Abstract
Due to their complexity, the separation of intact proteins from complex mixtures is an important step to comparative proteomics and the identification and characterization of the proteins by mass spectrometry (MS). In the study reported, we evaluated the use of non-porous-reversed-phase (np-RP) HPLC for intact protein separation prior to MS analyses. The separation system was characterized and compared to 1D-SDS-PAGE electrophoresis in terms of resolution and sensitivity. We demonstrate that np-RP HPLC protein separation is highly reproducible and provides intact protein fractions which can be directly analyzed by MALDI-TOF MS for intact molecular weight determination. An in-well digestion protocol was developed, allowing for rapid protein identification by peptide mass fingerprinting (PMF) and resulted in comparable or improved peptide recovery compared with in-gel digestion. The np-RP sensitivity of detection by UV absorbance at 214 nm for intact proteins was at the low ng level and the sensitivity of peptide analysis by MALDI-TOF MS was in the 10–50 pg level. A membrane protein fraction was characterized to demonstrate application of this methodology. Among the identified proteins, multiple forms of vimentin were observed. Overall we demonstrate that np-RP HPLC followed by MALDI-TOF MS allows for rapid, sensitive and reproducible protein fractionation and very specific protein characterization by integration of PMF analysis with MS intact molecular weight information.
Keywords: non-porous reversed phase HPLC, protein liquid chromatography, peptide mass fingerprint, intact protein mass spectrometry
INTRODUCTION
In the developing field of proteomics, the rapid separation, identification and characterization of proteins from complex samples is a challenging goal to ensure study of how changes in protein expression and their post-translational modifications (PTMs) can be correlated to a change at the genomic level, a particular disease or development state or a specific signaling pathway.[1][2] Proteomics has thus emerged as one of the most important “post-genomics” approaches to better understand gene and protein function since the completion of sequencing of the human genome.[3, 4] Proteome profiling has been used to directly study a disease at the protein level and represents a powerful means to investigate and to better understand the molecular etiology and phenotype of the disease and correlate the disease to the genome. Technologies such as mass spectrometry (MS) based proteomics allow for the direct comparison of proteome profiles and thus are utilized as important tools for identifying proteins and protein biomarkers, which are necessary for study, characterization and disease diagnosis.[5] Numerous proteomics studies involve analysis of extremely complex protein mixtures like serum, plasma, tissue homogenates or cellular lysates.[6, 7] These biological samples may contain large numbers of very diverse types of proteins expressed at very different concentrations, for example, plasma proteins which may span up to 10 orders of magnitude.[6] While these biological samples contain a wealth of information at the protein level, their complexity requires a substantial amount of sample processing, including fractionation, purification and multi-dimensional separation prior to MS and comparative proteomics analyses.[8, 9]
The most common technique to separate proteins prior to mass spectrometric analysis makes use of sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) running either in a 1- or 2- dimensional format (1DE, 2DE).[10][11] The 2-dimensional electrophoresis (2DE) strategy was invented and published simultaneously by Klose [12], O’Farrell [13] and Scheele [14] in 1975. In this technology, proteins are separated on the basis their isoelectric point (pI) by isoelectrofocusing (IEF) in the first dimension and then separated based on their molecular mass by gel electrophoresis in the second dimension. After locating a spot within the gel, it may be excised, digested and subjected for analysis, typically by matrix-assisted laser desorption/ionization (MALDI) time-of-flight (TOF) MS. [15] [16] The fractionation of the protein mixture leads to a decrease in the sample complexity and thus to more accurate MS identification of the proteins and identification of their PTMs. It also facilitates the detection of lower abundance proteins by reducing MS ion suppression effects of abundant species.[17] While 1DE and 2DE offer high resolution separations the technique remains labor intensive, requires significant operator skill and it is difficult to automate the fractionation, excision and digestion processes. Moreover, although 1DE and 2DE provide an estimate of molecular weight information, or protein pI information (2DE), this information is lost during the digestion process used to extract the protein from the gel.
Alternatives to 2DE separation have been investigated as a means to resolve proteins prior to MS characterization. Liquid chromatography techniques can be used for protein or peptide separations and have recently been improved to handle proteomic analyses of complex samples.[18] Diverse chromatography techniques have been extensively described in the literature as methods for protein/peptide separation. Cation exchange [19], anion exchange [20], reversed phase [21–27], biphasic ion-exchange [28], chromatofocusing [29–31] or size-exclusion [32] are all used for protein separation. As with electrophoresis, complex samples often need to be pre-fractionated [30] or separated by two dimensions of chromatography (2D HPLC), to obtain appropriately simplified samples for MS analysis.[28, 29, 32] These 2D HPLC technologies typically combine two of the chromatography techniques previously listed. Other two-dimensional approaches to protein fractionation have also been proposed combining, for example, continuous-elution gel electrophoresis and reversed phase HPLC [33] or anion exchange chromatography and 1D SDS-PAGE electrophoresis.[20, 28]
Another 2D chromatographic strategy termed multidimensional protein identification technology (MudPIT) has been extensively applied to proteomics analyses, although at a peptide level. It consists in, first, digesting the whole protein sample and then separating the peptide mixture by one or two dimensions of chromatography prior to LC-MS analysis.[34,35] Like SDS-PAGE electrophoresis, MudPIT strategies when applied to mixtures of proteins also result in a loss of intrinsic information about the intact proteins, this information, which could be used to facilitate the protein identification and a more complete characterization disappears in this case because digestion precedes the separation and the MS analyses of the proteins. Moreover, low abundance proteins from a complex mixture may be more difficult to identify because the ion suppression effect prevents the MS detection of low abundance ions.[17] Finally, the simultaneous presence of peptides originating from a multitude of proteins makes the identification and characterization of PTMs and their relationship with a specific protein very difficult.
The separation of intact proteins by liquid chromatography offers many advantages over the MudPIT strategy and the use of gel based methods. Proteins are kept intact throughout the separation and collection, so that several MS-based methods of analysis can be applied, such as the digestion of fractions for protein identification by PMF or the direct analysis of intact proteins by MS. In both cases, it is possible to more adequately characterize the PTMs of a protein when dealing with an intact protein as the starting point. The use of in-solution protein digestion is more facile and faster than in-gel digestion and can be easily automated. HPLC fractions can also be lyophilized and kept for further analysis and detector traces (usually UV absorbance) may be used for comparative proteomics and targeting interesting proteins. This strategy, however, requires the chromatographic separation to be highly reproducible, achieve adequate resolution and offer a method of detection sensitive enough to detect low abundance species.
In this report, we evaluate the use of np-RP HPLC for the fractionation of intact proteins as a separation technique coupled with intact protein characterization and PMF by MALDI-TOF MS for proteomics analysis of mixtures of proteins. We have compared the resolution of np-RP HPLC separation and the sensitivity of its associated UV detection at 214 nm with those of SDS-PAGE gel and Coomassie staining. Reproducibility of protein separation and linearity of detection were also measured in order to integrate this intact protein separation technology into our high-throughput proteomic workflow. Intact protein masses were determined for each fraction collected from the np-RP column by MALDI-TOF MS. Peptide recovery and sensitivity of analysis by MS after in-well digestion of the separated fractions was measured and compared with results obtained from in-gel digestion. As an application of this methodology, proteins obtained from a membrane fraction of bovine aortic endothelial cellular lysate were characterized.
MATERIALS AND METHODS
Protein standard mixture preparation
A protein mixture was prepared for use within the laboratory as a test standard for method and protocol development and quality control. The standard mixture was composed of nine proteins of varying molecular mass, pI and other physicochemical proprieties. Bovine insulin (Sigma, St. Louis, MO, I5500), bovine cytochrome (Sigma C3131), equine myoglobin (Sigma M0630), bovine β-lactoglobulin (Sigma L3908), glycine max trypsin inhibitor (Sigma T9003), rabbit GAPDH (Sigma G5262), bovine catalase (Sigma C40), aspergillus niger glucose oxidase (Sigma 49180) and equine ferritin (Calbiochem 341476) were first prepared as individual 1 mg/mL solutions in acetonitrile (ACN) (30%), isopropanol (5%), TFA (0.1%) and formic acid (3%). Five mL of each solution was pooled and the protein concentration was adjusted to 100 µg/mL of each protein. Aliquots of 100 µL were prepared (10 µg/protein/tube) and dried down for storage at −50 °C.
Cell culture and Membrane fractionation
Bovine Aortic Endothelial Cells (BAEC) were grown until confluence in an endothelial growth medium (Cambrex Bioproducts, Walkersville, MD) and made quiescent in basal medium for 24 h before lysis. Cells were harvested in a sucrose buffer (Tris HCl 10 mM and sucrose 0.3 M) and sonicated on ice four times for 10 s. The lysate was centrifuged 15 min at 15,000 × g at 4 °C, and the supernatant was subjected to ultra-centrifugation for 1 h at 100,000 × g at 4 °C. The supernatant constituted the cytosolic fraction. The pellets constituting the membrane fraction were dissolved in a detergent buffer (Tris HCl 25 mM pH 7.4, NaCl 150 mM, MgCl2 5 mM, 0.1% octyl-β-D-glucopyranoside) to constitute the membrane fraction. Both fractions were purified on PD-10 Sephadex-G25 columns (Amersham Biosciences, Piscataway, NJ) and membrane fractions were delipidated by methanol/chloroform extraction.
