Abstract
Purpose
To identify spontaneous Ca2+ sparks and global Ca2+ oscillations in microvascular smooth muscle cells (MVSM) within intact retinal arterioles and to characterize their spatiotemporal properties and physiological functions.
Methods
Retinal arterioles were mechanically dispersed from freshly isolated rat retinae and loaded with the Ca2+-sensitive dye Fluo-4. Changes in [Ca2+]i were imaged in MVSM cells in situ using confocal scanning laser microscopy in XY or line scan mode.
Results
XY scans revealed both discretely localised, spontaneous Ca2+ events resembling Ca2+ sparks, and more global and prolonged Ca2+ transients which sometimes led to cell contraction. In line-scans, Ca2+ sparks were similar to those previously described in other types of smooth muscle with an amplitude (ΔF/F0) of 0.81±0.04 (mean±SE), Full Duration Half Maximum (FDHM) of 23.62±1.15 ms, Full Width Half Maximum (FWHM) of 1.25±0.05μm and frequency of 0.56±0.06 s−1. Approximately 35% of sparks had a prolonged tail (>80ms), similar to Ca2+ ‘embers’ described in skeletal muscle. Sparks often summated to generate global and prolonged Ca2+ elevations on which Ca2+ sparks were superimposed. These sparks occurred more frequently (2.86±025 s−1) and spread further across the cell (FWHM=1.67±0.08μm), but were smaller (ΔF/F0 = 0.69±0.04).
Conclusions
Retinal arterioles generate Ca2+ sparks whose characteristics vary during different phases of the spontaneous Ca2+-signalling cycle. Sparks summate to produce sustained Ca2+-transients associated with contraction and thus may play an important excitatory role in initiating vessel constriction. This deserves further study, not least because Ca2+ sparks appear to inhibit contraction in many other smooth muscle cells.
Introduction
As with all vascular systems, blood flow through the retinal circulation depends on the perfusion pressure gradient across the vascular bed and the resistance to flow within it. Importantly, vascular resistance is inversely related to the fourth power of the radius of a blood vessel. Thus, a small change in diameter has a substantial influence on the blood flow. Variation in vessel diameter occurs frequently and may be considered the main regulatory mechanism controlling flow in the retinal circulation1. The retinal arterioles provide the major site of resistance to blood flow in the retina and thus have the greatest capacity for regulation of retinal perfusion2. Alterations in retinal arteriolar tone occur through the contraction or relaxation of the microvascular smooth muscle cells (MVSM) in the wall of the vessels. The retinal vasculature is not innervated3, and retinal arteriolar tone is mainly regulated by local factors, such as variations in pO2, pCO2 and pH as well as mediators released from neighboring endothelial and retinal cells (e.g. nitric oxide and endothelin-1)1. These local influences combine to ensure that blood flow to the retinal tissue is closely matched to metabolic demand.
Although some of the local factors responsible for regulating retinal vessel diameter have been identified, as listed above, the complex cellular mechanisms that underlie changes in retinal MVSM tone are poorly understood. MVSM contractility is known to be heavily dependent on changes in [Ca2+]i4.5 We have previously shown that retinal MVSM cells possess a variety of Ca2+ handling mechanisms which modulate cytosolic [Ca2+], including voltage-dependent and store-operated Ca2+ influx channels6, pools of releasable Ca2+ (the sarcoplasmic reticulum (SR) Ca2+ stores)7 and multiple Ca2+ efflux pathways8.
Our previous studies were largely restricted to the measurement of average retinal MVSM [Ca2+]i. The application of confocal Ca2+-imaging techniques have demonstrated, however, that intracellular [Ca2+]i signals are not homogenously distributed and rises in [Ca2+]i can differ in respect to their spatiotemporal properties and physiological functions. Both local and global Ca2+ signalling events have been observed in vascular smooth muscle cells and these may be generated spontaneously9 or in response to agonist exposure10,11. Localised Ca2+ transients are thought to result from the opening of clusters of ryanodine sensitive Ca2+ release channels on the SR and have been termed Ca2+ sparks. By activating Ca2+-activated K+ channels (KCa) to generate spontaneous outward currents (STOCs), Ca2+ sparks are thought to inhibit smooth muscle contraction in many tissues by increasing membrane polarisation and deactivating voltage-dependent Ca2+ channels, thus reducing Ca2+ influx12. In contrast to Ca2+ sparks, global Ca2+ oscillations have been implicated as mediators of constriction13. The frequency of these Ca2+ oscillations has been shown to increase with agonists that elevate inositol 1,4,5 trisphosphate (IP3) levels, suggesting that Ca2+ release through IP3 receptors on the SR contributes, at least in part, to their generation10,13.
