Abstract
Pharmacologic evidence suggests that the lipid products generated by one or more calcium-independent phospholipases A2 (iPLA2s) participate in the regulation of vascular tone through smooth muscle cell (SMC) Ca2+ signaling and the release of arachidonic acid. However, the recent identification of new members of the iPLA2 family, each inhibitable by (E)-6-(bromomethylene)-3-(1-naphthalenyl)-2H-tetrahydropyran-2-one, has rendered definitive identification of the specific enzyme(s) mediating these processes difficult. Accordingly, we used iPLA2β–/– mice to demonstrate that iPLA2β is responsible for the majority of thapsigargin and ionophore (A23187)-induced arachidonic acid release from SMCs. Both thapsigargin and A23187 stimulated robust [3H]arachidonate (AA) release from wild-type aortic SMCs that was dramatically attenuated in iPLA2β–/– mice (>80% reduction at 5 min; p < 0.01). Moreover, iPLA2β–/– mice displayed defects in SMC Ca2+ homeostasis and decreased SMC migration and proliferation in a model of vascular injury. Ca2+-store depletion resulted in the rapid entry of external Ca2+ into wild-type aortic SMCs that was significantly slower in iPLA2β-null cells (p < 0.01). Furthermore, SMCs from iPLA2β-null mesenteric arterial explants demonstrated decreased proliferation and migration. The defects in migration and proliferation in iPLA2β-null SMCs were restored by 2 μm AA. Remarkably, the cyclooxygenase-2-specific inhibitor, NS-398, prevented AA-induced rescue of SMC migration and proliferation in iPLA2β–/– mice. Moreover, PGE2 alone rescued proliferation and migration in iPLA2β–/– mice. We conclude that iPLA2β is an important mediator of AA release and prostaglandin E2 production in SMCs, modulating vascular tone, cellular signaling, proliferation, and migration.
Phospholipases A2 (PLA2s)2 catalyze hydrolysis of the sn-2 fatty acid substituent of glycerophospholipid substrates to yield a free fatty acid (e.g. arachidonic acid (AA)) and a 2-lysophospholipid as reviewed previously (1, 2). Both reaction products as well as their downstream metabolites possess potent biologic regulatory functions (3, 4). Thus, members of the PLA2 family initiate dual signaling pathways emanating from a single hydrolytic reaction. For example, AA can be converted to multiple eicosanoid products (e.g. prostaglandins, thromboxanes, leukotrienes, and epoxytrienes) (3), whereas lysophospholipids alter membrane dynamics modulating the activity of many transmembrane enzymes, regulate the electrophysiologic properties of multiple ion channels, and serve as metabolic nodes in signaling pathways (e.g. production of 2-arachidonoyl glycerol or AA from 2-arachidonoyl lysophosphatidylcholine) (5, 6). Furthermore, both eicosanoids and lysolipids interact with a diverse array of cellular receptors further amplifying the repertoire of biologic responses initiated by phospholipase A2 activity (7–9). Because many eicosanoids and lysolipids mediate alterations in vascular tone and inflammatory responses, the development of compounds that modulate their production has been an important pharmacologic objective. To this end, substantial efforts have focused on identifying the different types of phospholipases that contribute to eicosanoid production and cellular signaling.
The family of phospholipases A2 can be divided into four distinct subfamilies: secretory (sPLA2), platelet-activating factor-acetylhydrolase, cytosolic (cPLA2), and calcium-independent phospholipase A2 (iPLA2). The platelet-activating factor-acetylhydrolase PLA2 family exhibits substrate specificity for platelet-activating factor and oxidized phospholipids, whereas sPLA2s are low molecular weight enzymes that require millimolar calcium ion concentrations for catalysis (10). As the first intracellular phospholipase demonstrated to hydrolyze phospholipids at physiologic increments of intracellular Ca2+ concentrations (11), cPLA2α also preferentially hydrolyzes phospholipids containing AA at the sn-2 position (12), translocates to membrane bilayers during cellular activation (13), and is regulated by phosphorylation (14). Additional members of the cPLA2 family are encoded by five separate genes (15). The iPLA2s (16–19) do not require Ca2+ for either catalysis or membrane association. In addition, iPLA2s are inhibited by low micromolar concentrations of the suicide substrate (E)-6-(bromomethylene)-3-(1-naphthalenyl)-2H-tetrahydropyran-2-one (BEL) that does not inhibit phospholipid hydrolysis by sPLA2 or cPLA2 family members (20, 21). Recent in silico studies have theorized that the large majority of the intracellular serine lipases, including the cPLA2 and iPLA2 family members, likely originated from a common ancestral precursor that has a conserved structural motif containing three β-strands juxtaposed to an α-helix comprising a Walker motif that binds purine nucleotides (22). Within the calcium-independent phospholipase A2 family, only iPLA2β has been demonstrated to be regulated by purine-containing cofactors (i.e. ATP and acyl-CoA), implicating its importance in the integration of cellular lipid metabolism, signaling, and energy utilization (23, 24).
