Abstract
A simple, direct and accurate method for the determination of concentration and enrichment of free fatty acids in human plasma was developed. The validation and comparison to a conventional method are reported. Three amide derivatives, dimethyl, diethyl and pyrrolidide, were investigated in order to achieve optimal resolution of the individual fatty acids. This method involves the use of dimethylamine/Deoxo-Fluor to derivatize plasma free fatty acids to their dimethylamides. This derivatization method is very mild and efficient, and is selective only towards free fatty acids so that no separation from a total lipid extract is required. The direct method gave lower concentrations for palmitic acid and stearic acid and increased concentrations for oleic acid and linoleic acid in plasma as compared to methylester derivative after thin-layer chromatography. The [13C]palmitate isotope enrichment measured using direct method was significantly higher than that observed with the BF3/MeOH-TLC method. The present method provided accurate and precise measures of concentration as well as enrichment when analyzed with gas chromatography combustion-isotope ratio-mass spectrometry.
Keywords: dimethylamide, plasma free fatty acids, mass spectrometry, validation, GC-combustion isotope ratio, enrichment
1. Introduction
For many years, our laboratory has been interested in skeletal muscle utilization of lipids [1], [2], [3], [4]. A defect in the ability of skeletal muscle to oxidize plasma fatty acids could lead to an increase of intramyocellular lipid, which has been observed in obese as well as type 2 diabetic patients. Fatty acids are an important class of molecules in metabolism [5], [6], [7], [8]. Therefore, accurate measurement of plasma free fatty acids (FFA) has important physiological and clinical implications.
Since the percentage amount of free fatty acids in human plasma is low (3–10%) compared to fatty acids contained in plasma triacylglycerols, phospholipids or cholesterol esters, any in vitro lipolyic activity would cause an artifactual increase of FFA plasma content [9], [10]. Slight amounts of released of fatty acids from any of these ester lipid classes during sample preparation would distort the determined FFA concentration.
Methyl esters are used almost universally for gas chromatographic (GC) analysis of fatty acids [11], [12], [13]. The most common procedure used for measuring plasma FFA concentrations and fatty acid enrichment involves the following steps: a) Extraction of lipid from plasma; b) Isolation of FFA from the rest of lipids by thin-layer chromatography (TLC) and/or solid phase extraction (SPE); and c) Derivatization of FFA to fatty acid methyl esters (FAME). Boron trifluoride (BF3) in methanol is the most commonly used reagent for the derivatization step in spite of several disadvantages [14], [15]. Drawbacks of this procedure for the estimation of plasma FFA include: 1) A laborious sample preparation with TLC separation of FFAs from the others lipids after the extraction step; 2) The possibility of oxidative deterioration of polyunsaturated lipids during the TLC process; and 3) With the purification by SPE, multiple extractions at different pHs are required to obtain the FFA fraction. In addition to this method, there are reports in the literature on direct derivatization methods for the FFAs in plasma extracts under selective conditions [16], [17], [18]. However there are some questions concerning the selectivity and accuracy of these methods [9], [19].
To overcome these problems, a robust and gentle method for derivatization of fatty acid amides for GC analysis was developed. Amides are more hydrolytically stable and, due to higher polarity elute at higher temperature (Δ +10 to +20 °C) than their corresponding methyl ester. Both of these properties would be advantageous for assessment of short chain fatty acids [20]. Although amide derivatives such as pyrrolidides, picolinyl esters and dimethyloxazolines (DMOX) of fatty acids have been used for mass spectrometric determination of double bond positions in polyunsaturated fatty acids, these have not been widely adopted due to lack of a simple, mild and efficient derivatization methodology [21], [22], [23], [24], [25] with the exception of picolinyl esters derivatives [26].
We recently developed a very mild and efficient one-step procedure for the conversion of FFAs into their dimethyl, diethyl and pyrrolidide derivatives [27]. This procedure consists of the treatment of fatty acids and amines with bis(2-methoxyethyl)-amino-sulfur trifluoride (Deoxo-Fluor) at 0 °C. Reactions occur in 30 min and products are formed in high yields and purities.
In the current study, we report development of a method for the direct estimation of plasma FFA concentrations as well as their enrichment via derivatization to their dimethylamides. We have applied this method to analysis of plasma fatty acids by GC separation with flame ionization detection (GC-FID) as well with combustion-isotope ratio mass spectrometry (GC-C-IRMS) in 13C-enrichment experiments. The method involves the coupling of the extracted acids with dimethylamine using the Deoxo-Fluor reagent at 0 °C without prior separation of FFA from total lipid extracts (triacylglycerols, phospholipids and cholesterol esters).
2. Experimental
2.1. Chemicals
Dichloromethane, diisopropylethylamine, hexane, heptane, ethyl acetate (analytical-reagent grade), the Deoxo-Fluor reagent and all the fatty acids, (palmitic acid (C16:0), heptanoic acid (C17:0), stearic acid (C18:0), oleic acid (18:1n9), linoleic acid (18:2n6) 1,3-diolein, triolein, 1,2-diolein and cholesteryl pentadecanoate) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Apparatus
GC/FID
An Agilent GC-6890 system was equipped with a FID. A H2 flow rate of 30 mL/min and an air flow rate at 300 mL/min were used. The flow rate of carrier gas (He) was set at 2.5 mL/min. The temperature of the injection port and detector were set at 280 and 300 °C, respectively. The oven temperature was programmed to initiate at 160 °C for 2 min, then the temperature was raised to 200 °C at a rate of 20 °C/min, held there for 4 min, and finally increased to 270 °C at a rate of 5 °C/min and held there for 23 min. The injection volume was 1 µL in the split-less injection mode. A capillary column (SPD-1 fused-silica capillary, 30m × 0.53 mm I.D., 0.01 µm film thickness; Supelco, Bellefonte, PA, USA) was employed. Only results of major plasma FFAs (C16:0, C16:1, C18:0, C18:1 and C18:2) are reported.
