Abstract
Background
The authors tested the hypotheses that after hip arthroplasty, ambulation distance is increased and the time required to reach three specific readiness-for-discharge criteria is shorter with a 4-day ambulatory continuous lumbar plexus block (cLPB) than with an overnight cLPB.
Methods
A cLPB consisting of 0.2% ropivacaine was provided from surgery until the following morning. Patients were then randomly assigned either to continue ropivacaine or to be switched to normal saline. Primary endpoints included (1) time to attain three discharge criteria (adequate analgesia, independence from intravenous analgesics, and ambulation ≥ 30 m) and (2) ambulatory distance in 6 min the afternoon after surgery. Patients were discharged with their cLPB and a portable infusion pump, and catheters were removed on the fourth postoperative day.
Results
Patients given 4 days of perineural ropivacaine (n = 24) attained all three discharge criteria in a median (25th-75th percentiles) of 29 (24-45) h, compared with 51 (42-73) h for those of the control group (n = 23; estimated ratio = 0.62; 95% confidence interval, 0.45-0.92;P = 0.011). Patients assigned to receive ropivacaine ambulated a median of 34 (9-55) m the afternoon after surgery, compared with 20 (6-46) m for those receiving normal saline (estimated ratio = 1.3; 95% confidence interval, 0.6-3.0;P = 0.42). Three falls occurred in subjects receiving ropivacaine (13%),versusnone in subjects receiving normal saline.
Conclusions
Compared with an overnight cLPB, a 4-day ambulatory cLPB decreases the time to reach three predefined discharge criteria by an estimated 38% after hip arthroplasty. However, the extended infusion did not increase ambulation distance to a statistically significant degree.
ANALGESIA for hip arthroplasty is traditionally provided by a multimodal regimen of oral and intravenous analgesics within the United States.1 However, postoperative opioid use is associated with undesirable side effects,2,3 and inadequate analgesia often results which interferes with sleep, general activity, and physical therapy.4 The latter is of particular importance because early mobilization is associated with decreased venous thromboembolism and shorter hospitalization as well as improved quality-of-life outcomes.5-7 An epidural infusion is thus often added to help mitigate these impediments to recovery.1 An alternative analgesic option is a continuous lumbar plexus block (cLPB) via the posterior approach.8 This technique involves the percutaneous insertion of a catheter directly into the ipsilateral psoas muscle in the lower lumbar region.9 The catheter is then infused with local anesthetic, resulting in potent, unilateral analgesia free of systemic side effects.
Unlike femoral perineural infusion after total knee arthroplasty, cLPB for hip arthroplasty remains relatively uninvestigated—especially using the posterior approach. For example, data are unavailable from a randomized, masked, controlled trial even quantifying posterior cLPB analgesic potency and opioid sparing after hip arthroplasty. Similarly, any effect of a posterior cLPB on ambulation, hip flexion, sleep disturbances, or discharge readiness after hip arthroplasty remains unexamined and unknown. Previously published investigations involving hip arthroplasty provided posterior cLPB exclusively in hospitalized patients and are limited by one or more methodologic issues such as a retrospective study design; lack of randomization, adequately concealed treatment allocation (“blinding”), or placebo controls10; inadequate or missing sample-size estimates; or failure to specify primary endpoints a priori.11
An advantage of cLPB is that, unlike traditional intravenous opioid administration or epidural infusion, cLPB may be continued after discharge using a portable infusion pump.12 Consequently, ambulatory cLPB offers the potential of providing prolonged analgesia while simultaneously decreasing disability and hospitalization duration after hip arthroplasty and similar procedures. Use of ambulatory cLPB after hip arthroplasty has been reported just once, in a small series of subjects who were discharged home the day after surgery with their perineural infusions continuing for over 4 days.13 However, that investigation did not include a control group, and so the extent to which ambulatory cLPB influenced discharge readiness remains unknown.
Therefore, the primary objective of this dual-center, randomized, triple-masked (patients, investigators, and statisticians), placebo-controlled study was to test the hypotheses that, compared with an overnight cLPB, a 4-day ambulatory cLPB increases ambulation distance and shortens the time until three specific, predefined readiness-for-discharge criteria are met after hip arthroplasty. The three discharge-related criteria were (1) adequate analgesia, (2) independence from intravenous opioids, and (3) sufficient ambulation. These criteria were chosen because failure to meet one or more of them accounts for the majority of hospitalization days in hip arthroplasty patients at our institutions. Secondary endpoints included maximum passive hip flexion, average and worst resting and dynamic pain scores, oral and intravenous opioid requirements, sleep disturbances, and patient satisfaction.
Materials and Methods
Enrollment
The Institutional Review Board at each participating clinical center approved all study procedures (University of Florida, Gainesville, Florida, and University of California San Diego, San Diego, California). Patients to whom enrollment was offered included adults (aged 18-80 yr) scheduled to undergo primary, unilateral hip arthroplasty via a 15- to 25-cm curvilinear lateral skin incision centered over the greater trochanter (either hip resurfacing or hip replacement via the posterior approach with a posterior capsulotomy) who desired a cLPB for postoperative analgesia. Exclusion criteria included a history of opioid dependence or current chronic analgesic therapy (daily use > 4 weeks), allergy to study medications, known hepatic or renal insufficiency/disease, peripheral neuropathy, body mass index greater than 40 kg/m2, pregnancy, incarceration, or comorbidity that resulted in moderate or severe functional limitation. Participants provided written, informed consent, and because this was a multicenter trial, a Data Safety Monitoring Board (University of Florida and University of California San Diego) reviewed data and adverse events during enrollment.
Preoperative Management
Participating patients were placed in the lateral decubitus position with the operative hip up. Intravenous fentanyl and midazolam were titrated for patient comfort. The area that would be subsequently covered by the catheter dressing and tape was prepared with chlorhexidine gluconate and isopropyl alcohol (ChloraPrep One-Step; Medi-Flex Hospital Products, Inc., Overland Park, KS) and then shaved with a surgical hair clipper, if necessary. After sterile preparation (additional Chlora-Prep One-Step) and draping, a local anesthetic skin wheal was raised at the needle entry point using previously described landmarks.14 With the bevel directed caudad, a 102- or 152-mm, 18-gauge, insulated needle (Contiplex; B. Braun Medical Inc., Bethlehem, PA) was inserted with the long axis perpendicular to the skin. This needle was connected to a nerve stimulator (Stimuplex-DIG; B. Braun Medical) initially set at 1.2 mA, 0.1 ms, and 2 Hz. With gentle aspiration applied to aid in identification of a penetrated vessel, the needle was redirected, as needed, until quadriceps contractions and patellar motion were elicited with a stimulating current of 0.20-0.40 mA.