Early studies of intracellular iPLA2s identified a novel PLA2 activity in myocardial cytosol that was inhibited by Ca2+, although Ca2+ did not directly affect the activity of the purified enzyme (16, 17). This activity was subsequently demonstrated to be mediated by iPLA2β (18, 19). The Ca2+-dependent cytosolic inhibitor of iPLA2β was identified as calmodulin (CaM) (25), which binds to iPLA2β using canonical 1-9-14 and IQ CaM binding motifs near the C terminus of the enzyme (26). The physiologic importance of the regulation by Ca2+ and CaM was demonstrated by the finding that release of AA from vascular smooth muscle cell phospholipids can be induced by CaM antagonists (25).
In previous work, we demonstrated that BEL inhibits both thapsigargin (TG) and arginine vasopressin-induced release of AA from A-10 vascular smooth muscle cells (SMCs) by a mechanism that does not require an increase in cytosolic Ca2+ concentration, but rather results from calcium pool depletion and subsequent activation of iPLA2β through the release of calmodulin-mediated inhibition (27). We proposed that calcium store depletion-mediated activation of iPLA2β leads to the generation of lipid second messengers (eicosanoids and lysolipids) that activate capacitative calcium entry and recruit multiple downstream signaling pathways mediated by capacitative calcium influx (27). However, BEL has subsequently been found to inhibit many newly identified members of the iPLA2 family in addition to its known inhibition of some serine proteases (28–30). Accordingly, to unambiguously identify the role of iPLA2β in the TG- or ionophore-mediated release of AA in SMCs, it was necessary to demonstrate that AA release could be inhibited by genetic ablation of iPLA2β. To this end, we have recently generated iPLA2β-null mice by homologous recombination (31). Mice null for iPLA2β exhibit several phenotypes, including male sterility resulting from decreased sperm motility, but alterations in AA release in these mice have not been previously examined (31). In this report, we used iPLA2β–/– mice to unambiguously demonstrate the role of iPLA2β in SMC AA release in response to TG and ionophore stimulation and to identify alterations in SMC Ca2+ homeostasis, migration, and proliferation. We now report that iPLA2β is required for the release of AA during calcium pool depletion or ionophore stimulation in SMCs especially at early time points after stimulation and that the absence of iPLA2β alters Ca2+ signaling. Moreover, the absence of iPLA2β results in defects in mesenteric arterial SMC migration and proliferation, which were rescued through the addition of exogenous low micromolar amounts of AA and subsequently re-inhibited by the COX-2-specific inhibitor NS-398.
EXPERIMENTAL PROCEDURES
Materials—Reagents and media for cell culture were obtained from Invitrogen. Prostaglandin E2 (PGE2), buffer salts, recombinant rat platelet-derived growth factor-BB, and poly-l-lysine solution (0.01%) were purchased from Sigma-Aldrich Chemical Co. Fura-2/acetoxymethyl ester (Fura-2/AM), TG, ionophore A23187, arachidonyl trifluoromethylketone (AACOCF3), N-{(2S,4R)-4-(biphenyl-2-ylmethylisobutylamino)-1-[2-(2,4-difluorobenzoyl)benzoyl]pyrrolidin-2-ylmethyl}-3-[4-(2,4-dioxothiazolidin-5-ylidenemethyl)phenyl]acrylamide (Pyr), and the primary antibodies against smooth muscle α-actin and cyclooxygenase-2 were purchased from EMD Biosciences. BEL and a monoclonal PGE2 immunoassay kit were purchased from Cayman Chemical. Enantiomers of BEL were resolved and purified by high-performance liquid chromatography utilizing a Chirex column of 3,5-dinitrobenzoyl-(R)-phenylglycine attached to a silica matrix as previously described (32). 1-Oleoyl-2-hydroxy-sn-glycero-3-phosphate (LPA) was purchased from Avanti Polar Lipids. The LPA receptor antagonist, 1-bromo-3(S)-hydroxy-4-(palmitoyloxy)butyl phosphonate (BrP-LPA), was obtained from Echelon Biosciences (Salt Lake City, UT). [5,6,8,9,11,12,14,15-3H]AA was obtained from PerkinElmer Life Sciences. Other materials for Western blotting analyses, including primary monoclonal antibodies against α-actin and cPLA2α (to quantify total cPLA2α (residues 1–216)), polyclonal antibody against phosphorylated cPLA2α at Ser-505, and peroxidase-conjugated anti-mouse IgG, were purchased from Santa Cruz Biotechnology, Inc. and GE Biosciences. PCR reagents were obtained from Applied Biosystems and Qiagen, Inc.