Non porous reversed phase HPLC
Different amounts of the protein standard mixture or 100 µg of bovine endothelial cell membrane fraction were analyzed by HPLC, System Gold™, controlled by 32 Karat™ software and equipped with UV detection at 214 nm (Beckman Coulter, CA). Proteins were suspended in ACN 3% / TFA 0.1% and separated on a C18 non-porous reversed phase column (MICRA-Platinum ODS-I, Eprogen, Darien, IL). The separation was performed via gradient elution of two solvents: water / TFA 0.1% (A) and ACN / TFA 0.08% (B) at the constant flow rate of 0.75 mL/min and proteins were monitored by UV at 214 nm. Unless noted otherwise the gradient profile used for solvent B was the following: 0% for 2 min: 0 to 100 % in 30 min: 100% for 4 min: 100 to 0% in 2 min and 0% for 8 min, with a total run time of 45 min. The np-RP column was maintained at 50 °C with a column heater. The fractions were collected into 96-well plates using a FC 204 fraction collector (Gilson, Middleton, WI). Different manufacturers’ 96-well plates were tested and twin.tec PCR plates (Eppendorf®, Hamburg, Germany) were chosen for their low protein binding and low polymer contamination proprieties. Fractions were dried using a Speed-Vac® (SPD-1010, Thermo Electron Corporation, Waltham, MA) for 2 h without the application of heating. Proteins were then re-suspended in 200 µL of ACN 85% / TFA 0.1% and concentrated at the bottom of the wells in the Speed-Vac® for an additional 2 h. At this stage, proteins could be directly digested in-well or preserved dry at −80 °C for later analysis.
1D SDS-PAGE separation
SDS-PAGE separation of the protein standard mixture was performed to compare the resolution and the sensitivity of the two separation techniques (np-RP versus SDS-PAGE). Each protein of the standard mixture (100 ng and 1 µg) was suspended in 15 µL of 1× NuPAGE LDS Sample Buffer (Invitrogen, Carlsbad, CA) containing 1mM β-mercaptoethanol and the solution was boiled for 5 min. Samples were then loaded on a 10% Bis-Tris SDS-PAGE gel (Invitrogen) and separated at 150 V until the dye front reached the opposite side of the gel. Proteins were stained with Imperial Protein Stain (Pierce Biotechnology, Inc., Rockford, IL) for 4 h and destained overnight in distilled water.
In-well protein digestion
Fractions of the proteins from the np-RP HPLC were digested with modified trypsin (Promega, Madison, WI, Trypsin Gold TPCK treated) added directly to the collection well to simplify the process and obtain peptide mixtures ready for MS analysis. Proteins were re-suspended in 5 µL of ACN (30%) and 10 µL of the trypsin solution in 30 mM NH4HCO3 (pH 9) was added to each well, resulting in a solution of 20 mM NH4HCO3/ACN (10%). Approximate ratios of 1:10 trypsin were used for the in-well digests. Plates were incubated at 37 °C for 6 h and dried without heating in a Speed-Vac®. Plates were preserved dried at –80 °C. Prior to MALDI-TOF MS analysis, peptides were suspended into 10 µL of ACN (85%) / TFA (0.1%).
In-gel proteins digestion
The protein bands of interest were excised using a fresh scalpel and diced into 1 mm3 pieces. Gel pieces were further de-stained in 3×100 µL of 100 mM NH4HCO3 / ACN 50% (pH 9) and then washed three times with first 100 µL of 100 mM NH4HCO3 (pH 9), then 100 µL of 100 mM NH4HCO3 (pH 9) / ACN 50% and finally 100 µL of ACN . Proteins were then reduced by 20 mM DTT, 100 mM NH4HCO3 and ACN 5% for 1 hour at 55 °C. Cysteines were alkylated by 100 mM iodoacetamide in 100 mM NH4HCO3. Another washing process (same as after the destaining) was performed before the digestion. An approximate ratio of 1:10 of trypsin to protein (Promega Trypsin Gold, TPCK treated) was used to digest the proteins. Trypsin in 25 µL of 50 mM NH4HCO3 / ACN 5% was added to the gel pieces in an Eppendorf tube and the enzymatic reaction was carried overnight at 37 °C. Peptides were extracted in 100 µL of 20 mM NH4HCO3 by gentle vortexing for 20 min, then in 2×100 µL of TFA (1%) / ACN (50%), and 1× in 100 µL of ACN. Supernatants were collected and pooled at each step and the entire extraction process was performed in duplicate. Supernatants were then dried with a Speed-Vac® without heat. Peptides were suspended in 2 µL of ACN 15% / TFA 1% and 13 µL of distilled water prior to desalting by ZipTips™ (Millipore, Billerica, MA) purification. ZipTips™ were cleaned with 3×10 µL of ACN 50% / TFA 0.1%, then equilibrated with 3×10 µL of ACN 2% / TFA 0.1%. Peptides were adsorbed by pipetting the sample solution 20 times, then washed with 3×10 µL of ACN 2% / TFA 0.1%. Peptides were eluted with 10 µL of ACN 50% / TFA 0.1%, dried using the Speed-Vac® without heating and preserved dried at –80 °C. Prior to MALDI-TOF MS analysis, peptides were suspended into ACN 50% / TFA 0.1%.
MALDI-TOF MS analysis
MALDI-TOF MS analyses were used to determine both intact protein molecular weights and protein identities by PMF. Analysis was performed using a Reflex IV MALDI-TOF mass spectrometer (Bruker Daltonics, Billerica, MA). The sample was spotted on an AnchorChip™ target (Bruker Daltonics) with 1 µL of freshly prepared matrix solution. Sinapinic acid (Bruker Daltonics, part #201345) matrix was used for intact protein analysis and 2,5-dihydroxybenzoic acid (DHB) (Bruker Daltonics, part #201346) for PMF. External calibration was performed in each case using protein and peptide calibrants (Bruker Daltonics, part #202570). Signals from 100 to 200 laser shots were summed per mass spectrum. Mass accuracy was estimated at +/− 0.15 Da. Intact protein mass spectra were acquired using 2 separate sets of instrument parameters in order to achieve an expanded spectral range: m/z 1,000 to 30,000 or 60,000 (low to medium intact mass range) and m/z 5,000 to 90,000 m/z (medium to high intact mass range). Peptide masses were acquired with a range of ca. m/z 800 to 8,000.
Database search parameters
Singly charged monoisotopic peptide lists were generated and used as inputs for database searching using MoverZ software (ProteoMetrics, LCC, New York, NY), after external and internal calibration of spectra. Searches were performed against NCBInr and SwissProt database using MASCOT Peptide Mass Fingerprint database search software (www.matrixscience.com). The oxidation of methionine was included as possible modification as well as the alkylation of cysteines when appropriate. Up to two missed tryptic cleavages were considered, and the mass tolerance for the monoisotopic peptide masses was set to +/− 0.15 Da.
RESULTS AND DISCUSSION
This work focused on the evaluation and application of non-porous reversed phase (np-RP) HPLC for protein separation prior to intact protein characterization and peptide mass mapping by MS analyses. Np-RP HPLC material takes advantage of fast mass transfer kinetics to provide efficient separation of peptides and proteins, whereas traditional porous packing is often limited by a slow diffusion of biomacromolecules. [25] Non-porous stationary phases were developed in the 1980s [24, 36–38] and have been previously applied for the separation of proteins and peptides by reversed phase chromatography [23, 25–27, 39, 40] but no extensive characterization and comparison to existing separation techniques have been completed. For this purpose, a group of nine proteins exhibiting a wide range of isoelectric point (pI) and molecular mass was chosen to be used as a standard for the np-RP column characterization. Intact protein separation reproducibility and linearity of detection were investigated. Protein separation by np-RP HPLC was then compared to SDS-PAGE electrophoresis, in terms of resolution and the sensitivity of their associated detection technique (UV detection at 214 nm Coomassie stain respectively). Intact protein masses were determined for np-RP separated proteins by MALDI-TOF MS. Peptide recovery and sensitivity of analysis by MS after in-well digestion of the separated proteins was measured and compared with results obtained from in-gel digestion.
