increased the overall excitability of the neurons, did not (Holdefer et al. 1989). Bicuculline may also have increased the DOM through its blockade of apaminsensitive K+ channels. These Ca2+-dependent conductances increase the discharge rate of neurons in medial vestibular nucleus in response to injected current (Smith et al. 2002). Our similar results with SR95531 suggest that this was not the case. Finally, in addition to the PC inputs, interneurons and axon collateral inputs from GABA-containing projection cells to the inferior olive may also have been blocked (Czubayko et al. 2001; de Zeeuw et al. 1989). The same logic applies to these inputs as to those of PCs. If they are normally inhibited during movement, and thus through disinhibition, a source of the movement-related DOM, this effect would have been diminished by bicuculline. On the other hand, if these sources are normally excited during movement, removal of their influence could lead to larger CN bursts, but only provided there remains a source of movement-related input from a non-GABA projection. There is some inconclusive evidence of such a role for the interneurons from our limited AP5 experiments. In this case, the overall conclusion would remain the same, although the strength of the mossy fiber component might be somewhat overestimated.
Movement-related discharge was greater after PC inputs were blocked, and it occurred earlier with respect to reach. The earlier onset latencies were not simply a result of the modulation being easier to detect because the depth of modulation and onset latency were uncorrelated across neurons. However, it could reflect a greater sensitivity of CN neurons, mediated by t-type Ca2+ currents, to small depolarizing inputs. This would potentially allow MF collateral inputs to cross the threshold for eliciting a burst of action potentials earlier. These currents are thought to contribute to the spontaneous burst discharge in CN slices (Aizenman and Linden 1999; Czubayko et al. 2001).
How can the potent, excitatory drive in the absence of PC input be reconciled with the anatomical and microstimulation studies suggesting a weak MF input? There are several cellular mechanisms that the MF collateral system could use to increase its effect. Unlike NMDA receptors in other brain regions, the Mg2+ block in CN is relatively weak at resting membrane potentials, allowing them to play a potentially important role in normal synaptic signaling. The relatively long time constant of these currents could provide a more effective excitation for a relatively small number of excitatory synapses (Anchisi et al. 2001). Another possibility is that regulation of Ca2+-dependent K+ conductances may increase the firing response gain of CN neurons. This action has been demonstrated in medial vestibular nucleus and might contribute to behavioral gain changes in the vestibuloocular reflex (Smith et al. 2002). Neurons in CN may also make use of t-type Ca2+ currents to amplify MF collateral inputs. After deinactivation by hyperpolarization, a relatively small depolarization can trigger a large Ca2+ influx and a burst of action potentials. These t-channel–mediated bursts have been proposed to enhance signals in sensory thalamus (Fanselow et al. 2001; Guido and Weyand 1995). In the CN, t-type channels are preferentially located on more distal dendrites, allowing them to integrate inputs from both PCs and MF collaterals (Gauck et al. 2001).
In addition to these cellular mechanisms, it should be recognized that during behavior, large portions of the cerebral motor areas are excited, compared with those activated by microstimulation. The converging, excitatory inputs undoubtedly provide a more effective excitatory drive than microstimulation alone. It has also been proposed that extracerebellar drive to CN may be part of a recurrent loop, whereby motor-related excitatory drive is strengthened by positive feedback until sufficient populations of CN neurons are recruited for execution of a voluntary motor command (Allen and Tsukahara 1974; Houk et al. 1993). Our recordings were made in regions of the dentate that receive M1-related inputs from MF collaterals and, in turn, project to M1 through the ventrolateral thalamus (Hoover and Strick 1999; Shinoda et al. 1997). The nonphysiological activation resulting from electrical stimulation might be relatively ineffective for initiating this mechanism. The motor-related, extracerebellar drive demonstrated in the current results is consistent with this model.
Role of the cerebellar cortex
The robust behavioral modulation observed in CN after blocking Purkinje cell inputs raises the question, “What, then, does cerebellar cortex contribute to CN neuronal responses?” Inhibitory Purkinje cells may provide a precise spatiotemporal regulation of the extracerebellar drive restraining and tailoring the modulation of CN neurons to the specific task at hand (Houk et al. 1993). Bursts of PC discharge during movement have been shown to be positively correlated with limb EMG (Miller et al. 2002b). These bursts would serve to restrain and perhaps refine the larger-than-necessary, extracerebellar input to CN. A number of years ago, Thach speculated that the overall effect of PC inputs to CN might be either “augmenting” (through disinhibition) or “restraining,” and concluded by favoring the idea of restraint (Thach 1970). Earlier, Eccles and colleagues suggested that MF collaterals to CN might provide a, “somewhat nonspecific” excitatory input that would be “modified by the inhibitory action of the Purkinje cells” (Eccles et al. 1967). Our current finding of a persistent modulation in CN that actually increases after GABAA antagonists further supports these ideas. If the specificity of these mossy fiber inputs remains to be demonstrated conclusively, their strength is now clear.
