Abstract
Current vaccines against influenza virus elicit antibodies to the hemagluttinin and neuraminidase envelope proteins. Due to antigenic drift, these vaccines must be reformulated annually to include the envelope proteins predicted to dominate in the following season. By contrast, vaccination with the conserved nucleoprotein (NP) elicits immunity against multiple serotypes (heterosubtypic immunity). Vaccinating mice with DNA-based vectors allows antigen production by host cells, which present peptide epitopes to CD8 T cells. Such vaccines also induce significant titers of anti-NP antibodies, yet the involvement of antibodies in protection has largely been disregarded. To investigate how antibody responses might contribute to heterosubtypic immunity, we vaccinated C57BL/6 mice with recombinant (r)NP as a soluble protein. This approach induced high titers of NP-specific serum antibody, but only poorly detectable NP-specific T cell responses. Nevertheless, rNP immunization was effective at reducing morbidity and reducing viral titers after challenge with influenza virus. Importantly, antibody-deficient mice were not protected by this vaccination strategy. Furthermore, rNP-immune serum could transfer these protective effects to naïve hosts in an antibody-dependent manner. Therefore, antibody is essential for rNP-immune protection, strongly suggesting that NP-specific antibody can convey immunity to influenza virus. Thus, antibody to conserved, internal viral proteins, such as NP can provide an important mechanism of protection that may be utilized together with cytoxic T cells to elicit heterosubtypic immunity by future vaccines.
INTRODUCTION
Influenza virus causes acute respiratory illness that leads to ~94,000 hospitalizations (1) and 36,000 deaths annually in the United States (2). Vaccines against influenza have been available for many years, and are often highly effective at preventing infection as well as reducing morbidity and mortality associated with seasonal influenza outbreaks. Current vaccines are designed to elicit antibodies directed against the external glycoproteins of influenza: hemagglutinin (HA) and neuraminidase (NA). Neutralizing anti-HA antibodies prevent influenza virus infection of cultured epithelial cells (neutralization) and can passively protect mice from infection (3, 4). In fact, neutralizing antibody titers are considered to be the gold-standard correlate of vaccine-induced immunity, and are presumed to provide the mechanism for vaccine-induced protection (5–7). Despite the efficacy of neutralizing antibodies, their utility is limited, as they only protect against viral serotypes that express the same HA and NA proteins contained in the vaccine. Because mutations rapidly accumulate in the HA and NA proteins of influenza virus, particularly in the epitopes recognized by neutralizing antibodies, influenza vaccines must be reformulated each year to include the HA and NA proteins predicted to dominate in the following influenza season. Consequently, generating annual vaccines is cumbersome and costly, and if serotypes are not accurately predicted, the resulting immunity may not be very effective.
By contrast, vaccines that elicit immunity to conserved, often internal viral proteins, such as nucleoprotein (NP), provide some protection from multiple strains and subtypes of influenza virus. For example, mice vaccinated with influenza NP (as purified protein or using DNA expression vectors) have higher frequencies of NP-specific CD8 T cells before infection, as well as lower viral titers after challenge with H3N2 and H1N1 strains of influenza. This vaccination also protects from virus-induced lethality (8–13), including lethality induced by highly pathogenic H5N1 human isolates (14). T cell responses to conserved epitopes in these proteins are thought to be the main mechanism of protection, because restimulated T cells can transfer protection to naïve mice (15, 16), and because T cell depletion in the vaccinated mice can abrogate protection (14, 15). As a result, many investigations have focused on targeting antigens to the MHC class I pathway (e.g., using DNA-based vectors) to elicit CD8 T cell responses. Although CD4 and CD8 T cells can each contribute to protection elicited by vaccination with NP, T cells appear to be dispensable in some situations (13, 17), suggesting that other mechanisms, such as antibody production, may also contribute.
Both natural infection with influenza virus and vaccination with recombinant NP elicit NP-specific antibodies (18, 19). However, anti-NP antibodies were considered to be ineffective because they do not neutralize virus, and because passive transfer of such antibodies do not protect naïve immunodeficient scid recipient mice (4). However, it has recently been shown that immune complexes formed with anti-NP monoclonal antibodies can promote dendritic cell maturation, Th1 cytokine production, and anti-influenza CD8+ CTL responses in naïve immunocompetent recipients (20). Additionally, anti-NP IgG can stimulate complement-mediated lysis of infected P815 mastocytoma cells in vitro, due to expression of NP on the cell surface (21). Furthermore, non-neutralizing antibody to HIV-1 can promote complement-mediated virolysis (22) and non-neutralizing antibodies to Coxsackie B4 and poliovirus can induce secretion of the anti-viral cytokine IFNα from human monocytes (23–25). Based on these data, it is clear that non-neutralizing antibodies have the potential to elicit anti-viral responses. However, the anti-viral potential of non-neutralizing anti-NP antibodies in vivo, particularly in T cell-competent mice, has remained largely unexplored.
Interestingly, our laboratory recently showed that immune serum from C57BL/6 mice infected with an H3N2 strain of influenza virus can passively promote viral clearance and reduce morbidity in immune B cell-deficient μMT mice after challenge with a heterosubtypic H1N1 strain (19). Antibodies in H3N2-immune serum do not cross-react with the H1 or N1 proteins, and do not detect the external domain of M2 (M2e), suggesting that antibodies against surface epitopes of influenza virus are not involved in the protective effect. By contrast, H3N2-immune serum has high titers of antibody reactive with NP (19), consistent with previous observations (26–30). These data suggest that anti-NP antibodies may play an important role in mediating cross-reactive protection against multiple serotypes and subtypes of influenza.
Here, we show that antibody is necessary for NP immunization to confer protection in C57BL/6 mice, and that NP-immune serum can transfer protection to naïve recipient mice. Our results challenge the existing paradigm that T cell responses to conserved epitopes in internal proteins are the exclusive effectors of cross-reactive immunity to heterosubtypic strains of influenza, and strongly suggest that antibodies to these proteins are also an important component of the protective mechanism.