Separation of a protein standard mixture by np-RP HPLC
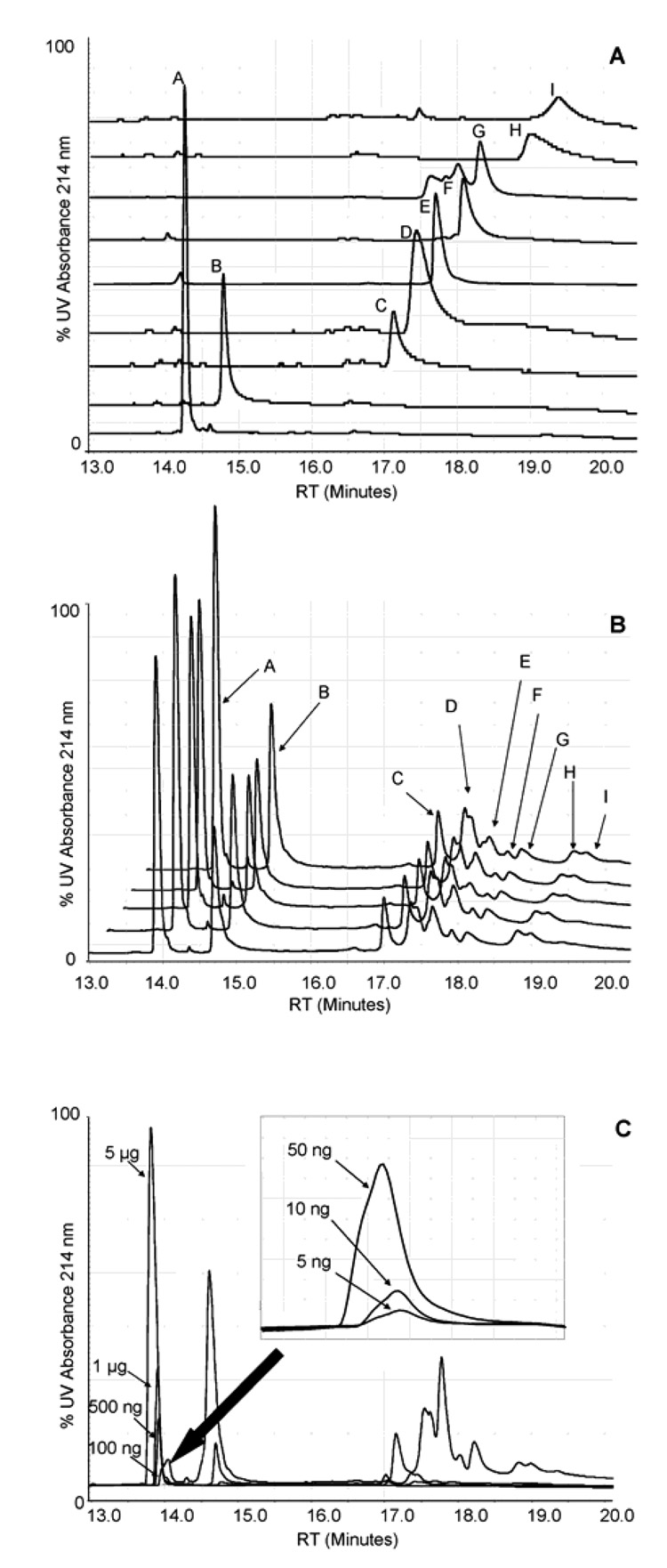
Each protein to be incorporated into the protein standard mixture was first run individually for verification of its identity and purity and measurement of its retention time. Mixtures containing 1 µg of each protein were then separated under the same conditions on the np-RP column (Figure 1). The same mixture was run five times consecutively, on the same column and under the same conditions, to evaluate the reproducibility of the separation (Figure 1B). Retention time and peak area averages, as well as standard deviations, were calculated for each protein and the retention time of each protein in the mixture was then compared to the individual runs (Table 1). Excellent reproducibility was observed for the retention time with generally less than 0.2% relative standard deviation for the entire set of proteins when run as a mixture. Peak area reproducibility was overall quite good with variations of less than 10 % RSD for the group of proteins. However, myoglobin (E), catalase (F) and ferritin (I) exhibited a higher variability of their peak area with 15.98, 17.08 and 10.18 % RSD respectively. This increase in variability may be the result of the lower degree of resolution of separation of the proteins and peak broadening of these proteins which made the peak area measurement more difficult. It is noted that the proteins analyzed may be made up of multiple isoforms, e.g. trypsin inhibitor and β-lactoglobulin, and/or may be post-translationally modified, e.g. ferritin and glucose oxidase, or contain a heme-group, e.g. catalase and myoglobin, all of these factors also may contribute to the heterogeneity of the protein and thus peak broadening and would make the peak area measurements more difficult.
Figure 1. Separation of a protein standard mixture by np-RP HPLC.
Individual protein chromatograms, 1 ug each, (A) from a protein standard were analyzed by a high pressure liquid chromatography System Gold, controlled by 32 Karat software and equipped with UV detection at 214 nm (Beckman Coulter, CA). Proteins were suspended in ACN 3% / TFA 0.1% and separated on a C18 non-porous reversed phase column. The separation was performed via different gradient elution of two solvents: distilled water/TFA 0.1% and ACN / TFA 0.08% at a constant flow rate of 0.75 mL/min and proteins were monitored at 214 nm. Values correspond to results shown in Table 1. (B) The protein mixture (1 µg of each protein) was separated five times consecutively on the same column and under the same conditions to evaluate the reproducibility of the system. Values correspond to results shown in Table 1. (C) UV214 nm detection sensitivity from 5 µg to 100 ng of a 9 protein mixture; from 50 ng to 5 ng of bovine insulin shown in inset.
Table 1.
Retention time and peak area reproducibility for np-RP HPLC of a nine protein standard.
| (ii)Individual | (iii) Protein Standard Mixture | |||||||
|---|---|---|---|---|---|---|---|---|
| (i) ID | Protein | RT (min) | RT (min) | SD (min) | RSD (%) | Area | SD (min) | RSD (%) |
| A | Insulin | 14.25 | 13.9 | 0.01 | 0.08 | 1358029 | 38432 | 2.83 |
| B | Cytochrome C | 14.78 | 14.68 | 0.01 | 0.07 | 880768 | 38363 | 4.36 |
| C | Trypsin Inhibitor | 17.12 | 17 | 0.02 | 0.12 | 282653 | 14830 | 5.25 |
| D | GAPDH | 17.43 | 17.35 | 0.01 | 0.07 | 552619 | 19302 | 3.49 |
| E | Myoglobin | 17.65 | 17.66 | 0.01 | 0.07 | 368721 | 58933 | 15.98 |
| F | Catalase | 18.08 | 17.92 | 0.01 | 0.04 | 109984 | 18785 | 17.08 |
| G | β-Lactoglobulin | 18.31 | 18.11 | 0.01 | 0.07 | 268237 | 16712 | 6.23 |
| H | Glucose oxidase | 18.99 | 18.98 | 0.01 | 0.07 | 168399 | 8602 | 5.11 |
| I | Ferritin | 19.39 | 19.38 | 0.04 | 0.19 | 133949 | 13630 | 10.18 |
Retention times and peak areas were determined for each of the 5 runs of 1 µg of each protein standard. Average and relative standard deviations were calculated and compared to values determined on the individual run of each protein.
Proteins correspond to results shown in Figure 1.
Retention times for each protein were calculated from individual runs shown in Figure 1A.
Values correspond to the nine protein mixture analysis shown in Figure 1B.
Shifts in retention times were also observed for some of the proteins when run as a mixture, especially insulin, which shifted from 14.25 min to 13.90 min (Table 1). While up to 100 µg of proteins could be loaded onto the column without a decrease in the resolution of separation, fronting of the peak shape was observed when increasing amounts of proteins were loaded. This phenomenon has been discussed in the literature and is associated with the reaction kinetics of the sample with the stationary phase of the column during the separation. [41][42]
We also observed that the peaks from GAPDH (D), myoglobin (E), catalase (F) and β-lactoglobulin (G) were less well defined when run as a mixture. We postulated that this phenomenon may be due to interactions of the proteins with one another, perhaps because they were not denatured before injection onto the column, as we suspended the standard protein mixture in a non-denaturing buffer (3% ACN / 0.1% TFA). Denaturation of the proteins prior to their separation by np-RP was achieved by incubating the mixture in 8M urea or 6M guanidine/HCl at room temperature and 37 °C, but no real changes in the separation pattern were observed, indicating that the column itself contributes to unfolding of the proteins by hydrophobic interactions and that prior denaturing did not enhance protein separation by np-RP HPLC.
Sensitivity of detection by UV at 214 nm
Decreasing amounts of the protein mixture were analyzed to investigate the sensitivity of UV detection at 214 nm (Figure 1C). Insulin, with a sharp well resolved peak, was detected with a sensitivity of 5 ng of protein. The linearity of the peak area for insulin spanned the range from 5 ng to 5 µg of protein (3 orders of magnitude) with an R-squared value of 0.999, demonstrating that the UV-detector’s response was quantitative and that the signal may be used to compare protein profiles of two distinct samples providing that sufficiently reproducible and resolved peaks are obtained. Cytochrome C also yielded good results as a well resolved peak was observed in the chromatogram. However, as the sensitivity of measurement by UV was proportional to the proteins’ resolution and peak shape, higher molecular weight proteins which were less than fully resolved due to peak broadening were visible by UV with a sensitivity in the high 10s of ng to the 100s of ng range.
Comparison of np-RP with SDS-PAGE
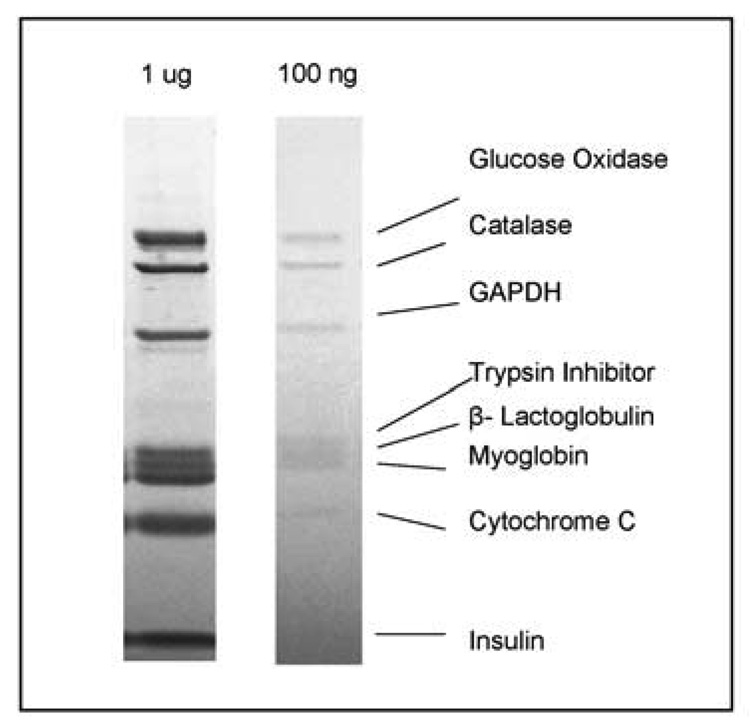
The resolution and reproducibility of separation and sensitivity of detection via np-RP HPLC was compared with the separation of the protein standard mixture using 1D SDS-PAGE. The protein standard mixture (1 µg or 100 ng of each protein) was separated by 1D SDS-PAGE electrophoresis and stained by Coomassie blue dye (Figure 2). It was observed that the order of the migration in the gel was different from the elution from the np-RP column. For instance, GAPDH (146 kDa) eluted relatively early from the np-RP column as a complex (Figure 1A) while it migrated within the gel respective of its 36-kDa monomer weight (Figure 2). Protein migration by SDS-PAGE electrophoresis is proportional to its molecular weight, whereas the reversed phase column separates proteins based on their hydrophobicity. Thus myoglobin (17.5 kDa) and catalase (60 kDa) are separated by only 0.26 minutes on the np-RP whereas trypsin inhibitor (20.1 kDa) and β-lactoglobulin (18.4 kDa) are resolved by more than one minute (Table 1). Ferritin (450 kDa), which is composed of 24 subunits of 2 kinds (heavy and light subunit), was not observed on the gel because the presence of the reducing agent in the sample buffer caused the disruption of ferritin disulfide bonds and its breakage into small polypeptides. However intact ferritin was observed and well resolved by the np-RP with a retention time of 19.39 minutes (Figure 1 and Table 1). Np-RP HPLC can thus be applied for the separation and characterization of large protein complexes which may not be resolved by SDS-PAGE electrophoresis, unless a non reducing conditions “native gel” is used.