Acknowledgments
GRANTS This work was supported in part by National Institutes of Health Grants P50MH-48185 and NS-43323.
References
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- Allen GI, Gilbert PF, Yin TC. Cerebral and peripheral inputs to interpositus neurons in monkey. Brain Res. 1976;105:337–341. doi: 10.1016/0006-8993(76)90430-3. [DOI] [PubMed] [Google Scholar]
- Allen GI, Gilbert PF, Yin TC. Convergence of cerebral inputs onto dentate neurons in monkey. Exp Brain Res. 1978;32:151–170. doi: 10.1007/BF00239724. [DOI] [PubMed] [Google Scholar]
- Allen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974;54:957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Anchisi D, Scelfo B, Tempia F. Postsynaptic currents in deep cerebellar nuclei. J Neurophysiol. 2001;85:323–331. doi: 10.1152/jn.2001.85.1.323. [DOI] [PubMed] [Google Scholar]
- Brodal P, Bjaalie JG. Salient anatomic features of the cortico-ponto-cerebellar pathway. Prog Brain Res. 1997;114:227–249. doi: 10.1016/s0079-6123(08)63367-1. [DOI] [PubMed] [Google Scholar]
- Bruckmoser P, Hepp-Reymond MC, Wiesendanger M. Cortical influence on single neurons of the lateral reticular nucleus of the cat. Exp Neurol. 1970;26:239–252. doi: 10.1016/0014-4886(70)90122-6. [DOI] [PubMed] [Google Scholar]
- Czubayko U, Sultan F, Thier P, Schwarz C. Two types of neurons in the rat cerebellar nuclei as distinguished by membrane potentials and intracellular fillings. J Neurophysiol. 2001;85:2017–2029. doi: 10.1152/jn.2001.85.5.2017. [DOI] [PubMed] [Google Scholar]
- de Zeeuw CI, Holstege JC, Ruigrok TJ, Voogd J. Ultrastructural study of the GABAergic, cerebellar, and mesodiencephalic innervation of the cat medial accessory olive: anterograde tracing combined with immunocyto-chemistry. J Comp Neurol. 1989;284:12–35. doi: 10.1002/cne.902840103. [DOI] [PubMed] [Google Scholar]
- Dias EC, Segraves MA. A pressure system for the microinjection of substances into the brain of awake monkeys. J Neurosci Methods. 1997;72:43–47. doi: 10.1016/s0165-0270(96)00154-9. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. Cerebellum as a Neuronal Machine. New York: Springer-Verlag; 1967. [Google Scholar]
- Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci USA. 2001;98:15330–15335. doi: 10.1073/pnas.261273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flumerfelt BA, Otabe S, Courville J. Distinct projections to the red nucleus from the dentate and interposed nuclei in the monkey. Brain Res. 1973;50:408–414. doi: 10.1016/0006-8993(73)90742-7. [DOI] [PubMed] [Google Scholar]
- Fortier PA, Kalaska JF, Smith AM. Cerebellar neuronal activity related to whole-arm reaching movements in the monkey. J Neurophysiol. 1989;62:198–211. doi: 10.1152/jn.1989.62.1.198. [DOI] [PubMed] [Google Scholar]
- Gauck V, Thomann M, Jaeger D, Borst A. Spatial distribution of low- and high-voltage-activated calcium currents in neurons of the deep cerebellar nuclei. J Neurosci. 2001;21:1–4. doi: 10.1523/JNEUROSCI.21-15-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W, Weyand T. Burst responses in thalamic relay cells of the awake behaving cat. J Neurophysiol. 1995;74:1782–1786. doi: 10.1152/jn.1995.74.4.1782. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Porter R, Rawson JA. The natural discharges of Purkinje cells in paravermal regions of lobules V and VI of the monkey’s cerebellum. J Physiol. 1977;271:515–536. doi: 10.1113/jphysiol.1977.sp012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Porter R, Rawson JA. Discharges of intracerebellar nuclear cells in monkeys. J Physiol. 1979;297:559–580. doi: 10.1113/jphysiol.1979.sp013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdefer RN, Miller LE, Chen LL, Houk JC. Functional connectivity between cerebellum and primary motor cortex in the awake monkey. J Neurophysiol. 2000;84:585–590. doi: 10.1152/jn.2000.84.1.585. [DOI] [PubMed] [Google Scholar]
