Abstract
In immunogold double-labeling of pea leaf thin sections with antibodies raised against ferredoxin-NADP reductase (EC 1.18.1.2, FNR) and antibodies directed against the A or B subunits of the NADP-linked glyceraldehyde-3-P dehydrogenase (GAPD) (EC 1.2.1.13), many small and large gold particles were found together over the chloroplasts. Nearest neighbor analysis of the distribution of the gold particles indicates that FNR and the NADP-linked GAPD are co-localized, in situ. This suggests that FNR might carry FADH2 or NADPH from the thylakoid membrane to GAPD, or that ferredoxin might carry electrons to FNR co-localized with GAPD in the stroma. Crystal structures of the spinach enzymes are available. When they are docked computationally, the proteins appear, as modeled, to be able to form at least two different complexes. One involves a single GAPD monomer and an FNR monomer (or dimer). The amino acid residues located at the putative interface are highly conserved on the chloroplastic forms of both enzymes. The other potential complex involves the GAPD A2B2 tetramer and an FNR monomer (or dimer). The interface residues are conserved in this model as well. Ferredoxin is able to interact with FNR in either complex.
Keywords: Co-localization, Ferredoxin-NADP reductase, Glyceraldehyde-3-P dehydrogenase, Nearest neighbor analysis, Photosynthetic CO2-fixation, Pisum sativum
1. Introduction
Ferredoxin-NADP reductase (EC 1.18.1.2, FNR) transfers electrons from the 1 e− donor ferredoxin to the 2 e− acceptor NADP+. The product, NADPH, is used by the Calvin cycle NADP-linked glyceraldehyde-3-P dehydrogenase (GAPD) (EC 1.2.1.13) and by other chloroplast enzymes. In 1993 Süss and coworkers (1993) identified both GAPD and FNR as components of a multienzyme fraction isolated from spinach chloroplasts, and suggested that they might interact. Consistent with those results, Srivastava et al. (2005) found both enzymes associated with the thylakoid membrane, apparently as peripheral proteins, in a proteomic investigation of the cyanobacterium Synechocystis, and Dani and Sainis (2005) found the two enzymes and all of the components of the electron transport system, except ferredoxin, in a complex isolated from Anacystis nidulans. The purpose of the present experiments was to determine whether FNR is co-localized with GAPD in situ in pea leaf chloroplasts, and whether the two enzymes can be docked, computationally, which would then suggest that direct transfer of NADPH from FNR to GAPD is feasible and might occur in vivo.
2. Methodology
2.1. Plant material
Pea (Pisum sativum L., var Little Marvel) plants were grown from seed in the University of Illinois at Chicago greenhouse as described previously (Anderson et al., 1995a). Seeds were purchased from Old's Seed Company, Madison, WI.
2.2. Antibodies
The anti-spinach FNR antibody (gift of Richard Malkin, University of California, Berkeley) was raised in rabbits against the isolated protein. The antibodies appeared to be monospecific (not shown). The apparent molecular mass of the antigen recognized by the anti-FNR antibody was 34 kDa on blots of both stroma and thylakoid proteins. The known molecular mass is 35 kDa. The anti-pea chloroplast GAPD subunit A and subunit B antibodies (provided by Bethyl Laboratories, Montgomery, TX) were raised against peptides representing unique regions of the chloroplast isozymes in sheep and chickens, respectively, and were affinity purified against the immunizing peptides. For details see Anderson et al. (2003). Immunoblots of chloroplast proteins probed with the anti-GAPD subunit A and B antibodies have been published. There was a trace of a second smaller stromal antigen, possibly a breakdown product, seen on the immunoblot of the stromal extract with the anti-GAPD subunit A antibody.
2.3. Fixation and immunolabeling
Thin sections were cut from pea leaf tissue that had been fixed in 1% acrolein, 0.1% glutaraldehyde and embedded in LR White resin, and were immunolabeled, as described previously (Anderson et al., 1995b, 2003). The grids were floated on solution containing both primary antibodies overnight. Exposure to the secondary antibodies was for 4 h the following morning. Details of the labeling experiments are given in Table 1. The secondary antibodies were immunogold-labeled IgG's obtained from Ted Pella, Inc., Redding, CA, and Electron Microscopy Sciences, Fort Washington, PA. Normal serum from the species used to elicit the secondary antibody was used in all of the blocking solutions.
Table 1.
Details of labeling
| Primary antibody pair | Figure | Primary antibody, dilution or concentration | Secondary antibodya | Gold particle size (nm) |
|---|---|---|---|---|
| R > FNR | 1 and 2a | 1 → 250 | D > R | 10 |
| S > GAPD A | 500 μg/ml | D > S | 20 | |
| 2b | 1 → 50 | D > R | 18 | |
| 100 μg/ml | D > S | 10 | ||
| R > FNR | 3a | 1 → 50 | G > R | 20 |
| Ck > GAPD B | 2.4 μg/ml | G > Ck | 10 | |
| 3b | 1 → 50 | G > R | 10 | |
| 2.4 μg/ml | G > Ck | 25 |
Ck, chicken; D, donkey, G, goat; R, rabbit; S, sheep.
2.4. Nearest neighbor analysis
We used the method of Anderson et al. (2003) for analysis of nearest neighbor distances on the micrographs from the double-labeling experiments. Briefly, for a population of two different non-interacting species the expression n/N = 1 - exp(−πr2ρ) gives the fraction n/N corresponding to position in an ordered list of samples with increasing nearest neighbor distance r, where n is the number of the measurement in rank order, N is the total number of measurements, r is the distance between nearest neighbors, and ρ is the species density (Anderson et al., 2003). A plot of -ln(1 − n/N) versus r2 produces a straight line, if the two species are distributed randomly. Where there is positive interaction the initial data points will be displaced towards the -ln(1 − n/N) axis and the curve will be biphasic. We measured the distance from the center of each large gold particle to the center of the nearest small gold particle using Scion Image (Scion Corporation, Frederick, MD) and plotted -ln(1 − n/N) against r2. Non-specific labeling will simply add to the data as a non-interacting species, and need not be corrected for.
2.5. Alignments
We used Clustal W (Thompson et al., 1994) to align the sequences of all available chloroplast NADP-linked glyceraldehyde-3-P dehydrogenases and FNR sequences. A conserved surface patch on FNR that corresponds roughly to the interaction surface, as modeled, was identified using the evolutionary trace report maker of Mihalek et al. 2006.
2.6. Model building
The structures of spinach GAPD A (PDB 1jn0) (Fermani et al., 2001) and FNR (PDB 1fnb) (Bruns and Karplus, 1995) were docked using FTDOCK (Gabb et al., 1997) and the best docked structures were selected and analyzed using InterProSurf (Negi et al., 2006). Taking into account that GAPD also occurs as a tetramer, we generated the docked structure of the spinach GAPD A2B2 tetramer (PDB 2pkq) (Fermani et al., 2007) with (a) FNR as a monomer, (b) FNR as a dimer, and (c) FNR complexed with ferredoxin. The best docked structures were selected using rpscore in FTDOCK. The final docked structures were energy minimized using NAMD (Phillips et al., 2005).
