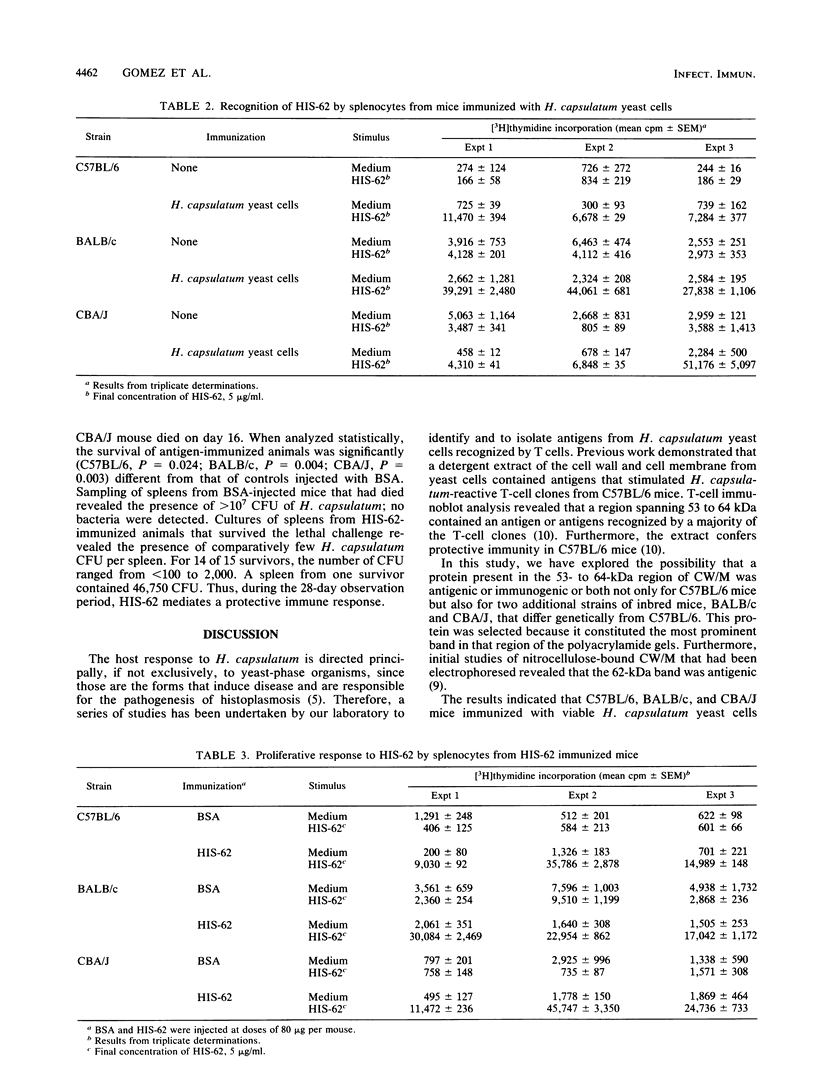
Abstract
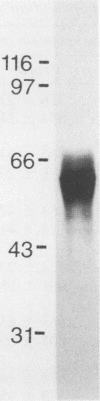
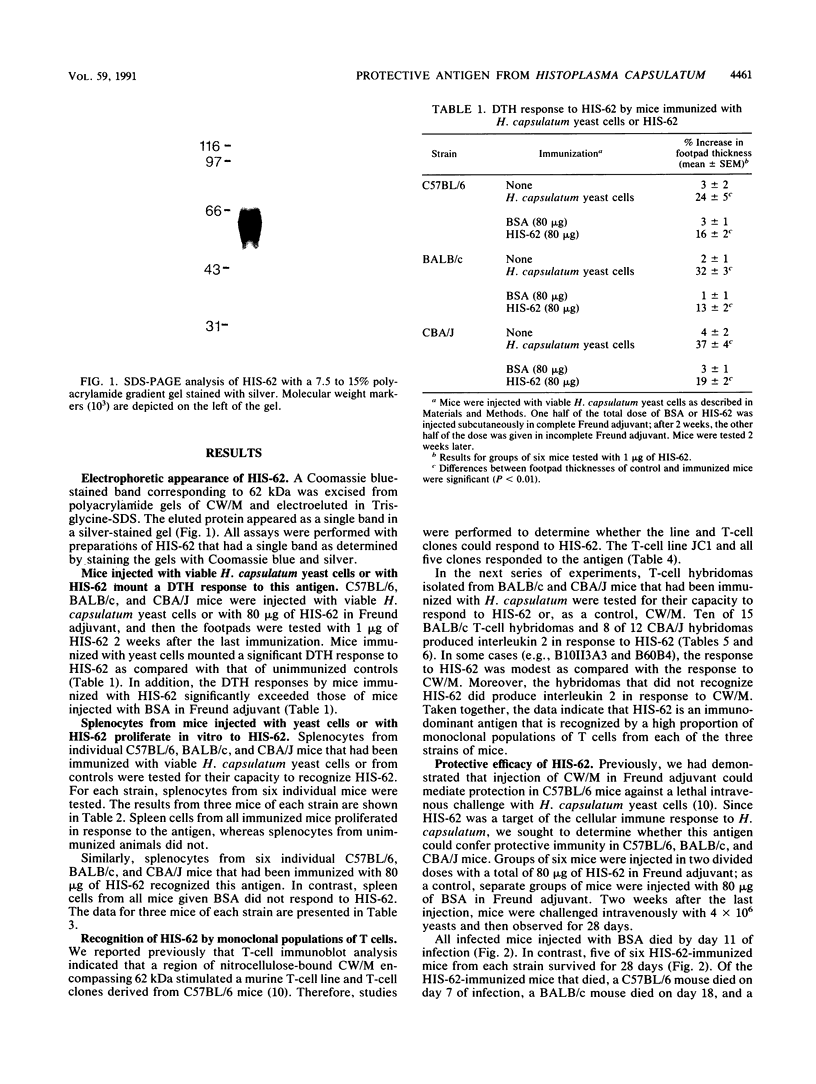
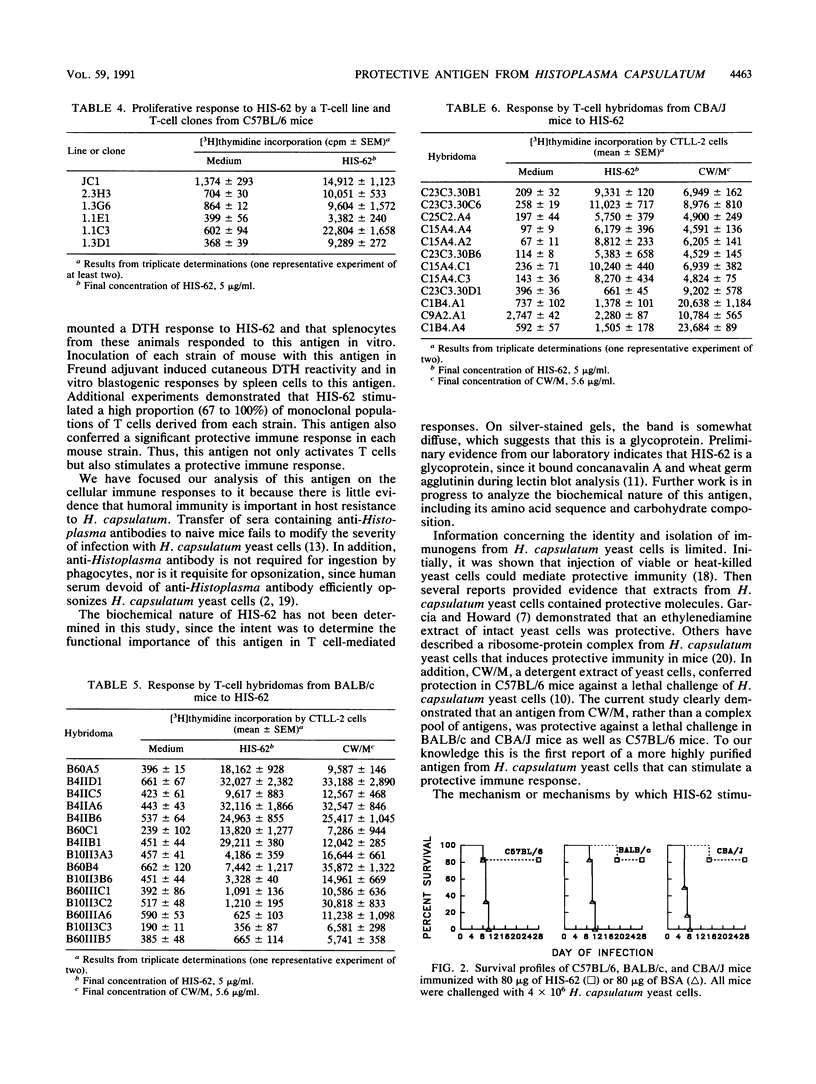
We reported previously that a detergent extract of the cell wall and cell membrane of Histoplasma capsulatum yeast cells contains antigens recognized by T cells. In T-cell immunoblot analysis, a region encompassing 62 kDa was stimulatory for an H. capsulatum-reactive T-cell line and T-cell clones derived from C57BL/6 mice. In this study, we isolated a 62-kDa band, termed HIS-62, from electrophoresed cell wall and cell membrane of H. capsulatum yeast cells and examined its antigenicity and immunogenicity. C57BL/6, BALB/c, and CBA/J mice that were immunized with viable H. capsulatum yeast cells mounted a delayed-type hypersensitivity response to HIS-62 that was stronger than that of normal controls. Spleen cells from each strain of mouse immunized with viable yeast cells proliferated vigorously in response to HIS-62; conversely, splenocytes from control animals did not recognize this antigen. A T-cell line and 5 of 5 T-cell clones from C57BL/6 mice, 10 of 15 BALB/c T-cell hybridomas, and 8 of 12 CBA/J T-cell hybridomas recognized HIS-62. A cutaneous delayed-type hypersensitivity response to the antigen was apparent in each strain of mouse that was injected with 80 micrograms of HIS-62 mixed with Freund adjuvant. In addition, spleen cells from HIS-62-immunized mice proliferated in vitro in response to this antigen. Vaccination of each strain of mouse with 80 micrograms of HIS-62 conferred protection against a lethal intravenous challenge with H. capsulatum yeast cells. Thus, HIS-62 appears to be an important target of the cellular immune response to H. capsulatum