Abstract
Background
Therapeutic hypothermia (TH) improves outcomes in comatose survivors of cardiac arrest. Few hospitals have protocol-driven plans that include TH. We implemented a series of process interventions designed to increase TH use and improve outcomes in patients successfully resuscitated from out-of-hospital cardiac arrest (OHCA) or in-hospital cardiac arrest (IHCA).
Methods and Results
Linked interventions including a TH order sheet, verbal and written feedback to individual providers, an educational program, TH “kit” and on-call consultants to assist with patient care and hypothermia induction were implemented between January 1, 2005 and December 31, 2007 in a large, university-affiliated, tertiary care center. We then completed a retrospective review of all patients treated for cardiac arrest during the study period.
Descriptive statistics, chi-squared analyses, or Fisher’s exact test were used as appropriate. A p value <0.05 was considered significant.
135 OHCA patients and 106 IHCA patients were eligible for post-arrest care. TH use increased each year in the OHCA group (from 6% to 65% to 76%; p<0.001) and IHCA group (from 0% to 36% to 53%; p=.02). A good outcome was achieved in 21% and 8% of comatose patients with OHCA and IHCA, respectively. Patients with OHCA and ventricular dysrhythmia were more likely to have a good outcome with TH treatment than without it (good outcome in 57% vs. 8%; p=.005).
Conclusion
Implementing a series of aggressive interventions increased appropriate TH use and was associated with improved outcomes in our facility.
Keywords: Cardiopulmonary resuscitation (CPR), Resuscitation, Hypothermia, Heart Arrest, Translational Research
INTRODUCTION
Sudden cardiac arrest is the leading cause of death in North America, affecting up to 450,000 persons each year.1 Overall survival rates for out-of-hospital cardiac arrest (OHCA) and in-hospital cardiac arrest (IHCA) are estimated to be 6.4% and 14%, respectively.2,3 Rates vary among cities and hospitals, perhaps because of differences in features of hospital care.4 Improved outcomes in survivors of cardiac arrest have been associated with induction of mild therapeutic hypothermia (TH) during the first day after cardiac arrest.5–9 Prior work is limited to OHCA patients or those with ventricular fibrillation or ventricular tachycardia (VF/VT) as the primary rhythm of arrest.
TH is the only post-arrest intervention associated with improved neurologic recovery in comatose survivors of cardiac arrest. In 2003, the American Heart Association issued an interim guideline recommending mild TH (cooling between 32°C and 34°C for 12 or 24 hours) be used to treat patients hospitalized for cardiac arrest associated with VF/VT.10 Although this recommendation was subsequently incorporated into the 2005 treatment guidelines,11 most survivors of cardiac arrest do not receive TH.8,12–15
In 2003, the University of Pittsburgh Medical Center (UPMC), a large health center in Pennsylvania, developed a standardized protocol for the use of TH in cardiac arrest survivors who were initially comatose after the event. The protocol was not regularly used. Therefore, in 2005 an institutional quality improvement (QI) program introduced a care package with multiple interventions of escalating intensity. The goal of these interventions was to increase the use of TH and to reduce variability in care delivered. In 2008, we retrospectively reviewed the medical records of patients treated between January 1, 2005 and December 31, 2007 to determine if TH use increased over the intervention period and whether outcomes were improved for patients who received TH.
METHODS
Setting
UPMC Presbyterian Hospital is a tertiary care facility with 152 critical care beds in 8 intensive care units, and uses a mature Rapid Response System (RRS) to respond to sudden critical health problems, including cardiac arrest.16 The overall unexpected cardiac arrest rate is <3 per 1000 admissions.17 Our retrospective review of the medical records was approved by the University of Pittsburgh Institutional Review Board.
Treatment Protocol
The Hypothermia Post-Cardiac Arrest Protocol (Appendix) was created in 2003. Patients were considered candidates for TH if they suffered cardiac arrest (defined as receiving defibrillation or chest compressions for pulseless arrhythmia), were intubated, could not follow verbal commands and had no contraindications for TH. Contraindications included: active bleeding, intracranial hemorrhage, early withdrawal of care or “comfort measures only” (CMO) status, traumatic injury, planned surgical intervention, or if their first score on the Glasgow Coma Score (GCS) was obscured by a sedative or paralytic agent. Patients with CMO or withdrawal of care status determined within the first 6 hours were considered “early” CMO or withdrawal of care.