3. Results
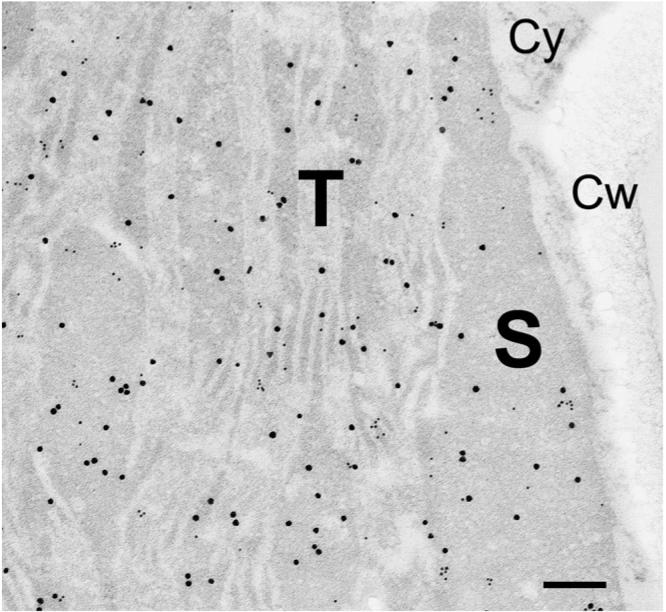
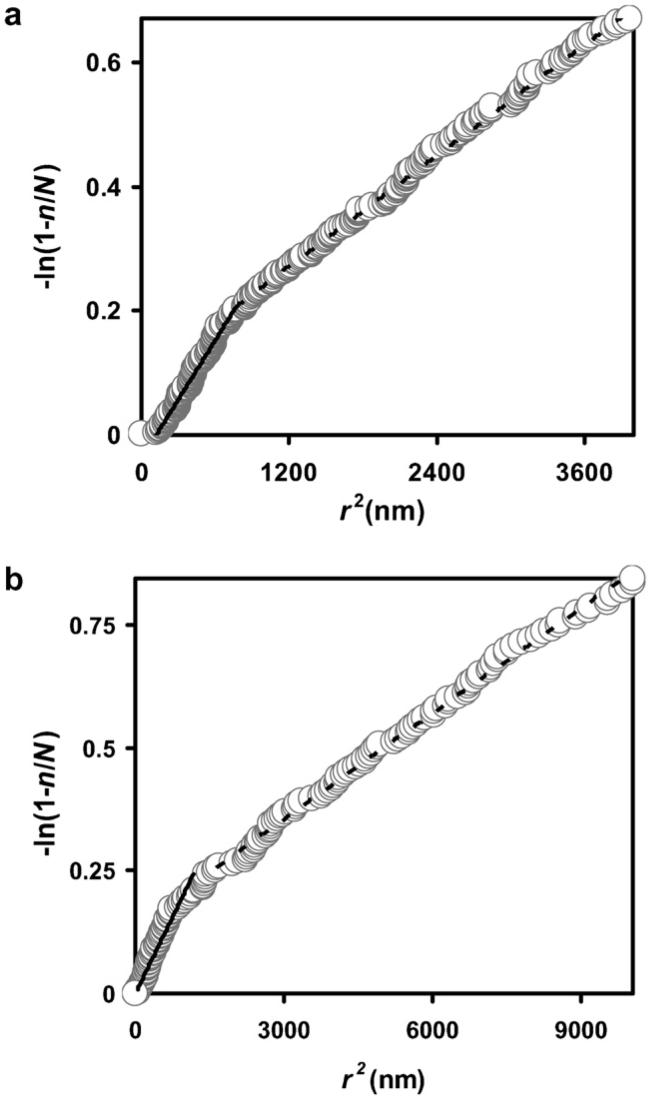
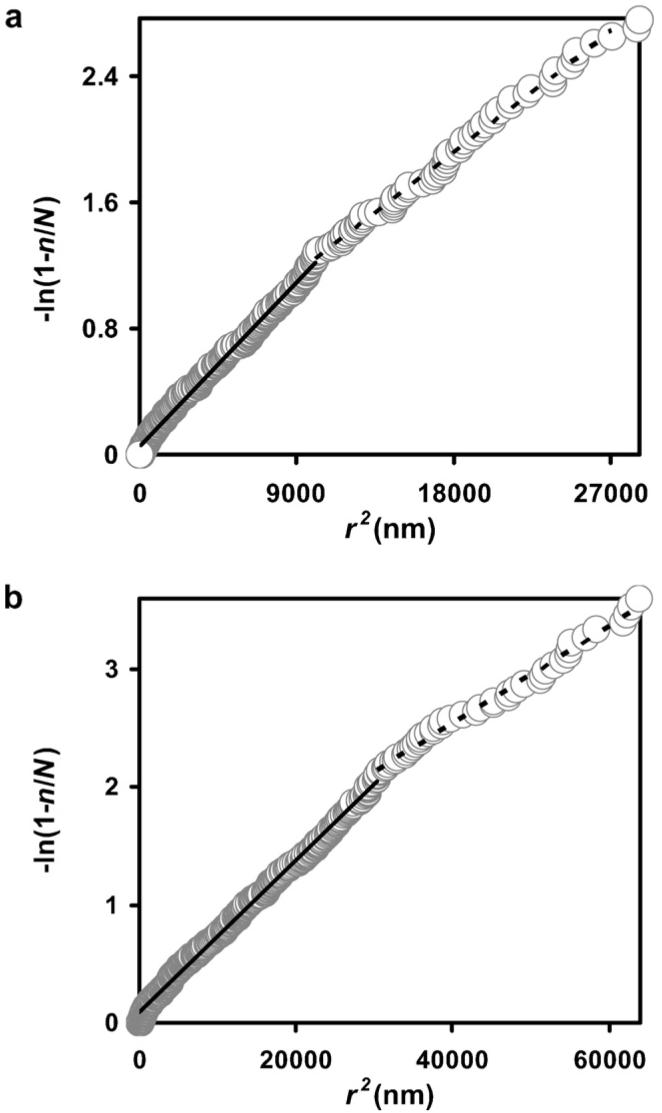
Fig. 1 shows a micrograph of a portion of a chloroplast in a leaf section doubly labeled with antibodies directed against GAPD subunit A and antibodies raised against FNR. There are many small and large gold particles occurring together, suggesting that the proteins might interact. When we measured nearest neighbor distances from gold particles marking GAPD subunit A to the gold particles marking FNR and plotted -ln(1 − n/N) against r2, the data did not fit a straight line (Fig. 2a). The curve is biphasic. At low values of r the data points climb the -ln(1 − n/N) axis. These data points represent the co-localized proteins. At higher values of r the data points describe a straight line with a less steep slope. This portion of the curve represents the protein molecules that are distributed randomly with respect to one another; they are not co-localized with the detected nearest neighbor. (Note that not all of the antigen molecules will be detected.) Similar results were found when the particle sizes were reversed (Fig. 2b). These experiments indicate that FNR is co-localized with subunit A of GAPD in the pea leaf chloroplast. Likewise, the B subunit of GAPD was distributed non-randomly with respect to FNR (Fig. 3a and b). Apparently part of the FNR population in these chloroplasts is located near GAPD A and GAPD B. The non-random distribution implies co-localization, but the enzymes are not necessarily adjacent to one another, and co-localized enzymes do not necessarily form a complex. Because they are co-localized, and because the product of one is the substrate for the other, there is a possibility that the enzymes might form a productive complex.