Subsequently, 15 ml D5W was injected in divided doses.13 The standard multiorifice perineural catheter that came packaged with the needle was then advanced 3 cm past the needle tip, and the needle was withdrawn over the catheter. If the catheter met more than minimal resistance at the needle tip, it was removed from the needle and replaced with a similar catheter, only with a single orifice at its tip (B. Braun Medical Inc.). The tip of this second catheter was advanced to the end of the needle and then held in place while the needle was withdrawn over the catheter. The catheter was advanced 2 cm after the needle tip had been withdrawn at least 3 cm from its original location. All catheters were tunneled subcutaneously 4 cm toward the contralateral side using a 16-gauge angiocatheter. The injection port was attached to the catheter, and the catheter was secured with sterile liquid adhesive, an occlusive dressing, tape, and an anchoring device on the ipsilateral shoulder.13
Fifteen milliliters mepivacaine, 2%, with 5 μg/ml epinephrine was slowly injected via the catheter with gentle aspiration every 2-3 ml. Catheter placement was considered successful if, within 30 min, the patient experienced a decreased sensation to cold temperature over the ipsilateral distal thigh and weakness with knee extension. Patients without a successful nerve block had their catheters replaced or were withdrawn from the study. In patients with a successful nerve block, 10 ml ropivacaine, 0.5%, with 25 μg epinephrine was injected via the catheter.
Intraoperative Management
Patients were given a standardized general anesthetic using sevoflurane, nitrous oxide, and oxygen during surgery. A 0.2% ropivacaine infusion was initiated via the perineural catheter at a basal rate of 8 ml/h, patient-controlled bolus dose of 4 ml, and lockout of 30 min. An intravenous hetastarch-based plasma volume expander (15 ml/kg) was administered before emergence.15 Fentanyl (25-μg increments) was given as needed during surgery; intravenous morphine sulfate was titrated to a respiratory rate of 12-14 just before emergence.
Postoperative Analgesics
In addition to the ropivacaine perineural infusion initiated in the operating room and continued until the morning after surgery, all patients were given 1 week of oral acetaminophen (975 mg every 6 h) and either aspirin (650 mg daily) or celecoxib (200 mg every 12 h). Deep vein thrombosis prophylaxis was provided with either enoxaparin (40 mg daily; University of California San Diego) or the previously mentioned aspirin (University of Florida) beginning the morning after surgery and continued for 2 or 6 weeks, respectively. For breakthrough pain, patients were instructed to depress the bolus button on their pump. Rescue opioid and route of administration were titrated to pain severity using a numeric rating scale of 0-10, with 0 equal to no pain and 10 being the worst imaginable pain (table 1).16
Table 1.
Protocol for Rescue Analgesic Administration
| NRS | Analgesic | Route | Dose, mg | Administration |
|---|---|---|---|---|
| NRS = pain on numeric rating scale (0-10, 0 = no pain and 10 = worst imaginable pain). | ||||
| Postanesthesia care unit (recovery room) | ||||
| 1-2 | Oxycodone* | Oral | 5 | If patient desired |
| 3-4 | Oxycodone* | Oral | 10 | Every 30 min |
| 5-6 | Morphine | Intravenous | 2 | Every 10 min |
| 7-10 | Morphine | Intravenous | 4 | Every 10 min |
| Orthopedic ward | ||||
| < 4 | Oxycodone* | Oral | 5 | If patient desired |
| 4-7 | Oxycodone* | Oral | 10 | Once |
| > 7 | Morphine | Intravenous | 2-4 | Every 10 min until NRS < 4 |
| Pain reassessed after 30 min | ||||
| < 4 | Oxycodone* | Oral | 5 | If patient desired |
| 4-10 | Morphine | Intravenous | 2-4 | Every 10 min until NRS < 4 |
Intravenous morphine (2 mg) was administered instead of oxycodone when oral intake was not tolerated.
Randomization and Intervention
Patients were allocated to treatment after confirmation of a successful initial surgical block preoperatively. Patients were randomly assigned to one of two groups— 0.2% ropivacaine or normal saline (placebo)—stratified by institution using computer-generated tables by the Investigational Drug Service of each participating center. Investigational Drug Service pharmacists prepared all perineural infusions. Investigators, patients, and all clinical staff were thus fully masked to treatment group assignments. At 06:00 on postoperative day (POD) 1, each patient's infusion pump which contained 0.2% ropivacaine was replaced with an infusion pump filled with study solution containing either additional 0.2% ropivacaine or normal saline (fig. 1).
Fig. 1.
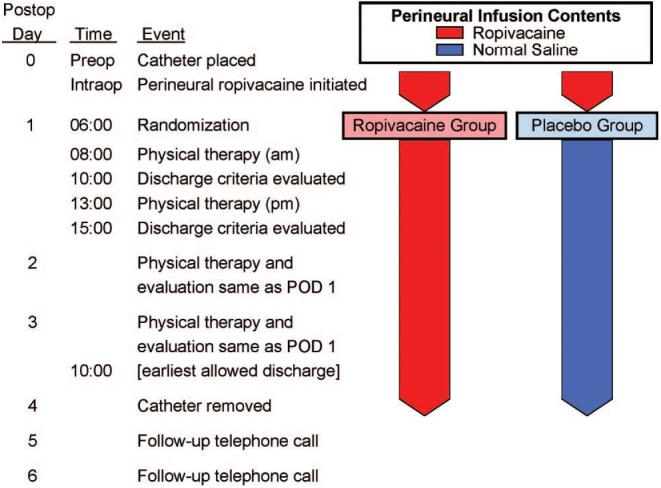
Study design overview. POD = postoperative day.
Pain scores were recorded every 4 h (except when patients were sleeping) and when patients requested analgesics. Patients underwent physical therapy twice daily beginning the morning after surgery at approximately 08:00 and 13:00, and thereafter until discharge (fig. 1). If the physical therapist believed subject ambulation was limited due to quadriceps weakness, the perineural infusion was stopped by turning off the infusion pump for 1 h and then restarted at half the previous basal rate. At 18:00 on POD 2 (36 h after randomization), a portable infusion pump (Pain Pump 2 Blockaid; Stryker Instruments, Kalamazoo, MI) containing 400 ml of the same study solution (basal 5 ml/h; bolus 4 ml; lockout 60 min) replaced the previous infusion pump. If the previous basal rate was less than 4 ml/h, the new pump was programmed for a 2-ml/h basal rate and 2-ml bolus volume (instead of the standard 5-ml/h basal with a 4-ml bolus).