TH was induced by rapid intravenous infusion of 1–2 L of 4°C saline solution and the use of cooling blankets. These measures could be supplemented by the administration of 4°C saline solution via nasogastric lavage, the use of ice packs, or endovascular cooling catheters. The target was to reach and maintain a core temperature between 32°C and 34°C until 24 hours after restoration of spontaneous circulation (ROSC). The preference of temperature monitoring sites was: pulmonary artery catheter, esophagus, bladder and rectum. Most patients were monitored with esophageal temperature probes. Sedation with propofol or benzodiazepines was recommended to prevent shivering. Induction of neuromuscular paralysis was an option, but discouraged because it could mask assessment of neurologic status or seizures.5,6
Additional physiologic goals were bundled with the TH protocol. Fluid infusion and use of vasopressors and inotropes were recommended to achieve a mean arterial pressure of ≥80mm Hg and a urine output of ≥0.5 mL/kg/hr. Use of insulin infusion to achieve glycemic control (<150 mg/dL) was encouraged.18 Cardiac catheterization was performed immediately if the patient had anginal symptoms prior to the arrest or current evidence of an acute coronary syndrome (e.g. ST segment elevation, left bundle branch block, or segmental wall motion abnormality on echocardiogram). Patients were evaluated for placement of an implantable cardioverter-defibrillator (ICD). Those who regained consciousness were evaluated for cognitive rehabilitation.
Process Interventions
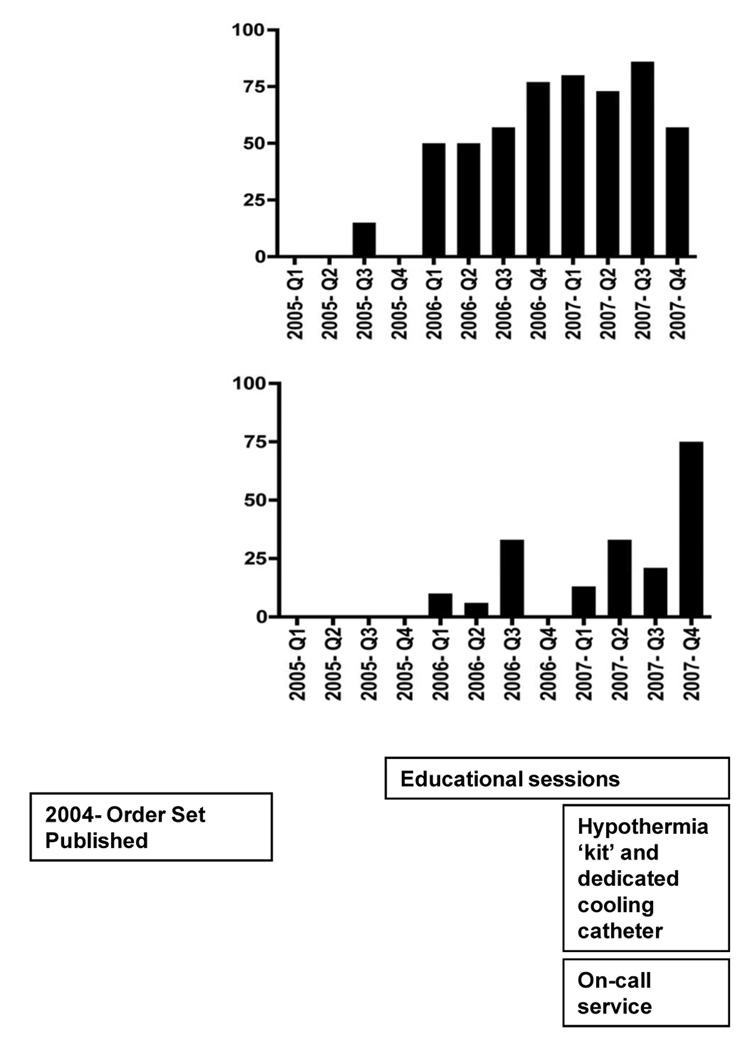
To increase the use of TH, we developed and implemented a set of 9 interventions. The timeline of these interventions are depicted in Figure 1.
