Abstract
Background
Lung graft dysfunction and rejection remain a significant cause of morbidity and mortality in transplant recipients. Thioredoxin-1 (Trx), a redox-regulatory protein, has been known to function as an antioxidant against oxidative injury in multiple organs including lungs. We examined whether priming of the donor lungs with Trx prior to transplantation attenuates acute lung injury.
Methods
Orthotopic left lung transplantation was performed from Lewis (donor) to Sprague-Dawley (recipient) rats using the cuff technique. For Trx priming, the donor lungs were perfused and stored in Perfadex solution with or without the presence of purified Trx prior to transplantation. Changes in bronchoalveolar (BAL) fluid analysis, allograft oxygen exchange function, nuclear factor kappa B (NF-kB)/DNA binding, myeloperoxidase (MPO) activities, and immunohisotologic evaluation of neutrophils, macrophages and cytotoxic T-cells (CD8+) infiltration were examined in one and/or five day post-transplant allograft (left) and native (right) lungs.
Results
BAL cell differential analysis showed significant increases in macrophages and neutrophils in one day post-transplant whereas lymphocyte infiltration was significantly increased in both one and five days post transplant allografts. MPO and NF-kB/DNA binding activities were increased over basal activities one and five days post transplant. Immunohistology staining of one and five day post transplant allografts revealed increased infiltration of macrophages, neutrophils, and CD8+ T cell subsets. Priming of donor lungs with Trx prior to transplantation improved O2 exchange and attenuated NF-kB/DNA binding activity and infiltration of macrophages, neutrophils, and CD8+ T cell subsets in one and five day post transplant allografts.
Conclusions
Priming of donor lungs with Trx prior to transplantation attenuates acute allograft injury in a rat model of lung transplantation. This protection appears to be associated with Trx’s antioxidant function that limits early I/R injury, NF-kB activation, and progressive infiltration of inflammatory and immune cells in allografts.
Keywords: Lung transplantation, Thioredoxin, Macrophage, Cytotoxic T-cell, NF-κB, MPO
INTRODUCTION
Lung transplantation is an accepted treatment for patients with progressive end-stage pulmonary disease (1–3). Despite improvements in preoperative and postoperative care, early graft dysfunction and rejection remain primary causes of increased mortality and morbidity after lung transplantation (4). A number of human and animal studies demonstrated that ischemia-reperfusion (I/R) injury is one of the major factors that play a critical role in enhancing early graft dysfunction via progressive infiltration of inflammatory and immune cells leading to generation of mediators and increased sequestration of circulating leukocytes (5–12). Increased oxidative stress and/or enhanced generation of multiple mediators including thioredoxin-1 (Trx) are implicated in progression of lung allograft rejection in canine and rat models (13, 14) and human lung transplant recipients (15). Increased levels of Trx and other biological mediators are directly or indirectly regulated by the Rel family transcription factor, nuclear factor kappa B (NF-kB), in multiple cells including inflammatory and immune cells (16–23). A recent report demonstrates increased population of cytotoxic CD8+ cytokine-expressing T cells in BAL of patients with chronic obstructive pulmonary disease (24). Persistent activation of NF-kB is required for increased expression of inflammatory mediators as well as for T-cell responses in vivo (25, 26). As such, activation of NF-kB and increased expression of biological risk factors including Trx appear to be a potent combination for progression of post-transplant allograft injury.
Trx is a 12 kDa cytosolic protein with a highly conserved active site containing two redox-sensitive cysteine residues (Trp-Cys-Gly-Pro-Cys-Lys). Although there are several isoforms of Trx, only cytosolic Trx translocates to the nucleus and plays a role in NF-kB activation and the regulation of gene expression (27, 28). Increased Trx expression has been shown to control a wide spectrum of biological activities ranging from cell growth to death (16–23, 27–31). This indicates that the levels of Trx and/or specific timing of Trx modulation are most likely associated with Trx-mediated diverse biological responses. Since nothing is known regarding potential benefits of Trx priming of donor lungs in context with limiting or preventing allograft injury in human and animal models of lung transplantation, the present study was designed to determine the role of Trx in pre-transplant priming of donor lungs and attenuation of post-transplant progression of allograft injury in a rat model of lung transplantation.
MATERIALS AND METHODS
Animals
Specific pathogen-free, male Lewis and Sprague-Dawley rats (250–300 g) were used and cared for in compliance with the “Guide for the Use of Laboratory Animals” published by NIH and used in this IACUC-approved protocol.
Surgical Procedures
Orthotopic left lung transplantation was performed from Lewis (donor) to Sprague-Dawley (recipient) rats using the cuff technique as described by Mizuta et al (32). All surgical procedures were performed under sterile conditions.