Primary Endpoints
Two hours after physical therapy sessions, each of the three discharge criteria were evaluated separately and scored as either fulfilled or unfulfilled by research nursing staff. The first primary endpoint was the time from surgical stop until all three of the criteria were fulfilled—without a reversion to unfulfilled status. For example, if a patient met all three criteria the morning of POD 1, subsequently met only two criteria later that afternoon, and again met all three criteria the following morning and thereafter, the primary endpoint would be the number of hours from the end of surgery until 10:00 on POD 2. The three specific readiness-for-discharge criteria were (1) adequate analgesia (numeric rating scale score < 4), (2) independence from intravenous opioids in the previous 12 h, and (3) ambulation of at least 30 m without a time limit.17
The second primary endpoint was the ambulatory distance during a Six-Minute Walking Test (6-MWT) the afternoon after surgery (7-8 h after randomization).18 The 6-MWT is used to measure the maximum distance that a patient can walk in 6 min on a 10-m level course, as previously described.18,19 Patients were allowed to continue ambulating after the initial 6 min, and the total distance and reasons for ambulation cessation were recorded.
The secondary endpoint of passive hip flexion was measured with the patient in the supine position during each physical therapy session before the 6-MWT using a goniometer. Maximums of 70° and 90° were permitted at the University of Florida and University of California, respectively.
Hospital Discharge
Patients were discharged home or to a rehabilitation center (if they lacked a capable caretaker at home) with their portable infusion pump and perineural catheter in situ. Patients were discharged at the discretion of the orthopedic surgeons after meeting the three main discharge criteria, but never before 10:00 on POD 3. Patients and their caretakers were provided with verbal and written catheter/pump instructions, the telephone and pager numbers of an investigator available at all times, and prescriptions for their outpatient oral medications that did not differ from the oral analgesics provided in the hospital. Patients were telephoned in the evenings through POD 6 for data collection (appendix 1) and infusion oversight (e.g., appearance of the catheter site/dressing).
In the evening of POD 4, patients' caretakers removed the perineural catheters with physician instructions provided by telephone. Enoxaparin was administered in the mornings (University of California San Diego patients), whereas catheter removal occurred in the evening, with the two events separated by approximately 8-10 h. However, this temporal relation was by coincidence and not by design.
Statistical Analysis
The study was powered for the two primary endpoints. Based on a pilot study,13 the planning distribution for time-to-discharge readiness for the ropivacaine (placebo) group was 6 h: 71% (29%); 30 h: 14% (29%); 45 h: 14% (14%); and 54 h: 0% (29%). To ensure 80% power at P = 0.05 (two-sided) for the Wilcoxon rank sum test, we planned for 25 patients randomized to each group on the basis of the formula of Shuster et al.20 As for planning parameters for the 6-MWT on the afternoon of POD 1, the sample size was calculated on the basis of an SD of approximately 19 m from the pilot study using the Wilcoxon rank sum test,13 where a study of 25 subjects per group would be sensitive to a difference of 0.83 SDs in medians, or approximately 16 m.
For consistency, all outcome variables (primary and secondary) were analyzed by the two-sided Wilcoxon rank sum test, which provides distribution-free P values and is highly robust against outliers (Statistical Analysis Software, Cary, NC). For descriptive purposes, Kaplan-Meier estimates were calculated and presented for several components of the primary endpoints. For convenient nomenclature, a two-sided P < 0.05 was considered significant. Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints.21 Significant findings in secondary outcomes should be viewed as suggestive, requiring confirmation in a future trial before considering them to be definitive.21
For the primary variables, effect sizes were estimated by the method of Hodges and Lehmann for a scale parameter.22 Essentially, this tests the null hypothesis that the distribution of an outcome variable under treatment A is the same as that of ρ times that of treatment B for every value of ρ. The values of ρ that cannot be rejected by a Wilcoxon rank sum test at P < 0.05, two-sided, form a 95% confidence interval for ρ. A value of ρ = 0.7, for example, is interpreted as the median (or any percentile) under treatment A is 70% of that of treatment B. For time to meeting discharge criteria, for example, this implies an expected 30% savings for A over B. Of note, the Hodges-Lehmann approach has the important feature that the 95% confidence interval excludes a value of ρ = 1 if and only if the P value from the Wilcoxon test is below 5%.
Results