Figure 2. Separation of a protein standard mixture by SDS-PAGE electrophoresis.
Samples containing 1 µg and 100 ng of each protein standard were separated on a 10% Bis-Tris SDS-PAGE electrophoresis gel at 150 V until complete. Proteins were stained with Imperial Protein Stain for 4 h and de-stained overnight in distilled water. Proteins correspond to those analyzed by np-RP HPLC shown in Figure 1 and Table 1.
The resolution of protein separation was generally better using 1DE electrophoresis, due to the high resolving power of SDS-PAGE. In an attempt to improve the resolution of separation by np-RP HPLC, different solvent profiles were tested. Increasing the separation time and the application of shallow gradients were shown to improve the resolution for some of the proteins (data not shown) but the increase in the separation time proved to be less favorable for adapting to high-throughput analyses. Additionally we explored several reversed phase columns from a variety of manufacturers, none of which resulted in improved resolution compared with the columns used for these experiments using the same separation time.
The sensitivity of detection via Coomassie stain was evaluated and compared with the sensitivity of detection of UV measured at 214 nm. When 100 ng of each protein were run on the SDS-PAGE gel, the staining method used was just sensitive enough to detect proteins larger than 10 kDa but not sufficient to detect the insulin band around 5700 Da. Small proteins are known to be difficult to detect by SDS-PAGE. Other stains, e.g. silver stain, may be commercially available and provide a better sensitivity, but may also be less compatible with further MS analysis. Sensitivity of detection using UV at 214 nm after np-RP HPLC is thus more sensitive than traditional Coomassie blue stain for low molecular mass species and can detect down to 5 ng of protein when the peak is sufficiently resolved (Figure 1C).
MALDI-TOF MS analysis of proteins separated by np-RP HPLC
MALDI-TOF MS is commonly used for PMF analysis of proteolytic digests of proteins in order to identify proteins via database searches. MALDI-TOF MS may also be used to characterize a protein by intact mass measurement. Post separation by np-RP HPLC, proteins were collected into fractions in 96 well plates, and then subjected to intact protein MS analysis. Because the proteins were separated under liquid conditions, they could be directly characterized as an intact entity by MS without the need of the proteolytic digestion which is necessary after SDS-PAGE electrophoresis to efficiently recover the proteins from a gel.
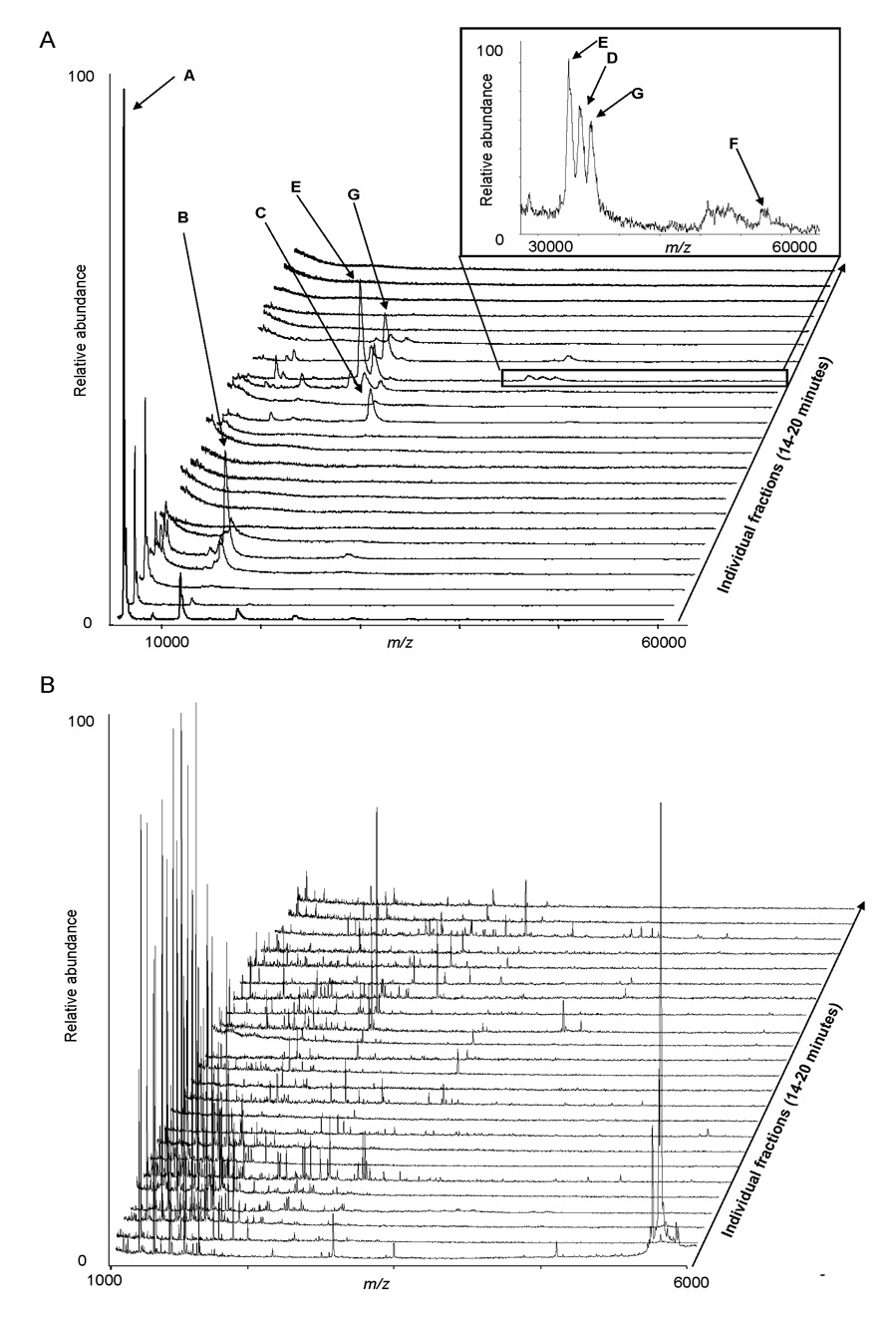
Intact molecular masses were obtained on the protein fractions obtained from np-RP HPLC (Figure 3A). Seven of the 9 proteins were detected with a mass corresponding to the theoretical mass of the protein or their respective sub-units (Table 2). This use of MALDI-TOF MS protein mass information acts as a means of analysis at the intact protein level and may be considered to represent a “second dimension” of protein separation within the vacuum phase of the mass spectrometer. By using intact protein MS analysis, we were able to resolve GAPDH, myoglobin and catalase which did not fully resolve when measured by UV at 214 nm in the chromatographic separation. This demonstrates the benefit of performing intact protein MS in order to characterize more efficiently the proteins before performing further analyses. Additionally, more complete information is gained when the chromatography results are taken in conjunction with the intact protein MS results. Proteins were observed to elute from the np-RP based on their hydrophobicity which is an intrinsic propriety of the protein or a protein-complex. Theoretical masses were obtained from the UniProt Knowledgebase (ca.expasy.org) and correspond well to the observed protein masses. Deviations in the observed mass compared with the theoretical mass values are due to isoforms, truncation and post-processing and, or post-translational modifications of the proteins; modifications which also will influence the np-RP HPLC analyses. Thus, when characterizing a protein using this methodology one may observe changes in the np-RP HPLC chromatogram and within the protein mass spectrum.
Figure 3. MALDI-TOF MS analyses of the 9 protein mixture after np-RP HPLC separation.
The protein mixture (1 µg of each protein) was separated by np-RP HPLC. 24 fractions were collected from 14 to 20 min into a 96-well plate and subjected either to intact MALDI-TOF MS (A) or to peptide mass mapping after tryptic digestion (B). Each MALDI-TOF spectrum corresponds to an individual fraction. Table 2 provides intact molecular weight information and PMF database search results and sequence coverage for each protein.
Table 2.
Np-RP HPLC MALDI-TOF MS versus SDS-PAGE/MALDI-TOF MS PMF results comparison.
| MW (Da)(ii) | In-gel digestion | In-well digestion | ||||||
|---|---|---|---|---|---|---|---|---|
| (i)ID | Protein | SwissProt Id. # | Theor. | Obs. | # peptides matched | Coverage (%) | # peptides matched | Coverage (%) |
| A | Insulin | P01317 | 5733 | 5740 | 1 | (iii) | 2 | (iii) |
| B | Cytochrome C | P62894 | 11572 | 11741 | 14 | 66 | 11 | 57 |
| C | Trypsin Inhibitor | P01071 | 20041 | 19432 | 8 | 33 | 10 | 37 |
| D | GAPDH tetramer |
P46406 | 35689 146 KDa |
34371 | 11 | 30 | 9 | 24 |
| E | Myoglobin | P68082 | 16951 | 16379 | 16 | 81 | 16 | 94 |
| F | Catalase tetramer |
P00432 | 59784 200 kDa |
56953 | 24 | 40 | 21 | 37 |
| G | β-Lactoglobulin dimmer |
P02754 | 18281 36 kDa |
17778 | 9 | 48 | 9 | 56 |
| H | Glucose oxidase dimmer |
P13006 | 63273 160 kDa |
Nd | 10 | 22 | 11 | 30 |
| I | Ferritin (LC) multimer |
P02791 | 19819 450 kDa |
Nd | (iv) | (iv) | 7 | 39 (v) |
The protein standard (1 µg of each protein) was separated by either np-RP HPLC or by SDS-PAGE electrophoresis. Corresponding fractions or bands were respectively in-well or in-gel digested with trypsin and resulting peptides were analyzed by MALDI-TOF MS. For samples obtained from np-RP HPLC intact MALDI-TOF MS spectra were analyzed and corresponding molecular weights measured. The list of the peaks corresponding to each spectrum was submitted to Mascot database search software for protein identification. The number of peptides matching and percentage of sequence coverage were determined.