Donor Lung Harvest and Storage
Donor lungs were isolated using previously described procedures (32). The lungs were then flushed with 20 ml preservation solution (Perfadex®, Vitrolife, Uppsala, Sweden) with or without human recombinant purified Trx (4 µg/ml, USB Corporation, Cleveland,OH) for 30 min. Immediately after flushing the lungs, the tracheostomy tube was clamped following inspiration to preserve the lungs in the inflated state. The heart-lung block was then removed and placed in ice-cold preservation solution for 4 hr. The left lung was prepared for transplantation with the placement of 14-gauge cuffs into the left main bronchus (MB) and left pulmonary vein (PV) and 16-gauge cuffs into the left PA, respectively. Tissue levels of Trx prior to transplantation were determined using lung homogenates as previously described (20). The endogenous lung contents of Trx with or without (control) Trx priming were 12.0 ± 3.1 ng/mg protein in control and 20.1 ± 2.4 ng/mg protein in Trx primed lungs (n = 4 in each set).
Orthotopic Left Lung Transplantation
Recipient animals were anesthetized, intubated and ventilated as described as above. After administration of atropine (0.25 mg/kg) intramuscularly, a left thoracotomy was performed through the fourth intercostal space. The left lung was mobilized by dividing the pulmonary ligament. The hilar structures were then dissected free. The left PA, PV, and MB were identified and clamped with microsurgical aneurysm clamps. A ventral incision was made in each of these structures. The cuffs on the donor lung structures were placed into the corresponding recipient structures. The anastomoses were secured with 6.0 silk ties. The clamp was removed, and the transplanted lung was reinflated. The surgical site was rinsed to assure there was no bleeding and the lung was placed inside the chest cavity. The chest was closed in layers after attaining complete hemostasis. After transplantation, rats were maintained for one or five days without administration of immunosuppressive agents. Biochemical and morphologic changes in one and five day post-transplant allograft were compared with native lungs unless otherwise indicated.
Left Lung Function Assessment
For allograft lung function, one day and five days post-transplant animals with or without Trx-priming were anesthetized and ventilated with 100% inspired oxygen. After dissection of the right hilum, microvascular clips were applied to the right main bronchus and right PA in order to ventilate and perfuse only the graft (left) lung. Four minutes after the occlusion, 0.5 ml of arterial blood were obtained from the left ventricle for measurement of PaO2 using an i-STAT blood gas analyzer (Heska, Fort Collins, CO). To determine PaO2 level in controls, identical procedures were employed using Sprague-Dawley rats without transplantation.
Biochemical and Histopathologic Characterization of Lung Transplant Model
After one or five days post-transplant, right (native) and left (transplanted) lungs were lavaged with 5 ml of isotonic saline solution. Lung tissues were collected separately for biochemical analyses (including wet/dry weight ratios, graft lung(g)/body weight(kg) ratio, total protein contents, cell differential analysis, and histologic analysis in day 1 and 5 day post-transplant allografts.
BAL Cell Differentiation
BAL fluids were centrifuged (1,300 × g, 5 min, 4°C) and cell suspensions used to prepare slides for differential counting of nucleated cells. Cytospin slides were stained with Leukostate (Fisher Diagnostics, Orlando, FL) and differential cell counts for neutrophils, macrophages, lymphocytes, and eosinophils were expressed as percent of total cell population.
NF-κB/DNA Binding Activity
Electrophoretic mobility shift assay was used to determine the DNA band shift using oligonucleotide (5'-AGA CCT GG ACT CTC CCT CCC AGC-3') representing specific NF-κB binding sequences in the Trx gene (20). The nuclear fractions were isolated from the native and allograft lung tissues, and the Trx-specific oligonucleotide NF-κB binding was determined using a 10-fold excess of unlabeled oligonuleotide as we previously described (20).
MPO Activity
The level of MPO activity as a marker of inflammation (33) was determined as previously described (34). In brief, lung tissue homogenates in 50 mM potassium phosphate buffer, pH 7.0 containing 2% protease inhibitor cocktail and 0.5% hexadecyltrimethylammonium bromide were centrifuged at 5000 × g (4° C) for 20 min, and 30 µl of supernatant mixed with 0.25 ml of the same phosphate buffer containing 0.167 mg/ml orthodianisidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance was monitored at 450 nm. Protein contents were determined by Bradford analysis. MPO activity was defined as the change in absorbance per minute per milligram of protein.
Histopathology Analysis
One and five day post-transplant lungs were fixed in 10% formalin and embedded in paraffin. Serial sections (5 µm) of native and allograft lungs were stained with hematoxylin and eosin (H&E). The histological analysis of the allografts was evaluated microscopically in a blinded fashion. The morphometric study was performed on H&E stained one and five day post-transplant allografts with or without Trx priming in a blinded fashion. The degree of injury was expressed as the percentage of damaged alveolar architecture compared to the native lung in ten randomly selected high power (200 ×) fields of micrographs.