The goal of the present study was to determine the spatiotemporal characteristics and functional relevance of spontaneous Ca2+-transients in retinal MVSM by obtaining the first images from retinal arterioles of both localised and generalised Ca2+ signalling events. Furthermore, we show that Ca2+ sparks can summate to produce sustained global Ca2+ oscillations, some of which are followed by cell contraction. This is consistent with a model in which local Ca2+ release events play an excitatory, rather than an inhibitory role in the retinal vasculature, and thus runs counter to current paradigms concerning the functional significance of Ca2+ sparks in vascular smooth muscle12. We also demonstrate the existence of two distinct populations of Ca2+ sparks for the same release sites during different phases of spontaneous signalling, presumably reflecting changes in localised Ca2+-release with different levels of cytoplasmic- and SR-[Ca2+].
Methods
Retinal microvessel preparation
Male Sprague-Dawley rats (200-300 g) were anaesthetized with CO2 and killed by cervical dislocation. Animal use conformed to the guidelines of the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and UK Home Office Regulations. Retinae were rapidly removed and arterioles devoid of surrounding neuropile isolated as previously described7.6 In brief, retinal quadrants were lightly triturated using a fire polished Pasteur pipette (internal tip diameter 0.3 mm) in a low Ca2+ Hanks' solution. Homogenates were centrifuged at 3000 rpm for 1 min, the supernatant aspirated off and the tissue washed again with low Ca2+ medium. The remaining fragments were incubated at 21°C in 1 ml of low Ca2+ Hanks' solution containing 10 μM Fluo-4 AM (Molecular Probes, Eugene, OR) and the suspension agitated every 15 min for 2 h. This prolonged incubation was necessary to facilitate adequate loading of the retinal MVSM cells with Fluo-4.
Homogenates were diluted with 10 volumes of low Ca2+ medium and the mixture vigorously triturated. Of this mixture, 1 ml was pipetted into a rotatable circular glass-bottomed recording bath on the stage of an inverted microscope (Nikon Eclipse TE300). Microvessels were anchored down with tungsten wire slips (50 μm diameter, 2 mm length) and superfused with normal Hanks' solution at 37°C. The recording bath was rotated so that the long axis of the arterioles was parallel to the x-axis of the microscope. Drug solutions were delivered via a 5-way micro-manifold with an exchange time of ∼ 1 s, as measured by switching to a dye solution.
Solutions
The bath solution had the following composition (in mM): 140, NaCl; 5, KCl; 5, D-glucose; 2, CaCl2; 1.3, MgCl2; 10, HEPES, pH 7.4 with NaOH. Low Ca2+ medium differed only in that it contained 0.1 mM CaCl2.
Ca2+ imaging and data analysis
Changes in [Ca2+]i were imaged in MVSM cell arrays with a confocal scanning laser microscope (Bio-Rad, MR-A1) used in X-Y mode at a rate of 1 image per 1.2s and in line scan mode at a rate of 500 scans/s14. Confocally imaged microvessels were excited at 488 nm and emitted light was filtered through a 530- to 560-nm band-pass filter. Data acquisition was controlled with Timecourse software (Lasersharp™; Biorad, US) and images were processed and analyzed with Image J (NIH, US). Confocal fluorescence data (F) were normalized using the average resting fluorescence (F0) for periods that exhibited no spontaneous elevations in [Ca2+]i.
Ca2+ sparks were measured within regions of interest (ROIs; 4μmx4μm boxes in XY mode and 4μmx120ms boxes in line scan mode), while global Ca2+ oscillations were averaged across the entire cell. Ca2+ events were defined as an increase in F/F0 of > 2 standard deviations above the mean resting fluorescence. The amplitudes of Ca2+ sparks and global Ca2+ oscillations were taken as the maximum increase in normalized fluorescence (ΔF/F0). Ca2+ event durations were measured along a line through the peak fluorescence as the time elapsed from reaching half the maximum amplitude during the rising phase, to falling back to that value during the decay, i.e., the full duration at half-maximal fluorescence (FDHM). Spatial spread was similarly defined as the distance in micrometers between the half-maximal fluorescence rise on either side of the peak fluorescence, i.e., the full width at half-maximum (FWHM). In some experiments using XY scan mode, cell area was calculated using the area calculator plug-in for Image J (Wayne Rasband, NIH).
Values are expressed as means ± SE. Comparisons were made with unpaired Student's t-tests, with p<0.05 considered significant.