Protein ID and intact mass values correspond to Figure 1 and Figure 3a, PMF information is from Figure 3b.
Molecular weights were obtained from the UniProt Knowledgebase (ca.expasy.org); masses of multimeric forms are estimated.
Because insulin possesses only two sites of trypsin cleavage, the percentage of coverage was not informative enough to be shown.
Ferritin (500 kDa), which is composed of 24 peptide chains was not detected on the gel, probably due to the presence of reducing agent in the sample buffer.
The percentage of sequence coverage of the in-well digestion is given for Ferritin light chain (20 kDa).
The proteins were also subjected to MALDI-TOF MS peptide mass fingerprint analysis after tryptic digestion of the collected fraction, following the protocol developed for this project. Figure 3B displays the compilation of the MALDI-TOF peptide mass spectra. Protein retention times observed by np-RP HPLC with UV absorbance at 214 nm correlated well with PMF identification of the proteins obtained from the fractions. Although some protein overlap between different fractions was observed, the PMF results from this experiment, presented in Table 2, show sufficient sequence coverage for protein identification using this methodology.
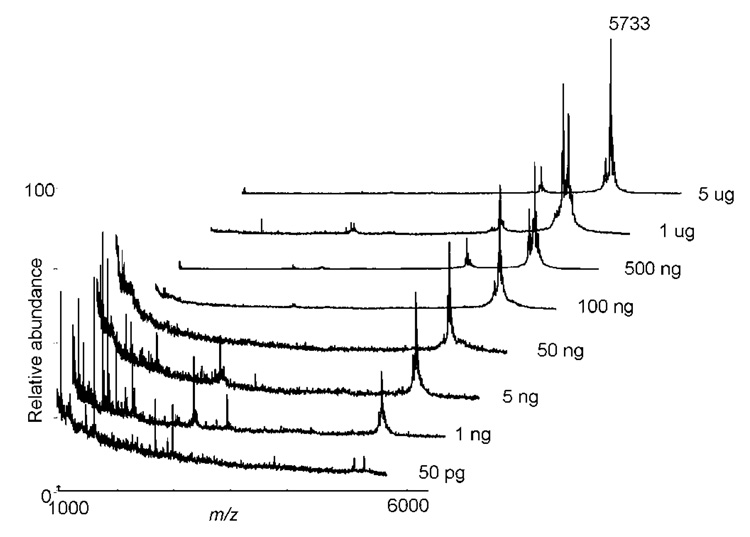
An estimate of the sensitivity of detection by intact protein MALDI-TOF MS was obtained on fractions corresponding to decreasing amounts of insulin separated by np-RP HPLC (Figure 4). Good MS signal was obtained on amounts of insulin varying over 5 orders of magnitude and sensitivity down to 50 pg (10 fmol) of insulin could be detected - improved results compared with detection limits and dynamic range typically observed with separations by 1DE methodology. These results also demonstrated that the absence of UV absorbance signal at 214 nm for protein amounts under 5 ng in Figure 1C is due to a lack of sensitivity of the detector and not to the loss of protein on the column.
Figure 4. MALDI-TOF MS analyses of np-RP HPLC separated intact insulin.
Decreasing amounts of the protein standard were separated by np-RP HPLC and fractions corresponding to insulin (13.75 to 14 min) were dried and analyzed by MALDI-TOF MS. Sensitivity of detection as shown was of the order of 50 pg.
Peptide detection sensitivity by MS after protein separation and in-well digestion was then evaluated. Fractions corresponding to decreasing amounts of cytochrome C separated by np-RP HPLC were digested by trypsin in the 96-well plates used for the fraction collection. Resulting peptides were analyzed by MALDI-TOF MS. Cytochrome C-related peptides were detected at concentrations of 500 pg (50 fmol) of protein (Figure 5), showing very good sensitivity of MS analysis. This result further demonstrates that even in the absence of detection by UV at 214 nm, proteins can be identified by MS and that the MS sensitivity is the limiting factor for protein identification using the np-RP HPLC MALDI-TOF MS instrumentation and methodology employed in this study. On the contrary, the reproducibility of SDS-PAGE electrophoresis is not sufficient to know exactly where a protein migrates, such that if the amount of protein within a gel is below the detection limit of the stain used, identifying proteins by MS after in-gel digestion may be very difficult.
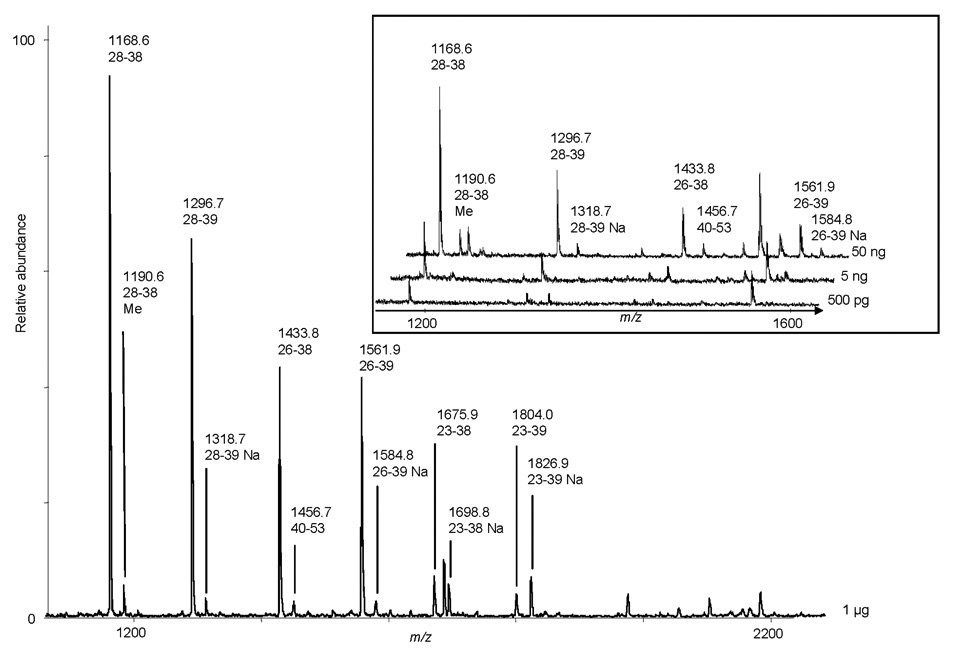
Figure 5. MALDI-TOF MS analyses of tryptic peptides from the np-RP HPLC fraction corresponding to cytochrome C.
Decreasing amounts of the protein standard were separated by np-RP HPLC and fractions corresponding to cytochrome C were collected, dried and digested with trypsin. Resulting peptides were analyzed by MALDI-TOF MS. Mass values shown correspond to theoretical peptides of cytochrome C; numbers indicate sequence. Inset shows m/z values for decreasing amounts of tryptic peptides from cytochrome C (50 ng–500 pg).
In-well versus in-gel digestion of separated proteins
To characterize our in-solution digestion protocol in terms of peptide recovery and sensitivity, we compared results from in-well digestion and in-gel digestion. For this purpose, 1 µg and 100 ng aliquots of protein standard were separated by 1D SDS-PAGE. Bands of interest were excised and processed following the in-gel digestion protocol. Peak lists were generated for all the spectra and submitted to database search using Mascot. Peptide mass fingerprinting results are presented in Table 2. Because insulin possesses only two sites for tryptic cleavage, the percentage of coverage was not informative enough to be displayed. No results were obtained for ferritin by in-gel digestion as ferritin was not observed in-gel. The percentage of sequence coverage for the in-well digestion of ferritin is provided for the ferritin light chain form of the protein.