GC-C-IRMS
A ThermoFinnigan model 2000 Trace GC coupled to a Deltaplus XL isotope ratio mass spectrometer with GC combustion III interface was used with a PAL autosampler (CTC Analytics, Zwingen). Carrier gas (He) flow was set at 1.5 mL/min. The temperature of the injection port was set at 280 °C and oven temperature was programmed to initiate at 160 °C for 2 min, then the temperature was raised to 200 °C (at a rate of 20 °C/min) and held for 4 min, and finally increased to 270 °C (at a rate of 5 °C/min) and held there for 23 min. The injection volume was 1 µL in the split-less injection mode using a capillary column (DB-5 fused-silica capillary, 30m × 0.25 mm I.D., 0.25 µm film thickness; Agilent Technologies, USA). After separation, the fatty acids were converted into CO2 and H2O using a combustion oven containing copper, nickel and platinum wires, heated at 940 °C. After water is removed, the CO2 molecules enter the source of the MS, are ionized and the masses of CO2 (m/z 44, 45 and 46) are analyzed by magnetic field mass spectrometry. An external CO2 reference gas (δ13 C= − 29.10‰) was used to obtain highly accurate isotopic compositions, or δ13 C values.
GC/EI-MS
The GC (Agilent GC-6890) system was equipped with a mass detector (quadrupolar 5973N) mass detector operating in 70V electron ionization (EI) mode. Carrier gas (He) flow was set at 0.8 mL/min. The temperature of the injection port was set at 250 °C and oven temperature was programmed to initiate at 140 °C for 2 min, the temperature was raised to 200 °C at a rate of 20 °C/min, held for 4 min, increased to 270 °C at a rate of 5 °C/min and held there for 23 min. The injection volume was 1 µL in the split-less injection mode. A capillary column (HP-5 fused-silica capillary, 30m × 0.25 mm I.D., 0.25 µm film thickness; Supelco, Bellefonte, PA, USA) was employed.
2.3. General Derivatization
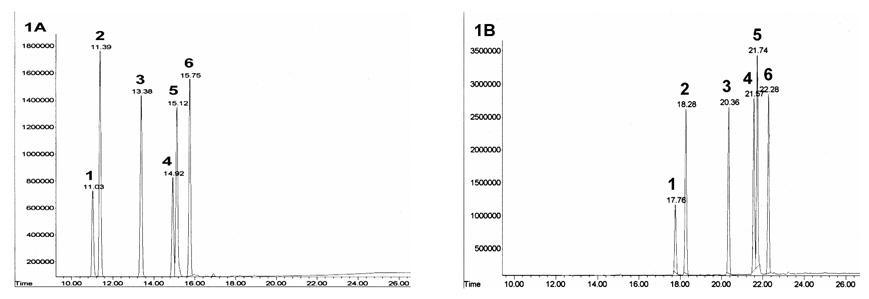
The free fatty acid (0.039 µmol), derivatizing amine (dimethylamine, diethylamine and pyrrolidides) (30 µL) and diisopropylethylamine (10 µL) were dissolved in CH2Cl2 (200 µL), cooled to 0 °C, and Deoxo-Fluor (10 µL) was added dropwise. After 30 min, the reaction was quenched with water and products were extracted into n-heptane. The organic layer was then dried in SpeedVac, reconstituted in 500 µL of heptane and transferred to glass autosampler vial (Fig. 1).
Fig. 1.
GC-FID chromatograms of the reaction products of 1: C16:1 (palmitoleic acid), 2: C16:0 (palmitic acid), 3: C17:0 (heptadecanoic acid), 4: C18:2 (linoleic acid), 5: C18:1 (oleic acid) and 6: C18:0 (stearic acid) with BF3/MeOH and dimethyl amine respectively. Conditions: GC-FID (Agilent GC-6890) the flow rate of carrier gas (He) was set at 5 mL/min. The oven temperature was programmed to initiate at 160 °C and held for 2 min. The temperature was raised to 200 °C at a rate of 20 °C and held for 4 min, and finally increased to 270 °C at a rate of 5 °C and held for 23 min. Column: SPD-1 fused-silica capillary column (30 m × 0.53 mm I.D., 0.10 µm film thickness; Supelco, Bellefonte, CA, USA). “A” Methyl ester derivatives; “B” Dimethylamide derivatives.
2.4. TLC/BF3 Method
This method has been previously described in detail [15], [28]. Briefly, C17:0 internal standard was added to plasma or water blanks, lipids were extracted with Dole reagent [isopropanol-heptane-hydrochloric acid (1M) (40:10:1, v/v)] dried and FFA were separated by TLC on silica gel plates using a heptane-ether-acetic acid [60:40:3] solvent system [29]. FFAs were visualized by spraying with 0.01% rhodamine 6G. TLC scrapings containing FFA were extracted with chloroform-methanol 3:1, dried and FAME were prepared by reaction with 14% BF3 in methanol.