Immunohistochemistry Evaluation
To detect macrophage/monocyte and cytotoxic T cell infiltration, immunohistochemical staining was performed as described previously (35). In brief, 5 µm sections of the fixed lung tissues were deparaffinized in toluene, hydrated in graded ethanol solution, and immersed in H2O2 (0.3% H2O2 in methanol) to block endogenous peroxidase activity. To determine the level of infiltration of macrophages, cytotoxic T cell subsets, and neutrophils, immunostaining of the native and allograft tissues was performed using affinity-purified mouse monoclonal anti-ED1 (1:500 dilution), mouse monoclonal anti-CD8 (1:500 dilution), and rat polyclonal anti-MPO antibodies (1:250 dilution) (BD Pharmingen, San Diego, CA), respectively. To retrieve the antigen in formalin fixed tissue, sections were treated with Trilogy antigen retrieval solution (Cell Marque, Hot Springs, AR) for 10 minutes at 90° C. After antigen retrieval, the sections were washed in phosphate buffered saline (PBS) and incubated with primary antibodies overnight at 4° C. Bound primary antibodies were detected with an anti-mouse peroxidase labeled secondary antibody, followed by mouse anti-peroxidase (DAKO, Carpinteria, CA). Brown color was generated using a diaminobenzidine substrate (DAB) (Sigma, St. Louis, MO) and counterstained with hematoxylin. Negative controls consisted of omission of the primary antibody with the appropriate pre-immune serum. The numbers of ED-1+, CD8+, and MPO-positive cells were counted in 10 randomly selected high-power microscopic fields (400 ×) per sample in a blinded fashion.
Statistical Analysis
Data are expressed as the mean ± SE. Groups were compared using analysis of variance, and Student t-test was used to compare the degree of allograft injury, MPO activity, and number of ED-1+, CD8+, and MPO+ cells between native and allograft lungs. P<0.05 was considered statistically significant.
RESULTS
Biochemical characterization of lung transplant model
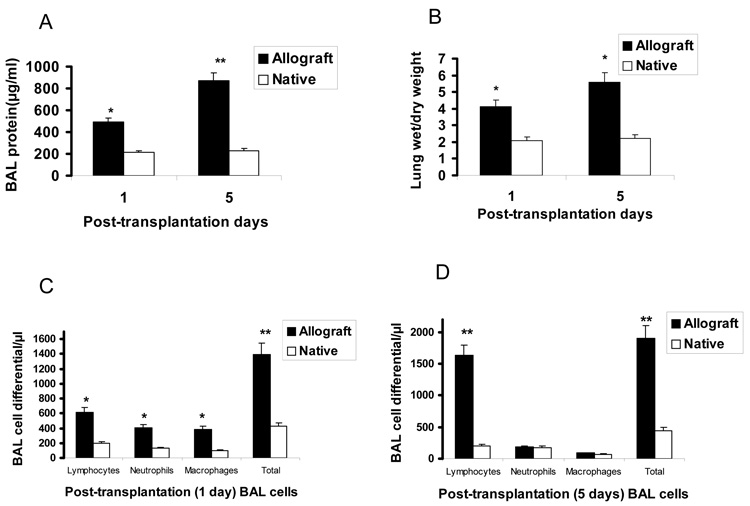
As shown in Fig. 1, there were significant increases in total protein contents (panel A) and wet/dry weight ratios (panel B) in one and five day post-transplant allografts (p < 0.05 for days 1 and 5 for both). Cell differential analysis showed significant increases in the total BAL cells in both 1 day (panel C) and day 5 (panel D) post transplant allografts. Day 1 post-transplant allografts showed significant increases in macrophages, neutrophils, and lymphocytes, whereas day 5 allografts showed significant infiltration of lymphocytes only (p < 0.05 for both days and cell types).
Fig. 1.
Biochemical characterization of native and one and five day post-transplant allografts. Panels A and B are total protein content of BAL and lung wet/dry weight, respectively. Panel C and D are BAL cell differential counts of one and five days post-transplant lungs, respectively. Data represent mean ± SE, n = 5 for each measurement, * P < 0.05, ** P < 0.01 vs native for all measurements.
Increased infiltration of inflammatory cells is associated with enhanced MPO activity in allografts
Basal MPO activity, i.e., prior to transplantation in donor lungs that were preserved for 4 hr total ischemic time (4 hr cold/1 hr warm), was 0.9 ± 0.03 (Δ OD/min/mg, n= 6). MPO activity was increased from basal level to 1.8 ± 0.7 (Δ OD/min/mg, n=4) at 1 day post-transplant and to 6.7 ± 0.9 (Δ OD/min/mg, n=4) in 5 day post-transplant allografts (p < 0.05 for both vs basal).
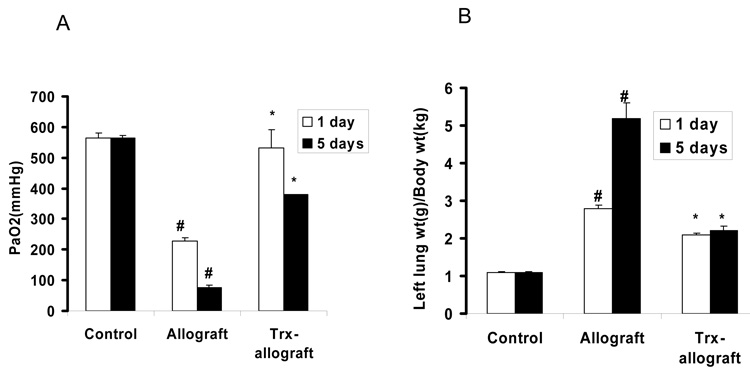
Trx priming of donor lungs improves allograft function
As shown in Fig. 2A, PaO2 was significantly reduced in one and five day post-transplant. In contrast, Trx priming of donor lungs prior to transplantation restored PaO2 in post transplant allografts close to PaO2 levels in control lungs. Fig. 2B shows a significant increase of lung wet weight to body weight ratio of allografts compared to controls, whereas Trx priming significantly reduced lung wet weight to body weight ratios compared to allografts.