Fig. 1.
Micrograph showing a portion of a chloroplast in a pea leaf section doubly labeled with antibodies directed against GAPD (20 nm particles) and with antibodies raised against FNR (10 nm particles). Bar = 200 nm. S, stroma; T, thylakoid; Cy, cytosol; Cw, cell wall. The maximum possible distance between the centers of two gold particles marking GAPD and FNR molecules that are in direct contact with one another would be about 86 nm (diameter of the two protein molecules, four IgG molecules and the radii of the two gold particles). Note high incidence of particles marking FNR over regions of the stroma distant from the thylakoid membranes.
Fig. 2.
(a) Plot of the negative log of 1 - fraction in the ordered list of measurements against the square of the distance between nearest neighbor gold particles marking GAPD subunit A and gold particles marking FNR, from the experiment in Fig. 1. There were 555 measurements. The first 271 data points are shown. 104 of the 555 gold particles marking GAPD A are non-randomly distributed with respect to gold particles marking FNR. 392 of the 555 particles marking GAPD A would be close enough to a particle marking FNR to represent adjacent proteins, if one assumes that the four antibodies and two antigens are arranged in linear fashion (i.e. maximum distance separating the two gold particles marking adjacent antigens). (b) Plot of the negative log of 1 - fraction in the ordered list of measurements against the square of the distance between nearest neighbor gold particles when the particle sizes were reversed. There were 284 measurements. The first 162 data points are shown. Fifty five of the 284 gold particles marking FNR are non-randomly distributed with respect to gold particles marking GAPD A, and 129 of the 284 particles would be close enough to the gold particles marking GAPD to indicate adjacent antigens (on the basis of the maximum possible distance separating the gold particles). In an earlier experiment (Anderson et al., 2003) antigens recognized by preimmune rabbit and sheep antisera were distributed randomly with respect to one another.
Fig. 3.
(a) Plot of the negative log of 1 - fraction in the ordered list of measurements against the square of the distance between nearest neighbor gold particles marking GAPD subunit B and gold particles marking FNR. There were 286 measurements. The first 268 data points are shown. Two hundred and seven of the 286 gold particles marking FNR are non-randomly distributed with respect to gold particles marking GAPD B, and 155 of the 286 would be close enough to the gold particles marking GAPD B to indicate adjacent antigens, assuming complete linearity of the antigen-antibody complexes. (b) Plot of the negative log of 1 - fraction in the ordered list of measurements against the square of the distance between nearest neighbor gold particles when the particle sizes and relative antibody concentrations were reversed. There were 477 measurements. The first 464 data points are shown. Four hundred and nineteen of the 477 gold particles marking GAPD B are non-randomly distributed with respect to gold particles marking FNR, and 197 of the 477 would be close enough to the gold particles marking FNR to indicate adjacent antigens, assuming complete linearity of the antibody-antigen complexes.
The structures of spinach GAPD A (PDB 1jn0) and FNR (PDB 1fnb) were docked. In the first docked structure, which involves a single GAPD subunit and FNR (Fig. 4a-c), the active sites are located close to one another, on opposite sides of the area of interaction. FNR can dimerize with a second FNR molecule and ferredoxin can dock to FNR (Fig. 4d). Inspection of the minimized structure reveals a series of arginine and lysine residues leading from one active site to the other, namely R191, R194 and R195 in GAPD and K116 and R117 in FNR (Fig. 5). The corresponding residues are arginines in all chloroplastic NADP-linked GAPD's for which sequence information is available (not shown). The residue corresponding to R117 is an arginine in all of the FNR's for which sequence information is available. In some species the residue corresponding to K116 is an arginine.
Fig. 4.
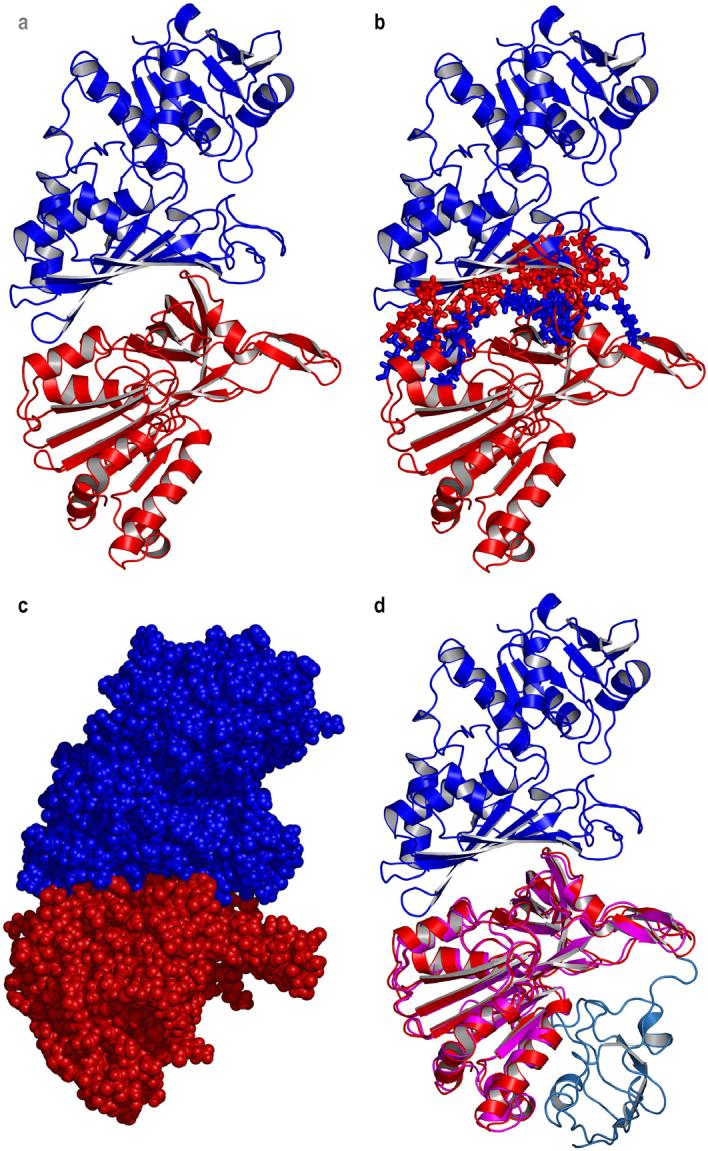
(a) Cartoon of the docked structures, based on PDB files 1jn0 (spinach GAPD) and 1fnb (spinach FNR). GAPD is shown in blue and FNR in red. The active sites are located on opposite sides of the area of interaction. (b) Interface residues, shown as red sticks for GAPD, and blue sticks for FNR. (c) Space filling model of the docked structures. (d) Cartoon of the docked GAPD and FNR structures overlaid on the structure of the maize FNR (pink), ferredoxin (light blue-green) complex in PDB file 1gaq. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Fig. 5.