The preprinted TH order set (Appendix) was updated and made available through the electronic medical record system.
The Condition A (IHCA) log and the emergency department (ED) logs were reviewed to identify attending physicians who treated patients after cardiac arrest. These physicians were sent e-mails to remind them that the TH order sheet was available for their use.
Educational sessions about TH were provided to physicians, nurses and medical trainees. They were trained to use the TH order sheet for any patient who required cardiopulmonary resuscitation, experienced ROSC, and was not following verbal commands. In June 2006, we began by working with staff members in the ED and providing them with verbal consultation and feedback about the care of patients after cardiac arrest. TH initiation in the ED increased to 75% after these sessions, leading the authors to expand this intervention. Subsequent efforts focused on providing training and offering feedback to staff members in the Division of Cardiology and the cardiac intensive care unit (CICU), since they treat the largest proportion of patients with OHCA. Educational efforts also involved encouraging the ED staff to preferentially send post-arrest patients to the CICU, rather than the first available ICU bed. This was intended to reduce treatment variability.
A TH “kit” that included the TH order set, a cooling blanket, and intravenous fluids was created and stocked in central supply for rapid deployment. Normal Saline and Lactated Ringer’s solution were placed in the medication refrigerators in all ICU’s and the ED to ensure cold fluids were available.
An endovascular cooling catheter and cooling apparatus were available from central supply should other measures fail to cool the patient.
In January 2007, a post-cardiac arrest service (PCAS) provided on-call physicians dedicated to the tasks of directing the resuscitation of patients after an OHCA or IHCA, resolving problems related to TH therapy, rendering bedside care during the period of hospitalization, and following the patients to ensure that secondary prevention measures were taken and evaluations for rehabilitation were performed. This service was separate from the RRS that provided initial resuscitation of patients with cardiac arrest.
The Medical Emergency Response Improvement Team was consulted to foster physician and nursing education and coordinate process implementation.
Members of the PCAS reviewed the medical charts of all patients with OHCA or IHCA to determine if TH and other target therapies had been delivered and to provide individual feedback to physicians as needed.
Referring physicians and Emergency Medical Service agencies were sent follow-up letters describing the care and outcomes for each of their patients as a continuous feedback activity.
Figure 1.
Flowchart of patients with cardiac arrests from 2005 to 2007. A. Out-of-hospital cardiac arrest (OHCA). B. In-hospital cardiac arrest (IHCA). CMO- comfort measures only; GCS- Glasgow Coma Score; TH- therapeutic hypothermia. A good neurologic outcome was defined as discharge to the patient’s home or to an acute rehabilitation center.
Analysis of Treatment Processes and Outcomes
Patients were eligible for inclusion if they were treated for OHCA or IHCA between January 1, 2005 and December 31, 2007. Exclusion criteria are outlined above.
Demographic information (age and gender), year, category (OHCA or IHCA), type of first rhythm, and first GCS after cardiac arrest were abstracted from the medical record. We also determined if treatment included TH, cardiac catheterization, placement of an ICD, and the patient’s disposition.
The primary process measure of interest was the use of TH, defined as the appropriate institution of TH in a post-arrest patient, n order to evaluate compliance with the protocol, we reviewed the duration of time at goal temperature (defined as ≤34° C). The proportion of patients receiving ≥6 hours of goal temperature and ≥12 hours of temperature were compared during the intervention period. The primary outcome measure of interest was good neurologic outcome. Similar to prior studies, we defined a good outcome as discharge to the patient’s home or to an acute rehabilitation center.6 All other outcomes were classified as bad. These criteria were used as detailed neurologic testing was not available for these patients. I
Descriptive statistics were used to report numbers and proportions of patient characteristics, treatment processes, and treatment outcomes. Chi-squared analyses or Fisher’s exact test were used to make group comparisons based on cardiac arrest year (2005, 2006, and 2007) and category (OHCA and IHCA). Multivariate logistic regression models were generated to determine predictors for use of TH, cardiac catheterization, and ICD placement. Candidate variables for predictors of each of these processes include age, gender, year, cardiac arrest location, and type of first rhythm. Additional variables for cardiac catheterization and ICD placement included ST segment elevation, new left bundle branch block and segmental wall motion abnormality on echocardiogram. The Hosmer-Lemeshow test was used to assess goodness of fit. All analyses were performed with STATA version 9.2 (College Station, TX), and a p value of <0.05 was considered significant.