- Holdefer RN, Norton TT, Godwin DW. Effects of bicuculline on signal detectability in lateral geniculate nucleus relay cells. Brain Res. 1989;488:341–347. doi: 10.1016/0006-8993(89)90727-0. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC, Keifer J, Barto AG. Distributed motor commands in the limb premotor network. Trends Neurosci. 1993;16:27–33. doi: 10.1016/0166-2236(93)90049-r. [DOI] [PubMed] [Google Scholar]
- Hua SE, Houk JC. Cerebellar guidance of premotor network development and sensorimotor learning. Learn Mem. 1997;4:63–76. doi: 10.1101/lm.4.1.63. [DOI] [PubMed] [Google Scholar]
- Li Q, Clark S, Lewis DV, Wilson WA. NMDA receptor antagonists disinhibit rat posterior cingulate and retrosplenial cortices: a potential mechanism of neurotoxicity. J Neurosci. 2002;22:3070–3080. doi: 10.1523/JNEUROSCI.22-08-03070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Cooper SE, Hacking A, Ghez C. Differential effects of deep cerebellar nuclei inactivation on reaching and adaptive control. J Neurophysiol. 2000;83:1886–1899. doi: 10.1152/jn.2000.83.4.1886. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Mason CR, Miller LE, Baker JF, Houk JC. Organization of reaching and grasping movements in the primate cerebellar nuclei as revealed by focal muscimol inactivations. J Neurophysiol. 1998;79:537–554. doi: 10.1152/jn.1998.79.2.537. [DOI] [PubMed] [Google Scholar]
- Matsunami K. Neuronal activity in nuclei pontis and reticularis tegmenti pontis related to forelimb movements of the monkey. Neurosci Res. 1987;5:140–156. doi: 10.1016/0168-0102(87)90030-7. [DOI] [PubMed] [Google Scholar]
- Miller LE, Holdefer RN, Houk JC. Early excitatory effects in cerebellar nuclei after M1 stimulation in the awake monkey. J Neurosci. 2002a;22 [Google Scholar]
- Miller LE, Holdefer RN, Houk JC. The role of the cerebellum in modulating voluntary limb movement commands. Arch Ital Biol. 2002b;140:175–183. [PubMed] [Google Scholar]
- Robinson FR, Houk JC, Gibson AR. Limb specific connections of the cat magnocellular red nucleus. J Comp Neurol. 1987;257:553–577. doi: 10.1002/cne.902570406. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Javitt DC, Steinschneider M, Mehta AD, Givre SJ, Vaughan HG, Jr, Arezzo JC. N-Methyl-d-aspartate enhancement of phasic responses in primate neocortex. Exp Brain Res. 1997;114:271–278. doi: 10.1007/pl00005635. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Izawa Y, Sugiuchi Y, Futami T. Functional significance of excitatory projections from the precerebellar nuclei to interpositus and dentate nucleus neurons for mediating motor, premotor and parietal cortical inputs. Prog Brain Res. 1997;114:193–207. doi: 10.1016/s0079-6123(08)63365-8. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sugiuchi Y, Futami T, Izawa R. Axon collaterals of mossy fibers from the pontine nucleus in the cerebellar dentate nucleus. J Neurophysiol. 1992;67:547–560. doi: 10.1152/jn.1992.67.3.547. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Oliver AP, Hoffer BJ, Bloom FE. Cyclic adenosine monophosphate and norepinephrine: effects on transmembrane properties of cerebellar Purkinje cells. Science. 1971;171:192–194. doi: 10.1126/science.171.3967.192. [DOI] [PubMed] [Google Scholar]
- Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol. 2002;87:2031–2042. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Wu H, Shinoda Y. Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat. J Comp Neurol. 1999;414:131–148. [PubMed] [Google Scholar]
- Thach WT. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970;33:537–547. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Tsukahara N, Bando T, Murakami F, Oda Y. Properties of cerebello-precerebellar reverberating circuits. Brain Res. 1983;274:249–259. doi: 10.1016/0006-8993(83)90702-3. [DOI] [PubMed] [Google Scholar]
- van Kan PLE, Gibson AR, Houk JC. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol. 1993;69:74–94. doi: 10.1152/jn.1993.69.1.74. [DOI] [PubMed] [Google Scholar]
- Wu HS, Sugihara I, Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol. 1999;411:97–118. doi: 10.1002/(sici)1096-9861(19990816)411:1<97::aid-cne8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]