2.5. Specificity of Derivatization to Free Fatty Acids
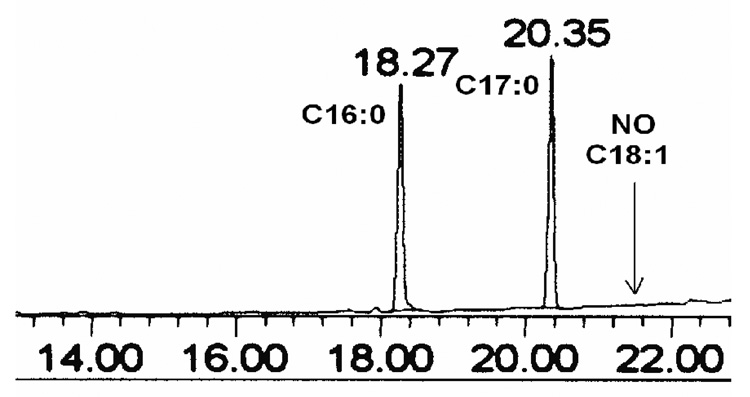
Fatty acid amide synthesis was specific to FFA. Fatty acid esters (triacylglycerols, phospholipids, etc.) present in biological samples are not hydrolyzed in the presence of Deoxo-Fluor reagent [27]. To test the selectivity of this method towards FFA, we conducted the following experiment: A standard palmitic acid (C16:0, 10 µg, 0.039, µmol) was mixed with the internal standard heptadecanoic acid (C17:0, 10 µg, 0.037, µmol) along with three acylglycerols 1,3-diolein, triolein and 1,2-diolein. We used 0.113 µmol of triacylglycerol equivalent to 0.339 µmol of fatty acid, and 0.171 µmol of each diacylglycerol, equivalent to 0.342 µmol of fatty acid. Therefore, the total amount of fatty acid from triacylglycerols was 1.023 mmoles, compared to 0.039 µmoles of the palmitate. Treatment with the reaction conditions described above gave C16:0 and C17:0 dimethylamide peaks upon analysis with GC-FID whereas no such peak was detected for C18:1 (Fig. 2). This result, clearly demonstrates our method is very mild and does not cause hydrolysis of esters (triacylglycerols, diacylglycerols, etc)
Fig. 2.
GC-FID chromatograms of a reaction products with dimethyl amine/Deoxo-Fluor for concentrations (0.0391and 0.0371µmol) of 1) C16:0, 2) C17:0 and 0.113 µmol of triolein (C18:1n9), shows no peak for 3) C18:1 at 21.74 min retention time.
2.6. Validation of the Analytical Method for GC-FID
For measures of linearity, repeatability and limits of detection and quantification, standard solutions (1.0 mg/mL) of C16:0, C16:1, C18:0, C18:1 and C18:2 were diluted separately with heptane to various concentrations (1–100 µg). Each was mixed separately with 10 µg of internal standard (C17:0). After their derivatization to dimethylamide derivatives, 1.0 µL of each mixture was subjected to GC analysis. Each concentration and recovery of each standard solution was determined in triplicate. Peak areas obtained from above analyses were analyzed with equation (1) to determine the internal response factor (IRF). Inter-day precision was estimated from the analysis of freshly prepared control samples (concentrations ranging from 6.20 µmol/L to 350 µmol-/L) on six separate days.
| Equation 1 |
The assay precision (coefficient of variation, C.V., in %) was assessed by expressing the standard deviation of the repeated measurements as a percentage of the mean value. Recovery of added fatty acids was estimated by adding a known quantity of FFA in a human plasma sample and calculating each percentage of recovery.
We compared our direct FFA determination method with TLC/BF3 method for plasma FFA determination. Plasma was prepared from the blood of one adult male volunteer as described above, transferred to separate storage tubes, and frozen. On three successive days, one of these tubes was thawed, and 3 replicate aliquots of 250 µl of this plasma were transferred to glass tubes. Internal standard (C17:0, 10 µg) was added to all the samples. The first groups of 3 replicates were subjected to FFAs determination by the published procedure with extraction, TLC purification and BF3/MeOH derivatization. The second group of aliquots was prepared using the dimethylamide derivatization described above following isolation of the FFAs by TLC. A third group of aliquots was prepared and analyzed according to the above direct procedure (that is extraction and derivatization of fatty acids into fatty acid dimethylamides). Analyses of FFA methyl esters as well as FFA dimethylamides were determined by GC-FID as described in the experimental section.
Analysis of variance (SAS 9.1 for Windows, Cary, NC) was used to determine whether there were differences in the determination of plasma FFA concentrations after TLC isolation with dimethylamide and BF3/MeOH methods and by direct dimethylamide method. To test for differences, we used Scheffe's multiple-comparison procedure. Values of P < 0.05 were considered statistically significant.
2.7. Isotope infusion
We also determined the appropriateness of this method to measure 13C enrichment of plasma palmitate during a euglycemic-hyperinsulinemic clamp (same samples as described in Table 4). After taking blood for baseline determinations, an intravenous priming dose of 0.085 mg/kg of NaH3CO3 was given. Then, a constant-rate continuous infusion of [13C]palmitate was begun (0.011 µmol. Kg−1 body wt.min−1) and continued during the entire period via a calibrated infusion pump (IVAC 560 pump; IVCA, San Diego, CA). The palmitate tracer (60 mg of the potassium salt of [13C]palmitate, 99% enrich; Cambridge Isotope Laboratories, Andover, MA) was dissolved in heated sterile water and passed through a 0.2µm filter into 25% warm human serum albumin (250 mL) to make a 0.670 mmol/l solution (mean ±SD, 0.668 ± 0.016 mmol/L).