RESULTS
rNP immunization reduces influenza-induced morbidity and enhances viral clearance
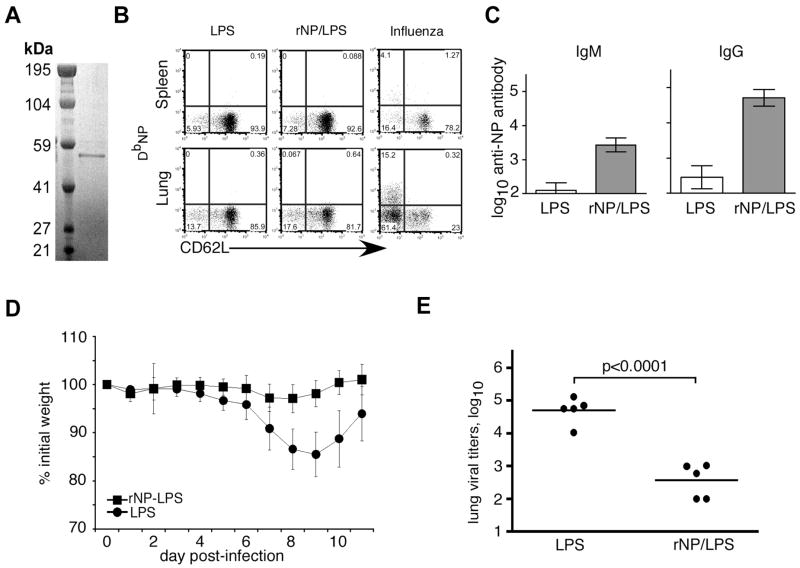
To induce high titers of NP-specific antibody, we purified soluble recombinant (r)NP to use as an immunogen. This protein runs as a single band of approximately 53 kDa on a reducing SDS-PAGE gel (Fig. 1A). We intraperitoneally (i.p.) immunized C57BL/6 mice with either 30 μg purified rNP plus 20 μg LPS as an adjuvant that is known to stimulate strong B cell responses, or with LPS alone on days 0 (prime) and 10 (boost). This vaccination alone did not induce an NP-specific CD8 T cell response that was detectable by MHC class I tetramer staining and flow cytometry at various times after boosting (Fig. 1B, and data not shown). However, the vaccination clearly induced high titers of NP-specific antibody in the serum as late as 39 days after priming (Fig. 1C). Thus, as expected, immunization with soluble rNP promotes a robust antibody response, but a limited CD8 T cell response.
Figure 1. Immunization with purified rNP protects C57BL/6 mice from influenza-induced morbidity and reduces viral titers.
(A) 500 ng of affinity-purified, sterile-filtered C-terminal 6Xhistidine-tagged rNP was fractionated using SDS-PAGE under reducing conditions, and stained with Coomassie Blue. (B) C57BL/6 mice were vaccinated i.p. with 30 μg rNP and 20 μg LPS (ν) or with LPS alone (λ) on days 0 and 10. T cells before challenge
(C) Sera from mice vaccinated as in panel B were assayed for NP-specific antibody by ELISA.
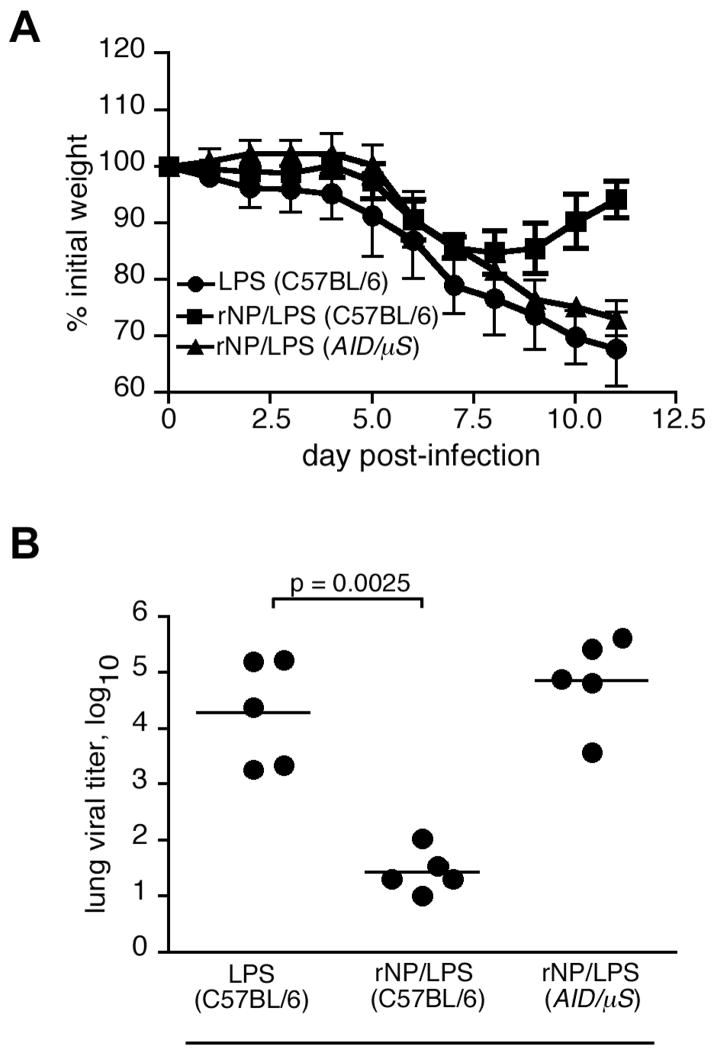
(D) Vaccinated mice were challenged i.n. on day 40 with 500 EIU PR8. Mice were weighed prior to infection, and the change in body weight was calculated as % of initial weight. Mean ± S.D., 5 mice/group. (E) Mice were immunized and infected as in panel D. Lung viral titers were assayed 8 days after influenza challenge.
To determine whether this apparently antibody-biased vaccine could still confer protection from a sublethal influenza virus challenge, the immunized mice were intranasally (i.n.) infected with a non-lethal dose of influenza PR8 virus (500 EIU, ~0.2 LD50) one month after the boost (day 40 after priming). Mice immunized with LPS alone lost ~15% body weight by day 7 post-infection, and had not yet recovered to their initial starting weight by day 11 (Fig. 1D). By contrast, mice vaccinated with rNP/LPS lost less than 5% of their initial weight, and fully recovered by day 11 (Fig. 1D). The reduced morbidity in rNP-vaccinated mice was associated with significantly lower viral titers in the lungs on day 8 after infection (Fig. 1E). Therefore, as previously described (11, 31), immunization of C57BL/6 mice with rNP provides some measure of protection from sublethal challenge.