During a 33-month period between July 2005 and February 2008, 50 patients enrolled, and all had a perineural catheter successfully positioned per protocol (table 2). All subjects exhibited a sensory and motor block 20 min after being given a local anesthetic bolus via the catheter. Three subjects requested study withdrawal after catheter placement, but before the intervention on POD 1. Among the remaining 47 subjects, 23 were randomly assigned to be switched from perineural ropivacaine to normal saline (placebo group) at 06:00 on POD 1, whereas 24 continued to receive perineural ropivacaine through POD 4 (ropivacaine group; fig. 1).
Table 2.
Population Data, Perineural Catheter Details, and Surgical Information
| Ropivacaine Group | Placebo Group | |
|---|---|---|
| Values are reported as median (25th-75th percentiles) or number of subjects. ASA = American Society of Anesthesiologists; UCSD = University of California San Diego; UF = University of Florida. | ||
| Enrolling center, UCSD/UF | 19/6 | 18/7 |
| Age, yr | 57 (48-71) | 59 (46-66) |
| Sex, F/M | 11/14 | 10/15 |
| Height, cm | ||
| Male patients | 178 (174-183) | 178 (174-183) |
| Female patients | 163 (159-164) | 159 (158-167) |
| Weight, kg | ||
| Male patients | 86 (81-100) | 91 (85-100) |
| Female patients | 66 (64-77) | 70 (65-78) |
| Body mass index, kg/m2 | 28 (24-31) | 28 (26-31) |
| ASA physical status | 2 (2-2) | 2 (2-2) |
| Minimum needle current, mA | 0.29 (0.24-0.32) | 0.24 (0.20-0.30) |
| Catheter inserted past needle tip, no. | 20 | 16 |
| Procedure, total arthroplasty/resurfacing | 19/6 | 18/7 |
| Intraoperative fentanyl, μg | 175 (100-250) | 250 (150-275) |
| Intraoperative morphine, mg | 5 (1-6) | 5 (0-10) |
| Surgery duration, min | 147 (132-168) | 132 (102-180) |
| Catheter insertion to intervention, h | 23 (21-23) | 23 (21-23) |
Primary Endpoints
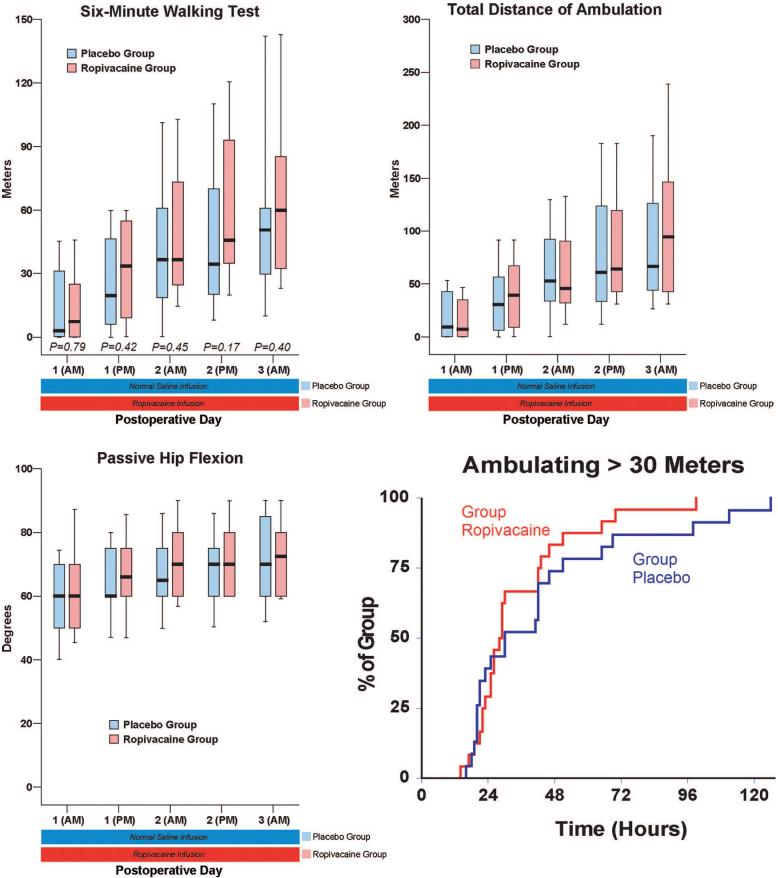
Patients given 4 days of perineural ropivacaine (n = 24) attained all three discharge criteria (fig. 2) in a median (25th-75th percentiles) of 29 (24-45) h, compared with 51 (42-73) h for those of the control group (n = 23; estimated ratio = 0.62; 95% confidence interval, 0.45-0.92; P = 0.011). This infers that perineural ropivacaine is associated with a 38% (95% confidence interval, 8-55%) reduction in time to meet discharge criteria as compared with placebo. Patients assigned to receive ropivacaine ambulated a median of 34 (9 -55) m in 6 min the afternoon after surgery, compared with 20 (6 - 46) m for those receiving normal saline (fig. 3; estimated ratio = 1.3; 95% confidence interval, 0.6 - 3.0; P = 0.42).
Fig. 2.
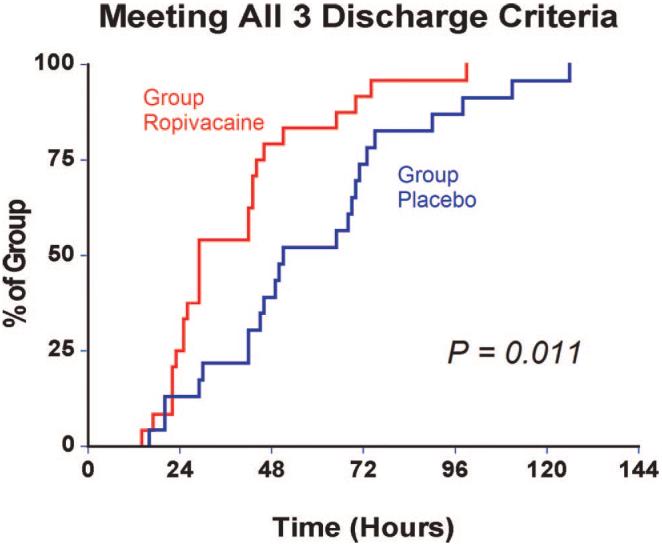
Effect of posterior lumbar plexus perineural ropivacaine infusion on the time to reach three predefined discharge criteria (adequate analgesia, independence from intravenous opioids, and the ability to ambulate at least 30 m) after hip arthroplasty. Data presented are Kaplan-Meier estimates of the cumulative percentages of patients meeting all three discharge criteria at each time point and subsequent time points. Data are for patients randomly assigned to the ropivacaine group (perineural ropivacaine from surgery through postoperative day 4) or the placebo group (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4).
Fig. 3.
Effects of posterior lumbar plexus perineural ropivacaine infusion on ambulation and passive hip flexion after hip arthroplasty. Kaplan-Meier estimates include the cumulative percentages of patients ambulating at least 30 m at each time point and subsequent time points. Other data are expressed as median (horizontal bar) with 25th-75th (box) and 10th-90th (whiskers) percentiles for patients randomly assigned to the ropivacaine group (perineural ropivacaine from surgery through postoperative day postoperative day 4) or the placebo group (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints. P values are provided where statistical comparisons were applied.
Secondary Endpoints
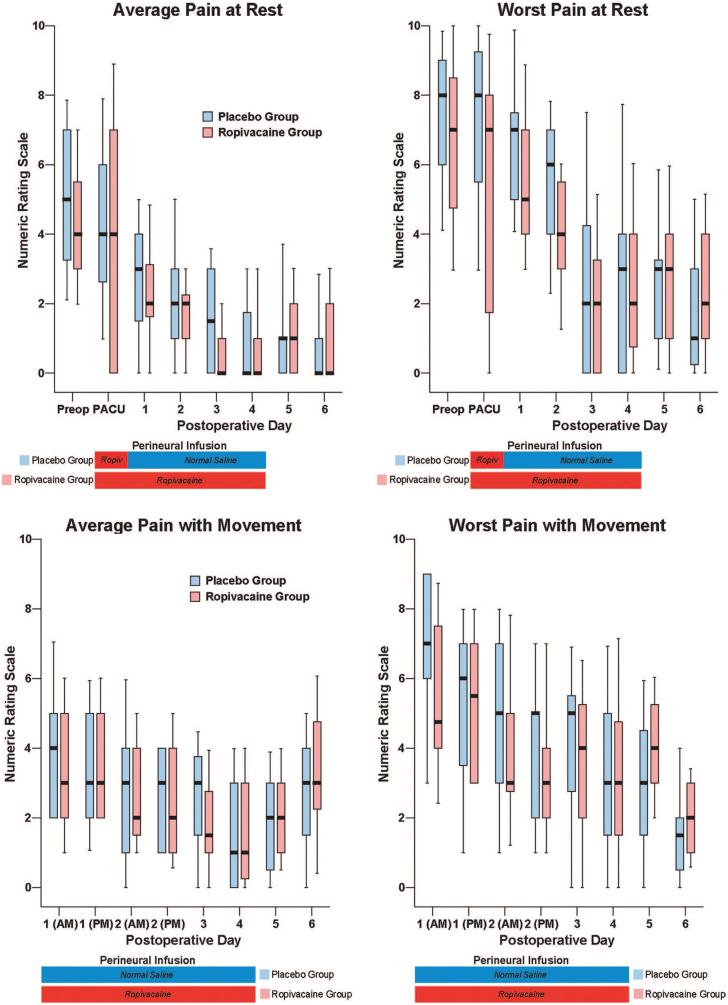
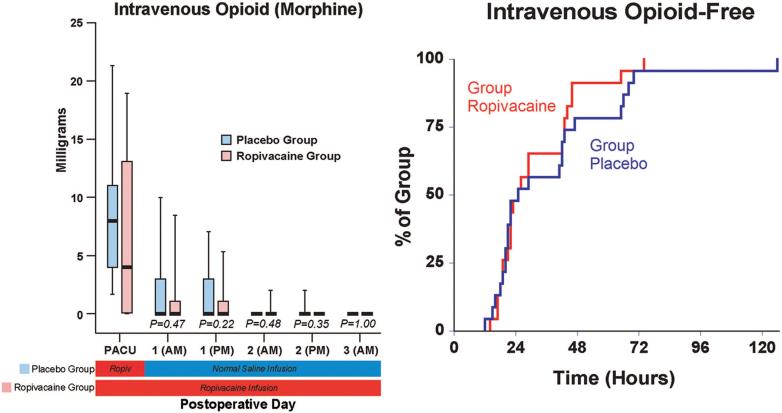
There were small differences overall in the average (baseline) resting and dynamic pain between the treatment groups, but the maximum (breakthrough) pain scores diverged to a greater degree (fig. 4). The differences in oral (table 3) and intravenous opioid requirements (fig. 5) appear small as well. Similarly, there were only minimal differences between groups in sleep disturbances (table 3), total ambulatory distance, and passive hip flexion (fig. 4). Although the differences between treatment groups were not particularly dramatic for the three discharge criteria we studied, when all three criteria were considered together—a primary endpoint of the study—providing a total of 4 days of perineural ropivacaine had a statistically and clinically significant impact on the time to reach the three criteria. Among the ropivacaine group patients, those who did not meet all three criteria usually ambulated less than 30 m and required intravenous morphine. In contrast, those in the placebo group who did not meet all three criteria usually ambulated less than 30 m or required intravenous morphine. This difference explains why even though there were only small differences between groups in meeting the individual discharge criteria at each time point (figs. 3 and 5), there was a much larger difference when all three criteria were evaluated for the primary endpoint (fig. 2).