Table 4.
Comparison of plasma FFA concentrations determined by dimethylamide direct method and with previously published method (BF3/MeOH- TLC)
| Time (min) |
Dimethylamide | BF3/MeOH-TLC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C16:0 (µM) |
C16:1 (µM) |
C18:0 (µM) |
C18:1 (µM) |
C18:2 (µM) |
Total FFA (µM) |
C16:0 (µM) |
C16:1 (µM) |
C18:0 (µM) |
C18:1 (µM) |
C18:2 (µM) |
Total FFA (µM) |
|
| BK | 130 | 20 | 38 | 146 | 69 | 403 | 149 | 20 | 46 | 144 | 74 | 433 |
| −30 | 127 | 20 | 38 | 145 | 69 | 399 | 146 | 19 | 47 | 145 | 75 | 431 |
| −15 | 127 | 19 | 38 | 145 | 68 | 396 | 150 | 18 | 46 | 144 | 72 | 430 |
| 0 | 134 | 20 | 33 | 139 | 67 | 393 | 151 | 18 | 46 | 136 | 68 | 419 |
| 150 | 32 | 6 | 9 | 22 | 15 | 83 | 41 | 5 | 11 | 21 | 15 | 93 |
| 165 | 32 | 5 | 9 | 22 | 15 | 82 | 41 | 5 | 11 | 20 | 15 | 92 |
| 180 | 29 | 5 | 9 | 22 | 15 | 80 | 38 | 5 | 11 | 21 | 15 | 89 |
| 270 | 22 | 3 | 7 | 10 | 7 | 49 | 30 | 3 | 7 | 8 | 8 | 55 |
| 285 | 21 | 3 | 6 | 9 | 7 | 46 | 30 | 3 | 8 | 9 | 8 | 57 |
| 300 | 22 | 3 | 6 | 10 | 7 | 48 | 29 | 3 | 6 | 8 | 7 | 54 |
2.9. Blood Specimens
Volunteers were recruited and medically screened. Participants were of stable weight, in good general health and with normal values for hematological, renal, thyroid and hepatic function. The University of Pittsburgh Institutional Review Board approved the investigation and all volunteers gave informed written consent. Plasma was collected under standardized conditions in a 10-mL tube containing potassium EDTA. The uncoagulated blood was then put on ice immediately and centrifuged at 2000 × g at 4 °C for 10 min. Plasma was separated from the cells, divided into two 1.5 mL aliquots, which were frozen within 3 h at −80 °C until analyzed.
2.10. Sample Preparation
Aliquots (250 µL) of plasma were spiked with 0.0371 µmol of heptadecanoic acid as internal standard. The samples were then processed according to the following procedure.
2.11. Extraction of Plasma Free Fatty Acids
Lipids were extracted from plasma using the Dole method [29]. Briefly, the solvent was prepared by mixing isopropanol-heptane-hydrochloric acid (1M) (40:10:1, v/v) and was thoroughly stirred before use. During the extraction procedure, lipids were protected against oxidation by adding 0.05 mg mL−1 butylated hydroxytoluene (BHT) to the solvent. Human plasma (250 µl) was mixed with 10 µl of the internal standard heptadecanoic acid (10 µg) in n-heptane, in a 13-mL Pyrex glass tube (Corning, NY, USA) with a PTFE lined screw cap. Extraction solvent (2.5 mL) was added, the tubes were thoroughly vortexed for 40 min and allowed to incubate at room temperature for 10 min. Heptane (4.0 mL) and water (2.0 mL) were added and the tubes were thoroughly vortexed for 5 min. The tube was centrifuged at 1000 × g for 10 min at 4 °C. The upper phase (heptane) was transferred into a fresh 13 × 100 mm screw top tube and dried in a SpeedVac centrifugal concentrator (Savant, Farmingdale, NY). The dried sample was then used for derivatization.
2.12. Derivatization Method
Briefly, the dried sample from above was diluted with CH2Cl2 (200 µL), and diisopropylethylamine and dimethylamine (10 and 30 µL respectively) were added. The mixture was cooled to 0 °C, and bis(2-methoxyethyl)amino-sulfur trifluoride (Deoxo-Fluor) (10 µL) was added. The resulting mixture was vortexed for 5 sec. After 5 min at 0 °C, the tubes were warmed to room temperature and kept there for 15 min. Water (2 mL) and heptane (4 mL) were added, the tubes were vortexed for 10min. The organic (upper) layer was collected and dried using a SpeedVac. The residue was resuspended in heptane (500 µL), which was transferred to an autosampler vial, from which 1 µL was injected into the GC-FID.
3. Results and discussion
3.1. GC Separation of Fatty Acids
To determine which of the fatty acid amide derivatives was most suitable for quantifying FFA as well as for closest resemblance to FAME, we prepared dimethyl, diethyl and pyrrolidide derivatives of C16:0, C16:1, C18:0, C18:1 and C18:2.