RESULTS
During the 3-year study period, 394 patients suffered cardiac arrest. Of these patients, 241 met the inclusion criteria for our analysis (Figure 2).
Figure 2.
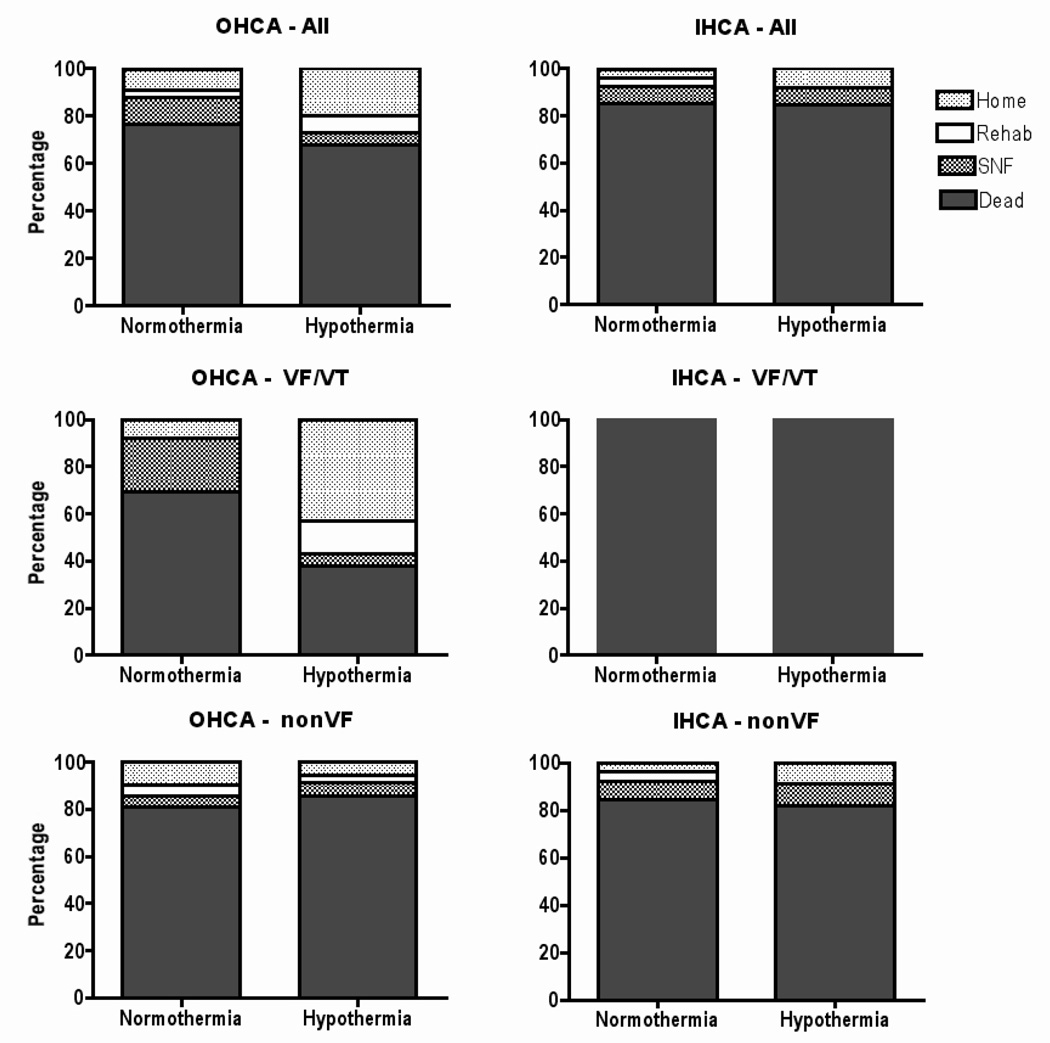
Outcomes of comatose survivors of cardiac arrest from 2005 to 2007, stratified by type of first rhythm and by category of cardiac arrest. OHCA indicates out-of-hospital cardiac arrest; IHCA, in-hospital cardiac arrest; VF, ventricular fibrillation; VT, ventricular tachycardia; rehab, acute rehabilitation center; and SNF, skilled nursing facility.