Fig. 4.
Effects of posterior lumbar plexus perineural ropivacaine infusion on postoperative pain after hip arthroplasty. Pain severity indicated using a numeric rating scale of 0-10, with 0 equal to no pain and 10 being the worst imaginable pain. Data are expressed as median (horizontal bar) with 25th-75th (box) and 10th-90th (whiskers) percentiles for patients randomly assigned to the ropivacaine group (perineural ropivacaine from surgery through postoperative day 4) or the placebo group (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints. For this reason, no statistical comparisons were applied to the data of this figure. PACU = postanesthesia care unit.
Table 3.
Oral Opioid Requirements and Sleep Disturbances
| Oral Opioid,* mg |
Difficulty Sleeping,† Subjects per Group |
At Least One Awakening,† Subjects per Group |
||||
|---|---|---|---|---|---|---|
| Postoperative Day/Night | Ropivacaine Group | Placebo Group | Ropivacaine Group | Placebo Group | Ropivacaine Group | Placebo Group |
| Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints. For this reason, no statistical comparisons were applied to the data of this table. | ||||||
| 0 | 0 (0-0) | 0 (0-0) | 3 | 6 | 3 | 6 |
| 1 | 20 (5-30) | 20 (25-30) | 3 | 2 | 3 | 2 |
| 2 | 10 (0-25) | 20 (10-30) | 1 | 3 | 1 | 4 |
| 3 | 0 (0-5) | 5 (0-30) | 4 | 3 | 4 | 3 |
| 4 | 0 (0-15) | 10 (0-20) | 4 | 7 | 4 | 7 |
| 5 | 10 (5-25) | 10 (0-20) | 4 | 3 | 3 | 3 |
| 6 | 10 (5-20) | 10 (0-15) | ‡ | ‡ | ‡ | ‡ |
Values are reported as median (25th-75th percentiles) for nonparametric data. Includes only immediate-release oxycodone provided for breakthrough pain for the previous 24 h as of 18:00 each day, with the exception of postoperative day 0, which includes only the postanesthesia care unit (recovery room).
As a result of surgical pain.
Data not collected.
Fig. 5.
Effects of posterior lumbar plexus perineural ropivacaine infusion on intravenous morphine consumption after hip arthroplasty. Kaplan-Meier estimates include the cumulative percentages of morphine-free patients at each time point and subsequent time points. Other data are expressed as median (horizontal bar) with 25th-75th (box) and 10th-90th (whiskers) percentiles for patients randomly assigned to the ropivacaine group (perineural ropivacaine from surgery through postoperative day 4) or the placebo group (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints. P values are presented where statistical comparisons were applied. PACU = postanesthesia care unit.
Ten subjects (42%) of the ropivacaine group had their basal ropivacaine infusion halved on POD 1 because of quadriceps weakness, versus 4 subjects (17%) of the placebo group. One of the 10 subjects in the ropivacaine group required a second halving of her basal rate because of continued quadriceps weakness. Hospitalization duration was a median of 3.0 (3.0-4.0) days in the ropivacaine group and 3.5 (3.0-4.0) days in the placebo group (P = 0.12). Satisfaction with postoperative analgesia was scored 10.0 (9.5-10.0) in the ropivacaine group and 9.0 (8.0 -10.0) in the placebo group (P = 0.02). There were five protocol violations and four adverse events (appendix 2).
Discussion
This investigation provides evidence that, compared with an overnight cLPB, a 4-day cLPB decreases the time to reach three predefined discharge criteria by an estimated 38%. Because the extended-duration cLPB may be provided on an ambulatory basis, prolonging the perineural infusion does not necessarily require prolonging hospitalization, and hospital duration of stay after hip arthroplasty may be shortened in some cases while still providing the benefits of cLPB. However, the appropriate subset of patients and incidence of complications associated with early discharge is currently unknown. Caution is warranted because, after hip arthroplasty, the median times to myocardial infarction and pulmonary embolism are 1 and 4 days, respectively.23 In addition, three subjects receiving perineural ropivacaine experienced a fall (13%), compared with no subjects receiving perineural normal saline. And, although the feasibility of converting hip arthroplasty into an overnight-stay procedure using ambulatory cLPB has been previously demonstrated,13 one small series of patients does not permit conclusions to be drawn regarding the relative safety of this practice. Therefore, additional study is required to define an appropriate subset of patients and assess the incidence of complications associated with earlier discharge after hip arthroplasty. Nonetheless, ambulatory cLPB may enable rapid hospital discharge to a skilled nursing facility or rehabilitation center where medical oversight would continue in case of an adverse event.