Results
Identification of Ca2+ sparks and global Ca2+ oscillations in retinal MVSM cells
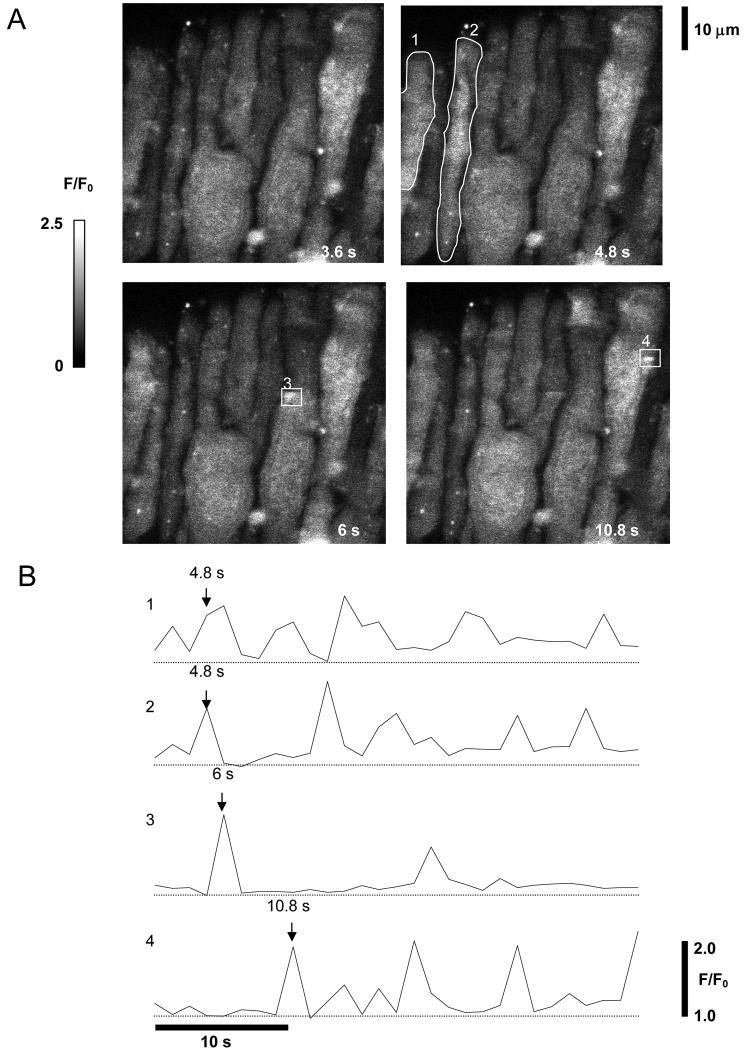
To explore whether distinct sub-cellular Ca2+ transients exist in rat retinal MVSM cells, changes in fluorescence intensity were monitored by laser scanning confocal microscopy in myocytes still embedded within their parent arterioles and loaded with the Ca2+ indicator dye Fluo-4. For the purposes of this study recordings were confined to retinal arteriole segments that were 35-40 μm in diameter and these represent the main trunk arterioles that emanate from the optic disk6. The wall of isolated retinal arterioles consisted of a monolayer of MVSM cells surrounding an intact endothelium (Fig 1). Under resting conditions, vascular myocytes within freshly dispersed retinal arterioles demonstrated considerable Ca2+ signaling activity. Two main types of spontaneous [Ca2+]i transients were observed, with different kinetics and spread. Brief and spatially localized events resembling Ca2+ sparks were often seen in close proximity to the cell membrane, as well as more prolonged global Ca2+ oscillations which usually spread across the full width of the cell (Fig 2).
Fig 1.
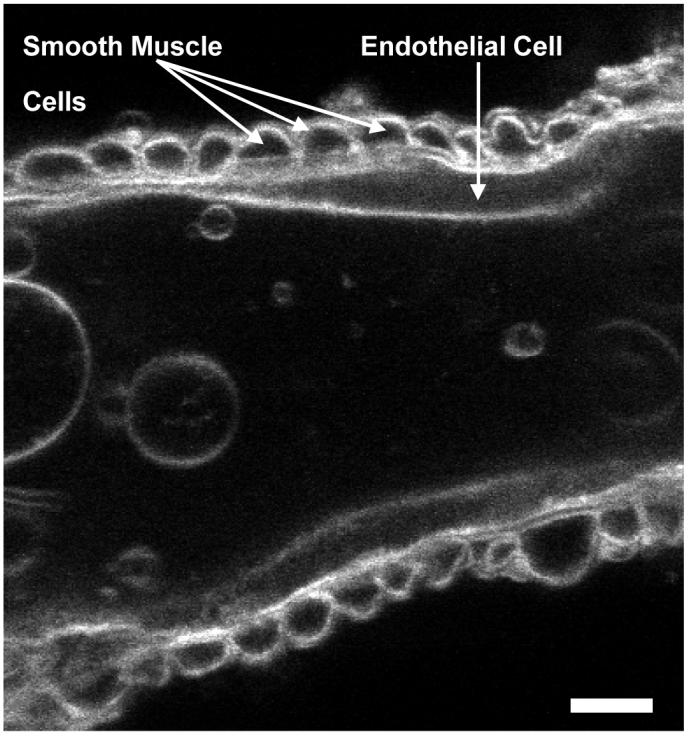
Luminal confocal section of an isolated retinal arteriole loaded for 10 min with the membrane-tracking dye di-4-ANEPPS (10μM; Molecular Probes). Scale bar = 10 μm.