Demographic and Clinical Characteristics
Of the 241 patients, 135 suffered OHCA and 106 suffered IHCA. Demographic and clinical characteristics are shown in Table 1. The OHCA group had significantly fewer VF/VT arrests in 2006 (30%) than in 2005 (55%) or 2007 (49%; p=0.002). The IHCA group had no significant differences over time in type of first rhythm noted. In 2005, 2006, and 2007, the proportions of patients remaining comatose after resuscitation in the OHCA group were 60%, 59% and 80%, respectively (p=0.06), and the proportions of patients remaining comatose after resuscitation in the IHCA group were 41%, 30%, and 47%, respectively (p=0.3).
Table 1.
Demographic and Clinical Characteristics of Patients With Out-of-Hospital Cardiac Arrest (OHCA) and In-Hospital Cardiac Arrest (IHCA) During the Intervention Period *
| Characteristic | 2005 | 2006 | 2007 | |||
|---|---|---|---|---|---|---|
| OHCA (n = 42) |
IHCA (n = 27) |
OHCA (n = 44) |
IHCA (n = 47) |
OHCA (n = 49) |
IHCA (n = 32) |
|
| Age, mean (SD), yr | 63 (16) | 63 (14) | 61 (15) | 62 (16) | 58 (16) | 57 (15) |
| Male, No. (%) | 21 (50) | 13 (48) | 24 (59) | 28 (60) | 31 (63) | 16 (50) |
| Rhythm, No. (%) | ||||||
| VF/VT | 23 (55) | 8 (30) | 13 (30) | 12 (26) | 24 (49) | 13 (41) |
| PEA | 11 (26) | 9 (33) | 19 (43) | 21 (45) | 4 (8) | 10 (31) |
| Asystole | 7 (17) | 6 (22) | 7 (16) | 10 (21) | 16 (33) | 6 (19) |
| Unknown | 1 (2) | 4 (15) | 5 (11) | 4 (8) | 5 (10) | 3 (9) |
| Comatose, No. (%) | 25 (60) | 11 (41) | 24 (59) | 14 (30) | 39 (80) | 15 (47) |
Abbreviations: PEA, pulseless electrical activity; VF, ventricular fibrillation; and VT, ventricular tachycardia.
Comparisons by group and year showed a significant difference in VF/VT in the OHCA group over time (p= .002).
Treatment Processes
Of the 241 patients, 130 were comatose after cardiac arrest. Table 2 depicts the use of TH, cardiac catheterization, and ICD’s in these patients during 2005, 2006, and 2007. The use of TH increased each year (3%, 35%, and 55%; p<0.001), and the use of cardiac catheterization also increased each year (17%, 38%, and 41%; p=0.05). Placement of ICD’s did not differ over time. An increase in the use of TH over the years was significant for both the OHCA group (from 6% to 65% to 76%; p<0.001) and the IHCA group (0%, 36%, and 53%; p=.02). Changes in the use of cardiac catheterization and ICD placement within these groups were not significant.
Table 2.
