Abstract
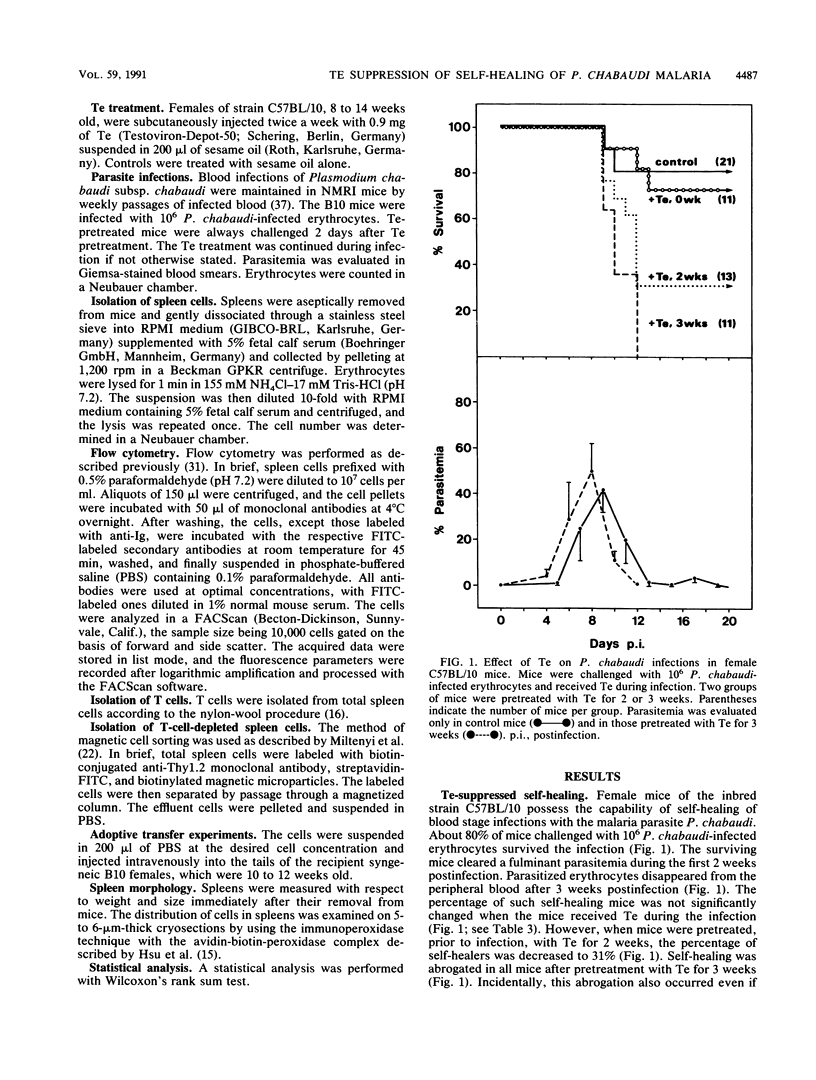
This study investigates the suppressive effect of testosterone (Te) on the self-healing of Plasmodium chabaudi malaria in female mice of the strain C57BL/10, and, in particular, the possible role of spleen cells in mediating this Te effect. Our data show the following. (i) About 80% of B10 mice infected with 10(6) P. chabaudi-infected erythrocytes are capable of self-healing the infections. This capability is progressively impaired and finally abrogated after pretreating the B10 mice with Te for 3 weeks. (ii) The spleen is Te responsive. This becomes evident in a reduction of total spleen cells from 1.05 x 10(8) to 0.54 x 10(8) on average after Te treatment for 3 weeks. Moreover, Te treatment causes an increase in the relative proportion of CD8+ cells by about 4% and a decrease of Ig+ cells by about 4.5%, as revealed by flow cytometry. (iii) Spleen cells mediate the suppressive Te effect as revealed by adoptive transfer experiments. The percentage of self-healing mice dramatically decreases to about 8% when they receive, just prior to infection, nucleated spleen cells isolated from mice treated with Te for 3 weeks. This suppressive effect can be transferred by T cells in particular but also by non-T cells, though to a lesser extent. (iv) The adoptively transferred cells mediate their suppressive effect on self-healing only if the recipient mice receive Te during infection. Our data suggest that spleen cells become functionally changed by the Te treatment for 3 weeks. Particularly T cells, but also non-T cells, gain P. chabaudi-specific suppressive activities, and the cells require a Te-induced factor(s) to mediate these activities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansar Ahmed S., Dauphinee M. J., Talal N. Effects of short-term administration of sex hormones on normal and autoimmune mice. J Immunol. 1985 Jan;134(1):204–210. [PubMed] [Google Scholar]
- Butcher G. A. Mechanisms of immunity to malaria and the possibilities of a blood-stage vaccine: a critical appraisal. Parasitology. 1989 Apr;98(Pt 2):315–327. doi: 10.1017/s0031182000062247. [DOI] [PubMed] [Google Scholar]
- Crane G. G. Hyperreactive malarious splenomegaly (tropical splenomegaly syndrome). Parasitol Today. 1986 Jan;2(1):4–9. doi: 10.1016/0169-4758(86)90067-0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dunkel L., Taino V. M., Savilahti E., Eskola J. Effect of endogenous androgens on lymphocyte subpopulations. Lancet. 1985 Aug 24;2(8452):440–441. doi: 10.1016/s0140-6736(85)92755-2. [DOI] [PubMed] [Google Scholar]
- Fujii H., Nawa Y., Tsuchiya H., Matsuno K., Fukumoto T., Fukuda S., Kotani M. Effect of a single administration of testosterone on the immune response and lymphoid tissues in mice. Cell Immunol. 1975 Dec;20(2):315–326. doi: 10.1016/0008-8749(75)90108-2. [DOI] [PubMed] [Google Scholar]
- Grossman C. J. Regulation of the immune system by sex steroids. Endocr Rev. 1984 Summer;5(3):435–455. doi: 10.1210/edrv-5-3-435. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Ross R. K., Pike M. C., Casagrande J. T. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982 Aug;42(8):3232–3239. [PubMed] [Google Scholar]
- Hoffmann E. J., Weidanz W. P., Long C. A. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect Immun. 1984 Mar;43(3):981–985. doi: 10.1128/iai.43.3.981-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kemp C. J., Drinkwater N. R. Genetic variation in liver tumor susceptibility, plasma testosterone levels, and androgen receptor binding in six inbred strains of mice. Cancer Res. 1989 Sep 15;49(18):5044–5047. [PubMed] [Google Scholar]