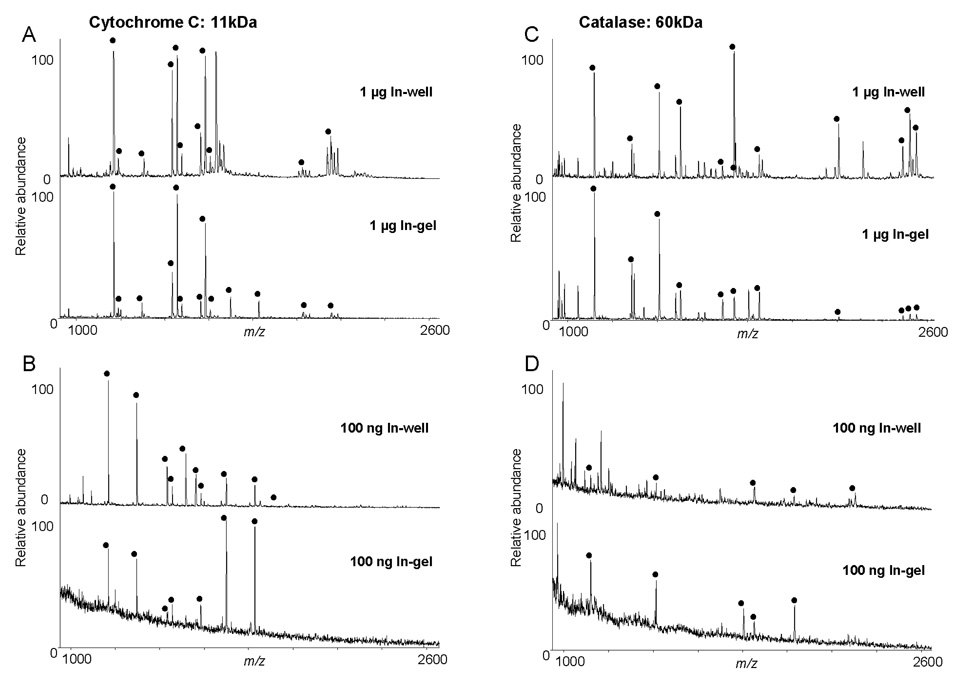
Bovine insulin was detected with poor S/N by MS after in-gel digestion of the 1 µg insulin band. On the contrary, the same amount of insulin collected after separation by np-RP HPLC produced very clear peaks by MALDI-TOF MS (Figure 3), showing that the relative recovery by in-well digestion after liquid separation was better that that obtained after in-gel separation. Large polypeptides like insulin tend to remain trapped in the gel and are difficult to extract. Moreover, we tested the protein recovery of the np-RP column by re-analyzing the standard mixture after a first separation by np-RP HPLC and obtained better than 80% recovery of the proteins. Several methods have been attempted to increase the recovery of intact proteins extracted from polyacrylamide gels. For example, sonication [43] or NaOH extraction [44] have been used, but recovery rates remained low (about 50%) and were limited to the range of about 30 ng, which is higher than the results which we obtained for insulin by np-RP HPLC. In general, the quality of mass spectra from the in-well digested proteins were superior to those of the spectra obtained from the in-gel digests of the proteins. Slightly improved sequence coverage was observed for myoglobin and trypsin inhibitor by in-well digestion, however, for glucose oxidase and GAPDH very similar results were obtained using the two methodologies (Table 2). The use of np-RP HPLC for protein separation did facilitate the identification of the multimeric protein complex ferritin as well as bovine insulin , a protein which is be too small to be adequately detected by SDS-PAGE electrophoresis and as shown here, too large to be extracted efficiently from the gel.
Separation and characterization of bovine endothelial membrane proteins
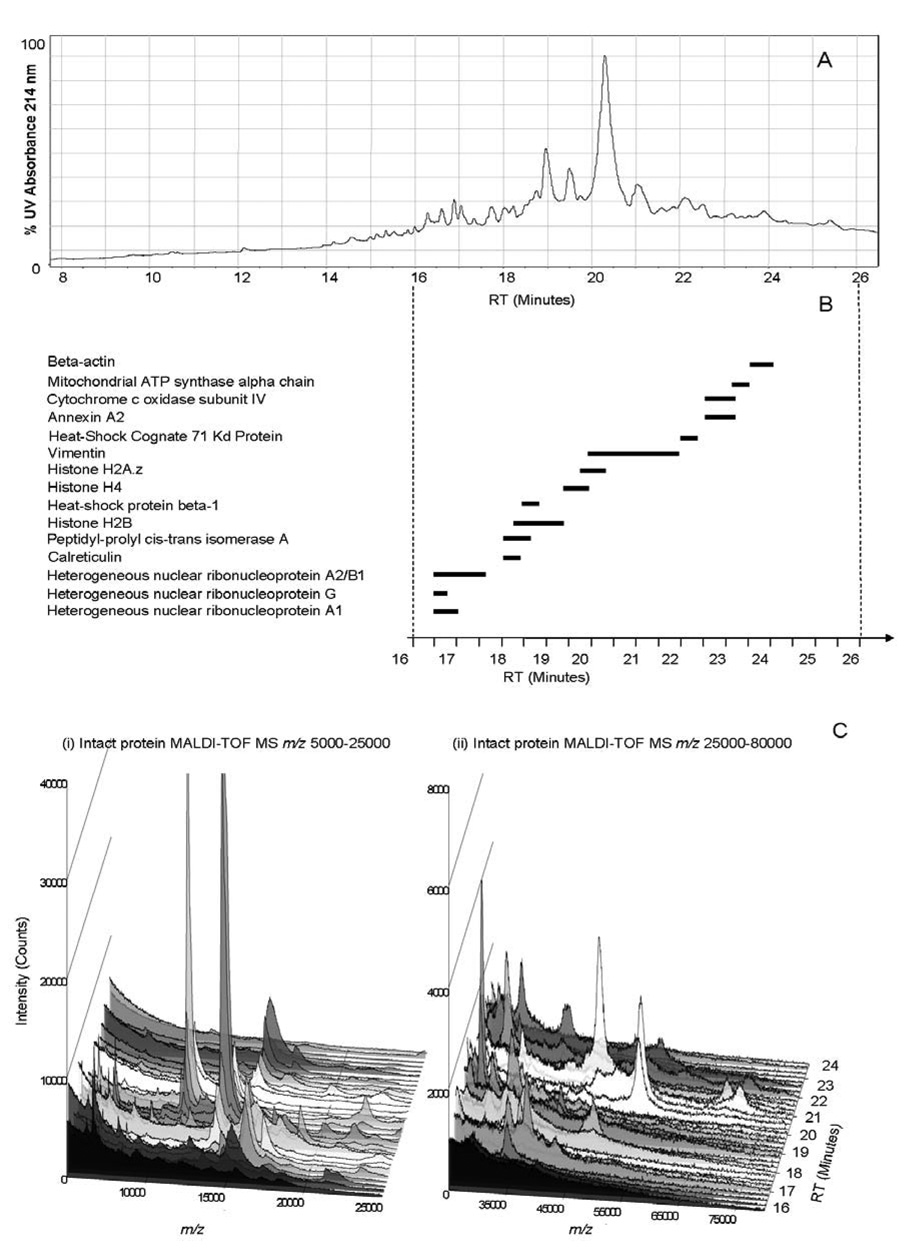
To demonstrate an application of the protein characterization methodology by np-RP HPLC MALDI-TOF MS using a real biological system, 100 µg of an endothelial cell membrane protein fraction were characterized (Figure 7A). Twenty-four fractions were collected and analyzed by PMF, in addition to analysis of the intact protein molecular weights by MALDI-TOF MS (Figure 7B, 7C). This use of intact protein MS as a second dimension of protein separation revealed that a large number of proteins were present in each fraction collected from the np-RP column due to the original sample complexity (Figure 7C). Even though this sample was quite complex, minimal overlap was observed between the adjacent fractions showing sufficient separation and resolution of the method and any such overlap was clearly resolved via the intact mass measurement of the protein fractions.
Figure 7. Endothelial cell membrane protein fraction separation by np-RP HPLC: UV214 nm and intact protein MS results.
100 µg of bovine endothelial cell membrane mixture were separated by np-RP HPLC. (A) Chromatogram from HPLC: UV214 nm. Twenty four fractions were analyzed either by intact MALDI-TOF MS or by peptide mass mapping. In-well trypsin digested fractions were spotted on a MALDI target and analyzed by MALDI-TOF MS. A list of the peaks corresponding to each spectrum was generated by MoverZ software (ProteoMetrics) and then submitted to Mascot (Matrixscience) database search software for protein identification. (B) MALDI-TOF MS results. The most abundant proteins are plotted following the retention time of the fractions where they were identified, corresponding to the HPLC UV-trace. (C) Intact endothelial cell membrane proteins observed by MALDI-TOF MS. Twenty four fractions collected after np-RP HPLC from 16 to 24 minutes were spotted on a MALDI target and analyzed by intact protein MALDI-TOF MS. Spectra are presented using SigmaPlot software (Systat Software Inc.). (i) Spectra recorded over the range m/z 5,000 to 25,000. (ii) Spectra recorded over the range m/z 25,000 to 80,000. Peptide mass fingerprint database search results and intact protein mass values are found in Table 3.
The collected proteins were then digested by trypsin and PMF analysis was performed using MALDI-TOF MS. Peak lists for each fraction were generated and submitted to MASCOT database search software. Correlating the PMF results with the intact mass measurements obtained on each of the fractions greatly improved protein identification (Table 3). Generally, PMF results showed good sequence coverage and good Mascot scores for the first protein identified within each fraction. However, the PMF analyses was limited to the identification of a restricted number of proteins within one sample, usually 2 or 3 of the top scoring proteins obtained from the database search. In these cases, the intact protein measurements allowed us to further characterize the proteins that differed in mass from one to another. Thus, some of the lower abundance proteins which were detected by intact MALDI-TOF MS were not identified by PMF because of the ion suppression effect of higher abundance species within the fraction. In other cases, identification of proteins with poorer Mascot scores, due of the complexity of each fraction, was facilitated and confirmed by the intact mass analysis. Intact proteins were observed varying in molecular mass from 8 kDa for mitochondrial ATP synthase e-chain, to 71 kDa for HSP70 (Table 3). The observed masses differed sometimes by several kDa from the identified protein’s theoretical mass because each expressed protein may exist in several isoforms and may undergo post processing such as truncation and, or may be post-translationally modified.
Table 3.