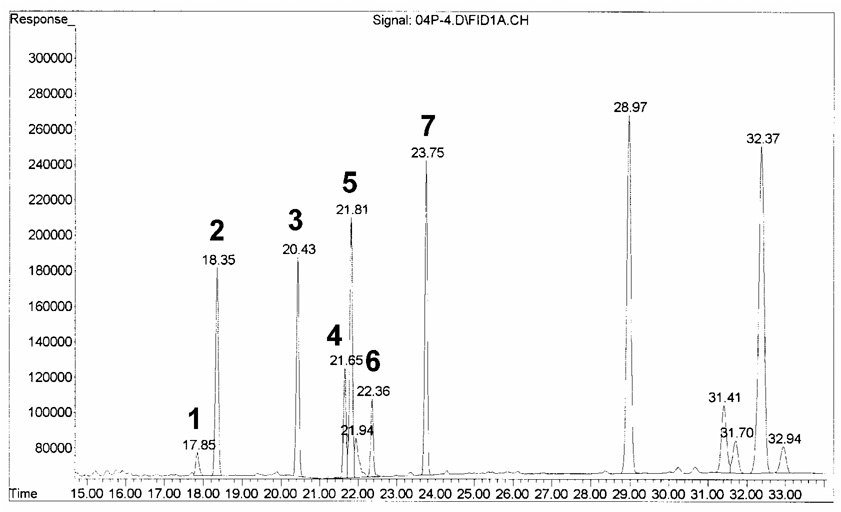
As shown the Fig 1, the separation followed the typical trend for the linear alkanes, with retention time proportional to chain length. The elution was linear with the number of carbons for saturated amides. Under identical chromatographic conditions, the five major FFAs in human plasma eluted as methyl esters, dimethylamides, diethylamides and pyrrolidides in 16, 23, 25 and 29 min, respectively (Table 1). The separations between the methyl esters, dimethylamides and diethylamides of oleic (C18:1) and linoleic (C18:2) acids were approximately the same. Dimethylamides of fatty acids elute from the GC column first due to its lower molecular weight among the amide derivatives of FFAs. Diethylamide derivatives of FFAs eluted from the GC column approximately two minutes later due to one extra carbon atom. Due to interference of the reagent (diethylamine) with C18:1, this derivative was discarded. Furthermore, the pyrrolidide derivatization was eliminated due to the long retention time and they show overlapping peaks with fatty acid esters (triacylglycerols/phospholipids). Therefore, dimethylamide derivatization was selected for direct analysis of FFA in plasma. Representative chromatograms of the five major plasma standard FFA methyl ester derivatives and dimethylamide derivatives are shown in Fig. 1A–1B. All other components in the lipid extract elute from the GC column well after elution of the FFA and hence do not interfere with the FFA quantification. Therefore, it is important to include a temperature program (time and temperature ramp) to remove all remaining components of the total lipid extract to eliminate possible interference for the next sample (Fig. 3; components eluting after 25 minutes).
Table 1.
Comparison of retention times (min) of FAME and FFA amides derivatives
| Derivatives | Retention time for C16:1 (min) | Retention time for C16:0 (min) | Retention time for C18:2 (min) | Retention time for C18:1 (min) | Retention time for C18:0 (min) |
|---|---|---|---|---|---|
| Methyl ester | 11.03 | 11.39 | 14.92 | 15.12 | 15.75 |
| Dimethylamide | 17.76 | 18.28 | 21.57 | 21.74 | 22.28 |
| Diethylamide | 19.98 | 20.46 | 23.49 | 23.64 | 24.15 |
| Pyrrolidide | 23.79 | 24.21 | 27.57 | 27.76 | 28.49 |
Fig. 3.
GC-FID chromatogram of a plasma sample. 1: C16:1, 2: C16:0, 3: C17:0, 4: C18:2, 5: C18:1, 6: C18:0 and 7: reagent peak.
3.2. Limit of detection and quatification
The limit of detection (LOD) and the limit of quantification (LOQ) corresponding to signal-to-noise values of 3 and 10 (according to the IUPAC rules and Miller and Miller suggestion [30], [31]), respectively, (in the range of 1.69–3.15 pmol) were evaluated for the FFAs. The data in Table 2 demonstrating linearity, repeatability, low limit of quantification and nearly 100% recovery of added FFA to a plasma sample support the suitability of the present method for its application in physiological studies.
Table 2.
Linearity, precision, accuracy, limit of detection and quantification, and recovery of five major FFAs
| Validation parameters | Analyte (C16:0) | Analyte (C16:1) | Analyte (C18:0) | Analyte (C18:1) | Analyte (C18:2) |
|---|---|---|---|---|---|
| Linearity interval (µmol/l) | 0–250 | 0–50 | 0–100 | 0–350 | 0–50 |
| (r2 = 0.985) | (r2 = 0.988) | (r2 = 0.990) | (r2 = 0.997) | (r2 = 0.971) | |
| Inter-day repeatability RSD, % concentration, (µmol/l) | 3.4 % (31.3) | 2.7 % (6.25) | 3.9 % (12.5) | 2.5 % (43.75) | 2.4 % (6.25) |
| 3.6 % (62.5) | 2.6 % (12.5) | 4.0 % (25) | 2.6 % (87.5) | 2.6 % (12.5) | |
| 3.1 % (125) | 2.4 % (25) | 4.2 % (50) | 3.1 % (175) | 2.3 % (25) | |
| 3.7 % (250) | 2.8 % (50) | 3.9 % (100) | 2.9 % (350) | 2.0 % (50) | |
| Limit of detection (pmol) | (1.95) | (2.09) | (1.69) | (1.84) | (2.21) |
| Limit of quantification (pmol) | (2.93 ) | (3.15) | (2.64) | (2.73) | (2.86) |
| Recovery: added known quantity in (µmol/l) (and recovery in %) | 35.7 (96.2) | 8.9 (99.2) | 35.7 (96.2) | 35.7 (96.2) | 35.7 (96.2) |
| 71.4 (96.4) | 17.9 (96.6) | 71.4 (96.4) | 71.4 (96.4) | 71.4 (96.4) | |
| 142.9 (96.5) | 35.7 (103.1) | 142.9 (96.5) | 142.9 (96.5) | 142.9 (96.5) | |
| 285.7 (97.0) | 71.4 (99.0) | 285.7 (97.0) | 285.7 (97.0) | 285.7 (97.0) | |
Accuracy and precision of the dimethylamide (direct) method at different FFA concentrations on three successive triplicate standards. Recovery studies were conducted by adding known quantities of FFA to a human plasma sample.