Fig 2.
Ca2+ sparks and waves imaged in fluo-4 loaded retinal MVSM cells. A. Four time frames from a series of XY images of a retinal arteriole. The smooth muscle cells are oriented at right angles to the long axis of the vessel. Fluorescence relative to basal values (F/F0) is represented using a greyscale, as indicated on the calibration bar on the left. Four regions of interest (ROI) are marked and the average fluorescence within each of these regions through the course of the 40s experiment is plotted against time in B. The timepoint at which each frame was captured is indicated by labelled arrows. Both small localised events (ROIs 3 & 4), and generalised Ca2+ oscillations (ROIs 1 & 2), were seen. (A movie showing the spontaneous Ca2+ rises in this vessel is included as supplementary material online : Movie 1.
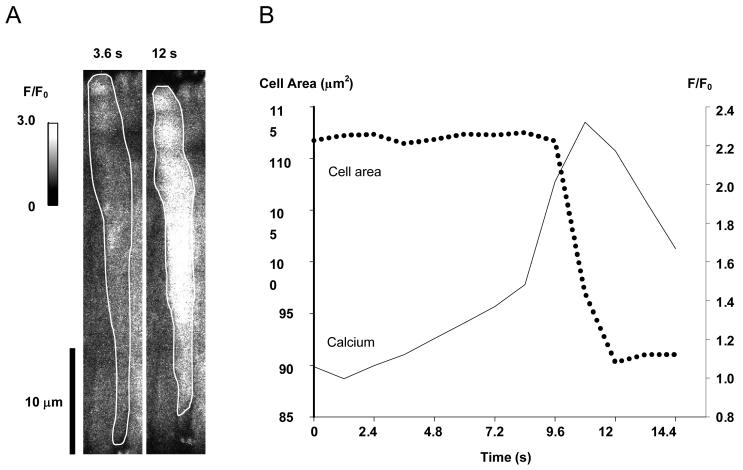
Global Ca2+ oscillations precede retinal MVSM contraction
In arterial smooth muscle, global Ca2+ oscillations have been recognized as the main driving force underlying vasoconstriction13. Likewise we observed that spontaneous global Ca2+ oscillations in retinal MVSM cells could trigger contractile responses. In the example shown (Fig 3) a global Ca2+ oscillation was associated with a 19% reduction in MVSM cell area. Although it is apparent, therefore, that a prolonged global increase in [Ca2+]i can trigger retinal MVSM contraction, this was relatively uncommon. In 6 vessels (82 cells), only 18 out of 103 global Ca2+ oscillations were followed by a decrease in cell area. The amplitude of the [Ca2+]i increases were no higher in cells which contracted than in those which did not (p=0.55), so this variability probably reflects differences in the sensitivity of the individual cells to [Ca2+]i. Mechanical contraction was never observed in response to individual Ca2+ sparks (6 vessels, 82 cells, 163 sparks).
Fig 3.
Global Ca2+ oscillations can induce retinal MVSM cell contraction. A. The outline of a retinal arteriolar smooth muscle cell imaged prior to and during a global Ca2+ oscillation. B. Changes in calcium (as F/F0) and cell area are plotted against time for the same cell.