rNP vaccination alters the kinetics of the CD8 T cell response to influenza virus
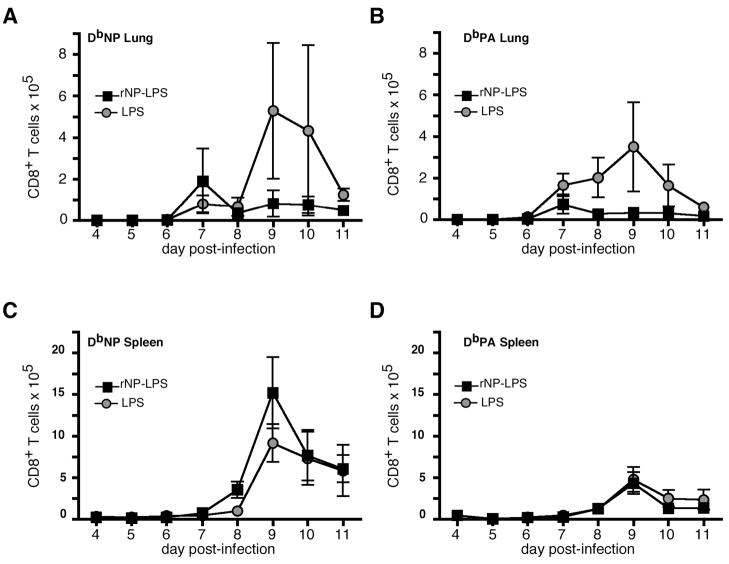
Although we immunized our mice in a manner that would bias the immune response to produce high-titer antibody, it remained possible that soluble rNP could be cross-presented on MHC class I to generate a memory CD8 T cell population that was undetectable before virus challenge. We therefore used flow cytometry to enumerate the influenza-specific CD8 T cell response after virus infection. On day 7 after challenge infection, there was a modestly greater number of NP-specific CD8 T cells in the lungs of rNP/LPS-vaccinated mice than in the lungs of LPS-vaccinated mice (Fig. 2A). However, after day 7, NP-specific CD8 T cells in the lungs of rNP-vaccinated mice declined, whereas the number of these cells in the lungs of LPS-vaccinated controls continued to increase through days 9 and 10 (Fig. 2A). Acidic polymerase (PA)-specific CD8 T cell responses also peaked earlier (day 7), although at lower numbers in the lungs of rNP/LPS-vaccinated mice than in LPS-vaccinated controls (Fig. 2B). Similarly to NP-specific cells, the PA response was dampened in rNP-immune mice subsequent to day 7. With little apparent acceleration of the CD8 T cell response in the lung of rNP-immune mice, we also examined the spleen. NP-specific CD8 T cells appeared one day earlier and peaked at higher numbers in rNP-immune mice relative to LPS-vaccinated controls (Fig. 2C). By contrast, the kinetics and magnitude of the splenic PA-specific CD8 T cell response was unaffected by rNP vaccination (Fig. 2D). Therefore, at the site of infection, the numbers of NP-specific CD8 T cells were increased at an early timepoint, suggesting a modest recall T cell response. However, the early viral clearance (Fig. 1C, and data not shown), and thereby limiting antigen, likely prevents further expansion of these cells and expansion of newly responding influenza-specific CD8 T cells in the lung.
Figure 2. rNP immunization alters the kinetics of the CD8 T cell response after viral challenge.
C57BL/6 mice were vaccinated intraperitoneally with 30 μg rNP and 20 μg LPS (ν) or with LPS alone (λ) on days 0 and 10, and challenged with 500 EIU PR8 on day 40. NP-specific (A) and PA-specific (B) CD8 T cells in the lung were measured by flow cytometry on the indicated day subsequent to challenge infection. NP-specific (C) and PA-specific (D) CD8 T cells were measured in the spleen. Mean ± S.D., 5 mice/group.
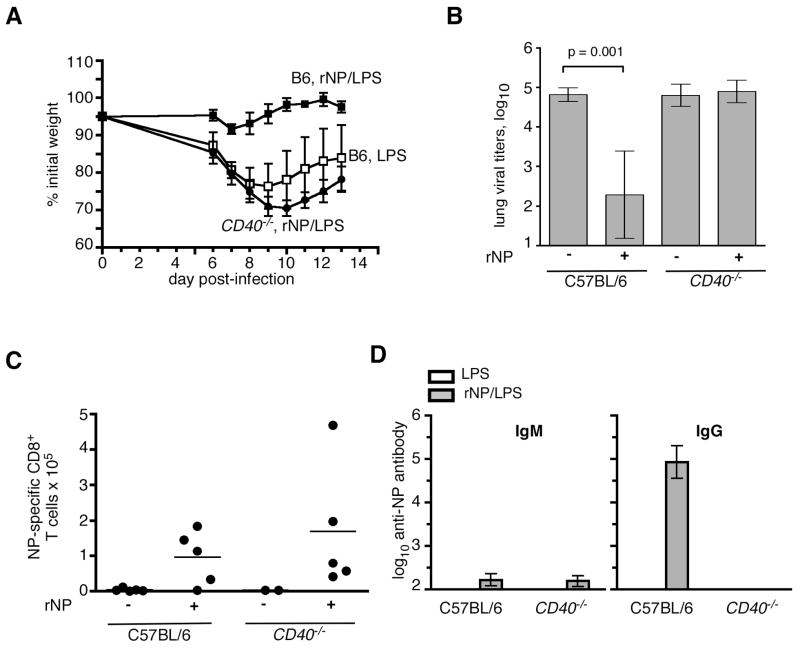
Our laboratory previously showed that CD40 expression is required for optimal CD8 T cell responses to influenza virus {lee, et al}. Thus, if CD8 T cells were contributing to rNP-immune protection, we would expect that rNP immunization would be ineffective in the absence of CD40 expression. Indeed, whereas C57BL/6 mice vaccinated with rNP/LPS lost very little weight after challenge infection, rNP-immune CD40−/− mice lost as much weight as LPS-vaccinated C57BL/6 mice (Fig. 3A). Furthermore, rNP vaccination did not reduce lung viral titers in CD40−/− mice as in the controls (Fig. 3B). Thus, a protective immune response to vaccination with rNP/LPS requires CD40. However, the early NP-specific CD8 T cell response in the lung was observed in rNP-immune CD40−/− mice as well as in rNP-immune C57BL/6 mice, in comparison with LPS-immunized controls (Fig. 3C). Therefore, although an early CD8 T cell recall response occurs in the lung of rNP-immune mice, this response alone appears to be insufficient to protect mice that do not express CD40.
Figure 3. rNP-elicited protection from influenza requires CD40.
(A) C57BL/6 mice were vaccinated i.p. with 30 μg rNP and 20 μg LPS (ν) or with LPS alone (□) on days 0 and 10. CD40−/− mice were also vaccinated i.p. with 30 μg rNP and 20 μg LPS (λ) on days 0 and 10. All groups were challenged i.n. with 500 EIU PR8 on day 40, and relative body weights were determined. Mean ± S.D., 5 mice/group. (B) Viral titers were assayed in the lung at day 8 post-infection in mice vaccinated with LPS ± rNP, as indicated. Mean ± S.D., 5 mice/group. (C) NP-specific CD8 T cells in the lung were determined by flow cytometry on day 7 post-infection. (D) Serum titers of NP-specific IgM and IgG were measured by ELISA in vaccinated mice on day 39 (one day prior to infection). Mean ± S.D., 5 mice/group.