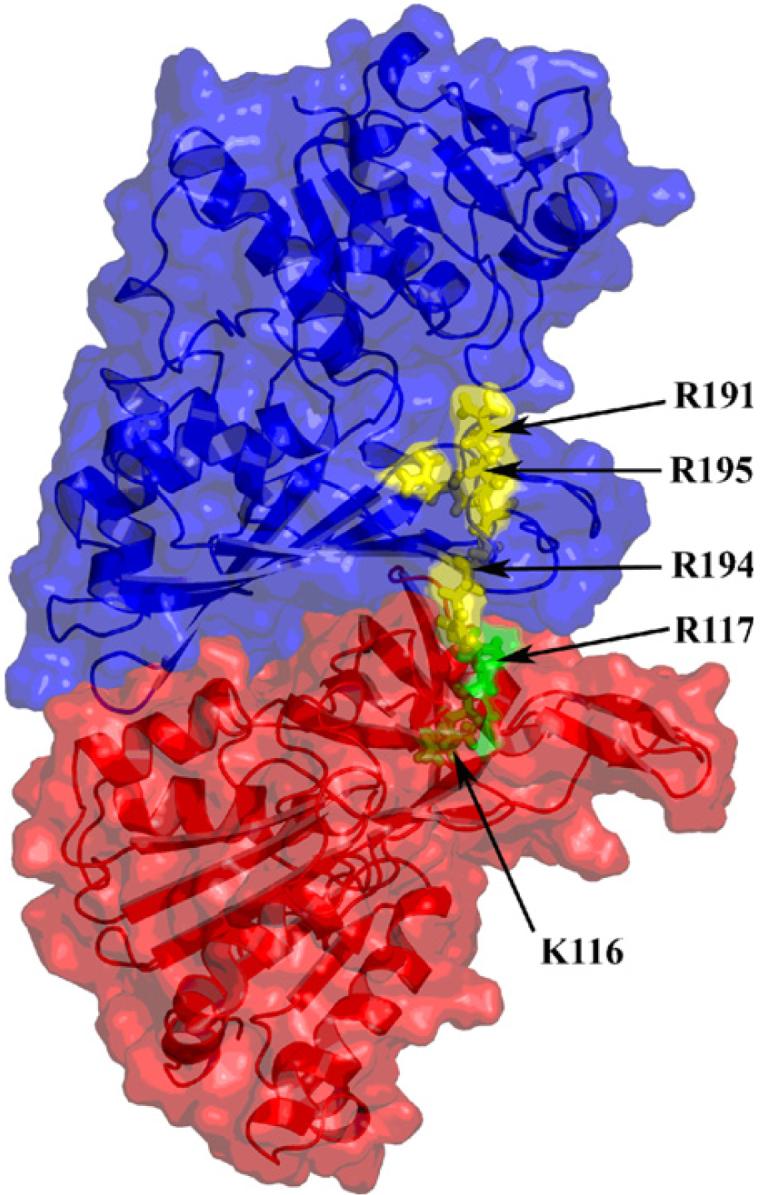
Basic residues connecting the FNR active site (in green, residues K116 and R117) and the GAPD active site (in yellow, residues R191, R194, and R195). NAPD binds into the active site cleft above and opposite R191. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
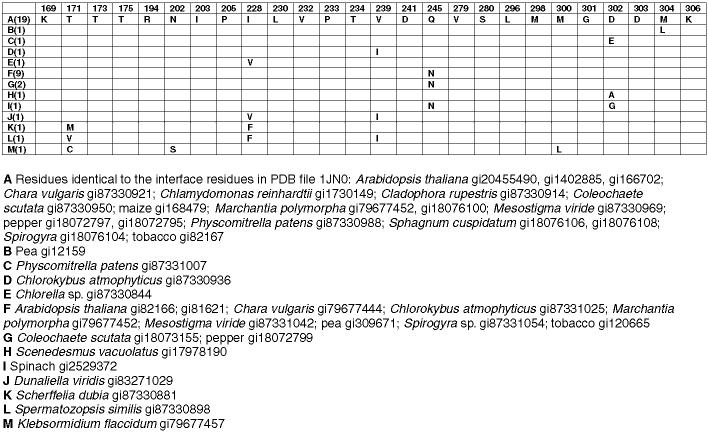
We aligned all of the available sequences of plant FNRs and chloroplastic GAPDs. FNR occurs in plastids in roots, embryos, and green leaves. The residues located at the interface in the model in Fig. 4 are completely conserved in 16 angiosperm chloroplastic FNRs including the enzymes from 13 dicots and 3 monocots (Fig. 6). Substitutions (one conservative, one non-conservative) were found in two monocot chloroplastic enzymes. The interface residues were not conserved in the enzymes from algae, in the embryo enzymes, or, with two exceptions, in the enzymes designated as root FNRs. In the case of the chloroplastic NADP-linked GAPD the interface residues are also involved in the subunit interface in the GAPD tetramer. They are conserved in all eukaryotic species for which sequences are available (Fig. 7).
Fig. 6.
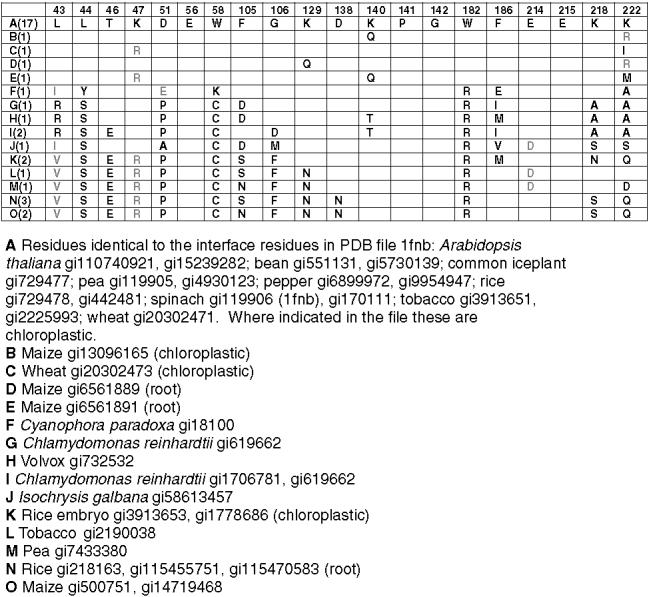
Conserved residues at putative interface in FNR in the model in Fig. 4. Number of sequences containing indicated residues in parentheses in left column. Completely redundant sequences within species not included. Conservative substitutions in gray. Only substitutions are shown.