and induces a protective immune response in mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Wright S. D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987 Jan 1;165(1):195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe G. S., Jr, Brunner G. D. Functional analysis of Histoplasma capsulatum-reactive T-cell hybridomas. Infect Immun. 1990 Jun;58(6):1538–1544. doi: 10.1128/iai.58.6.1538-1544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe G. S., Jr Protective immunity in murine histoplasmosis: functional comparison of adoptively transferred T-cell clones and splenic T cells. Infect Immun. 1988 Sep;56(9):2350–2355. doi: 10.1128/iai.56.9.2350-2355.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe G. S., Jr, Smith J. G., Sonnenfeld G., Denman D., Bullock W. E. Development and characterization of Histoplasma capsulatum-reactive murine T-cell lines and clones. Infect Immun. 1986 Dec;54(3):714–722. doi: 10.1128/iai.54.3.714-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. P., Howard D. H. Characterization of antigens from the yeast phase of Histoplasma capsulatum. Infect Immun. 1971 Aug;4(2):116–125. doi: 10.1128/iai.4.2.116-125.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A. M., Bullock W. E., Taylor C. L., Deepe G. S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988 Jul;56(7):1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez A. M., Rhodes J. C., Deepe G. S., Jr Antigenicity and immunogenicity of an extract from the cell wall and cell membrane of Histoplasma capsulatum yeast cells. Infect Immun. 1991 Jan;59(1):330–336. doi: 10.1128/iai.59.1.330-336.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Khardori N., von Behren L., Chaudhary S., McConnachie P., Strano A., Tewari R. P. Cellular mediators of anti-histoplasma immunity: I. Protective immunity and cellular changes in spleens of mice immunized by sublethal infection with yeast cells of histoplasma capsulatum. Mykosen. 1986 Mar;29(3):103–115. [PubMed] [Google Scholar]