Antibody is required for rNP-immune protection
Because CD40 expression is required for rNP-immune protection, but not for early CD8 T cell responses in our system (Fig. 3A–C), we hypothesized that another CD40-regulated effector function was necessary. CD40 is essential for effective induction of germinal center reactions to protein antigens {refs} and for the accompanying class-switched, high-affinity, and long-lived antibody responses (32). In fact, rNP-immune CD40−/− mice had little to no NP-specific IgG in the serum (Fig. 3D). These results show that protection induced by rNP correlates with serum titers of NP-specific antibody.
To determine whether the loss of protection in the CD40−/− mice was due to the failure to generate antibody, we crossed mice with a mutation in activation-induced cytidine deaminase (Aid −/−) {cell 102:553} with mice lacking the secretory form of IgM (μS −/− mice) {JI 160:4776}. Because Aid −/− mice cannot isotype switch their antibody genes, and μS−/− mice cannot secrete IgM, the resulting AID/μS mice have B cells, but cannot secrete antibody of any isotype. We vaccinated C57BL/6 mice and AID/μS mice with rNP/LPS or with LPS alone, challenged them with influenza virus on day 40. Figure 4A shows that, even after vaccination and influenza infection, AID/μS mice do not generate any NP-specific antibodies compared to vaccinated and infected C57BL/6 mice. As observed earlier, rNP-immune C57BL/6 mice had significantly lower viral titers than LPS-vaccinated controls on day 8 post-infection (Fig. 4B). However, rNP-immune, antibody-deficient AID/μS mice had viral titers that were as high as those in LPS-vaccinated control mice. Importantly, rNP-immune AID/μS mice still had an enhanced NP-specific CD8 T cell response that was still detectable at day 8 post-infection, when the modest recall response in the rNP-immune C57BL/6 mice had declined (Fig. 4C). It is likely that the higher antigen load (Fig. 4B) extends the expansion of existing memory T cells in the AID/μS mice, whereas the antibody in rNP-immune C57BL/6 mice prevents further expansion by promoting viral clearance. These results directly demonstrate that antibodies are essential for rNP-elicited protection from influenza virus.
Figure 4. rNP vaccination-mediated reduction in viral titers requires antibody.
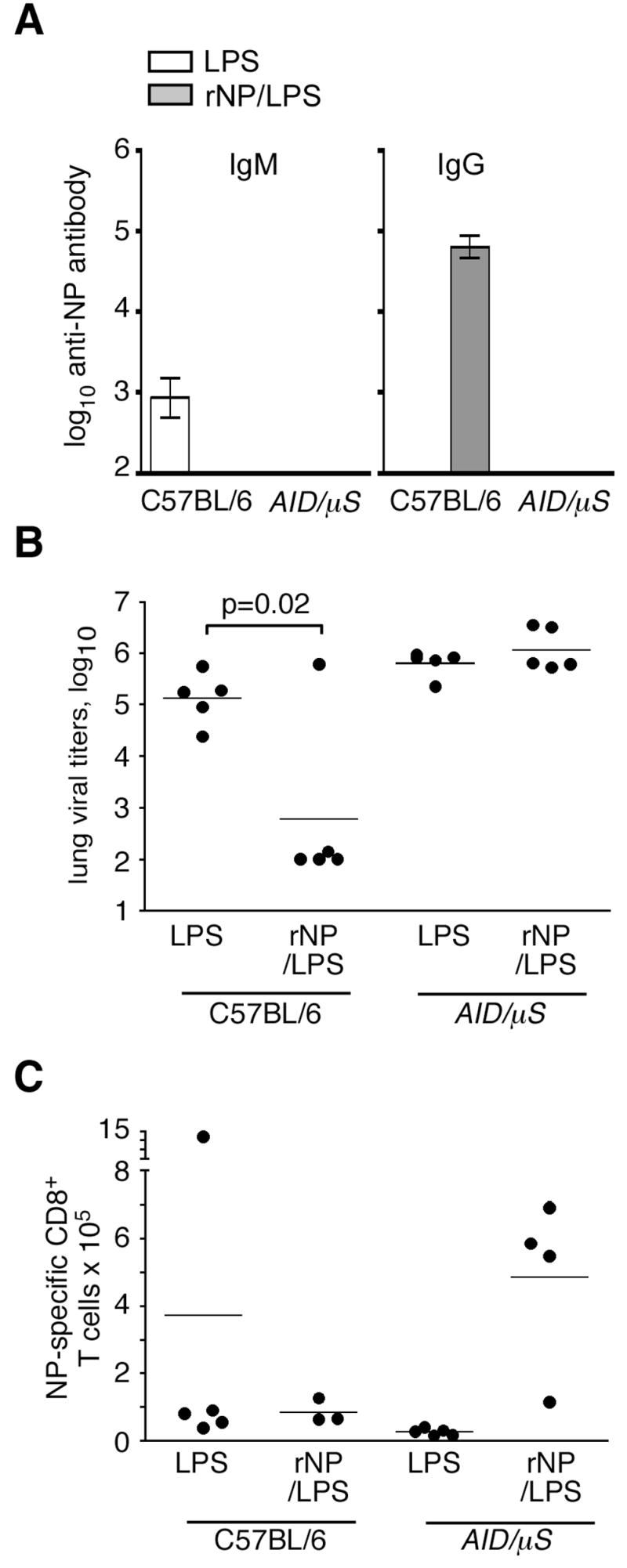
C57BL/6 and antibody-deficient AID/μS mice were i.p.-immunized with either 30 μg rNP and 20 μg LPS or with LPS alone on days 0 and 10. All groups were subsequently challenged i.n. with 500 EIU PR8 on day 40. (A) Titers of NP-specific IgM and IgG were measured by ELISA in serum from mice at day 8 post influenza infection. Mean ± S.D., 5 mice/group. The low titers of anti-NP IgM in C57BL/6 mice are not consistently detected among experiments performed at this timepoint. (B) Lung viral titers were measured on day 8 after infection. (C) NP-specific CD8 T cells in the lung were determined by flow cytometry on day 8 post-infection.