Fig. 7.
Residues corresponding to the residues in spinach chloroplast GAPD at the interface in the putative GAPD-FNR complex in the model in Fig. 4. Number of sequences containing indicated residues in parentheses in left column. Completely redundant sequences within species not included. Conservative substitutions in gray. Only substitutions are shown.
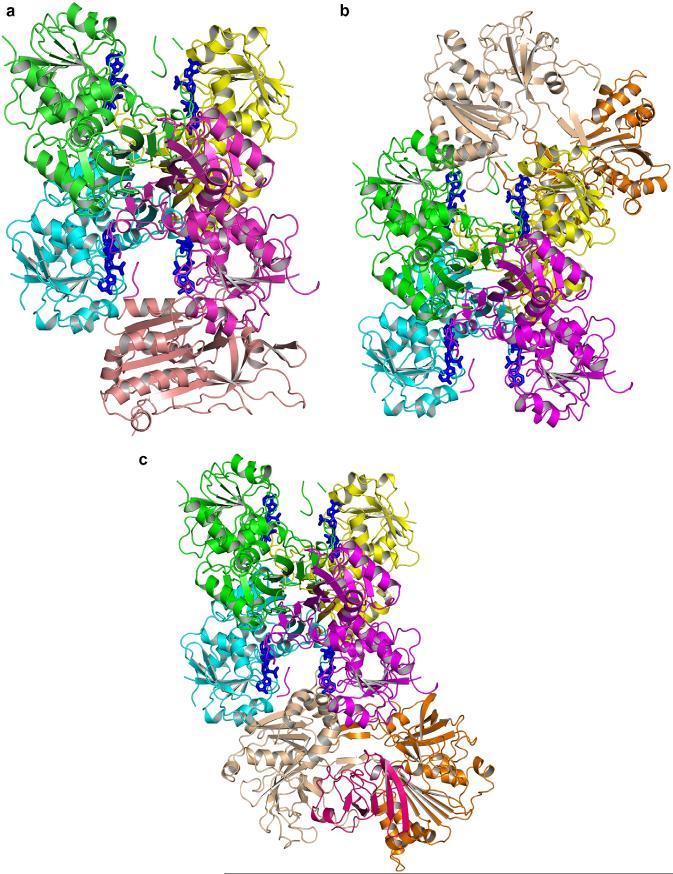
A second possible model was found when the GAPD tetramer and the FNR monomer were docked (Fig. 8a). This model will accommodate two FNR monomers on opposite sides of the GAPD tetramer and FNR dimers (Fig. 8b). Ferredoxin can dock to the FNR molecules (Fig. 8c). As in the first model, there are charged residues positioned to facilitate transfer of pyridine nucleotide between the active sites. Here the pyridine nucleotide is almost caged between the two enzymes. Many of the interface residues are conserved in angiosperm GAPDs and FNRs (Figs. 9-12).
Fig. 8.
(a) Cartoon showing the GAPD A2B2 tetramer (green, yellow, cyan, and magenta) docked to FNR (wheat). The structures are based on PDB files 1pkq and 1fnb. NADP in blue bound to GAPD. (b) Cartoon showing the GAPD A2B2 tetramer docked to an FNR dimer (wheat, orange). (c) Cartoon showing the GAPD A2B2 tetramer docked to the FNR (wheat), ferredoxin (pink) complex in PDB file 1ewy. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Fig. 9.
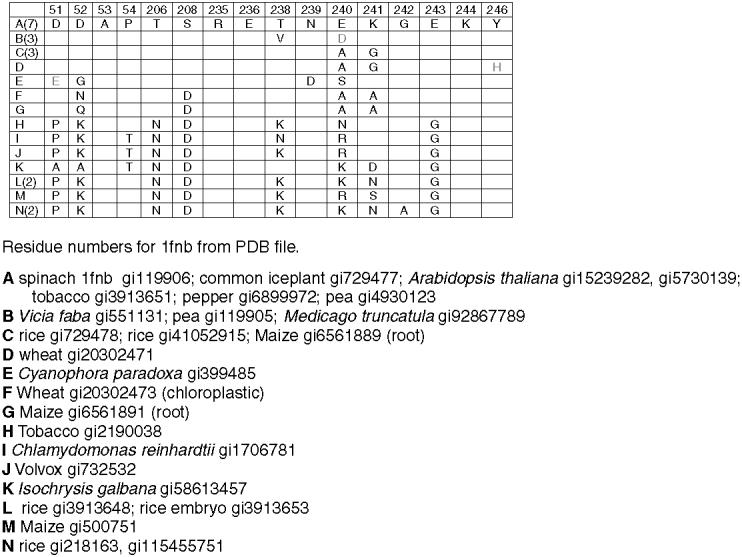
Conserved residues at putative interface with GAPD B subunit in FNR in the model in Fig. 8a. Number of sequences containing indicated residues in parentheses in left column. Completely redundant sequences within species not included. Conservative substitutions in gray. Only substitutions are shown.
Fig. 12.