Fig. 2.
The effect of Trx on allograft gas exchange function and the changes of allograft (left lung) wet weight to body weight ratios. Panel A shows PaO2 levels in one and five day post-transplant allografts ventilated for 5 min with 100% oxygen with or without Trx priming. A separate group of Sprague-Dawley rats was used to determine control levels of PaO2 in single left lung ventilation without transplantation using identical procedures (Control). Panel B shows the ratio of left lung (allograft with or without Trx priming or normal left lung as control) wet weight (g) to body weight (kg). .# p<0.05 vs control for respective days in both panels A and B; * P<0.05 vs allograft for respective days in both panels A and B (n=3 to 5 for each group).
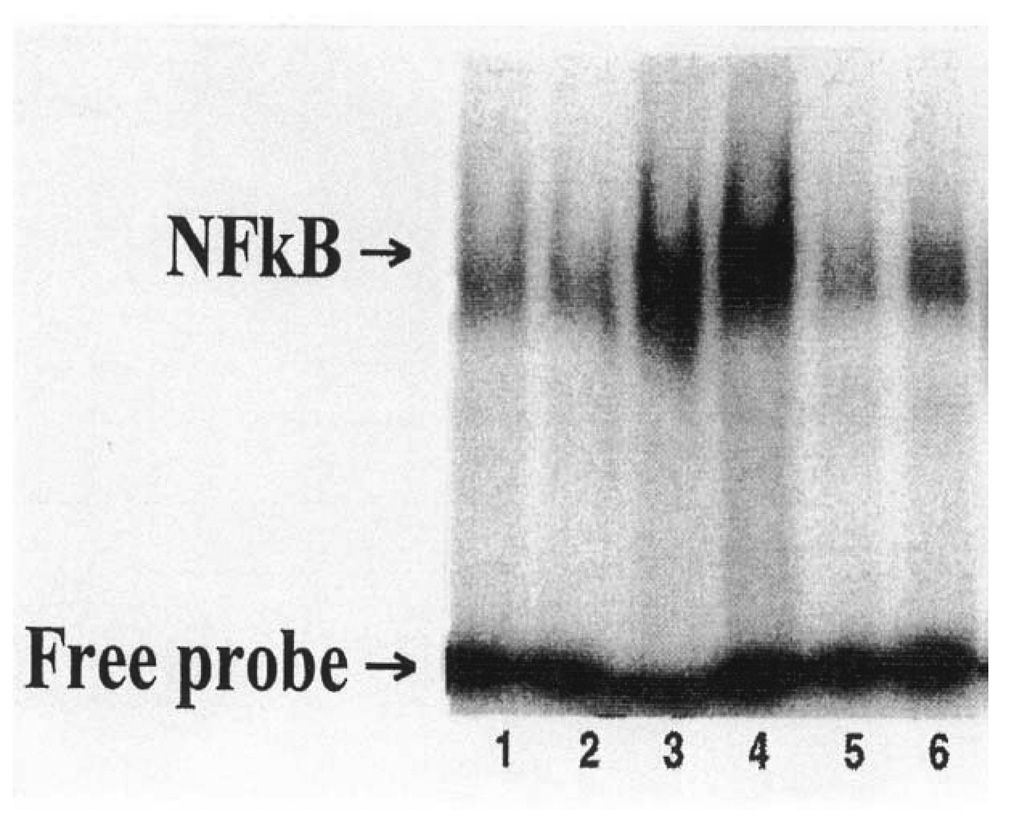
Trx priming of donor lungs attenuated NF-kB activation in post-transplant allografts
Figure 3 shows increased NF-kB/DNA binding activity in one and five day post-transplant allografts. Priming donor lungs with Trx attenuated NF-kB/DNA binding activity in both the one and five day post-transplant allografts.
Fig. 3.
The effects of Trx priming of donor lungs on NF-kB/DNA binding activity in native and allograft transplants. Donor lungs were primed with an infusion of Perfadex with or without (control) Trx (4 µg/ml) for 4 hr as described in Materials and Methods. Transplanted and native lungs were isolated one and five days post-transplant. NF-kB/DNA binding activity was monitored using an oligonucleotide representing specific NFkB binding sequences on the Trx gene. Lanes 1 and 2 = native lungs; Lanes 3 and 4 = one and five days post-transplant allografts; and Lanes 5 and 6 = Trx-primed one and five days post-transplant allografts. Lanes 1, 3, and 5 = one day post transplant, and Lanes 2, 4, and 6 = five day post-transplant allografts. Data shown are from one of two gel shift blots with similar results.