Line scan imaging reveals that Ca2+ sparks give rise to global Ca2+oscillations
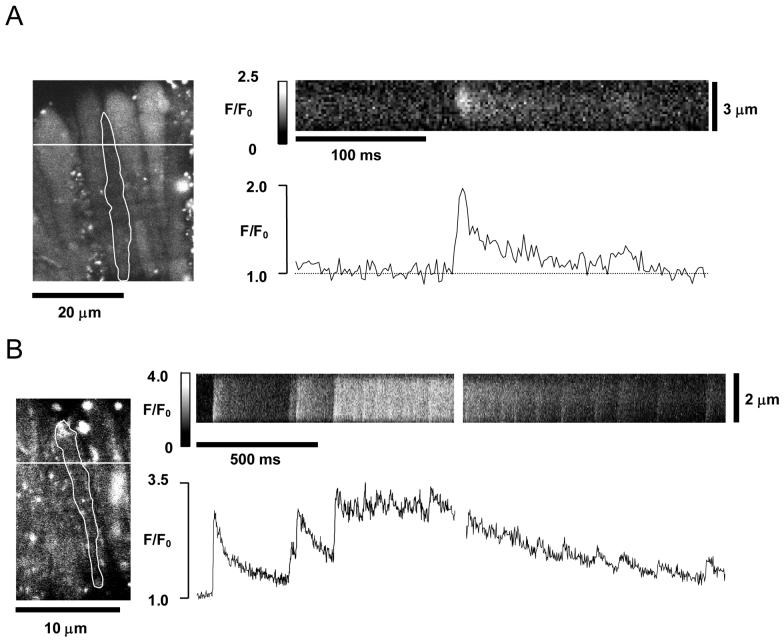
Although spontaneous [Ca2+]i transients could be visualized in retinal MVSM cells using XY scan imaging, this approach did not allow adequate temporal resolution of rapid, localized [Ca2+]i changes within a cell. Consequently, it appears from Fig 2B that both Ca2+ sparks and global Ca2+ oscillations have a similar time course. To improve temporal resolution the line-scan mode of confocal imaging was used. Analysis was limited to vessel segments exhibiting no frame-to-frame movement of the cells. A typical example of an image obtained by scanning a line orientated at right angles to the long axis of a cell is shown in Fig 4A. A brief, localised Ca2+ spark can be clearly seen in the image. This spontaneously localized Ca2+ event had a peak amplitude (ΔF/F0) of 0.95, a FDHM of 14 ms and was restricted to a relatively small area (FWHM of 1.57 μm). Interestingly some Ca2+ sparks (≅ 35% of recorded sparks in 7 cells) were associated with a prolonged tail (>80 ms) similar to that seen in the example in Fig 4A. These events resemble the Ca2+-‘embers’ or ‘glows’ recently described in skeletal muscle cells15. Ca2+ embers were ‘site dependent’ and in some sites practically all Ca2+ sparks displayed prolonged tails.
Fig 4.
Linescan images of Ca2+ sparks and global Ca2+ oscillations. A. The left-hand panel shows the shaded outline of a single retinal myocyte in a XY fluo-4 image of a retinal vessel. The position of the transverse scanline is marked, and the resulting line-scan image showing spontaneous changes in fluorescence for the selected cell is seen in the horizontal frame. Average fluorescence for this frame is plotted against time on the graph below it. A brief, localised Ca2+ spark is seen spreading laterally from the centre of the cell. B. Transverse line-scan image from another vessel (see cell outline and scanline position in left-hand panel) in which sparks summated to give a longer lasting global Ca2+ oscillation. The gap in the linescan image and fluorescence v. time plot results from the time taken for the image to be downloaded by the computer between acquisition cycles.
It has been previously demonstrated in ileal myocytes that spatiotemporal recruitment of ‘elementary’ Ca2+ sparks may give rise to cell wide elevations in [Ca2+]i.16 Using line scan imaging it was possible to visualise the initiation site of some global Ca2+ oscillations in retinal MVSM cells. In the example shown in Fig 4B it is evident that a global Ca2+ oscillation was initiated from a site where spontaneous Ca2+ sparks were also observed. The temporal profile of the global Ca2+ oscillation consists of a series of step-like increases in fluorescence resulting from the summation of consecutive Ca2+ sparks. It is also striking that even after the global Ca2+ oscillation reached its maximum, Ca2+ sparks persist throughout both the plateau and declining phases.
Analysis of spatiotemporal properties uncovers two populations of Ca2+ sparks
The spatiotemporal properties of Ca2+ sparks and global Ca2+ oscillations in retinal MVSM cells were characterized from line-scan images of 60 cells in 6 vessels, and the data are summarized in Table 1. Ca2+ sparks were separated into two groups, namely, ‘basal’ sparks that arose from resting fluorescence values (F/F0 of 0.95-1.05) and those that were superimposed upon global Ca2+ oscillations (Ca2+ sparks on oscillations). At 0.81, the mean spark amplitude under basal conditions was nearly 6x the SD of the background signal noise (noise SD=0.138). Global Ca2+ oscillations were similar in amplitude to basal Ca2+ sparks, but were nearly 100-times longer in duration (as measured from the FDHM) and occurred less frequently (Table 1). Differences in the spatiotemporal properties of basal Ca2+ sparks and Ca2+ sparks overlaying Ca2+ oscillations were also observed. The latter were smaller in amplitude, increased in width and increased in frequency (Table 1).