Physical Therapy
In contrast to discharge readiness, the results for our other primary endpoint—ambulation distance the afternoon after surgery as measured by the 6-MWT—were comparable (fig. 3). The theory underlying our hypothesis was that postoperative pain limits postoperative range of motion and ambulation, and therefore, optimizing analgesia would improve ambulation ability and distance. Although cLPB somewhat decreased pain during movement (fig. 4), this did not translate into improved range of motion or ambulatory distance measured with the 6-MWT. Multiple variables influence ambulatory ability in addition to the quality of analgesia,24 including cardiovascular status25,26; physical characteristics such as age, sex, height, and weight27; and general health status.28 It remains unknown whether these other variables neutralized any benefits of improved analgesia, whether cLPB-induced motor block resulted in decreased ambulation ability, or whether pain is simply not a significant limiting factor of ambulation after hip arthroplasty.
It nonetheless remains noteworthy that a subset of patients benefited from an extended-duration ambulatory cLPB even though the differences in medians between groups were minimal after POD 2. For example, the median requirements for oral opioids on POD 3 were 0 and 5 mg for the ropivacaine and placebo groups, respectively, but the 75th-90th percentiles were 5-18 versus 30-48 mg, respectively, demonstrating a clinically important cLPB opioid-sparing effect for a subset of patients. Similar subgroup differences in intravenous opioid requirements and ambulatory distance resulted in 11 subjects (46%) receiving prolonged perineural saline reaching all three discharge criteria after POD 2, compared with only 4 subjects (17%) receiving perineural ropivacaine. These data suggest that there is no single optimal infusion duration for all patients after hip arthroplasty. And for patients who benefit from more than 1-2 days of cLPB, ambulatory infusion provides a potential treatment modality to extend benefits without requiring prolonged hospitalization. Providing retrospective statistical subgroup analysis was not appropriate for this trial,29 but further prospective investigation seems warranted.
Patient Safety
In addition, the use of low-molecular-weight heparin for deep vein thrombosis prophylaxis may obviate the epidural option because of the unacceptably high risk of epidural hematoma.30 There are case reports of patients with a cLPB receiving low-molecular-weight heparin developing a retroperitoneal hematoma.31,32 These reports have led some healthcare providers to manage patients with a psoas compartment catheter in a similar way as those having neuraxial block when thromboprophylaxis is ordered,31 although this practice has been questioned by others.33 The American Society of Regional Anesthesia consensus statement on neuraxial anesthesia and anticoagulation notes that, “conservatively, the [recommendations]... may be applied to plexus and peripheral techniques. However, this may be more restrictive than necessary”; and “additional information is needed to make definitive recommendations.”30
Of concern are three patients of the current study who experienced a fall during infusion: All three were receiving perineural ropivacaine, although only one described a weak quadriceps muscle as an instigating factor (appendix 2). To what degree the cLPB was a contributing factor for these three cases remains unknown because our study was not powered to detect such (presumably) rare complications. In one series of 338 outpatients with single-injection ropivacaine (0.5%) psoas compartment blocks, there were no falls identified at 1 and 7 days.34 However, these outpatients had smaller procedures than hip arthroplasty and were, on average, younger with fewer comorbidities.34 In a randomized, placebo-controlled trial involving ambulatory continuous femoral nerve blocks after anterior cruciate ligament reconstruction, 4 of 233 patients reported falling after returning home.35 All 4 had received a single-injection femoral nerve block with levobupivacaine, 3 of these had a levobupivacaine perineural infusion as well, and none of the patients who received a placebo block and infusion reported a fall. The relationship between cLPB and balance/proprioception/strength deserves further investigation. Related to this issue, the fact that 42% of the ropivacaine group (vs. 17% of the placebo group) required a decrease in their basal infusion rate to enable ambulation suggests that an initial basal rate of 8 ml/h is too high for many patients when using 0.2% ropivacaine. However, the optimal local anesthetic concentration, basal rate, bolus volume, and lockout period remain undetermined, and initially providing a lower rate than 8 ml/h may result in decrease cLPB benefits for a subset of patients.
Study Limitations
The control (“placebo”) group received a single-injection psoas compartment block followed by an overnight cLPB rather than simply opioids as is common practice within the United States.36 Although the postoperative questionnaire included validated measures such as the numeric rating scale for pain assessment,16 the instruments used to assess sleep quality and analgesia satisfaction have not been previously validated (appendix 1). This study also excluded patients who had taken opioids daily for more than the previous 4 weeks. Although precise figures are unavailable, undoubtedly a large percentage of patients undergoing hip arthroplasty have received more than a month of opioids daily, and whether the results of the current study remain applicable to this patient subset remains unknown.
In summary, compared with an overnight cLPB, a 4-day ambulatory cLPB decreases the time to reach three predefined discharge criteria by an estimated 38% (95% confidence interval, 8-55%) after hip arthroplasty. However, the extended infusion did not increase ambulation distance the afternoon after surgery to a statistically significant degree. Given that the subjects all remained hospitalized at least until the third postoperative day, and the three falls associated with perineural ropivacaine, it is emphasized that additional study is required to define an appropriate subset of patients and assess the incidence of complications associated with earlier discharge after hip arthroplasty.
Acknowledgments
Supported by National Institutes of Health grant No. GM077026 from the National Institute of General Medical Sciences, Bethesda, Maryland; the Foundation of Anesthesia Education and Research, Rochester, Minnesota; National Institutes of Health grant Nos. RR00082 and RR000827 from the National Center for Research Resources, Bethesda, Maryland; the Departments of Anesthesiology, University of Florida, Gainesville, Florida, and University of California San Diego, San Diego, California; Stryker Instruments, Kalamazoo, Michigan; and B. Braun Medical, Bethlehem, Pennsylvania. Dr. Sessler is supported by National Institutes of Health grant No. GM061655 from the National Institute of General Medical Sciences, Bethesda, Maryland, and the Joseph Drown Foundation, Los Angeles, California. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of these entities. B. Braun Medical and Stryker Instruments provided funding and donated portable infusion pumps and perineural catheters for this investigation. These two companies had no input into any aspect of study conceptualization, design, and implementation; data collection, analysis, and interpretation; or manuscript preparation. Drs. Enneking and Mariano conduct continuous peripheral nerve block workshops for Stryker Instruments. None of the other authors has any personal financial interest in this research. Preliminary results of this investigation were submitted as an abstract to the Annual Meeting of the American Society of Anesthesiologists, Orlando, Florida, October 18-22, 2008.