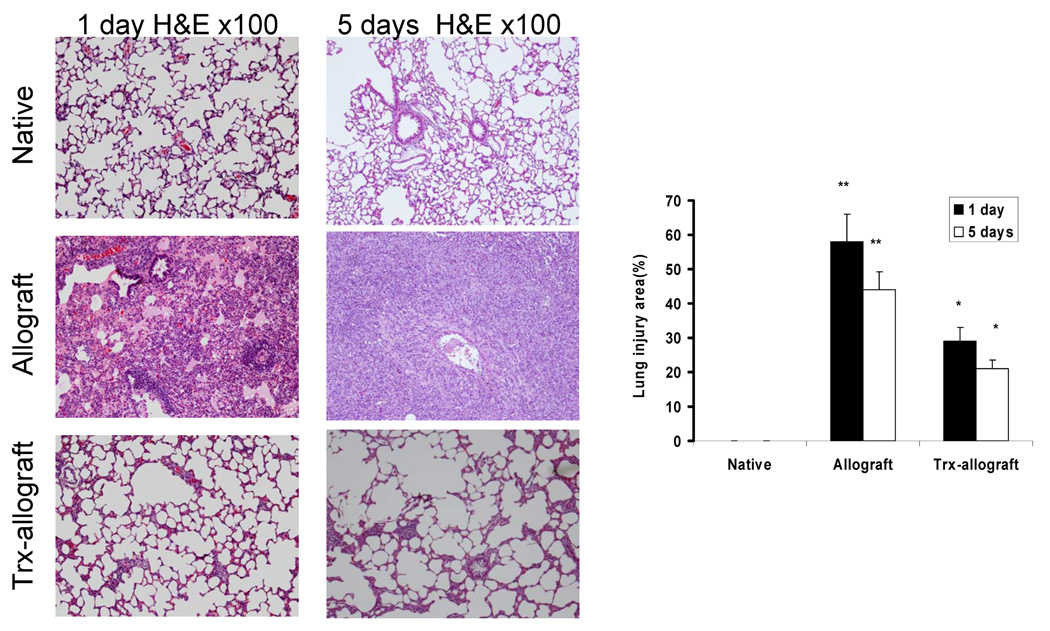
Trx priming attenuates morphologic changes in one and five day post-transplant allografts
Fig. 4 shows quantitative analysis of fractional areas of native, allograft and Trx-allograft H&E stained tissues. Lung injury area in one- and five-day post-transplant allografts was 2–3-fold higher than in Trx-primed allograft (p < 0.01 vs allograft).
Fig. 4.
The effect of Trx on morphologic changes in one day(left panel) and five day (middle panel) post-transplantation allografts. A representative micrograph from three separate experiments shows extensive perivascular edema and lymphocytic infiltration in the allograft without Thx priming (middle panel) compared to native lung (upper panel). Trx priming significantly attenuated the allograft injury (lower panel).Right panel shows a quantitative analysis of the relative lung injury area in the micrographs determined as described in Materials and Methods. The native lungs were set at 0% injury area. **p < 0.01 vs native; *p<0.05 vs allograft, n=3 for each measurement.
Trx attenuates increased infiltration of inflammatory and immune cells in allografts
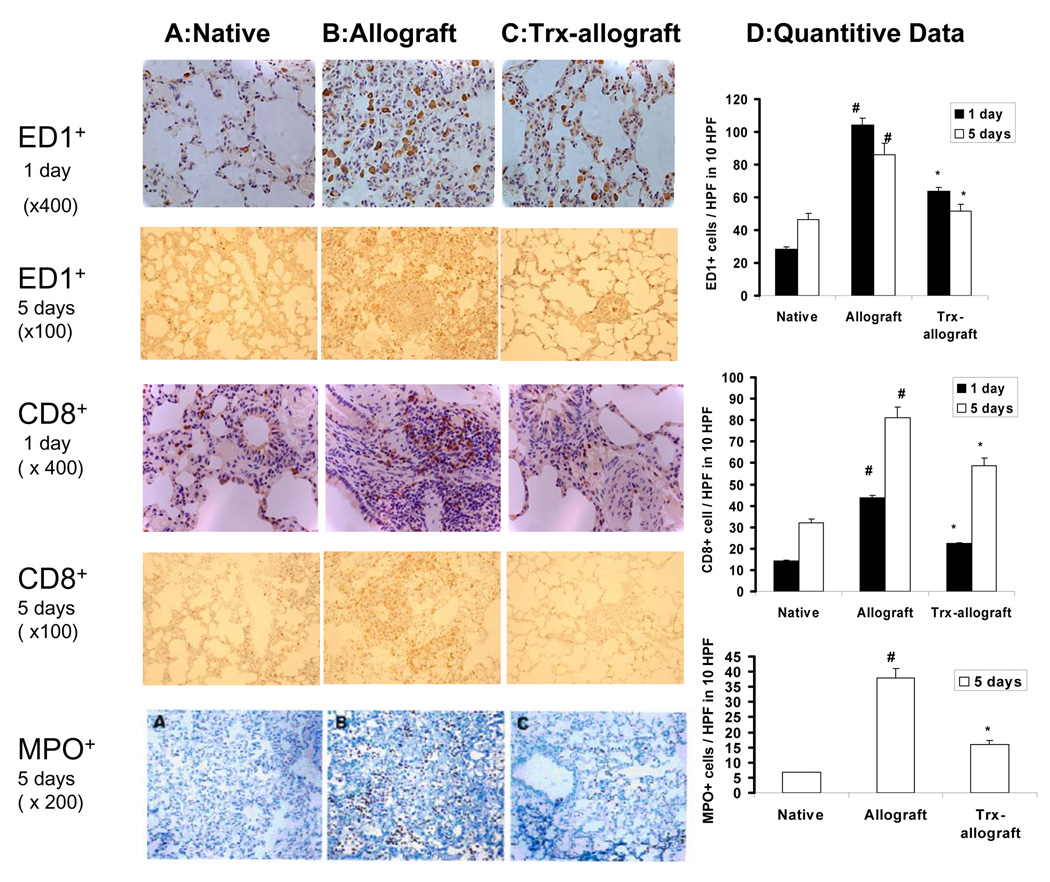
Micrographs in Fig. 5 show immunostaining by ED-1 and CD8 antibodies in native, one- or five-day post-transplant allografts, and Trx primed allograft lungs. One- and five- day post-transplant allograft without Trx priming show extensive infiltration of ED-1+ (macrophages) and CD8+ (cytotoxic T cells) cells compared to limited immnunostaining of ED-1+ and CD8+ cells in native lung. Similarly, five days post-transplant, allografts show extensive MPO staining compared to native lungs. Trx priming of donor lungs attenuated infiltration of macrophages and cytotoxic T cells in one- and five-days post-transplant allografts and MPO staining in five days post-transplant allografts.
Fig. 5.
The effect of Trx on the infiltration of macrophages, cytotoxic T lymphocytes (CD8+ T cell subset), and neutrophils. Representative micrograph from two separate experiments shows immunostaining of ED1+ macrophages (upper two rows) and CD8+ cytotoxic T cell subsets (middle row) were extensively increased in one day post transplant allograft (Panel B) compared to native lungs (Panel A). Trx priming of donor lungs attenuated infiltration of CD8+ T cells and macrophages in one day and five day post-transplant allografts (Panel C). The bottom row shows limited immnunostaining of MPO in native lungs (panel A). In contrast, five day post-transplant allograft without Trx priming show extensive MPO staining (Panel B) which is attenuated in Thx primed allografts (Panel C). Panel D shows a quantitative analysis of ED-1, CD8+, and MPO+ cells from 10 randomly selected high power field (HPF) areas of micrographs. # P<0.05 vs native for respective days; * P<0.05 vs allograft for respective days.
DISCUSSION