3.3. Specificity of Dimethylamide Direct Method to Free Fatty Acids
We also performed an experiment in which cholesteryl pentadecanoate was added to each of three replicate plasma specimens before determination of FFA by our direct method. We did not detect any peak for pentadecanoic acid (the product of hydrolysis of cholesteryl pentadecanoate is pentadecaoic acid) in the GC-FID chromatogram (data not shown). This result, along with our results from the experiments with triacylglycerol and diacylglycerols clearly demonstrates our method is very mild and does not cause hydrolysis of lipid esters in plasma (Fig. 2).
3.4. Comparison of Free Fatty Acid Determination Procedures
We compared the determination of FFA concentration using BF3/MeOH and dimethylamide derivatization after isolation of the FFA by TLC with the direct, dimethylamide derivatization procedure. The concentrations found for each fatty acid methyl ester and dimethylamide derivatives were nearly identical following TLC (Table 3). However, the direct method gave lower concentrations for C16:0 and C18:0 and increased concentrations for C18:1 and C18:2 in plasma as compared to either derivative after TLC. Concentration differences observed for FFA were significant by Scheffe's multiple-comparison procedure (p <0.05) only for C16:0, C18:0, C18:1, and C18:2 (Table 3). This result is similar to that reported by Patterson et al. who described that TLC/BF3 causes an increase in concentration of C16:0 and C18:0 (Patterson et al. plasma FFA using TLC gave: C16:0 +13%, C16:1 – −3%, not significant, C18:0 +38%, C18:1 − 3% NS, C18:2 − 18%. Our results: using TLC gave: C16:0 +16%; C16:1 not significant, C18:0 = +14% C18:1 − 13%, C18:2 − 14%) compared with a direct methylation with iodomethane followed by solid phase extraction (SPE) [28].
Table 3.
Comparison of plasma FFA concentrations determined after TLC isolation with BF3/MeOH and dimethylamide and by direct dimethylamide method.
| Free Fatty Acid | BF3/MeOH-TLC (µM) | Dimethylamide-TLC (µM) | Dimethylamide (µM) |
|---|---|---|---|
| C16:0 | 74 ± 2a | 73 ± 4a | 63 ± 1b |
| C16:1 | 7 ± 4a | 7 ± 6a | 7 ± 2a |
| C18:0 | 25 ± 5a | 25 ± 3a | 22 ± 1b |
| C18:1 | 77 ± 6a | 76 ± 4a | 87 ± 2b |
| C18:2 | 49 ± 3a | 49 ± 5a | 57 ± 2b |
Values shown are mean ±SD for FFA concentrations in plasma on three successive triplicates. Means with different superscripts are significantly different (p<0.05).
The comparison of change during a hyperinsulinemic euglycemic clamp in five major plasma FFA concentrations level were observed by both the dimethylamide direct method and BF3/MeOH/TLC. Blood samples were collected at baseline, −30, −15, 0, 150, 165, 180, 270, 285 and 300 min of insulin infusion for measurements of plasma FFA (Table 4). Table 4 shows that the total amounts of FFAs were approximately the same, but the measured concentration of each individual fatty acid varied between methods. Most importantly, as this was a study examining palmitate kinetics, the C16:0 concentrations were consistently higher over all timepoints when determined with the BF3/MeOH-TLC method, as described above for plasma samples and by Patterson et al [28].
3.5. Validation of the Analytical Method for Isotope Enrichment by GC-C-IRMS
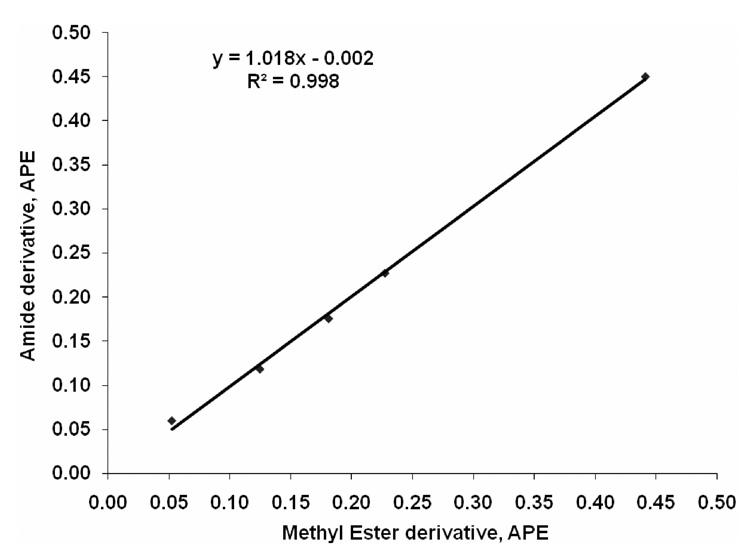
We added known amounts of [13C]palmitate to unlabeled palmitate to produce an enrichment standard curve (0%, 0.05%, 0.10%, 0.15%, 0.20%, 0.40%). The measured enrichment using the dimethylamide derivative was nearly identical to the expected enrichment (y = 1.0277x + 0.0093; R2 = 0.997). Comparison of the calculated vs. peak areas measured showed a mean difference of 0.002% APE (in the range of 0.05–0.40 APE). This demonstrated that even low enrichments could be accurately determined ±0.002% APE. Moreover, the isotope enrichment determined using our new method gave nearly identical results to that obtained using the standard method of conversion to methyl esters with BF3/MeOH with a slope near unity and an intercept of 0 (Fig. 4).