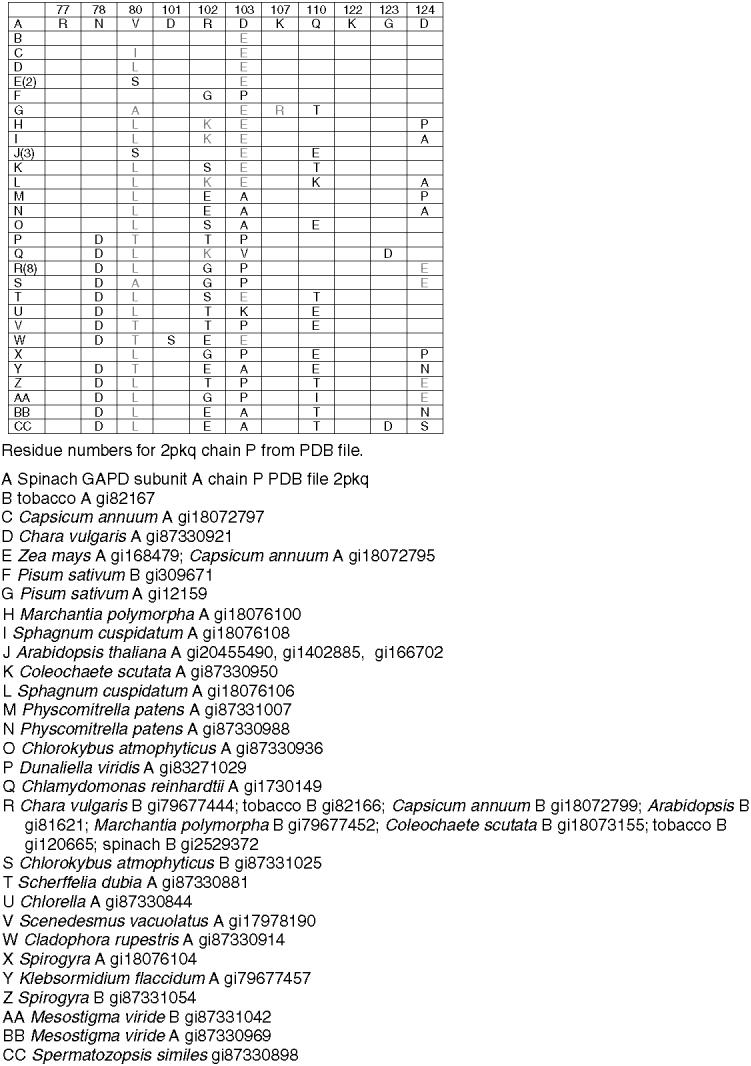
Residues corresponding to the residues in spinach chloroplast GAPD A at the interface with FNR in the putative GAPD-FNR complex in Fig. 8a. Number of sequences containing indicated residues in parentheses in left column. Completely redundant sequences within species not included. Conservative substitutions in gray. Only substitutions are shown.
4. Discussion
Higher plant chloroplastic GAPDs are tetramers composed of very similar A and B subunits. The B subunit contains two redox-sensitive cysteine residues in a C-terminal extension (Qi et al., 2001; Sparla et al., 2002; Fermani et al., 2007). Both subunits appear to be co-localized with FNR in the chloroplasts in these pea leaf sections (Figs. 1-3). Both subunits are also co-localized with the Calvin cycle enzymes P-glycerate kinase (Anderson et al., 2003), triose-P isomerase, and aldolase (Anderson et al., 2005), but only the B subunit appears to be co-localized with transketolase (Anderson et al., 2006). Both subunits are co-localized with thioredoxin m, but only the A subunit is co-localized with thioredoxin f (Anderson and Carol, unpublished). The Chlamydomonas A4 enzyme forms a complex with phosphoribulokinase and CP12 and a low resolution structure based on cryoelectron imaging is available (Mouche et al., 2002).
FNR occurs both free in the stroma (Okutani et al., 2005; Lintala et al., 2007) and docked to the thylakoid membrane (Onda and Hase, 2004; Okutani et al., 2005; Lintala et al., 2007). It has been identified as one of the proteins in the Arabidopsis thylakoid membrane proteome (Friso et al., 2004). There are apparently two different membrane docking sites (Matthijs et al., 1986), one of which is the cytochrome b6f complex, and it has been suggested that FNR participates in cyclic phosphorylation (Zhang et al., 2001). FNR also binds to Tic62 on the inner envelope membrane and may be involved in redox-regulation of protein import into the chloroplast (Küchler et al., 2002). We found many gold particles marking FNR over the thylakoid membranes, and over the stroma, far from the thylakoid membranes, in these thin sections. GAPD appears to be co-localized with FNR in both places (Fig. 1).
Consistent with the notion that these enzymes might interact, it was possible to build two different models of the potential GAPD-FNR complex (Figs. 4 and 8). The first involves a single GAPD monomer and FNR. Both GAPD and FNR are known to occur in complexes as monomers. A single monomer of the NAD-linked glyceraldehyde-3-P dehydrogenase is present in the mammalian Oct-1 CoActivator in S phase (OCA-S) complex that is involved in the activation of the histone H2B promoter (Zheng et al., 2003). A single FNR monomer is associated with the cytochrome b6f complex in the chloroplast thylakoid membrane (Zhang et al., 2001). Sedimentation velocity experiments indicate that FNR exists in solution as a monomer (Sheriff et al., 1980), and there is evidence that it exists in vivo as a dimer (Lintala et al., 2007). The nascent GAPD and FNR polypeptides that form the two enzymes are transported into the chloroplast as monomers. It is not unreasonable to suggest that the newly imported GAPD and FNR monomers might combine to form a GAPD-FNR functional heterodimer in the chloroplast. A second FNR molecule could combine with the FNR in the complex.
Consistent with the modeled interaction, the proposed interface residues in the chloroplastic FNR species are highly conserved in both monocots and dicots (Fig. 6). In the root and embryo forms of the enzyme, which would not be expected to form a complex with GAPD, only some of these residues are conserved. The subcellular location of the algal FNR species is not noted in the sequence files. The residues corresponding, as aligned, to the spinach enzyme interface residues are not as highly conserved as in the angiosperm chloroplastic enzymes, but conservation is greater than in the angiosperm root and embryo enzymes.
The GAPD residues located at the interface with FNR in the GAPD-FNR heterodimer model (Fig. 4) are the same residues present at the interface between the subunits in the GAPD tetramer. Not surprisingly, they are very highly conserved in the enzymes from green plants and algae (Fig. 7). In the plant and animal NAD-linked GAPDs there is a different set of highly conserved residues located at these same positions (not shown). It is reasonable to expect that in complexes where GAPD is present as a monomer that these residues and this highly conserved region could be involved in interaction with other protein species.
In the second model the GAPD A2B2 tetramer interacts with FNR (Fig. 8a). This complex could form after the GAPD subunits have oligomerized into a tetramer. The association with FNR might be transitory. This model, also, can accommodate FNR dimers (Fig. 8b). The interface residues are not as highly conserved as in the case of the GAPD monomer model (Figs. 9-12).
NADPH and NADP+ are negatively charged. The basic arginine and lysine residues connecting the two sites in the first model would provide a positively charged path for the transit of the pyridine nucleotides (Fig. 5). NADPH and NADP+ could be continually cycled back and forth between the two enzymes. In the second complex (Fig. 8) the pyridine nucleotide would be essentially trapped between the two enzymes with little opportunity to escape into the surrounding stroma. Here, too, there are positively charged residues on the surface of the complex between the active sites (not shown).
In either complex NADPH would be protected from other NADPH-utilizing enzymes, such as malate dehydrogenase, glutamate dehydrogenase, and the two reductases involved in fatty acid synthesis. The CO2-fixing Calvin cycle would have first call on the available reduced pyridine nucleotide, with only spill-over NADPH remaining for other stromal syntheses. FNR is inactivated by NADPH (Zanetti and Forti, 1966). Continual removal of NADPH by GAPD would reduce or eliminate that inactivation.