The results of the present study using a rat model of lung transplantation demonstrate for the first time that priming of donor lungs with purified human recombinant Trx prior to transplantation attenuates early acute injury and progressive infiltration of inflammatory and immune cells in the allografts. Our results provide experimental evidence that pre-transplant administration of Trx in donor lungs attenuates NF-kB activation, improves lung oxygen exchange, and limits progressive infiltration of macrophages, neutrophils, and cytotoxic T lymphocyte (CD8+ cell subset) in the allografts. Although the precise mechanisms of Trx-mediated protection against allograft injury remain to be determined, Trx’s antioxidant function involving scavenging of ROS during the early phase of organ preservation and transplantation may be critical in limiting the progression of graft injury via NF-kB activated cellular events. For example, a number of human and animal studies have described I/R injury as one of the major factors that plays a critical role in enhancing early graft dysfunction, leading to progressive infiltration of inflammatory and immune cells at the site of inflammation in lung transplant recipients (4–7). I/R associated oxidative stress is also known to regulate the activity of the redox-sensitive transcription factor NF-kB in multiple cells, including inflammatory and immune cells (7–12, 22, 23).
Our study demonstrates that priming of donor lungs prior to transplantation attenuates activation of NF-kB in the allograft. Activation of the NF-kB complex by multiple stress factors including ROS is a secondary event that involves its translocation to the nucleus resulting in DNA binding, activation, and gene expression (36, 37). The reduction of cysteine residue 62 of the p50 subunit by Trx in the nucleus is critical for the binding of NF-kB to its target site in DNA which results in gene expression (38, 39). Paradoxically, cytosolic Trx inhibits NF-kB activation by reducing the dissociation of the inhibitory subunit IkB (40). Similarly, extracellular and cytosolic Trx appear to function as the first line of defense against initiation of I/R injury in multiple organs (17, 30, 31). This suggests that extracellular, cytosolic, and nuclear levels of Trx may be critical to regulating NF-kB-mediated responses in biological systems. For example, increased extracellular and cytosolic Trx may scavenge ROS and prevent activation of NF-kB. In contrast, increased nuclear translocation of Trx may cause NF-kB activation. Although priming donor lungs increased extracellular Trx levels, in this study we have not examined the cytosolic and nuclear levels of Trx. Our data involving attenuation of NF-kB activity in post-transplant allografts supports the notion that priming donor lungs with Trx prior to transplantation most likely associated with both the increased extracellular and the cytoplasm levels of Trx.