Procedures Used in the Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest (OHCA) and In-Hospital Cardiac Arrest (IHCA) During the Intervention Period *
| Procedure | 2005 | 2006 | 2007 | |||
|---|---|---|---|---|---|---|
| OHCA (n = 25) |
IHCA (n = 11) |
OHCA (n = 26) |
IHCA (n = 14) |
OHCA (n = 39) |
IHCA (n = 15) |
|
| Therapeutic Hypothermia, No. (%) | 2 (8) | 0 (0) | 21 (81) | 5 (36) | 33 (85) | 8 (53) |
| Emergent cardiac catheterization, No. (%) | 6 (24) | 0 (0) | 12 (46) | 3 (21) | 19 (49) | 3 (20) |
| Placement of implantable cardioverter-defibrillator, No. (%) | 2 (8) | 0 (0) | 3 (12) | 1 (7) | 6 (15) | 0 (0) |
Comparisons by group and year showed a significant difference in the use of therapeutic hypothermia over time in the OHCA group (p<.001) and the IHCA group (p= .016)
In 2007, the PC AS was consulted for 49 patients. A larger proportion of the OHCA group (73%) than the IHCA group (41%) was evaluated by this service.
Based on multivariate logistic regression, TH was more likely to be used in men than women (Odds Ratio [OR], 3.47; 95% CI 1.22, 9.88), in patients treated with OHCA than IHCA (OR 8.93; 95% CI 2.82, 22.22), in patients treated in 2006 than in those treated in 2005 (OR 91.1; 95% CI 14.6, 567.4) and in patients treated in 2007 than 2005 (OR 124.7; 95% CI 21.5, 723.1). TH was less likely to be used in patients with an unknown rhythm (OR 0.11; 95% CI 0.02, 0.57). This model had good fit with a Hosmer-Lemeshow value of 0.94.
Cardiac catheterization was more likely to be used in patients with OHCA than in those with IHCA (OR 4.34; 95% CI 1.42, 13.3), in patients treated in 2006 than 2005 (OR 6.94; 95% CI 1.84, 26.1), and in patients treated in 2007 than 2005 (OR 6.19; 95% CI 1.81, 21.2). It was less likely to be used in patients with pulseless electrical activity than VF/VT (OR 0.20; 95% CI 0.06, 0.65) or in patients with asystole rather than VF/VT (OR 0.10; 95% CI 0.03, 0.33). It was least likely to be used in patients with an asystolic OHCA (OR 0.03, 95% CI 0.003, 0.29). This model also had good fit with a Hosmer-Lemeshow value of 0.43.
Multivariate logistic regression found no predictors of the use of ICD placement.
In the 79 patients where detailed temperature data were available (2005- N=2; 2006 N=34; 2007 N=43), maintenance at goal temperature for ≥6 hours increased during the study period (2005-0%, 2006- 79%, 2007- 72%; p=0.045). There was a trend toward maintaining goal temperature for ≥12 hours (2005- 0%, 2006- 74%, 2007- 60%; p=0.076).
Treatment Outcomes
Figures 2 and 3 show the outcomes for patients in the study. The outcome was good in 19 of 90 comatose patients with OHCA (21%) but only 3 of 40 patients with IHCA (7.5%; p=0.06). Twice as many good outcomes were achieved in comatose patients receiving TH (16 of 69, 23%) than in those comatose patients not receiving TH (6 of 61, 10%; p=0.04). This difference was most pronounced in the comatose VF/VT cohort. Comatose patients with VF/VT-related OHCA were more likely to achieve a good outcome with TH treatment than without TH treatment (good outcome in 57% vs. 8%; p=0.005).
DISCUSSION
In our large, university-affiliated, tertiary care hospital, we found a significant difference in the processes and outcomes of comatose survivors of cardiac arrest after we instituted a set of 9 increasingly aggressive interventions. These included sending reminders to reinforce the use of an established treatment protocol and creating a TH order sheet, education program, TH “kit”, and on-call PCAS.