Fig. 4.
Comparison of dimethylamide and methylester derivative for [13C]palmitate enrichment.
Based on the measurement of quality control samples, we determined that the within day precision (n = 3) for GC-C-IRMS analyses was at least ±0.002% APE; the between day precision (n = 5, 4 month period) was at least ±0.01% APE.
The palmitate isotope enrichment measured using the current method was consistently higher across all timepoints than that observed with the BF3/MeOH-TLC method (Table 5). This finding would be expected based on the increased concentration of palmitate with the BF3/MeOH-TLC procedure (Table 4). This same phenomenon, higher concentration and lower enrichment observed with the BF3/MeOH-TLC procedure, was also observed by Patterson et al. [31].
Table 5.
Comparison of plasma C16 enrichment determined by dimethylamide direct method with previously published method (BF3/MeOH-TLC)
| Time (min) | Dimethylamide | BF3/MeOH-TLC | ||||||
|---|---|---|---|---|---|---|---|---|
| R 13C/12C | d 13C/12C [per mil] vs. PDB | AT% 13C/12C [%] | APE% | R 13C/12C | d 13C/12C [per mil] vs. PDB | AT% 13C/12C [%] | APE% | |
| BK | 0.0111 | −15.71 | 1.0940 | 0.0109 | −22.49 | 1.0811 | ||
| −30 | 0.0154 | 367.25 | 1.5132 | 0.419 | 0.0150 | 336.65 | 1.4798 | 0.399 |
| −15 | 0.0156 | 386.68 | 1.5343 | 0.440 | 0.0152 | 350.76 | 1.4952 | 0.414 |
| 0 | 0.0158 | 403.06 | 1.5522 | 0.458 | 0.0152 | 351.78 | 1.4963 | 0.415 |
| 150 | 0.0258 | 1292.02 | 2.5109 | 1.417 | 0.0221 | 965.02 | 2.1604 | 1.079 |
| 165 | 0.0260 | 1310.36 | 2.5305 | 1.437 | 0.0216 | 917.93 | 2.1097 | 1.029 |
| 180 | 0.0237 | 1104.94 | 2.3107 | 1.217 | 0.0214 | 906.74 | 2.0977 | 1.017 |
| 270 | 0.0255 | 1265.08 | 2.4821 | 1.388 | 0.0203 | 806.29 | 1.9894 | 0.908 |
| 285 | 0.0261 | 1321.17 | 2.5420 | 1.448 | 0.0204 | 814.40 | 1.9981 | 0.917 |
| 300 | 0.0257 | 1288.26 | 2.5069 | 1.413 | 0.0201 | 793.05 | 1.9751 | 0.894 |
3.6. GC-MS Analysis of Enrichment
We compared our dimethylamide derivative method with the methyl ester method for the [13C]palmitate using GC-EI-MS. The percentage of M+• in the case of methylester derivative was ≥98%, as expected. In the case of dimethylamide, M+• was ≥84% along with M−1 of 15 %. This finding is not surprising given that nitrogen-containing fatty acid derivatives are susceptible to removal of hydrogen atoms from random positions on the hydrocarbon chain during the electron ionization process [32]. Although amide derivatives are ideal for mass spectrometric analysis of double bond position [21], [22], [23], [24], [25], [26], [33] they are not suitable for analysis of enrichment by GC-EI-MS.
4. Conclusions
In this report, we describe a new, improve, simple and efficient procedure for measuring plasma free fatty acid concentrations by quantitative GC-FID and fatty acid [13C]palmitate isotopic enrichment by GC-C-IRMS. This method involves the use of dimethylamine/Deoxo-Fluor to derivatize plasma FFAs to their dimethylamides. This new method is specific for free fatty acids without contributions from esterified (triacylglycerols, diacylglycerols, phospholipids and cholesterol ester) fatty acids, thus no separation step (TLC or SPE) is required.
Our derivatization and GC method reported here have a number of advantages over existing methods and are suitable for analysis of large numbers of plasma samples from in vivo metabolic studies. Compared to other methods, our method is equally sensitive (limit of detection < 2 pmol) and reproducible (C.V. < 5%) but significantly more rapid. The greatly reduced time for analysis makes our method more suitable for analysis of large samples numbers.
In summary, we report an optimized method for GC/FID and GC-C-IRMS quantitation and enrichment of individual FFAs in human plasma. Our GC method separates and accurately quantifies the five major fatty acids present in human plasma (i.e., those that contribute more than 2% of total lipid) as well as enrichment with GC-C-IRMS with a high degree of sensitivity, reproducibility and enrichment values.