Table 1.
| Ca2+ sparks | Ca2+ Sparks on Oscillations |
Oscillations | |||||
|---|---|---|---|---|---|---|---|
| n | n | p | n | ||||
| Amplitude (ΔF/F0) | 0.81 ± 0.04 | 102 | 0.69 ± 0.04 | 78 | * | 0.93 ± 0.04 | 162 |
| Spread (FWHM) | 1.25 ± 0.05 μm | 96 | 1.67 ± 0.08 μm | 65 | *** | n/a | n/a |
| Duration (FDHM) | 23.6 ± 1.15 ms | 102 | 22.2 ± 1.12 ms | 78 | ns | 1992 ± 0.06 ms | 162 |
| Frequency | 0.56 ± 0.06 s−1 | 60 cells |
2.86 ± 0.25 s−1 | 50 cells |
*** | 0.13 ± 0.01 s−1 | 35 cells |
Basic properties of Ca2+ sparks and global Ca2+ oscillations in retinal MVSM cells. It was generally not possible to measure the FWHM for oscillations since the majority of these events spread across the full cell width. With the exception of frequency data, ‘n’ represents the number of Ca2+ events analyzed from a minimum of 6 vessels. The statistical significance for comparisons between sparks from baseline and sparks superimposed on oscillations as follows:
(p>0.05)
(p<0.05)
(p<0.001).
Discussion
In this study we describe the first visualization of spontaneous subcellular Ca2+ transients in retinal arteriolar smooth muscle cells. Two distinct Ca2+ signaling events were seen, discretely localized, spontaneous near-membrane Ca2+ sparks and more global and prolonged Ca2+ oscillations. Most studies on elementary Ca2+ signaling events in arterial smooth muscle have used single, isolated myocytes, even though the ultimate goal is to understand how function is regulated in intact vessels10,17. A major advantage of the technique described here is the use of intact arteriole segments in which the physiological relationships between the retinal MVSM cells, basal lamina and endothelium are preserved. Our technique also allows simultaneous imaging of subcellular Ca2+ signals in a number of MVSM cells, thus allowing cell to cell variation to be assessed while increasing the amount of data that can be collected in a single experiment.
Ca2+ sparks are thought to result from transient local release of Ca2+ from intracellular stores and have been described in cardiac18, skeletal19 and several smooth muscle cell preparations12, including arteriolar smooth muscle cells20. In smooth muscle cells Ca2+ spark amplitudes are known to be quite variable, with the average peak increase in [Ca2+]i ranging from 50-200 nM12. In retinal MVSM cells, the average spark amplitude (ΔF/F0 of 0.81; Table 1) equates to an elevation in [Ca2+]i of ∼80 nM as determined using the pseudoratiometric calculation of Cheng et al (1993)18, assuming an in situ dissociation constant for Fluo-4 of 1000 nM21 and a resting Ca2+ level in retinal arterioles of 66 nM7. The average frequency (0.56 s−1), duration (23.6 ms; FDHM) and spatial spread (1.25μm FWHM) of Ca2+ sparks in retinal MVSM are all quite similar to those in other smooth muscle cells, with reported values for these parameters ranging from 0.5 to 1 s−1 12, 30 to 65 ms22 and 1.2 to 2.3 μm23,24, respectively. There are, however, some reports of more prolonged Ca2+ sparks (100-600 ms) in tracheal25 and urinary bladder smooth muscle cells26, while the events observed in human cerebral arterial smooth muscle cells appeared to spread further, with an average FWHM of 8.2 μm 27.
Our records provide clear evidence that Ca2+ sparks in retinal MVSM cells may fuse to produce cell wide global Ca2+ oscillations that can lead to cell contraction. These findings are of particular interest since they imply that Ca2+ sparks in retinal arterioles are principally excitatory in nature, whereas it has been proposed that Ca2+ sparks exert a predominantly inhibitory effect in vascular smooth muscle, providing a negative feedback mechanism which favors decreased Ca2+ influx and vasodilatation12. Interestingly, only a small proportion of global Ca2+ oscillations actually led to retinal MVSM cell contraction. Ca2+ ions regulate nearly every cell function and sub-cellular Ca2+ transients are known to cause a pulsatile activation of Ca2+ dependent enzymes28 as well as driving changes in gene expression29. Consequently, those Ca2+ oscillations that failed to initiate excitation-contraction coupling are still likely to be physiologically relevant.
Detailed analysis revealed two distinct populations of Ca2+ sparks, with those superimposed on global Ca2+ oscillations displaying an increased frequency and spread, but reduced amplitude, when compared with sparks originating at the same release sites but from basal [Ca2+]i levels. Ca2+ sparks are thought to be generated by the opening of ryanodine receptor linked channels (RyRs) on the SR12, and elevations in cytosolic Ca2+ are known to increase RyR open probability30. This may well explain increased spark frequency during global Ca2+ oscillations. Likewise, increased spatial spread may also be accounted for by an overall increase in the open probability of RyRs, since this would favour recruitment of release sites. Since global Ca2+ oscillations in smooth muscle cells are known to involve Ca2+ store release13, the smaller amplitudes of the Ca2+ sparks seen during such oscillations in retinal MVSM cells may reflect a reduction in SR Ca2+ content. Clearly, further studies are now required to unravel the precise mechanisms through which localized Ca2+ release is modified during Ca2+ oscillations, as well as to determine the functional implications of such modifications.