Our interventions resulted in an 11-fold increase in the use of TH and a 2-fold increase in the use of cardiac catheterization for comatose patients in the OHCA cohort. They were also associated with improved outcomes in this cohort, especially in patients with VF/VT-related OHCA. While these interventions increased the use of TH from 0% to 53% in the IHCA cohort, they did not significantly improve outcomes. Our small sample size may be one reason for this finding. We note that many of these interventions began in the ED and this may account for the rapid increase in TH use in the OHCA cohort and slower increase of TH use in the IHCA cohort. Differences in the underlying pathology may explain the lack of improved outcomes in the IHCA cohort. Specifically, patients resuscitated from IHCA have been reported to be more likely than patients resuscitated from OHCA to die from multisystem organ failure and less likely to die from anoxic encephalopathy.19
In contrast to earlier studies, which described the use of standardized care for patients who had OHCA and were being treated in one designated ICU,7,9 our study applied a protocol-driven plan to patients who experienced either OHCA or IHCA and were being treated in various ICU settings. Care of patients after ROSC is not typically provided by a dedicated specialist or at dedicated referral hospitals. Consequently, individual physicians have few opportunities to treat these patients and may lack standard protocols. Variability in the care that patients receive is thought to account for variations in patient survival rates of different hospitals and cities.4 We implemented a physician service that provided clinical care, assisted design of the treatment protocol, and championed the care plan throughout the hospital. We believe that our data support the value of following a standardized care plan for patients with cardiac arrest. They also support the value of making system improvements to ensure that best practices are followed, including practices concerning the optimal duration of hypothermia, optimal glucose management, and optimal blood pressure management.20 Because great effort was required to implement a change of care in this tertiary care hospital, these data may support referral of post-cardiac arrest patients to specialized centers.
Our study had several limitations that deserve mention. First, because our QI initiative included a package of care, we cannot attribute our results to any one intervention. Our data cannot establish that it was hypothermia per se that was responsible for improved outcomes. These outcomes support the benefit of a care package. Second, the patients included in our analysis did not undergo systematic assessments for neurologic recovery. However, in the 49 patients treated by the PCAS, a CPC was determined prior to discharge. Of the subjects discharged to home (N=12), 10/12 were classified as a CPC 1 and 2/12 were classified as a CPC 2. Subjects discharged to acute rehabilitation (N=2) were classified as CPC 3 in 2/2 cases. Subjects discharged to a skilled nursing facility (N=2) were classified as CPC 2 in ½ and CPC 3 in ½ cases. Subjects discharged to long term acute care facilities were classified as CPC 4 in 3/3 cases. Therefore, we believe our surrogate measurement approximates the CPC in many of these patients. We do not have data on the baseline cognitive status of the patients and thus do not know if discharge to long-term care represented a partial or complete return to baseline. We have previously reported the limitations of a chart review to obtain neurologic status.21 Third, our analysis is based on records from a single hospital, limiting the generalizability of these results. Our hospital has a mature and established RRS that has contributed to a dramatic reduction in the rate of IHCA.16 Our sample of patients with IHCA may not be generalizable to hospitals that lack a similar system.
Future studies need to determine whether our intervention strategies can be successfully exported to other hospital systems. If these results translate beyond our hospital, they may save thousands of lives per year. In addition, the progressive escalation of interventions described here may be a useful model for implementing other new therapies for medical problems that are not frequently encountered.
CONCLUSIONS
A systematic series of system enhancements improved neurologic outcomes after cardiac arrest in a large, academic, tertiary care facility despite a large number of critical care settings. These enhancements were also associated with an increase in the use of TH and cardiac catheterization for OHCA patients and an increase in the use of TH in IHCA patients. Optimal care for cardiac arrest patients required a multidisciplinary effort that included hospital administrators and practitioners in emergency medicine, cardiology, critical care medicine, and rehabilitation.