- Kemp C. J., Leary C. N., Drinkwater N. R. Promotion of murine hepatocarcinogenesis by testosterone is androgen receptor-dependent but not cell autonomous. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7505–7509. doi: 10.1073/pnas.86.19.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M., Nawa Y., Fujii H. Inhibition by testosterone of immune reactivity and of lymphoid regeneration in irradiated and marrow reconstituted mice. Experientia. 1974 Nov 15;30(11):1343–1345. doi: 10.1007/BF01945220. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Morton J. I., Weyant D. A., Siegel B. V., Golding B. Androgen sensitivity and autoimmune disease. I. Influence of sex and testosterone on the humoral immune response of autoimmune and non-autoimmune mouse strains to sheep erythrocytes. Immunology. 1981 Dec;44(4):661–669. [PMC free article] [PubMed] [Google Scholar]
- Rifé S. U., Márquez M. G., Escalante A., Velich T. The effect of testosterone on the immune response. 1. Mechanism of action on antibody-forming cells. Immunol Invest. 1990 Jun;19(3):259–270. doi: 10.3109/08820139009041841. [DOI] [PubMed] [Google Scholar]
- Sayles P. C., Wassom D. L. Immunoregulation in murine malaria. Susceptibility of inbred mice to infection with Plasmodium yoelii depends on the dynamic interplay of host and parasite genes. J Immunol. 1988 Jul 1;141(1):241–248. [PubMed] [Google Scholar]
- Schuurs A. H., Verheul H. A. Effects of gender and sex steroids on the immune response. J Steroid Biochem. 1990 Feb;35(2):157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Lyanga J. J., Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982 Oct;38(1):80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Skamene E. Murine malaria: resistance of AXB/BXA recombinant inbred mice to Plasmodium chabaudi. Infect Immun. 1985 Feb;47(2):452–456. doi: 10.1128/iai.47.2.452-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet E., Melis M., Van Ewijk W. Monoclonal antibodies to stromal cell types of the mouse thymus. Eur J Immunol. 1984 Jun;14(6):524–529. doi: 10.1002/eji.1830140608. [DOI] [PubMed] [Google Scholar]
- Weinstein Y., Berkovich Z. Testosterone effect on bone marrow, thymus, and suppressor T cells in the (NZB X NZW)F1 mice: its relevance to autoimmunity. J Immunol. 1981 Mar;126(3):998–1002. [PubMed] [Google Scholar]
- Weiss L. The spleen in malaria: the role of barrier cells. Immunol Lett. 1990 Aug;25(1-3):165–172. doi: 10.1016/0165-2478(90)90109-4. [DOI] [PubMed] [Google Scholar]
- Wunderlich F., Helwig M. Plasmodium chabaudi malaria: red blood cells with altered membrane proteins in immune mice. Eur J Cell Biol. 1987 Jun;43(3):499–500. [PubMed] [Google Scholar]
- Wunderlich F., Marinovski P., Benten W. P., Schmitt-Wrede H. P., Mossmann H. Testosterone and other gonadal factor(s) restrict the efficacy of genes controlling resistance to Plasmodium chabaudi malaria. Parasite Immunol. 1991 Jul;13(4):357–367. doi: 10.1111/j.1365-3024.1991.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Wunderlich F., Mossmann H., Helwig M., Schillinger G. Resistance to Plasmodium chabaudi in B10 mice: influence of the H-2 complex and testosterone. Infect Immun. 1988 Sep;56(9):2400–2406. doi: 10.1128/iai.56.9.2400-2406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich F., Stübig H., Königk E. Development of Plasmodium chabaudi in mouse red blood cells: structural properties of the host and parasite membranes. J Protozool. 1982 Feb;29(1):60–66. doi: 10.1111/j.1550-7408.1982.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Wyler D. J. The spleen in malaria. Ciba Found Symp. 1983;94:98–116. doi: 10.1002/9780470715444.ch7. [DOI] [PubMed] [Google Scholar]
- van Vliet E., Melis M., van Ewijk W. The influence of dexamethasone treatment on the lymphoid and stromal composition of the mouse thymus: a flowcytometric and immunohistological analysis. Cell Immunol. 1986 Dec;103(2):229–240. doi: 10.1016/0008-8749(86)90086-9. [DOI] [PubMed] [Google Scholar]