The authors thank the following individuals for their invaluable assistance: Linda Berry, R.N. (Patient Care Resource Manager, Department of Orthopaedics and Rehabilitation, Shands Hospital, Gainesville, Florida); Beverly Morris, R.N., C.N.P., M.B.A. (Educator, Department of Nursing, University of California San Diego, San Diego, California); Patrick Olsen, R.N.,.B.S.N. (Nurse Manager), Claire Hardy, R.N. (Assistant Nurse Manager), and Maureen Benetti, M.S., A.P.R.N., B.C. (Recruitment Director, Hillcrest Hospital, San Diego, California); Eugene Spadoni, P.T. (Physical Therapist, Department of Physical Therapy, Shands Hospital); Lisa Dacey, P.T., Takae Annaka, P.T., and Kerrie Olexa, P.T. (Physical Therapists, Department of Physical Therapy, Hillcrest Hospital); Tami DeMartinis, P.T., and Lynn Loxterman, P.T. (Physical Therapists, Department of Physical Therapy, Thornton Hospital, La Jolla, California); Rosalita Maldonado, B.S., and Marina Nekhendzy (Research Coordinators, Department of Anesthesiology, University of California San Diego); Jennifer Woodard, B.S. (Research Coordinator, Department of Anesthesiology, University of Florida, Gainesville, Florida); and the entire staffs of the Shands Hospital Regional Anesthesia Induction Area, University of Florida General Clinical Research Center, and Hillcrest Hospital Orthopedic Ward (San Diego, California).
Appendix 1: Nightly Telephone Questionnaire
Pain Scores (Postoperative days 3-6)
“Please answer the following questions regarding your surgical pain in the last 24 h using a scale of 0-10, 0 being no pain at all and 10 being the worst pain you can imagine.”
“While resting in bed, what was the worst pain you have felt?”
“While resting in bed, what was the average pain you have felt?”
“While walking, what was the worst pain you have felt?”
“While walking, what was the average pain you have felt?”
Opioid Use (If patient was discharged postoperative day 3)
“Since you left the hospital, how many of your oxycodone tablets have you taken? These are the pills you take if your infusion pump does not decrease your pain enough.”
(Postoperative days 4-6)
“Since we last spoke, how many of your oxycodone tablets have you taken? These are the pills you take if your infusion pump does not decrease your pain enough.”
Sleep Quality (Postoperative days 1-6)
“Did you have difficulty sleeping last night because of pain?”
“Did you awaken last night because of pain?”
If “yes,” then: “How many times did you awaken last night because of pain?” (If ≥10 awakenings or complete insomnia, score = 10)
Satisfaction (Postoperative day 4 only)
“What has been your satisfaction with your pain control following your surgery, using a scale of 0-10, 0 being very unsatisfied and 10 being very satisfied?”
Appendix 2: Protocol Violations and Adverse Events
One subject from the placebo group experienced a vasovagal episode on POD 3 at home, was readmitted, underwent a negative workup for instigating conditions, and was discharged home the following day without negative sequelae. Subjects from the ropivacaine group had their catheter inadvertently dislodged (evening POD 2), occlusive dressing inadvertently removed with subsequent purposeful catheter removal (morning POD 4), and catheter purposefully removed as requested by patient (morning of POD 3). One subject from the placebo group had her infusion pump tubing disconnected from the catheter, and the catheter was subsequently purposefully removed out of concern for sterility (morning of POD 4). For purposes of analysis, these subjects were retained in their respective treatment group per the intention-to-treat principle.11 One subject from the ropivacaine group requested study withdrawal the afternoon of POD 1 in the belief that the perineural infusion was causing her nausea.
Three subjects from the ropivacaine group experienced a fall during the infusion period. The first ambulated 13-18 m twice on POD 1 without apparent quadriceps weakness, self-administered local anesthetic boluses every 30 min after her afternoon therapy session, and then fell immediately upon attempting to stand without assistance that evening (she described her thigh as “numb” when she fell, which it had not been previously). A second subject ambulated over 30 m on five occasions over the course of 3 days without apparent quadriceps weakness, was discharged home on POD 3, lost her balance, then experienced what she described as a “slow, controlled fall” onto her buttocks, and was readmitted for one night. The third subject had experienced weak quadriceps on POD 1, and her basal infusion and bolus dose volumes were halved per study protocol, after which she ambulated over 30 m five times without difficulty. However, she experienced dizziness on POD 3 and fell that evening when attempting to walk without assistance, had her catheter removed the following day, and was discharged home without further incident. The patient attributed her fall to the dizziness (presumed etiology: anemia) which did not recur. None of these three falls resulted in physical injury.
References
- 1.Anderson FA, Jr, Hirsh J, White K, Fitzgerald RH., Jr Temporal trends in prevention of venous thromboembolism following primary total hip or knee arthroplasty 1996-2001: Findings from the Hip and Knee Registry. Chest. 2003;124:349S–56S. doi: 10.1378/chest.124.6_suppl.349s. [DOI] [PubMed] [Google Scholar]
- 2.Roberts GW, Bekker TB, Carlsen HH, Moffatt CH, Slattery PJ, McClure AF. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg. 2005;101:1343–8. doi: 10.1213/01.ANE.0000180204.64588.EC. [DOI] [PubMed] [Google Scholar]
- 3.Matheny JM, Hanks GA, Rung GW, Blanda JB, Kalenak A. A comparison of patient-controlled analgesia and continuous lumbar plexus block after anterior cruciate ligament reconstruction. Arthroscopy. 1993;9:87–90. doi: 10.1016/s0749-8063(05)80350-0. [DOI] [PubMed] [Google Scholar]
- 4.Strassels SA, Chen C, Carr DB. Postoperative analgesia: Economics, resource use, and patient satisfaction in an urban teaching hospital. Anesth Analg. 2002;94:130–7. doi: 10.1097/00000539-200201000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Leali A, Fetto J, Moroz A. Prevention of thromboembolic disease after non-cemented hip arthroplasty: A multimodal approach. Acta Orthop Belg. 2002;68:128–34. [PubMed] [Google Scholar]
- 6.Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279:847–52. doi: 10.1001/jama.279.11.847. [DOI] [PubMed] [Google Scholar]
- 7.Pour AE, Parvizi J, Sharkey PF, Hozack WJ, Rothman RH. Minimally invasive hip arthroplasty: What role does patient preconditioning play? J Bone Joint Surg Am. 2007;89:1920–7. doi: 10.2106/JBJS.F.01153. [DOI] [PubMed] [Google Scholar]