rNP-immune serum protects μMT mice from influenza-derived morbidity and enhances viral clearance in an antibody-dependent manner
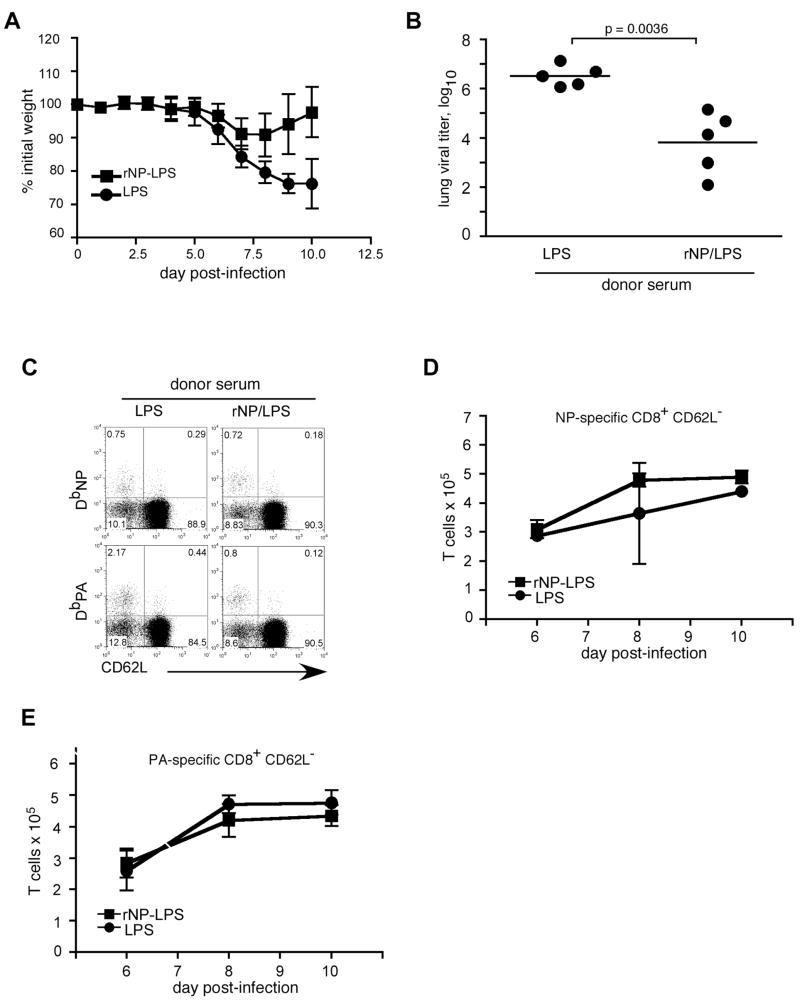
Although a previous study demonstrated that transfer of monoclonal anti-NP antibodies to scid mice did not protect from influenza virus (4), it was possible that these antibodies would be protective if T cells were also present. To test this possibility, we transferred serum from rNP- vaccinated C57BL/6 donors to μMT mice, and challenged the B cell-deficient recipients with influenza virus the following day. Whereas recipients of LPS-immune serum continued to lose up to 25% of initial body weight through day 10 after infection, μMT mice receiving rNP-immune serum lost only about 10% of their body weight, and began to recover by day 8 (Fig. 5A). Moreover, lung viral titers on day 10 were reduced by ~100-fold in recipients of rNP-immune serum relative to those in mice that received control serum (Fig. 5B). Therefore, rNP-immune serum can convey protection against influenza challenge in T cell-competent μMT hosts.
Figure 5. rNP-immune serum protects μMT mice against influenza-induced morbidity.
Serum was obtained on day 40 from C57BL/6 mice vaccinated with either 30 μg rNP and 20 μg LPS (ν) or with LPS alone (λ) at days 0 and 10. 200 μl sera were injected i.p. into naïve μMT mice 1d prior to influenza PR8 infection (500 EIU). (A) Mice were weighed daily and relative body weights were determined. Mean ± S.D., 5 mice/group. (B) Lung viral titers were determined on day 10 post-infection. (C) Flow cytometry of lung cells was performed on day 8 post-infection. Values shown are percentage of the CD8+ gate. (D) Kinetics of the NP-specific CD8 T cell response in the spleen. Mean ± S.D., 5 mice/group. The total number of NP-specific CD8 T cells per spleen was calculated from flow cytometry data as in panel C.
We next tested whether the transfer of rNP-immune serum affected CD8 T cell responses. The frequency of NP-specific CD8 T cells in the spleen was not significantly different in recipients of rNP-immune serum compared with control serum, and the frequency of PA-specific CD8 T cells was slightly lower in mice that received rNP-immune serum (Fig. 5C). Furthermore, the overall kinetics and magnitude of the CD8 T cell response in these mice remained largely unaffected (Fig. 5D). Thus, the protection provided by rNP-immune serum does not correlate with a detectable modification of the CD8 T cell response.
To demonstrate that the protection conveyed by rNP-immune serum transfer is antibody-mediated, we immunized C57BL/6 and antibody-deficient AID/μS mice with rNP/LPS, transferred serum from these animals to naïve μMT recipients, and challenged them with influenza virus the following day. Recipients of rNP-immune serum from C57BL/6 mice lost only about 15% of their initial body weight, and were recovering by day 11 post-infection; however, mice that received serum from rNP-immune AID/μS mice still lost >25% body weight and showed no recovery - effects comparable to recipients of C57BL/6 control serum (LPS) (Fig. 6A). Additionally, rNP-immune serum from the AID/μS donors failed to reduce lung viral titers (Fig. 6B). These results clearly demonstrate that the protection against influenza infection conveyed by rNP-immune serum transfer is dependent upon antibody. Collectively, these findings establish that antibody is a crucial effector underlying the protection elicited by rNP vaccination. Furthermore, the results reveal a novel and important role for non-neutralizing polyclonal antibody to internal, conserved viral proteins in vaccine-mediated protection.
Figure 6. Protection of μMT mice by transfer of rNP-immune serum requires antibody.
(A) Serum was obtained from rNP/LPS-vaccinated C57BL/6 mice (ν), rNP/LPS-vaccinated AID/μS mice (σ), and LPS-vaccinated C57BL/6 mice (λ) 40 days post-vaccination. 200 μl of serum were transferred to μMT mice 1 day prior to i.n. challenge with 500 EIU influenza PR8. (A) Weight loss was measured daily and relative body weights were determined. Mean ± S.D., 5 mice/group. (B) Viral titers were measured in the lungs of the recipient mice on d10 post- infection.