Under basal conditions, many sparks in retinal MVSM cells had protracted tails similar to the Ca2+ embers of skeletal muscle cells15. We have previously described prolonged, spontaneous Ca2+ release events in isolated smooth muscle cells during store-overload14, but no such events have been reported in untreated smooth muscle, or indeed any intact tissue. In skeletal muscle cells, embers are thought to reflect direct RyR opening by voltage sensors15 but, since the RyRs are not believed to be under direct voltage control in smooth muscle, it is unclear what mechanism generates Ca2+ embers in retinal MVSM cells. It seems likely, however, that events in which Ca2+ release is prolonged well beyond the average channel-open time associated with RyRs in lipid bilayer experiments31 may have important consequences, perhaps increasing the likelihood of spark summation and the initiation of global Ca2+ oscillations. These findings also underline the fact that the study of intact tissues may reveal subtleties of signaling behaviour not apparent in isolated cells or molecules.
A possible limitation of our current model is the use of non-pressurized retinal arterioles. Passive stretching of the retinal vessel wall during increases in intraluminal pressure may lead to an elongation of the MVSM cells and thereby provoke changes in the spatiotemporal properties of the spontaneous Ca2+ signals. Increases in retinal MVSM cell length could modulate spontaneous Ca2+ sparks and global oscillations through the activation of stretch-activated currents32 or via stretch-induced gating of RyRs33. In rat cerebral arteries, pressurization increases the frequency of Ca2+ sparks and global Ca2+ oscillations, but other spatiotemporal features such as amplitudes and rise times are similar to those seen in non-pressurized vessels34.
In summary, the application of high resolution imaging to intact retinal arterioles has allowed us to visualize sub-cellular Ca2+-signaling events in retinal MVSM cells. These cells are the primary effectors of retinal arteriolar tone and the data from the present study takes us a step closer to elucidating the basic mechanisms involved in the regulation of local blood flow in the retina. Understanding how such control is achieved will be fundamental to the development of novel therapeutic strategies aimed at restoring adequate blood flow in disease states such as diabetic retinopathy35 and glaucoma36.
Supplementary Material
Acknowledgements
We thank Fight for Sight (UK), The Wellcome Trust, The Juvenile Diabetes Research Foundation (US) and the British Heart Foundation for financial support.
References
- 1.Delaey C, Van D,V. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–256. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 2.Hill DW. The regional distribution of retinal circulation. Ann R Coll Surg Engl. 1977;59:470–475. [PMC free article] [PubMed] [Google Scholar]
- 3.Ye XD, Laties AM, Stone RA. Peptidergic innervation of the retinal vasculature and optic nerve head. Invest Ophthalmol Vis Sci. 1990;31:1731–1737. [PubMed] [Google Scholar]
- 4.Guibert C, Beech DJ. Positive and negative coupling of the endothelin ETA receptor to Ca2+-permeable channels in rabbit cerebral cortex arterioles. J Physiol. 1999;514(Pt 3):843–856. doi: 10.1111/j.1469-7793.1999.843ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill MA, Zou H, Potocnik SJ, et al. Invited review: arteriolar smooth muscle mechanotransduction: Ca(2+) signaling pathways underlying myogenic reactivity. J Appl Physiol. 2001;91:973–983. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- 6.Curtis TM, Major EH, Trimble ER, et al. Diabetes-induced activation of protein kinase C inhibits store-operated Ca2+ uptake in rat retinal microvascular smooth muscle. Diabetologia. 2003;46:1252–1259. doi: 10.1007/s00125-003-1178-5. [DOI] [PubMed] [Google Scholar]
- 7.Scholfield CN, Curtis TM. Heterogeneity in cytosolic calcium regulation among different microvascular smooth muscle cells of the rat retina. Microvasc Res. 2000;59:233–242. doi: 10.1006/mvre.1999.2227. [DOI] [PubMed] [Google Scholar]
- 8.Gormley BA, Scholfield CN. Mechanism of calcium extrusion in microvascular smooth muscle in the rat retina. J Physiology. 2004:555P. [Google Scholar]
- 9.Bolton TB, Gordienko DV, Pucovsky V, et al. Calcium release events in excitation-contraction coupling in smooth muscle. Novartis Found Symp. 2002;246:154–168. doi: 10.1002/0470853050.ch12. [DOI] [PubMed] [Google Scholar]
- 10.Mauban JR, Lamont C, Balke CW, et al. Adrenergic stimulation of rat resistance arteries affects Ca(2+) sparks, Ca(2+) waves, and Ca(2+) oscillations. Am J Physiol Heart Circ Physiol. 2001;280:H2399–H2405. doi: 10.1152/ajpheart.2001.280.5.H2399. [DOI] [PubMed] [Google Scholar]