Np-RP HPLC MALDI-TOF MS of a membrane protein fraction characterized by PMF and intact MALDI-TOF MS.
| Fraction RT (min) | Protein ID | SwissProt / NCBInr identification # | Theoretical MW (Da) | Experimental MW (Da) | Mascot Score | # Peptides match | Coverage (%) |
|---|---|---|---|---|---|---|---|
| 16.5 | Heterogeneous nuclear ribonucleoprotein A1 | gi|76617606 | 38780 | 39958 | 64 | 9 | 33 |
| 16.75 | Heterogeneous nuclear ribonucleoprotein G | gi|76658693 | 32173 | 34299 | 61 | 12 | 37 |
| Heterogeneous nuclear ribonucleoprotein A2/B1 | gi|76662781 | 37407 | nd | 37 | 6 | 24 | |
| Heterogeneous nuclear ribonucleoprotein A1 | gi|76617606 | 38780 | nd | 41 | 7 | 31 | |
| 17 | Heterogeneous nuclear ribonucleoprotein A2/B1 | gi|76662781 | 37407 | 37515 | 148 | 21 | 57 |
| Heterogeneous nuclear ribonucleoprotein G | gi|76658693 | 32173 | 34348 | 45 | 9 | 33 | |
| 17.25 | Heterogeneous nuclear ribonucleoprotein A2/B1 | gi|76662781 | 37407 | 36137 | 137 | 16 | 57 |
| 17.5 | Heterogeneous nuclear ribonucleoprotein A2/B1 | gi|76662781 | 37407 | 36156 | 57 | 7 | 29 |
| 18 | Calreticulin | P52193 | 48009 | 48153 | 78 | 12 | 22 |
| Peptidyl-prolyl cis-trans isomerase A | P62935 | 17727 | 17683 | 40 | 5 | 35 | |
| 18.25 | Peptidyl-prolyl cis-trans isomerase A | P62935 | 17727 | 17706 | 47 | 6 | 47 |
| ATP synthase e chain, mitochondrial | Q00361 | 8184 | 8538 | 38 | 5 | 52 | |
| Histone H2B | P62808 | 13767 | 13769 | 40 | 7 | 40 | |
| 18.5 | Heat-shock protein beta-1 | Q3T149 | 22379 | 22316 | 57 | 8 | 53 |
| ATP synthase e chain, mitochondrial | Q00361 | 8184 | 8492 | 52 | 7 | 38 | |
| 18.75 | Histone H2B | P62808 | 13767 | 13779 | 48 | 9 | 56 |
| 40S ribosomal protein S7 | gi|76666230 | 41894 | nd | 44 | 10 | 24 | |
| 19 | Histone H2B | P62808 | 13797 | 13752 | 138 | 15 | 78 |
| Keratin, type II cytoskeletal | P05786 | 42369 | 41258 | 32 | 6 | 19 | |
| ATP synthase D chain, mitochondrial | P13620 | 18550 | 18128 | 34 | 4 | 32 | |
| 19.25 | Histone H2B | P62808 | 13767 | 13738 | 80 | 12 | 52 |
| Ezrin | P31976 | 68586 | nd | 37 | 12 | 20 | |
| Hydroxyindole O-methyltransferase | P10950 | 37900 | 38661 | 32 | 7 | 15 | |
| 19.5 | Histone H4 | P62803 | 11229 | 11301 | 201 | 18 | 79 |
| Histone H2A.z | P0C0S4 | 13414 | 13762 | 44 | 6 | 62 | |
| Cytochrome P450 | P46194 | 58052 | 38366 | 32 | 9 | 18 | |
| 19.75 | Histone H4 | P62803 | 11229 | 11306 | 68 | 9 | 62 |
| Histone H2A.1b | P0C0S9 | 13952 | 13932 | 42 | 6 | 58 | |
| Histone H2A.z | P0C0S4 | 13414 | 13365 | 42 | 6 | 55 | |
| 20 | Vimentin | P02543 | 51513 | nd | 94 | 13 | 39 |
| Glyceraldehyde-3- phosphate dehydrogenase | P10096 | 35714 | 35983 | 25 | 5 | 15 | |
| 20.25 | Vimentin | P02543 | 53513 | 53915 | 305 | 32 | 71 |
| Peptidyl-prolyl cis-trans isomerase B precursor | P80311 | 22687 | 22537 | 27 | 4 | 31 | |
| Histone H2A.1b | P0C0S9 | 13952 | 14029 | 29 | 3 | 51 | |
| 20.5 | Vimentin | P02543 | 53513 | 53482 | 311 | 33 | 67 |
| Histone H2A.1b | P0C0S9 | 13952 | 13983 | 30 | 3 | 44 | |
| 20.75 | Vimentin | P02543 | 53513 | 53722 | 379 | 36 | 70 |
| Keratin, type I cytoskeletal 19 | P08728 | 43858 | nd | 36 | 5 | 17 | |
| 21 | Vimentin | P02543 | 53513 | 53764 | 238 | 29 | 66 |
| 40S ribosomal protein SA | P26452 | 32747 | 32806 | 45 | 6 | 30 | |
| Gamma-aminobutyricacid receptor | P10063 | 51113 | nd | 38 | 7 | 19 | |
| 21.25 | Vimentin | P02543 | 53513 | 53902 | 233 | 27 | 67 |
| Recoverin | P21457 | 23188 | 23337 | 30 | 4 | 32 | |
| Moesin | gi|76659545 | 67488 | 68128 | 39 | 10 | 20 | |
| 21.5 | Vimentin | P02543 | 53513 | 53753 | 177 | 20 | 48 |
| Moesin | gi|76659545 | 67488 | 68073 | 50 | 10 | 14 | |
| Histone H3.1 | P68432 | 15263 | 15303 | 25 | 4 | 24 | |
| 21.75 | Vimentin | P02543 | 53513 | 53807 | 87 | 12 | 31 |
| Platelet-activating factor acetylhydrolase 2 | P79106 | 43837 | 43887 | 35 | 5 | 17 | |
| 60S ribosomal protein L7 | Q58DT1 | 29150 | 29329 | 49 | 9 | 29 | |
| 22 | Heat shock cognate 71 kDa protein | P19120 | 71195 | 71324 | 60 | 12 | 19 |
| Vimentin | P02543 | 53513 | 53926 | 62 | 11 | 29 | |
| Histone H3.1 | P68432 | 15263 | 15328 | 32 | 7 | 34 | |
| 22.25 | Annexin A2 | P04272 | 38457 | 38857 | 136 | 15 | 31 |
| Cytochrome c oxidase subunit IV | P00423 | 19559 | nd | 38 | 6 | 37 | |
| NADH-ubiquinone oxidoreductase subunit B17.2 | O97725 | 17079 | 17113 | 36 | 5 | 44 | |
| 22.5 | Annexin A2 | P04272 | 38457 | 38799 | 53 | 8 | 52 |
| Dehydrogenase/reductase SDR family member 4 | Q8SPU8 | 27368 | 28018 | 42 | 6 | 28 | |
| Collagen alpha-3(IV) chain | Q28084 | 47554 | nd | 33 | 6 | 20 | |
| 22.75 | Annexin A2 | P04272 | 38457 | 38827 | 101 | 13 | 44 |
| Cytochrome c oxidase subunit IV | P00423 | 19559 | 19927 | 38 | 6 | 37 | |
| NADH-ubiquinone oxidoreductase subunit B17.2 | O97725 | 17079 | nd | 36 | 5 | 44 | |
| Ras GTPase-activating protein 4 | gi|76653884 | 83561 | nd | 67 | 13 | 20 | |
| 23.25 | ATP synthase alpha chain heart isoform, mitochondrial precursor | P19483 | 59683 | nd | 149 | 20 | 43 |
| 23.5 | 60S ribosomal protein L10a | Q5E9E6 | 24684 | 24928 | 59 | 9 | 40 |
| Beta Actin | P60712 | 41710 | nd | 37 | 7 | 21 | |
| 23.75 | Beta Actin | P60712 | 41710 | nd | 47 | 6 | 22 |
Intact molecular mass and PMF database search results obtained from a bovine endothelial cell membrane sample. One hundred µg total proteins was separated by np-RP HPLC, collected into a 96-well plate and in-well digested with trypsin. Fractions were analyzed by MALDI-TOF MS. The list of the peaks corresponding to each spectrum was submitted to Mascot database search software for protein identification. Identified protein molecular weights were compared and matched with experimental protein masses obtained from intact MALDI-TOF MS analysis, whose spectra are displayed in Figure 7C. (nd: not detected)
Figure 7B shows a schematic of the overlap of the most abundant proteins identified. Vimentin, a regulated intermediate filament protein which is known to interact strongly with the plasma membrane [45], was observed in abundance and was eluted from the np-RP column between 20 and 22 minutes (Figure 7A). The detected molecular mass of vimentin was observed to increase through the fractions (Table 3), indicating differences in the proteins mass due to isoforms and, or PTMs.
Several histones were also identified in the sample based on good Mascot scores obtained from PMF results and the correlated molecular weights of measured on the intact proteins (Table3). Histones are abundant nuclear proteins which were co-purified during the membrane isolation process. Histones are interesting proteins which have already been found to be present in the surface of lymphocytes [46], suggesting a possible role of these proteins in cellular recognition. Interestingly, we were able to separate and identify several different forms of histones: H2B, H4 and H2A.z, H2A.1b and H3.1 by np-RP HPLC. Similar to membrane proteins, histones have a very basic pI (around 10–12) and are difficult to separate and analyze by gel electrophoresis. Proteins from the ribosomal membrane were also largely represented due to their very high abundance within the sample. The larger peak width of some identified proteins reflects the separation of different isoforms or post-translationally modified forms of the protein. Full characterization of these species will require further analysis, including MALDI-MS/ MS analysis at the peptide level, and are the subject of future research.
CONCLUSIONS
This study focused on evaluating the use of non-porous reversed phase protein chromatography coupled with MALDI-TOF MS as an alternative to SDS-PAGE methodology for protein identification via intact protein mass measurement and PMF. The np-RP HPLC MALDI TOF MS methodology developed here was proven to be very reproducible in terms of protein separation and to exhibit an linearity of UV detection up to 3 orders of magnitude. The detection limit of the UV detector was estimated at 5 ng for well-resolved proteins. Intact proteins were analyzed by MALDI-TOF MS for molecular weight estimation and a sensitivity of 50 pg (10 fmol) of intact protein was achieved by MALDI-TOF MS after np-RP HPLC separation. Protein fractions were also digested by trypsin following a rapid and robust protocol which enabled us to detect peptides at concentrations of 500 pg (50 fmol) and provided for high quality mass spectra which yielded good database search results. The integrated np-RP HPLC MALDI-TOF-MS methodology developed here was shown to be as efficient, or better than 1D-SDS-PAGE separation and in-gel digestion in terms of peptide recovery and sequence coverage via database search. Characterization of a membrane protein sample obtained from a biological sample was used as an application of this proteomics methodology. The intact masses of the proteins present within each fraction were measured and correlated well with the results of protein identification obtained from PMF analysis of the digested proteins. Overall, we demonstrated that the np-RP HPLC MALDI-TOF-MS system allows proteins from a complex sample to be rapidly fractionated and characterized through a combination of the intact molecular weight information obtained by MS and the information obtained from PMF database search after in-solution digestion.