DISCUSSION
Current influenza vaccines are designed to elicit neutralizing antibody responses to external, mutation-prone molecules such as HA and NA. However, these vaccines must be reformulated annually to account for antigenic drift, and they fail to provide significant protection when the HA and NA proteins of the circulating virus are substantially different than those in the vaccine. An alternative strategy is to vaccinate with highly conserved internal influenza proteins, such as NP. Our data confirm that NP vaccination alleviates morbidity and reduces viral load when the vaccinated C57BL/6 mice are challenged with live influenza virus. Although we immunized with a soluble protein antigen, we observed a modest acceleration of the NP-specific CD8 T cell response in the lung and spleen, suggesting that the antigen was cross-presented and generated a memory precursor pool. However, a rapid CD8 T cell expansion may not be entirely responsible for protection in these experiments, as CD40−/− and AID/μS mice were not protected by rNP vaccination, despite the presence of responding NP-specific memory CD8 T cells in the lung. Instead, these mice are defective in anti-NP antibody responses. Furthermore, transferred rNP-immune serum reduces influenza-induced morbidity and viral load in an antibody-dependent fashion in otherwise naïve μMT recipients. Together, these data indicate that antibodies play an unexpectedly important role in immune protection elicited by vaccination with rNP.
Despite high titers of specific antibody generated by NP vaccination (9, 14, 17, 31, 35), the potential for these antibodies to facilitate influenza resistance has been underappreciated. In part, this disregard is due to early studies that failed to show protective effects of NP-specific antibodies in lymphopenic scid (4) and in BALB/c recipients (9). These results solidified the paradigm that non-neutralizing antibodies to NP do not contribute to protective immunity from influenza virus. We find that B cell-deficient μMT mice can be protected by heterosubtypic immune serum (19), and more specifically, by rNP-immune serum in an antibody-dependent manner (Fig. 6 and 7). Compared with control mice such as C57BL/6, μMT mice are much more susceptible to the pathogenic effects of influenza, likely due to poor antibody responses to the virus (36), as well as a lack of influenza-reactive natural antibody (37). Thus, the protective effects that we transfer with a single dose of rNP-immune sera are readily observable in the absence of endogenous antibody responses. By contrast, intact recipient mice may require larger amounts of anti-NP antibody comparable to the titers observed in actively immunized C57BL/6 (Fig. 3) and BALB/c mice, which are each protected by NP vaccination [(9), and data not shown]. The failure to protect lymphopenic scid recipient mice with monoclonal anti-NP antibody in a previous report (4) may be due to the limited specificity and single isotype of the clone used, and possibly due to the absence of T cells in the recipient mice. Nonetheless, our data add to growing evidence that non-neutralizing antibodies can contribute to protection from influenza challenge {Bender, 1994 #64;Rangel-Moreno, 2008 #183;Nguyen, 2001 #206;Sambhara, 2001 #185}.
Another reason that the protective potential of anti-NP antibodies has been dismissed is the assumption that effective antibody must neutralize virus and prevent its attachment to host cells. However, the Fc region of an antibody can activate a variety of anti-viral effector functions {Huber, 2006 #106;Leopold, 2006 #187;Palmer, 2000 #76;Hober, 2001 #75;Chehadeh, 2005 #211;Muckelbauer, 1995 #214;Muckelbauer, 1997 #213}. In fact, non-neutralizing anti-influenza antibodies, including anti-NP, have been shown to induce complement-mediated cytolysis (21), increased T cell responses associated with enhanced dendritic cell function (20), and reduced viral replication in culture (39). Therefore, NP-immune antibody in our model may be promoting early viral clearance through some combination of these mechanisms.
Influenza NP is internal to the virion and internal to influenza-infected cells {j gen virol 83:723}. Thus, it is not readily apparent how anti-NP antibodies in vaccinated mice would encounter this antigen. Nonetheless, anti-NP antibodies are eventually generated during natural influenza virus infection (18, 19), indicating that this antigen is somehow exposed to the humoral immune system. This exposure may be via NP released from dying infected cells (49) or by its expression on the plasma membrane (50, 51). Therefore, interaction of vaccine-induced anti-NP antibodies with this NP early in the infection likely triggers downstream effector mechanisms that blunt virus replication, slow progression of the infection, and reduce morbidity.
Our data clearly demonstrate that anti-NP antibodies are essential for rNP-derived protection, and that rNP-immune serum can convey this protection to naïve recipients. Importantly, these results show that a humoral immune response to a single, conserved, internal protein of influenza virus makes a significant contribution to protection. This information greatly enhances our understanding of how current influenza vaccines could be improved to provide cross-protective immunity in humans. If long-lived, this cross-protection would considerably reduce the effort and cost of providing annual immunizations, the cost of caring for infected individuals, and possibly provide a level of population immunity that would curtail the spread of pandemic influenza.
MATERIALS AND METHODS
Protein production and purification
NP protein was produced and purified as previously described {Rangel-Moreno, 2008 #183}. Briefly, the NP gene of influenza A/PR8/34 was cloned into the pTricHis2c plasmid for expression in E. coli. 6X His-tagged rNP protein was affinity-purified via the ProBond purification system (Invitrogen). Resulting protein was dialyzed into PBS and sterile-filtered.
Mice and procedures
All mice were on the C57BL/6 background and were bred and maintained at the Trudeau Institute. C57BL/6, B6.129P2-Cd40tm1Kik/J (CD40−/−), and B cell deficient B6.129S2-Igh-6tm1Cgn/J (μMT) mice were obtained from the Jackson Laboratory. AID−/− mice were obtained from Dr. Rachael Gerstein at the University of Massachusetts. Mice lacking the secretory exon of IgM (μS −/− mice) were obtained from Dr. Ronald Corley at Boston University. Aid −/− and μS −/− mice were intercrossed to generate antibody-deficient AID/μS mice. Mice were immunized i.p. with combinations of 20 μg LPS ± 30 μg rNP at days 0 and 10. For influenza infections, mice were anaesthetized with isofluorane USP (Webster Veterinary) and 500 EIU (0.2 LD50) influenza PR8 were administered intranasally in 100 μl sterile PBS. All procedures involving live animals were approved by the Trudeau Institute Institutional Animal Care and Use Committee, and were performed in accordance with guidelines set by the National Research Council.
Virus foci assay
Madin-Darby Canine Kidney cells were grown in 96-well, flat-bottom plates until just confluent and then washed with HBSS. Homogenized lung samples were diluted in Zero Serum Media (Diagnostic Hybrids) supplemented with 4 μg/ml trypsin and applied to washed Madin Darby Canine Kidney cells. Plates were centrifuged for 1.5 h at 800 ×g, washed, and cultured overnight in Zero Serum Media/trypsin at 33°C. The medium was removed, and the cells were fixed with 80% acetone and allowed to dry. The wells were rehydrated with PBS, containing 2% FBS and 0.01% NaN3, and probed with mouse anti-influenza A antibody (Chemicon International). The primary antibody was detected with biotinylated goat anti-mouse IgG (Chemicon International) followed by alkaline phosphatase-conjugated streptavidin (DakoCytomation). Viral foci were developed by incubating for 30 min with 5-bromo-4-chloro-3-indolyl phosphate and Nitro Blue Tetrazolium tablets (Sigma Fast BCIP/NBT from Sigma-Aldrich) dissolved in H2O. The resulting foci were counted under a dissecting microscope. Data were analyzed for significance by Student’s t test.