The significance of NF-kB inhibition in lung allograft injury has been reported. For example, pyrrolidinedithiocarbamate (PDTC), a potent inhibitor of NF-kB, given to lung allografts during organ preservation improved lung function 4 hr after transplantation in porcine lung graft (41). However, the long term and cytotoxic effects of non-specific inhibitors such as PDTC on graft survival are unknown. Although attenuation of lung injury 5 days post-transplant using NF-kB decoy transfection has also been reported in a rat model of lung transplantation, the clinical implications of the inhibitory effects of an NF-kB decoy lasting 1–2 weeks are not clearly defined (42). This prolonged inhibitory effect may be critical since NF-kB has been known to regulate the transcription of an exceptionally large number (>900) of genes, some of which may be potentially beneficial for graft survival.
Early activation of polymorphonuclear leukocytes and alveolar macrophages under oxidative stress contributes to vascular dysfunction promoting increased infiltration of inflammatory and immune cells in the graft. Increased trafficking or infiltration of inflammatory cells activates NF-kB and enhances generation of potent proinflammatory mediators responsible for progression of allograft injury. Our strategy of priming donor lungs with Trx during the preservation phase offers a novel approach that limits early oxidative injury and prevents activation of NF-kB rather than inhibition of NF-kB. In addition, clinically, donor lungs can be primed at the site of procurement, and stored in preservation media until transplantation. The overall implications of Trx priming can be significant for preventing early injury and extending survival of allografts not only in lung transplant patients but also for other solid organ transplant recipients.
ACKNOWLEDGMENTS
We thank Dr. Subbarao Mandalapu for transplant surgery training. The study was supported in-part by the Department of Veterans Affairs Merit Review program and by National Heart, Lung, and Blood Institute Grant HL-67886 (to J.M.Patel).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Trulock EP, Edwards LB, Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart-lung transplantation report--2006. J Heart Lung Transplant. 2006;25:880–892. doi: 10.1016/j.healun.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.DeMeo DL, Ginns LC. Lung transplantation at the turn of the century. Annu Rev Med. 2001;52:185–201. doi: 10.1146/annurev.med.52.1.185. [DOI] [PubMed] [Google Scholar]
- 3.Mendeloff EN, Meyers BF, Sundt TM, et al. Lung transplantation for pulmonary vascular disease. Ann Thorac Surg. 2002;73:209–217. doi: 10.1016/s0003-4975(01)03082-x. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Bavaria JE, Palevsky HI, et al. Primary graft failure following lung transplantation. Chest. 1998;114:51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–1011. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Hosenpud JD, Bennett LE, Keck BM, et al. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official report--1999. J Heart Lung Transplant. 1999;18:611–626. doi: 10.1016/s1053-2498(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 7.Perrot Mde, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 8.Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Briscoe DM, Alexander SI, Lichtman AH. Interactions between T lymphocytes and endothelial cells in allograft rejection. Curr Opin Immunol. 1998;10:525–531. doi: 10.1016/s0952-7915(98)80218-5. [DOI] [PubMed] [Google Scholar]
- 10.Smith DM, Agura ED, Ausloos K, et al. Graft-vs-host disease as a complication of lung transplantation. J Heart Lung Transplant. 2006;25:1175–1177. doi: 10.1016/j.healun.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Cottini SR, Lerch N, de PM, et al. Risk factors for reperfusion injury after lung transplantation. Intensive Care Med. 2006;32:557–563. doi: 10.1007/s00134-006-0096-7. [DOI] [PubMed] [Google Scholar]
- 12.Ng CS, Wan S, Arifi AA, Yim AP. Inflammatory response to pulmonary ischemia-reperfusion injury. Surg Today. 2006;36:205–214. doi: 10.1007/s00595-005-3124-2. [DOI] [PubMed] [Google Scholar]
- 13.Wada H, Muro K, Hirata T, et al. Rejection and expression of thioredoxin in transplanted canine lung. Chest. 1995;108:810–814. doi: 10.1378/chest.108.3.810. [DOI] [PubMed] [Google Scholar]
- 14.Patel JM, Chen S, Block ER. Thioredoxin expression is increased after lung transplantation in rats. Am J Respir Crit Care Med. 2001;163:A333. [Google Scholar]
- 15.Patel JM, Hu H, Lu L, et al. Thioredoxin as a biomarker for graft rejection in lung transplant recipients. Biomarkers. doi: 10.1080/13547500802061822. (In press) [DOI] [PubMed] [Google Scholar]