Acknowledgements
This investigation was supported by the grant (DK046204) from the National Institute of Diabetes and Digestive and Kidney Diseases. We also thank Professor B.W. Day and Ms. Kelly McCoy for reviewing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Menshikova EV, Ritov VB, Toledo FGS, Ferrell RE, Goodpaster BH, Kelley DE. Am. J. Physiol. 2005;288:E818. doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- 2.Kelley DE. J. Clinical Inves. 2005;115:1699. doi: 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. J. Gerontology, Series A: Biological Sciences and Medical Sciences. 2006;61A:534. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritov VB, Menshikova EV, Kelley DE. J. Chromatogr. B. 2006;831:63. doi: 10.1016/j.jchromb.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Campbell PJ, Carlson MG, Hill JO, Nurjhan N. Am. J. Physiol. 1992;263:E1063. doi: 10.1152/ajpendo.2006.263.6.E1063. [DOI] [PubMed] [Google Scholar]
- 6.Campbell PJ, Carlson MG, Nurjhan N. Am. J. Physiol. 1994;266:E600. doi: 10.1152/ajpendo.1994.266.4.E600. [DOI] [PubMed] [Google Scholar]
- 7.Carlson MG, Oeser A, Swift L. Diabetes. 1997;46:221A. [Google Scholar]
- 8.Arab L. J. Nutr. 2003;133:925S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 9.Ingalls ST, Xu Y, Hoppel CL. J. Chromatogr. B. 1995;666:1. doi: 10.1016/0378-4347(94)00555-j. [DOI] [PubMed] [Google Scholar]
- 10.Diem K, Lenter C, editors. Scientific Tables. Basle: Ciba-Geigy; 1975. p. 600. [Google Scholar]
- 11.Darbre A. In: Handbook of Derivatives for Chromatography. Blau K, King G, editors. London: Heyden and son; 1977. pp. 39–103. [Google Scholar]
- 12.Stoffel W, Chu F, Ahrens EH., Jr Anal. Chem. 1959;31:307. [Google Scholar]
- 13.Shantha NC, Napolitano G. J. Chromatogr. 1992;624:37. doi: 10.1016/0021-9673(92)85673-h. [DOI] [PubMed] [Google Scholar]
- 14.Morrison WR, Smith LM. J. Lipid Res. 1964;5:600. [PubMed] [Google Scholar]
- 15.Wolfe RR. Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- 16.Lin C, Blank EW, Ceriani RL, Baker N. Lipids. 1991;26:548. doi: 10.1007/BF02536602. [DOI] [PubMed] [Google Scholar]
- 17.Lepage G, Roy CC. J. Lipid Res. 1986;27:114. [PubMed] [Google Scholar]
- 18.Lepage G, Roy CC. J. Lipid Res. 1988;29:227. [PubMed] [Google Scholar]
- 19.Hallaq Y, Becker TC, Manno CS, Laposata M. Lipids. 1993;28:355. doi: 10.1007/BF02536323. [DOI] [PubMed] [Google Scholar]
- 20.Parise RA, Beumer JH, Kangani CO, Holleran JL, Eiseman JL, Smith NF, Covey JM, Perrine SP, Egorin MJ. J. Chromatogr. B. 2008;862:168. doi: 10.1016/j.jchromb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson BA. Prog. Chem. Fats Lipids. 1978;16:279. doi: 10.1016/0079-6832(78)90048-4. [DOI] [PubMed] [Google Scholar]
- 22.Vetter W, Walther W, Vecchi M. Helv. Chim. Acta. 1971;54:1559. doi: 10.1002/hlca.19670500721. [DOI] [PubMed] [Google Scholar]
- 23.Andersson BA, Heimermann WH, Holman RT. Lipids. 1974;9:443. doi: 10.1007/BF02534269. [DOI] [PubMed] [Google Scholar]
- 24.Kuklev DV, Smith WL. Lipid Res. 2003;44:1060. doi: 10.1194/jlr.D200046-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton JTG, Christie WW. Chem. Phys. Lipids. 2000;105:93. doi: 10.1016/s0009-3084(99)00133-4. [DOI] [PubMed] [Google Scholar]
- 26.Destaillats F, Angers P. J. Am. Oil Chem. Soc. 2002;79:253. [Google Scholar]
- 27.Kangani CO, Kelley DE. Tetrahedron Lett. 2005;46:8917. doi: 10.1016/j.tetlet.2005.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. J. Lipid Res. 1999;40:2118. [PubMed] [Google Scholar]
- 29.Dole VP, Meinertz H. J. Biol. Chem. 1960;235:2595. [PubMed] [Google Scholar]
- 30.Moore DS, Ure TAM, Butler LRP. Pure Appl. Chem. 1976;45:99. [Google Scholar]
- 31.Miller JC, Miller JN. Statistics for Analytical Chemistry. Ellis Horwood Limited; 1988. p. 115. [Google Scholar]
- 32.Christie WW. Lipids. 1998;33:343. doi: 10.1007/s11745-998-0214-x. [DOI] [PubMed] [Google Scholar]
- 33.Kangani CO, Kelley DE, Evans RW. Rapid Commun. Mass Spectrom. 2007;21:2129. doi: 10.1002/rcm.3071. [DOI] [PubMed] [Google Scholar]