Flow cytometry
Mice were sacrificed at the indicated times after infection, tissues were removed and mechanically disrupted by passage through wire mesh. Resultant cell suspensions were RBC-lysed and mesh-filtered. Cells were incubated in 3% FBS in PBS containing 10 sg/ml 2.4G2 to block Fc receptor binding, followed by staining with fluorochrome-conjugated CD8, CD62L (BD Biosciences), and MHC Class I tetramers presenting NP366–374 or PA224–233 peptides (Trudeau Institute Molecular Biology Core Facility). Samples were analyzed with a FacsCalibur flow cytometer (BectonDickinson).
Serum collection and ELISAs
Peripheral blood was obtained from either euthanized mice by severing the renal artery and pipetting into a 1.5-ml tube or from live mice via the lateral tail vein. After clotting for 30 min at 37°C, the precipitate was pelleted in a microcentrifuge, and the serum was collected. NP-specific ELISAs were performed by coating plates with 2 μg/ml rNP. Serum samples were diluted in 3-fold serial dilutions in PBS with 10 μg/ml BSA and 0.1% Tween 20 before incubation on coated plates. Bound antibody was detected with HRP-conjugated goat anti-mouse IgM or goat anti-mouse IgG (Southern Biotechnology Associates).
Acknowledgments
The authors thank Drs. Rachel Gerstein and Ronald Corley for providing mutant mice and Drs. Frances Lund, Ravi Misra, and Javier Rangel-Moreno for helpful discussions and for reading the manuscript. Supported by NIH AI061511 and AI072689 to T.D. Randall, and by the Trudeau Institute. The authors have no conflicting financial interests.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. Jama. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69:2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser J. A one-size-fits-all flu vaccine? Science. 2006;312:380–382. doi: 10.1126/science.312.5772.380. [DOI] [PubMed] [Google Scholar]
- 6.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194(Suppl 2):S111–118. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- 7.Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Wraith DC, Vessey AE, Askonas BA. Purified influenza virus nucleoprotein protects mice from lethal infection. J Gen Virol. 1987;68(Pt 2):433–440. doi: 10.1099/0022-1317-68-2-433. [DOI] [PubMed] [Google Scholar]
- 9.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 10.Endo A, Itamura S, Iinuma H, Funahashi S, Shida H, Koide F, Nerome K, Oya A. Homotypic and heterotypic protection against influenza virus infection in mice by recombinant vaccinia virus expressing the haemagglutinin or nucleoprotein of influenza virus. J Gen Virol. 1991;72(Pt 3):699–703. doi: 10.1099/0022-1317-72-3-699. [DOI] [PubMed] [Google Scholar]
- 11.Tamura S, Miyata K, Matsuo K, Asanuma H, Takahashi H, Nakajima K, Suzuki Y, Aizawa C, Kurata T. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J Immunol. 1996;156:3892–3900. [PubMed] [Google Scholar]
- 12.Tite JP, Hughes-Jenkins C, O’Callaghan D, Dougan G, Russell SM, Gao XM, Liew FY. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. II. Protection from influenza infection and mechanism of protection. Immunology. 1990;71:202–207. [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein SL, Stack A, Misplon JA, Lo CY, Mostowski H, Bennink J, Subbarao K. Vaccination with DNA encoding internal proteins of influenza virus does not require CD8(+) cytotoxic T lymphocytes: either CD4(+) or CD8(+) T cells can promote survival and recovery after challenge. Int Immunol. 2000;12:91–101. doi: 10.1093/intimm/12.1.91. [DOI] [PubMed] [Google Scholar]
- 14.Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, Nabel GJ. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23:5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 15.Ulmer JB, Fu TM, Deck RR, Friedman A, Guan L, DeWitt C, Liu X, Wang S, Liu MA, Donnelly JJ, Caulfield MJ. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998;72:5648–5653. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu TM, Guan L, Friedman A, Schofield TL, Ulmer JB, Liu MA, Donnelly JJ. Dose dependence of CTL precursor frequency induced by a DNA vaccine and correlation with protective immunity against influenza virus challenge. J Immunol. 1999;162:4163–4170. [PubMed] [Google Scholar]
- 17.Bender BS, Bell WE, Taylor S, Small PA., Jr Class I major histocompatibility complex-restricted cytotoxic T lymphocytes are not necessary for heterotypic immunity to influenza. J Infect Dis. 1994;170:1195–1200. doi: 10.1093/infdis/170.5.1195. [DOI] [PubMed] [Google Scholar]
- 18.Sukeno N, Otsuki Y, Konno J, Yamane N, Odagiri T, Arikawa J, Ishida N. Anti-nucleoprotein antibody response in influenza A infection. Tohoku J Exp Med. 1979;128:241–249. doi: 10.1620/tjem.128.241. [DOI] [PubMed] [Google Scholar]
- 19.Rangel-Moreno J, Carragher DM, Misra RS, Kusser K, Hartson L, Moquin A, Lund FE, Randall TD. B Cells Promote Resistance to Heterosubtypic Strains of Influenza via Multiple Mechanisms. J Immunol. 2008;180:454–463. doi: 10.4049/jimmunol.180.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng B, Zhang Y, He H, Marinova E, Switzer K, Wansley D, Mbawuike I, Han S. Rectification of age-associated deficiency in cytotoxic T cell response to influenza a virus by immunization with immune complexes. J Immunol. 2007;179:6153–6159. doi: 10.4049/jimmunol.179.9.6153. [DOI] [PubMed] [Google Scholar]
- 21.Yewdell JW, Frank E, Gerhard W. Expression of influenza A virus antigens on the surface of infected P815 cells. J Immunol. 1981;126:1814–1819. [PubMed] [Google Scholar]
- 22.Huber M, Fischer M, Misselwitz B, Manrique A, Kuster H, Niederost B, Weber R, von Wyl V, Gunthard HF, Trkola A. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 2006;3:e441. doi: 10.1371/journal.pmed.0030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chehadeh W, Bouzidi A, Alm G, Wattre P, Hober D. Human antibodies isolated from plasma by affinity chromatography increase the coxsackievirus B4-induced synthesis of interferon-alpha by human peripheral blood mononuclear cells in vitro. J Gen Virol. 2001;82:1899–1907. doi: 10.1099/0022-1317-82-8-1899. [DOI] [PubMed] [Google Scholar]