Figure 6. In-well versus in-gel digestion of separated proteins.
Cytochrome C (A and B) and catalase (C and D) (1 µg or 100 ng) were separated from the whole protein standard by either by np-RP HPLC or by SDS-PAGE electrophoresis. Corresponding fractions or bands were respectively in-well or in-gel digested with trypsin and resulting peptides were analyzed by MALDI-TOF MS. Spectra corresponding to peptides from cytochrome C and catalase were aligned and peaks which matched the theoretical digest of the protein are highlighted (●) for each protein. Values correspond to results shown in Table 2.
ACKNOWLEDGMENTS
Funding was from NIH-NHLBI Contract N01 HV28178 and NIH grants P41 RR10888 and S10 RR15942. The authors thank Beckman Coulter for their assistance with the ProteomeLab™ PF 2D system and Richard A. Cohen, and Nicolas Clavreul for providing the endothelial cell membrane sample and Hua Huang for her assistance and advice.
ABBREVIATIONS
- np-RP
non-porous reversed phase
- MALDI
matrix-assisted laser desorption ionization
- TOF
time-of-flight
- MS
mass spectrometry
- PMF
peptide mass fingerprint
- 1D SDS-PAGE
one-dimensional sodium-dodecyl sulfate polyacrylamide gel electrophoresis
REFERENCES
- 1.Tyers M, Mann M. Nature. 2003;422:193–197. doi: 10.1038/nature01510. [DOI] [PubMed] [Google Scholar]
- 2.Pandey A, Mann M. Nature. 2000;405:837–846. doi: 10.1038/35015709. [DOI] [PubMed] [Google Scholar]
- 3.Abbott A. Nature. 1999;402:715–720. doi: 10.1038/45350. [DOI] [PubMed] [Google Scholar]
- 4.Venter JC, Adams MD, Myers EW, Li PW, et al. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 5.Aebersold R, Mann M. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 6.Anderson NL, Anderson NG. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 7.Omenn GS, States DJ, Adamski M, Blackwell TW, Menon R, et al. Proteomics. 2005;5:3226–3245. doi: 10.1002/pmic.200500186. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Gong Y, Wang Y, Wu S, et al. Proteomics. 2005;5:3423–3441. doi: 10.1002/pmic.200401226. [DOI] [PubMed] [Google Scholar]
- 9.Cho SY, Lee EY, Lee JS, Kim HY, et al. Proteomics. 2005;5:3386–3396. doi: 10.1002/pmic.200401310. [DOI] [PubMed] [Google Scholar]
- 10.Bichsel VE, Liotta LA, Petricoin EF., 3rd Cancer J. 2001;7:69–78. [PubMed] [Google Scholar]
- 11.Arrell DK, Neverova I, Van Eyk JE. Circ Res. 2001;88:763–773. doi: 10.1161/hh0801.090193. [DOI] [PubMed] [Google Scholar]
- 12.Klose J. Humangenetik. 1975;26:231–243. doi: 10.1007/BF00281458. [DOI] [PubMed] [Google Scholar]
- 13.O'Farrell PH. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 14.Scheele GA. J Biol Chem. 1975;250:5375–5385. [PubMed] [Google Scholar]
- 15.Hillenkamp F, Karas M, Beavis RC, Chait BT. Anal Chem. 1991;63:1193A–1203A. doi: 10.1021/ac00024a002. [DOI] [PubMed] [Google Scholar]
- 16.Hillenkamp F, Karas M. Methods Enzymol. 1990;193:280–295. doi: 10.1016/0076-6879(90)93420-p. [DOI] [PubMed] [Google Scholar]
- 17.Krause E, Wenschuh H, Jungblut PR. Anal Chem. 1999;71:4160–4165. doi: 10.1021/ac990298f. [DOI] [PubMed] [Google Scholar]
- 18.Neverova I, Van Eyk JE. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815:51–63. doi: 10.1016/j.jchromb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Zurbriggen K, Schmugge M, Schmid M, Durka S, et al. Clin Chem. 2005;51:989–996. doi: 10.1373/clinchem.2005.047985. [DOI] [PubMed] [Google Scholar]
- 20.Wu S, Tang XT, Siems WF, Siems WF, Bruce JE. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;822:98–111. doi: 10.1016/j.jchromb.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Martosella J, Zolotarjova N, Liu H, Nicol G, Boyes BE. J Proteome Res. 2005;4:1522–1537. doi: 10.1021/pr050088l. [DOI] [PubMed] [Google Scholar]
- 22.Neverova I, Van Eyk JE. Proteomics. 2002;2:22–31. [PubMed] [Google Scholar]
- 23.Wall DB, Lubman DM, Flynn SJ. Anal Chem. 1999;71:3894–3900. doi: 10.1021/ac990120t. [DOI] [PubMed] [Google Scholar]
- 24.Maa YF, Horvath C. J Chromatogr. 1988;445:71–86. doi: 10.1016/s0021-9673(01)84509-x. [DOI] [PubMed] [Google Scholar]
- 25.Banks JF, Gulcicek EE. Anal Chem. 1997;69:3973–3978. doi: 10.1021/ac970226t. [DOI] [PubMed] [Google Scholar]
- 26.Lee WC. J Chromatogr B Biomed Sci Appl. 1997;699:29–45. doi: 10.1016/s0378-4347(97)00179-5. [DOI] [PubMed] [Google Scholar]
- 27.Itoh H, Nimura N, Kinoshita T, Nagae N, Nomura M. Anal Biochem. 1991;199:7–10. doi: 10.1016/0003-2697(91)90261-q. [DOI] [PubMed] [Google Scholar]
- 28.Ottens AK, Kobeissy FH, Wolper RA, Haskins WE, et al. Anal Chem. 2005;77:4836–4845. doi: 10.1021/ac050478r. [DOI] [PubMed] [Google Scholar]
- 29.Sheng S, Chen D, Van Eyk JE. Mol Cell Proteomics. 2005 doi: 10.1074/mcp.T500019-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Chen EI, Hewel J, Brunhilde Felding-Habermann B, Yates JR., 3rd Mol Cell Proteomics. 2005 doi: 10.1074/mcp.T500013-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Billecke C, Malik I, Movsisyan A, Sulghani S, et al. Mol Cell Proteomics. 2005 doi: 10.1074/mcp.M500124-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Opiteck GJ, Ramirez SM, Jorgenson JW, Moseley MA., 3rd Anal Biochem. 1998;258:349–361. doi: 10.1006/abio.1998.2588. [DOI] [PubMed] [Google Scholar]
- 33.Meng F, Cargile BJ, Patrie SM, Johnson JR, et al. Anal Chem. 2002;74:2923–2929. doi: 10.1021/ac020049i. [DOI] [PubMed] [Google Scholar]
- 34.Fujii K, Nakano T, Kawamura T, Usui F, et al. J Proteome Res. 2004;3:712–718. doi: 10.1021/pr030007q. [DOI] [PubMed] [Google Scholar]
- 35.Coldham NG, Woodward MJ. J Proteome Res. 2004;3:595–603. doi: 10.1021/pr034129u. [DOI] [PubMed] [Google Scholar]
- 36.Jilge G, Janzen R, Giesche H, Unger KK, et al. J Chromatogr. 1987;397:71–80. doi: 10.1016/s0021-9673(01)84990-6. [DOI] [PubMed] [Google Scholar]
- 37.Janzen R, Unger KK, Giesche H, Kinkel JN, Hearn MT. J Chromatogr. 1987;397:81–89. doi: 10.1016/s0021-9673(01)84991-8. [DOI] [PubMed] [Google Scholar]
- 38.Janzen R, Unger KK, Giesche H, Kinkel JN, Hearn MT. J Chromatogr. 1987;397:91–97. doi: 10.1016/s0021-9673(01)84992-x. [DOI] [PubMed] [Google Scholar]
- 39.O'Neil KA, Miller FR, Barder TJ, Lubman DM. Proteomics. 2003;3:1256–1269. doi: 10.1002/pmic.200300446. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Wall D, Lubman DM. Rapid Commun Mass Spectrom. 1998;12:1994–2003. doi: 10.1002/(SICI)1097-0231(19981230)12:24<1994::AID-RCM423>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Snyder LR, Kirkland JJ. Introduction to modern liquid chromatography. New York: Wiley; 1979. p. xix.p. 863. [Google Scholar]
- 42.Brogle K, Ornaf RM, Wu D, Palermo PJ. J Pharm Biomed Anal. 1999;19:669–678. doi: 10.1016/s0731-7085(98)00290-8. [DOI] [PubMed] [Google Scholar]
- 43.Retamal CA, Thiebaut P, Alves EW. Anal Biochem. 1999;268:15–20. doi: 10.1006/abio.1998.2977. [DOI] [PubMed] [Google Scholar]
- 44.Jin Y, Manabe T. Electrophoresis. 2005;26:2823–2834. doi: 10.1002/elps.200410421. [DOI] [PubMed] [Google Scholar]
- 45.Ketis NV, Hoover RL, Karnovsky MJ. J Cell Physiol. 1986;128:162–170. doi: 10.1002/jcp.1041280205. [DOI] [PubMed] [Google Scholar]
- 46.Watson K, Edwards RJ, Shaunak S, Parmelee DC, et al. Biochem Pharmacol. 1995;50:299–309. doi: 10.1016/0006-2952(95)00142-m. [DOI] [PubMed] [Google Scholar]