- 16.Rancourt RC, Lee RL, O'Neill H, et al. Reduced thioredoxin increases proinflammatory cytokines and neutrophil influx in rat airways: modulation by airway mucus. Free Radic Biol Med. 2007;42:1441–1453. doi: 10.1016/j.freeradbiomed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Rad Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Ueno Y, Okamoto T. Oxidoreductive regulation of nuclear factor kappa B. Involvement of a cellular reducing catalyst thioredoxin. J Biol Chem. 1993;268:11380–11388. [PubMed] [Google Scholar]
- 19.Piette J, Piret B, Bonizzi G, et al. Multiple redox regulation in NF-kappaB transcription factor activation. Biol Chem. 1997;378:1237–1245. [PubMed] [Google Scholar]
- 20.Zhang J, Velsor LW, Patel JM, et al. Nitric oxide-induced reduction of lung cell and whole lung thioredoxin expression is regulated by NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 1999;277:L787–L793. doi: 10.1152/ajplung.1999.277.4.L787. [DOI] [PubMed] [Google Scholar]
- 21.Harper R, Wu K, Chang MM, et al. Activation of nuclear factor-kappa b transcriptional activity in airway epithelial cells by thioredoxin but not by N-acetyl-cysteine and glutathione. Am J Respir Cell Mol Biol. 2001;25:178–185. doi: 10.1165/ajrcmb.25.2.4471. [DOI] [PubMed] [Google Scholar]
- 22.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 23.Finn PW, He H, Ma C, et al. Molecular profiling of the role of the NF-kappaB family of transcription factors during alloimmunity. J Leukoc Biol. 2002;72:1054–1062. [PubMed] [Google Scholar]
- 24.Barczyk A, Pierzchala W, Kon OM, et al. Cytokine production by bronchoalveolar lavage T lymphocytes in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2006;117:1484–1492. doi: 10.1016/j.jaci.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Finn PW, Stone JR, Boothby MR, et al. Inhibition of NF-kappaB-dependent T cell activation abrogates acute allograft rejection. J Immunol. 2001;167:5994–6001. doi: 10.4049/jimmunol.167.10.5994. [DOI] [PubMed] [Google Scholar]
- 26.Boothby MR, Mora AL, Scherer DC, et al. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 28.Powis G, Briehl M, Oblong J. Redox signalling and the control of cell growth and death. Pharmacol Ther. 1995;68:149–173. doi: 10.1016/0163-7258(95)02004-7. [DOI] [PubMed] [Google Scholar]
- 29.Okubo K, Kosaka S, Isowa N, et al. Amelioration of ischemia-reperfusion injury by human thioredoxin in rabbit lung. J Thorac Cardiovasc Surg. 1997;113:1–9. doi: 10.1016/S0022-5223(97)70393-3. [DOI] [PubMed] [Google Scholar]
- 30.Wada H, Hirata T, Decampos KN, et al. Effect of the combination of human thioredoxin and L-cysteine on ischemia-reperfusion injury in isolated rat lungs. Eur Surg Res. 1995;27:363–370. doi: 10.1159/000129422. [DOI] [PubMed] [Google Scholar]
- 31.Hattori I, Takagi Y, Nakamura H, et al. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxid Redox Signal. 2004;6:81–87. doi: 10.1089/152308604771978372. [DOI] [PubMed] [Google Scholar]
- 32.Mizuta T, Kawaguchi A, Nakahara K, et al. Simplified rat lung transplantation using a cuff technique. J Thorac Cardiovasc Surg. 1989;97:578–581. [PubMed] [Google Scholar]
- 33.Loria V, Dato I, Graziani F, et al. Myeloperoxidase: A new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators of Inflammation. 2008;2008:1–4. doi: 10.1155/2008/135625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 35.Mu W, Ouyang X, Agarwal A, et al. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol. 2005;16:3651–3660. doi: 10.1681/ASN.2005030297. [DOI] [PubMed] [Google Scholar]
- 36.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NFkB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 37.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-kB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 38.Mattthews JR, Wakasugi N, Virelizier JL, et al. Thioredoxin regulates the DNA binding activity of Nf-kappa-B by reducing a disulfide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi T, Uedo Y, Okamato T. Oxidoreductive regulation of nuclear factor kappa-B involvement of a cellular reducing catalyst thioredoxin. J Biol Chem. 1993;268:11380–11388. [PubMed] [Google Scholar]
- 40.Hirota K, Murata M, Sachi Y. Dinstinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 41.Ross SD, Kron IL, Gangemi JJ, Shockey KS, Stoler M, Kern JA, Tribble CG, Laubach VE. Attenuation of lung reperfusion injury after transplantation using inhibitor of nuclear factor-kB. Am. J Physiol Lung Cell Mol Physiol. 2000;279:L528–L536. doi: 10.1152/ajplung.2000.279.3.L528. [DOI] [PubMed] [Google Scholar]
- 42.Ohmori K, Takeda S, Miyoshi S, et al. Attenuation of lung injury in allograft rejection using NFkB decoy transfection-novel strategy for use in lung transplantation. Eur J Card Thorac Surg. 2005;27:23–27. doi: 10.1016/j.ejcts.2004.09.016. [DOI] [PubMed] [Google Scholar]