- 24.Palmer P, Charley B, Rombaut B, Daeron M, Lebon P. Antibody-dependent induction of type I interferons by poliovirus in human mononuclear blood cells requires the type II fcgamma receptor (CD32) Virology. 2000;278:86–94. doi: 10.1006/viro.2000.0627. [DOI] [PubMed] [Google Scholar]
- 25.Hober D, Chehadeh W, Bouzidi A, Wattre P. Antibody-dependent enhancement of coxsackievirus B4 infectivity of human peripheral blood mononuclear cells results in increased interferon-alpha synthesis. J Infect Dis. 2001;184:1098–1108. doi: 10.1086/323801. [DOI] [PubMed] [Google Scholar]
- 26.Scherle PA, Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986;164:1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scherle PA, Gerhard W. Differential ability of B cells specific for external vs. internal influenza virus proteins to respond to help from influenza virus-specific T-cell clones in vivo. Proc Natl Acad Sci U S A. 1988;85:4446–4450. doi: 10.1073/pnas.85.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein SL, Lo CY, Misplon JA, Lawson CM, Hendrickson BA, Max EE, Subbarao K. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain-deficient mice. J Immunol. 1997;158:1222–1230. [PubMed] [Google Scholar]
- 29.Sealy R, Surman S, Hurwitz JL, Coleclough C. Antibody response to influenza infection of mice: different patterns for glycoprotein and nucleocapsid antigens. Immunology. 2003;108:431–439. doi: 10.1046/j.1365-2567.2003.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall D, Sealy R, Sangster M, Coleclough C. TH cells primed during influenza virus infection provide help for qualitatively distinct antibody responses to subsequent immunization. J Immunol. 1999;163:4673–4682. [PubMed] [Google Scholar]
- 31.Cox RJ, Mykkeltvedt E, Robertson J, Haaheim LR. Non-lethal viral challenge of influenza haemagglutinin and nucleoprotein DNA vaccinated mice results in reduced viral replication. Scand J Immunol. 2002;55:14–23. doi: 10.1046/j.1365-3083.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 32.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 33.Altstein AD, Gitelman AK, Smirnov YA, Piskareva LM, Zakharova LG, Pashvykina GV, Shmarov MM, Zhirnov OP, Varich NP, Ilyinskii PO, Shneider AM. Immunization with influenza A NP-expressing vaccinia virus recombinant protects mice against experimental infection with human and avian influenza viruses. Arch Virol. 2006;151:921–931. doi: 10.1007/s00705-005-0676-9. [DOI] [PubMed] [Google Scholar]
- 34.Saha S, Yoshida S, Ohba K, Matsui K, Matsuda T, Takeshita F, Umeda K, Tamura Y, Okuda K, Klinman D, Xin KQ. A fused gene of nucleoprotein (NP) and herpes simplex virus genes (VP22) induces highly protective immunity against different subtypes of influenza virus. Virology. 2006;354:48–57. doi: 10.1016/j.virol.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Scheepers K, Becht H. Protection of mice against an influenza virus infection by oral vaccination with viral nucleoprotein incorporated into immunostimulating complexes. Med Microbiol Immunol. 1994;183:265–278. doi: 10.1007/BF00198460. [DOI] [PubMed] [Google Scholar]
- 36.Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, Lund FE, Randall TD. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 37.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen HH, van Ginkel FW, Vu HL, McGhee JR, Mestecky J. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J Infect Dis. 2001;183:368–376. doi: 10.1086/318084. [DOI] [PubMed] [Google Scholar]
- 39.Sambhara S, Kurichh A, Miranda R, Tumpey T, Rowe T, Renshaw M, Arpino R, Tamane A, Kandil A, James O, Underdown B, Klein M, Katz J, Burt D. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol. 2001;211:143–153. doi: 10.1006/cimm.2001.1835. [DOI] [PubMed] [Google Scholar]
- 40.Leopold PL, Wendland RL, Vincent T, Crystal RG. Neutralized adenovirus-immune complexes can mediate effective gene transfer via an Fc receptor-dependent infection pathway. J Virol. 2006;80:10237–10247. doi: 10.1128/JVI.00512-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 42.Chehadeh W, Lobert PE, Sauter P, Goffard A, Lucas B, Weill J, Vantyghem MC, Alm G, Pigny P, Hober D. Viral protein VP4 is a target of human antibodies enhancing coxsackievirus B4- and B3-induced synthesis of alpha interferon. J Virol. 2005;79:13882–13891. doi: 10.1128/JVI.79.22.13882-13891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishiko H, Shimada Y, Yonaha M, Hashimoto O, Hayashi A, Sakae K, Takeda N. Molecular diagnosis of human enteroviruses by phylogeny-based classification by use of the VP4 sequence. J Infect Dis. 2002;185:744–754. doi: 10.1086/339298. [DOI] [PubMed] [Google Scholar]
- 44.Muckelbauer JK, Kremer M, Minor I, Tong L, Zlotnick A, Johnson JE, Rossmann MG. Structure determination of coxsackievirus B3 to 3.5 A resolution. Acta Crystallogr D Biol Crystallogr. 1995;51:871–887. doi: 10.1107/S0907444995002253. [DOI] [PubMed] [Google Scholar]
- 45.Muckelbauer JK, Rossmann MG. The structure of coxsackievirus B3. Curr Top Microbiol Immunol. 1997;223:191–208. doi: 10.1007/978-3-642-60687-8_9. [DOI] [PubMed] [Google Scholar]
- 46.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas PG, Brown SA, Yue W, So J, Webby RJ, Doherty PC. An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. Proc Natl Acad Sci U S A. 2006;103:2764–2769. doi: 10.1073/pnas.0511185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinshaw VS, Olsen CW, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virelizier JL, Allison AC, Oxford JS, Schild GC. Early presence of ribonucleoprotein antigen on surface of influenza virus-infected cells. Nature. 1977;266:52–54. doi: 10.1038/266052a0. [DOI] [PubMed] [Google Scholar]
- 51.Stitz L, Schmitz C, Binder D, Zinkernagel R, Paoletti E, Becht H. Characterization and immunological properties of influenza A virus nucleoprotein (NP): cell-associated NP isolated from infected cells or viral NP expressed by vaccinia recombinant virus do not confer protection. J Gen Virol. 1990;71(Pt 5):1169–1179. doi: 10.1099/0022-1317-71-5-1169. [DOI] [PubMed] [Google Scholar]