- 8.Turker G, Uckunkaya N, Yavascaoglu B, Yilmazlar A, Ozcelik S. Comparison of the catheter-technique psoas compartment block and the epidural block for analgesia in partial hip replacement surgery. Acta Anaesthesiol Scand. 2003;47:30–6. doi: 10.1034/j.1399-6576.2003.470106.x. [DOI] [PubMed] [Google Scholar]
- 9.Pandin PC, Vandesteene A, d'Hollander AA. Lumbar plexus posterior approach: A catheter placement description using electrical nerve stimulation. Anesth Analg. 2002;95:1428–31. doi: 10.1097/00000539-200211000-00060. [DOI] [PubMed] [Google Scholar]
- 10.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 11.Todd MM. Clinical research manuscripts in ANESTHESIOLOGY. ANESTHESIOLOGY. 2001;95:1051–3. doi: 10.1097/00000542-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: A review. Anesth Analg. 2005;100:1822–33. doi: 10.1213/01.ANE.0000151719.26785.86. [DOI] [PubMed] [Google Scholar]
- 13.Ilfeld BM, Gearen PF, Enneking FK, Berry LF, Spadoni EH, George SZ, Vandenborne K. Total hip arthroplasty as an overnight-stay procedure using an ambulatory continuous psoas compartment nerve block: A prospective feasibility study. Reg Anesth Pain Med. 2006;31:113–8. doi: 10.1016/j.rapm.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Capdevila X, Macaire P, Dadure C, Choquet O, Biboulet P, Ryckwaert Y, d'Athis F. Continuous psoas compartment block for postoperative analgesia after total hip arthroplasty: New landmarks, technical guidelines, and clinical evaluation. Anesth Analg. 2002;94:1606–13. doi: 10.1097/00000539-200206000-00045. [DOI] [PubMed] [Google Scholar]
- 15.Arellano R, Gan BS, Salpeter MJ, Yeo E, McCluskey S, Pinto R, Irish J, Ross DC, Doyle DJ, Parkin J, Brown D, Rotstein L, Witterick I, Matthews W, Yoo J, Neligan PC, Gullane P, Lampe H. A triple-blinded randomized trial comparing the hemostatic effects of large-dose 10% hydroxyethyl starch 264/0.45 versus 5% albumin during major reconstructive surgery. Anesth Analg. 2005;100:1846–53. doi: 10.1213/01.ANE.0000152008.04333.53. [DOI] [PubMed] [Google Scholar]
- 16.Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–7. doi: 10.1016/s0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 17.Enloe LJ, Shields RK, Smith K, Leo K, Miller B. Total hip and knee replacement treatment programs: A report using consensus. J Orthop Sports Phys Ther. 1996;23:3–11. doi: 10.2519/jospt.1996.23.1.3. [DOI] [PubMed] [Google Scholar]
- 18.ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 19.Ilfeld BM, Le LT, Meyer RS, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Berry LF, Spadoni EH, Gearen PF. Ambulatory continuous femoral nerve blocks decrease time to discharge readiness after tricompartment total knee arthroplasty: A randomized, triplemasked, placebo-controlled study. ANESTHESIOLOGY. 2008;108:703–13. doi: 10.1097/ALN.0b013e318167af46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuster JJ, Chang M, Tian L. Design of group sequential trials with ordinal categorical data based on the Mann-Whitney-Wilcoxon test. Sequential Analysis. 2004;23:413–26. [Google Scholar]
- 21.Mariano ER, Ilfeld BM, Neal JM. “Going fishing”: The practice of reporting secondary outcomes as separate studies. Reg Anesth Pain Med. 2007;32:183–5. doi: 10.1016/j.rapm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Statist. 1963;34:598–611. [Google Scholar]
- 23.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. ANESTHESIOLOGY. 2002;96:1140–6. doi: 10.1097/00000542-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Carli F, Mayo N, Klubien K, Schricker T, Trudel J, Belliveau P. Epidural analgesia enhances functional exercise capacity and health-related quality of life after colonic surgery: Results of a randomized trial. ANESTHESIOLOGY. 2002;97:540–9. doi: 10.1097/00000542-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt GH, Thompson PJ, Berman LB, Sullivan MJ, Townsend M, Jones NL, Pugsley SO. How should we measure function in patients with chronic heart and lung disease? J Chronic Dis. 1985;38:517–24. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 27.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–4. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 28.Bautmans I, Lambert M, Mets T. The six-minute walk test in community dwelling elderly: Influence of health status. BMC Geriatr. 2004;4:6. doi: 10.1186/1471-2318-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine: Reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 30.Horlocker TT, Wedel DJ, Benzon H, Brown DL, Enneking FK, Heit JA, Mulroy MF, Rosenquist RW, Rowlingson J, Tryba M, Yuan CS. Regional anesthesia in the anticoagulated patient: Defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation) Reg Anesth Pain Med. 2003;28:172–97. doi: 10.1053/rapm.2003.50046. [DOI] [PubMed] [Google Scholar]
- 31.Weller RS, Gerancher JC, Crews JC, Wade KL. Extensive retroperitoneal hematoma without neurologic deficit in two patients who underwent lumbar plexus block and were later anticoagulated. ANESTHESIOLOGY. 2003;98:581–5. doi: 10.1097/00000542-200302000-00044. [DOI] [PubMed] [Google Scholar]
- 32.Klein SM, D'Ercole F, Greengrass RA, Warner DS. Enoxaparin associated with psoas hematoma and lumbar plexopathy after lumbar plexus block. ANESTHESIOLOGY. 1997;87:1576–9. doi: 10.1097/00000542-199712000-00040. [DOI] [PubMed] [Google Scholar]
- 33.Chelly JE, Greger JR, Casati A, Gebhard R, Ben David B. What has happened to evidence-based medicine? ANESTHESIOLOGY. 2003;99:1028–9. doi: 10.1097/00000542-200310000-00044. [DOI] [PubMed] [Google Scholar]
- 34.Klein SM, Nielsen KC, Greengrass RA, Warner DS, Martin A, Steele SM. Ambulatory discharge after long-acting peripheral nerve blockade: 2382 blocks with ropivacaine. Anesth Analg. 2002;94:65–70. doi: 10.1097/00000539-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Williams BA, Kentor ML, Bottegal MT. The incidence of falls at home in patients with perineural femoral catheters: A retrospective summary of a randomized clinical trial. Anesth Analg. 2007;104:1002. doi: 10.1213/01.ane.0000256006.46703.7f. [DOI] [PubMed] [Google Scholar]
- 36.Biboulet P, Morau D, Aubas P, Bringuier-Branchereau S, Capdevila X. Postoperative analgesia after total-hip arthroplasty: Comparison of intravenous patient-controlled analgesia with morphine and single injection of femoral nerve or psoas compartment block. A prospective, randomized, double-blind study. Reg Anesth Pain Med. 2004;29:102–9. doi: 10.1016/j.rapm.2003.11.006. [DOI] [PubMed] [Google Scholar]