Supplementary Material
Acknowledgements
Dr. Rittenberger is supported by Grant Number 1 KL2 RR024154-02 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Dr. Rittenberger is also supported by an unrestricted grant from the National Association of EMS Physicians/Zoll EMS Resuscitation Research Fellowship. We are indebted to Ms. Sharon Maddox for her editorial assistance with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics 2008 update. A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Stiell IG, Laupacis A, Pham B, DeMaio VJ, Wells GA. A cumulative meta-analysis of the effectiveness of defibrillator-capable emergency medical services for victims of out-of-hospital cardiac arrest. Ann Emerg Med. 1999;34(4 Pt 1):517–525. [PubMed] [Google Scholar]
- 3.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report on 14720 cardiac arrests from the national registry of cardiopulmonary resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 4.Langhalle A, Tyvold SS, Lexow K, Hapnes SA, Sunde K, Steen PA. In-hospital factors associated with improved outcome after out-of-hospital cardiac arrest. A comparison between four regions in Norway. Resuscitation. 2003;56(3):247–263. doi: 10.1016/s0300-9572(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 5.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 6.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 7.Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardized treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Oksanen T, Pettila V, Hynynen M, Varpula T Intensium Consortium Study Group. Therapeutic hypothermia after cardiac arrest: implementation and outcome in Finnish intensive care units. Acta Anaesthesiol Scand. 2007;51(7):866–871. doi: 10.1111/j.1399-6576.2007.01365.x. [DOI] [PubMed] [Google Scholar]
- 9.Werling M, Thoren AB, Axelsson C, Herlitz J. Treatment and outcome in post-resuscitation care after out-of-hospital cardiac arrest when a modern therapeutic approach was introduced. Resuscitation. 2007;73(1):40–45. doi: 10.1016/j.resuscitation.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Nolan JP, Morley PT, Vanden Hoek TL, et al. International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the international liaison committee on resuscitation. Circulation. 2003;108(1):118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 11.American Heart Association. Part 7.5: Postresuscitation support. Circulation. 2005;112 24 Suppl:IV-84–IV-88. [Google Scholar]
- 12.Wolfrum S, Radke PW, Pischon T, Wilich SN, Schunkert H, Kurowski V. Mild therapeutic hypothermia after cardiac arrest- a nationwide survey on the implementation of ILCOR guidelines in German intensive care units. Resuscitation. 2007;72(2):207–213. doi: 10.1016/j.resuscitation.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Laver SR, Padkin A, Atalla A, Nolan JP. Therapeutic hypothermia after cardiac arrest: a survey of practice in intensive care units in the United Kingdom. Anaesthesia. 2006;61(9):873–877. doi: 10.1111/j.1365-2044.2006.04552.x. [DOI] [PubMed] [Google Scholar]
- 14.Merchant RM, Soar J, Skrifvars MB, et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34(7):1935–1940. doi: 10.1097/01.CCM.0000220494.90290.92. [DOI] [PubMed] [Google Scholar]
- 15.Abella BS, Rhee JW, Huang K, Vanden Hoek TL, Becker LB. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64(2):181–186. doi: 10.1016/j.resuscitation.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 16.DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons TL. Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251–254. doi: 10.1136/qshc.2003.006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galhotra S, DeVita MA, Simmons RL, Dew MA. Medical Emergency Response Improvement Team (MERIT). Mature rapid response system and potentially avoidable cardiopulmonary arrests in hospital. Qual Saf Health Care. 2007;16(4):260–265. doi: 10.1136/qshc.2007.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Losert H, Sterz F, Roine RO, et al. Strict normoglycaemic blood glucose levels in the therapeutic management of patients within 12h after cardiac arrest might not be necessary. Resuscitation. 2008;76(2):214–220. doi: 10.1016/j.resuscitation.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 20.Gazmuri RJ, Nadkarni VM, Nolan JP, et al. Scientific knowledge gaps and clinical research priorities for cardiopulmonary resuscitation and emergency cardiovascular care identified during the 2005 international consensus conference on ECC and CPR science with treatment recommendations: A consensus statement from the International liaison committee on Resuscitation (American Heart Association, Australian Resuscitation Council, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa and the New Zealand Resuscitation Council); the American Heart Association Emergency Cardiovascular Care Committee; the Stroke Council; and the Cardiovascular Nursing Council. Circulation. 2007;116(21):2501–2512. doi: 10.1161/CIRCULATIONAHA.107.186228. [DOI] [PubMed] [Google Scholar]
- 21.Raina KD, Callaway C, Rittenberger JC, Holm MB. Neurological and functional status following cardiac arrest: Method and tool utility. Resuscitation. 2008 doi: 10.1016/j.resuscitation.2008.06.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.