- Konigsberg W. H., Henderson L. Removal of sodium dodecyl sulfate from proteins by ion-pair extraction. Methods Enzymol. 1983;91:254–259. doi: 10.1016/s0076-6879(83)91022-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Gootee L., Bucher C., Bullock W. E. Inhibition of intracellular growth of Histoplasma capsulatum yeast cells by cytokine-activated human monocytes and macrophages. Infect Immun. 1991 Feb;59(2):737–741. doi: 10.1128/iai.59.2.737-741.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASLAW S., SCHAEFER J. Survival of Histoplasma capsulatum in experimental histoplasmosis in mice. Proc Soc Exp Biol Med. 1956 Mar;91(3):412–414. doi: 10.3181/00379727-91-22279. [DOI] [PubMed] [Google Scholar]
- Schnur R. A., Newman S. L. The respiratory burst response to Histoplasma capsulatum by human neutrophils. Evidence for intracellular trapping of superoxide anion. J Immunol. 1990 Jun 15;144(12):4765–4772. [PubMed] [Google Scholar]
- Tewari R. P., Sharma D. K., Mathur A. Significance of thymus-derived lymphocytes in immunity elicited by immunization with ribosomes or live yeast cells of Histoplasma capsulatum. J Infect Dis. 1978 Nov;138(5):605–613. doi: 10.1093/infdis/138.5.605. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Hancock K., Maddison S. E., Beatty A. L., Moss D. M. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA). J Immunol. 1984 May;132(5):2607–2613. [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J Immunol. 1984 May;132(5):2593–2597. [PubMed] [Google Scholar]