If NADPH is channeled from FNR to GAPD in the chloroplast, then either FNR carries FADH2 or NADPH from the thylakoid membrane to GAPD, or ferredoxin carries electrons from the thylakoid membrane to FNR co-localized with GAPD, in the stroma. Notably, there are crystal structures of ferredoxin docked with FNR (1EWY, Morales et al., 2000; 1GAQ, Kurisu et al., 2001). The ferredoxin interface region is distinct from the GAPD interface region. Either of the models proposed here can accommodate docking of ferredoxin to FNR, as in those structures (Figs. 4d and 8c). It seems possible that the three proteins might form a complex and that electrons might be transferred from ferredoxin to FNR, and from FNR (via NADPH) to GAPD, within that complex. Clearly, channeling of NADPH from FNR directly to GAPD, both in the stroma and at the thylakoid membrane, is a possibility. Interestingly, Cámara-Artigas and coworkers recovered, and were able to crystallize, spinach GAPD A from an immobilized ferredoxin column eluate (Cámara-Artigas et al., 2006). Since GAPD does not bind to ferredoxin, and FNR does bind to ferredoxin and has been recovered from similar columns, it seems possible that it was the GAPD-FNR complex that bound to the ferredoxin column.
Within the chloroplast, the NADP-linked GAPD is apparently co-localized with at least five Calvin cycle enzymes, and with thioredoxin and FNR (this paper). It seems possible that this same region might be involved in interaction with one or more of these enzymes, as well as with FNR, in the chloroplast.
Fig. 10.

Conserved residues at putative interface with GAPD A subunit in FNR in the model in Fig. 8a. Number of sequences containing indicated residues in parentheses in left column. Completely redundant sequences within species not included. Conservative substitutions in gray. Only substitutions are shown.
Fig. 11.
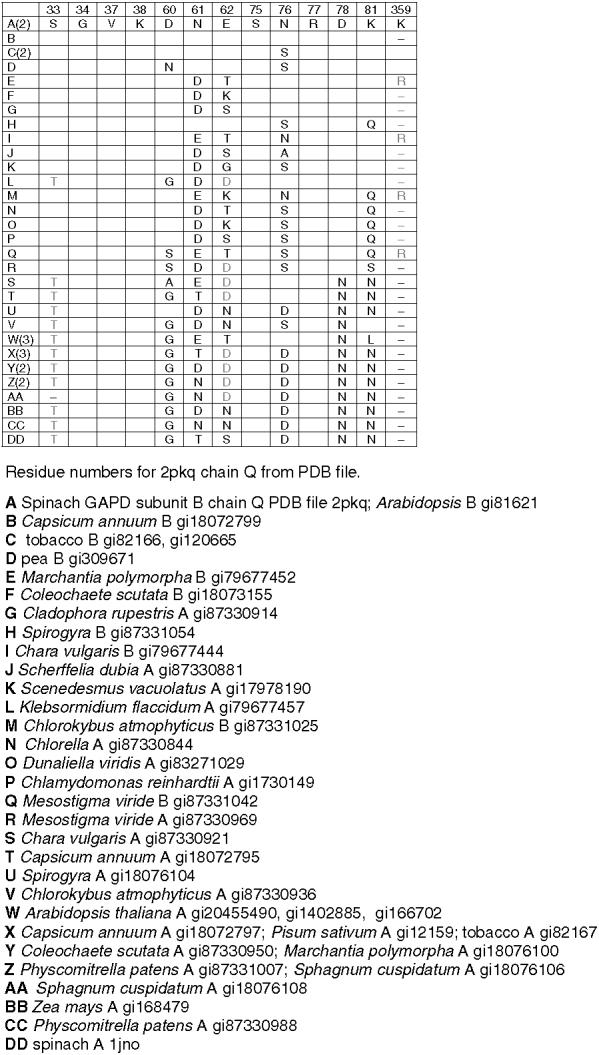
Residues corresponding to the residues in spinach chloroplast GAPD B at the interface with FNR in the putative GAPD-FNR complex in Fig. 8a. Number of sequences containing indicated residues in parentheses in left column. Completely redundant sequences within species not included. Conservative substitutions in gray. Only substitutions are shown.
Acknowledgments
This work was supported in part by National Science Foundation Grant MCB-0079913 to L.E.A. and National Institutes of Health Grant AI064913 to W.B. We thank Richard Malkin, University of California—Berkeley, for generously providing the anti-FNR antibody, Alex Dong Li for initial advice on sequence alignment, and Joanna Chrostowski, for assistance with the immunoblots.
References
- Anderson LE, Goldhaber-Gordon IM, Li D, Tang X-Y, Xiang M, Prakash N. Enzyme-enzyme interaction in the chloroplast: glyceraldehyde-3-phosphate dehydrogenase, triose phosphate isomerase and aldolase. Planta. 1995a;196:245–255. doi: 10.1007/BF00201381. [DOI] [PubMed] [Google Scholar]
- Anderson LE, Wang X, Gibbons JT. Three enzymes of carbon metabolism or their antigenic analogs in pea leaf nuclei. Plant Physiol. 1995b;108:659–667. doi: 10.1104/pp.108.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JB, Carol AA, Brown VK, Anderson LE. A quantitative method for assessing co-localization in immunolabeled thin section electron micrographs. J. Struct. Biol. 2003;143:95–106. doi: 10.1016/s1047-8477(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Anderson LE, Gatla N, Carol AA. Enzyme co-localization in pea leaf chloroplasts: glyceraldehyde-3-P dehydrogenase, triose-P isomerase, aldolase and sedoheptulose bisphosphatase. Photosyn. Res. 2005;83:317–328. doi: 10.1007/s11120-005-0790-2. [DOI] [PubMed] [Google Scholar]
- Anderson LE, Chrostowski J, Carol AA. Enzyme co-localization with transketolase, xylulose-5-P 3-epimerase and phosphoriboisomerase in pea leaf chloroplasts. Plant Sci. 2006;171:686–698. [Google Scholar]
- Bruns CM, Karplus PA. Refined crystal structure of spinach ferredoxin reductase at 1.7 A resolution: oxidized, reduced and 2′phospho-5′-AMP bound states. J. Mol. Biol. 1995;247:125–145. doi: 10.1006/jmbi.1994.0127. [DOI] [PubMed] [Google Scholar]
- Cámara-Artigas A, Hirasawa M, Knaff DB, Wang MT, Allen JP. Crystallization and structural analysis of GADPH from Spinacia oleracea in a new form. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006;62:1087–1092. doi: 10.1107/S174430910604200X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani DN, Sainis JK. Isolation and characterization of a thylakoid membrane module showing partial light and dark reactions. Biochim. Biophys. Acta. 2005;1669:43–52. doi: 10.1016/j.bbamem.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Fermani S, Ripamonti A, Sabatino P, Zanotti G, Scagliarini S, Sparla F, Trost P, Pupillo P. Crystal structure of the non-regulatory A(4)isoform of spinach chloroplast glyceraldehyde-3-phosphatedehydrogenase complexed with NADP. J. Mol. Biol. 2001;314:527–542. doi: 10.1006/jmbi.2001.5172. [DOI] [PubMed] [Google Scholar]