- 11.Jaggar JH, Nelson MT. Differential regulation of Ca(2+) sparks and Ca(2+) waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1528–C1539. doi: 10.1152/ajpcell.2000.279.5.C1528. [DOI] [PubMed] [Google Scholar]
- 12.Jaggar JH, Porter VA, Lederer WJ, et al. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 13.Iino M, Kasai H, Yamazawa T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. EMBO J. 1994;13:5026–5031. doi: 10.1002/j.1460-2075.1994.tb06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White C, McGeown JG. Inositol 1,4,5-trisphosphate receptors modulate Ca2+ sparks and Ca2+ store content in vas deferens myocytes. Am J Physiol Cell Physiol. 2003;285:C195–C204. doi: 10.1152/ajpcell.00374.2002. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez A, Kirsch WG, Shirokova N, et al. The spark and its ember: separately gated local components of Ca(2+) release in skeletal muscle. J Gen Physiol. 2000;115:139–158. doi: 10.1085/jgp.115.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordienko DV, Bolton TB, Cannell MB. Variability in spontaneous subcellular calcium release in guinea-pig ileum smooth muscle cells. J Physiol. 1998;507(Pt 3):707–720. doi: 10.1111/j.1469-7793.1998.707bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol. 1998;274:C1755–C1761. doi: 10.1152/ajpcell.1998.274.6.C1755. [DOI] [PubMed] [Google Scholar]
- 18.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 19.Tsugorka A, Rios E, Blatter LA. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995;269:1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- 20.Burdyga T, Shmygol A, Eisner DA, et al. A new technique for simultaneous and in situ measurements of Ca2+ signals in arteriolar smooth muscle and endothelial cells. Cell Calcium. 2003;34:27–33. doi: 10.1016/s0143-4160(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 21.Thomas D, Tovey SC, Collins TJ, et al. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 2000;28:213–223. doi: 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- 22.Remillard CV, Zhang WM, Shimoda LA, et al. Physiological properties and functions of Ca(2+) sparks in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L433–L444. doi: 10.1152/ajplung.00468.2001. [DOI] [PubMed] [Google Scholar]
- 23.Furstenau M, Lohn M, Ried C, et al. Calcium sparks in human coronary artery smooth muscle cells resolved by confocal imaging. J Hypertens. 2000;18:1215–1222. doi: 10.1097/00004872-200018090-00007. [DOI] [PubMed] [Google Scholar]
- 24.Pabelick CM, Sieck GC, Prakash YS. Invited review: significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J Appl Physiol. 2001;91:488–496. doi: 10.1152/jappl.2001.91.1.488. [DOI] [PubMed] [Google Scholar]
- 25.Sieck GC, Kannan MS, Prakash YS. Heterogeneity in dynamic regulation of intracellular calcium in airway smooth muscle cells. Can J Physiol Pharmacol. 1997;75:878–888. [PubMed] [Google Scholar]
- 26.Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca(2+) sparks to BK(Ca) channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2001;280:C481–C490. doi: 10.1152/ajpcell.2001.280.3.C481. [DOI] [PubMed] [Google Scholar]
- 27.Wellman GC, Nathan DJ, Saundry CM, et al. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33:802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- 28.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 29.Cartin L, Lounsbury KM, Nelson MT. Coupling of Ca(2+) to CREB activation and gene expression in intact cerebral arteries from mouse : roles of ryanodine receptors and voltage-dependent Ca(2+) channels. Circ Res. 2000;86:760–767. doi: 10.1161/01.res.86.7.760. [DOI] [PubMed] [Google Scholar]
- 30.Saftenku E, Williams AJ, Sitsapesan R. Markovian models of low and high activity levels of cardiac ryanodine receptors. Biophys J. 2001;80:2727–2741. doi: 10.1016/S0006-3495(01)76241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 32.Kirber MT, Walsh JV, Jr., Singer JJ. Stretch-activated ion channels in smooth muscle: a mechanism for the initiation of stretch-induced contraction. Pflugers Arch. 1988;412:339–345. doi: 10.1007/BF01907549. [DOI] [PubMed] [Google Scholar]
- 33.Ji G, Barsotti RJ, Feldman ME, et al. Stretch-induced calcium release in smooth muscle. J Gen Physiol. 2002;119:533–544. doi: 10.1085/jgp.20028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaggar JH. Intravascular pressure regulates local and global Ca(2+) signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 35.Schmetterer L, Wolzt M. Ocular blood flow and associated functional deviations in diabetic retinopathy. Diabetologia. 1999;42:387–405. doi: 10.1007/s001250051171. [DOI] [PubMed] [Google Scholar]
- 36.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.