- Fermani S, Sparla F, Falini G, Martelli PL, Casadio R, Pupillo P, Ripamonti A, Trost P. Molecular mechanism of thioredoxin regulation in photosynthetic A2B2-glyceraldehyde-3-phosphate dehydrogenase. Proc. Natl. Acad. Sci. USA. 2007;104:11109–11114. doi: 10.1073/pnas.0611636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso G, Giacomelli L, Ytterberg AJ, Peltier JB, Rudella A, Sun Q, van Wijk KJ. In-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: new proteins, new functions, and a plastid proteome database. Plant Cell. 2004;16:478–499. doi: 10.1105/tpc.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabb HA, Jackson RM, Sternberg MJE. Modelling protein docking using shape complementarity, electrostatics and biochemical information. J. Mol. Biol. 1997;272:106–120. doi: 10.1006/jmbi.1997.1203. [DOI] [PubMed] [Google Scholar]
- Küchler M, Decker S, Hormann F, Soll J, Heins L. Protein import into chloroplasts involves redox-regulated proteins. EMBO J. 2002;21:6136–6145. doi: 10.1093/emboj/cdf621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisu G, Kusunoki M, Katoh E, Yamazaki T, Teshima K, Onda Y, Kimata-Ariga Y, Hase T. Structure of the electron transfer complex between ferredoxin and ferredoxin-NADP+ reductase. Nat. Struct. Biol. 2001;8:117–121. doi: 10.1038/84097. [DOI] [PubMed] [Google Scholar]
- Lintala M, Allahverdiyeva Y, Kidron H, Piippo M, Battchikova N, Suorsa M, Rintamäki E, Salminen TA, Aro EM, Mulo P. Structural and functional characterization of ferredoxin-NADP+-oxidoreductase using knock-out mutants of Arabidopsis. Plant J. 2007;49:1041–1052. doi: 10.1111/j.1365-313X.2006.03014.x. [DOI] [PubMed] [Google Scholar]
- Matthijs HCP, Coughlan SJ, Hind G. Removal of ferredoxin:NADP+ oxidoreductase from thylakoid membranes, rebinding to depleted membranes, and identification of the binding site. J. Biol. Chem. 1986;261:2154–2158. [PubMed] [Google Scholar]
- Mihalek I, Res I, Lichtarge O. Evolutionary trace report_maker: a new type of service for comparative analysis of proteins. Bioinformatics. 2006;22:1656–1657. doi: 10.1093/bioinformatics/btl157. [DOI] [PubMed] [Google Scholar]
- Morales R, Kachalova G, Vellieux F, Charon MH, Frey M. Crystallographic studies of the interaction between the ferredoxin-NADP+ reductase and ferredoxin from the cyanobacterium Anabaena: looking for the elusive ferredoxin molecule. Acta Crystallogr., Sect. D. 2000;56:1408–1412. doi: 10.1107/s0907444900010052. [DOI] [PubMed] [Google Scholar]
- Mouche F, Gontero B, Callebaut I, Mornon JP, Boisset N. Striking conformational change suspected within the phosphoribulokinase dimer induced by interaction with GAPDH. J. Biol. Chem. 2002;277:6743–6749. doi: 10.1074/jbc.M106401200. [DOI] [PubMed] [Google Scholar]
- Negi SS, Kolokoltsov AA, Schein CH, Davey RA, Braun W. Determining functionally important amino acid residues of the E1 protein of Venezuelan equine encephalitis virus. J. Mol. Model. 2006;12:921–929. doi: 10.1007/s00894-006-0101-7. [DOI] [PubMed] [Google Scholar]
- Okutani S, Hanke GT, Satomi Y, Takao T, Kurisu G, Suzuki A, Hase T. Three maize leaf ferredoxin:NADPH oxidoreductases vary in subchloroplast location, expression, and interaction with ferredoxin. Plant Physiol. 2005;139:1451–1459. doi: 10.1104/pp.105.070813. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda Y, Hase T. FAD assembly and thylakold membrane binding of ferredoxin: NADP+ oxidoreductase in chloroplasts. FEBS Lett. 2004;564:116–120. doi: 10.1016/S0014-5793(04)00325-4. [DOI] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J. Comp. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JF, Isupov MN, Littlechild JA, Anderson LE. glyceraldehyde-3-P dehydrogenase contains a single disulfide bond located in the C-terminal extension to the B subunit. J. Biol. Chem. 2001;276:35247–35252. doi: 10.1074/jbc.M103855200. [DOI] [PubMed] [Google Scholar]
- Sheriff S, Teller DC, Herriott JR. Ferredoxin-NADP+ oxidoreductase is active as a monomer with molecular weight 33,000-36,000. Arch. Biochem. Biophys. 1980;205(2):499–502. doi: 10.1016/0003-9861(80)90132-0. [DOI] [PubMed] [Google Scholar]
- Sparla F, Pupillo P, Trost P. The C-terminal extension of glyceraldehyde-3-phosphate dehydrogenase subunit B acts as an autoinhibitory domain regulated by thioredoxins and nicotinamide adenine dinucleotide. J. Biol. Chem. 2002;277:44946–44952. doi: 10.1074/jbc.M206873200. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Pisareva T, Norling B. Proteomic studies of the thylakoid membrane of Synechocystis sp. PCC 6803. Proteomics. 2005;5:4905–4916. doi: 10.1002/pmic.200500111. [DOI] [PubMed] [Google Scholar]
- Süss KH, Arkona C, Manteuffel R, Adler K. Calvin cycle multienzyme complexes are bound to chloroplast thylakoid membranes of higher plants in situ. Proc. Natl. Acad. Sci. USA. 1993;90:5514–5518. doi: 10.1073/pnas.90.12.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti G, Forti G. Studies on the triphosphopyridine nucleotide-cytochrome f reductase of chloroplasts. J. Biol. Chem. 1966;241:279–285. [PubMed] [Google Scholar]
- Zhang H, Whitelegge JP, Cramer WA. Ferredoxin:NADP+ oxidoreductase is a subunit of the chloroplast cytochrome b6f complex. J. Biol. Chem. 2001;276:38159–38165. doi: 10.1074/jbc.M105454200. [DOI] [PubMed] [Google Scholar]
- Zheng L, Roeder RG, Luo Y. S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell. 2003;114:255–266. doi: 10.1016/s0092-8674(03)00552-x. [DOI] [PubMed] [Google Scholar]