Isolation and Culture of Mouse SMCs—All animal studies were approved by the Washington University Animal Studies Committee. Aortic and mesenteric arterial SMCs were isolated from wild-type and iPLA2β-null mice essentially using minor modifications of a previously described method (33, 34). Briefly, to isolate mesenteric arterial SMCs, the subxiphoid abdominal region of asphyxiated mice (age 10–14 weeks) was incised, and the mesenteric arcade was exposed. Mesenteric arteries (∼100-μm diameter) were separated after removing fatty tissues under stereomicroscopic visualization. Exposed arterioles were excised and placed in a culture dish containing DMEM, and residual fatty tissue attached to the isolated arterioles was removed. The lumen of the arteriole was gently compressed and scraped with tweezers to remove blood clots and to remove the endothelium. Micro-dissected tissue sections were placed on 35-mm poly-l-lysine (0.01%)-coated tissue culture dishes, and the sections were covered with poly-l-lysine-coated glass coverslips and incubated in DMEM/F-12 (1:1) medium containing 2 mm glutamine, 1% antibiotic/antimycotic supplement, 20% heat-inactivated fetal bovine serum, and platelet-derived growth factor-BB (20 ng/ml) under an atmosphere of 5% CO2 at 37 °C. To isolate aortic SMCs, excised aortae were scraped to remove blood clots and adventitia. The aortae were incised longitudinally, and the inner surface was scraped to remove the endothelium. Aortic SMCs were cultured as described above for mesenteric arterial SMCs. Confluent primary cells were detached from the plates with 0.25% Trypsin/EDTA. All experiments were conducted using primary and first passage cells.
Migration and Proliferation of Mouse Smooth Muscle Cells—Mesenteric arterial SMCs were isolated from wild-type and iPLA2β-null mice, and the primary cells were cultured in the presence of 2 μm AA, 2 μm lysophosphatidic acid, 20 μm non-selective COX inhibitor indomethacin, 10 μm COX-2-specific inhibitor NS-398, 5 μm PGE2, 10 μm BrP-LPA, or vehicle alone in the culture medium, which was changed every 2 days. In some experiments, AA and PGE2 were added to cells incubated with the COX-2 inhibitor NS-398. Images of cells were obtained by using a Zeiss inverted digital microscope (10× air objective), and the number of migrating/proliferating cells was counted (at 21 days post-isolation). The path-independent migration distance of each cell from the nearest explant edge was determined by Intelligent Imaging Innovations software.
Analyses of iPLA2β mRNA Expression by Aortic SMCs by Reverse Transcription-PCR—Total RNA was extracted from first passage subcultured aortic SMCs using RNA-STAT-60™ (Tel-Test, Inc.) according to the manufacturer's instructions, and cDNA was prepared using a TaqMan® Reverse Transcription PCR kit. The following iPLA2β-specific primers were used: F1, 5′-CTGCAGAATTCCATGTCGAAAGATAACATGGAG-3′; R1, 5′-CCGAAGCGGCCGCTCCTTCATACGGAAGTACAC-3′; F2, 5′-ATGATTATCAGCATGGACAGCA-3′; and R2, 5′-ACACAGGTTACAGGCACTTGAGG-3′. PCR was performed for 30 cycles of 53 °C (30 s), 72 °C (2 min), and 94 °C (30 s), and products were analyzed by 1% agarose gel electrophoresis. The F1–R1, F2–R1, and F1–R2 primer sets generated predicted amplification products of 431, 683, and 898 bp, respectively, from wild-type template.
Quantitative PCR—Quantitative PCR was performed as previously described (35). The following mouse forward and reverse primers and probes, respectively, were purchased from Applied Biosystems: iPLA2γ:5′-GAGGAGAAAAAGCGTGTGCTACTTC-3′, 5′-GGTTGTTCTTCTTAAGGCCTGAA-3′, and 5′-TCTGTTATCAATACTCACTCTTGCAATA-3′; iPLA2β:5′-CCTTCCATTACGCTGTGCAA-3′, 5′-GAGTCAGCCCTTGGTTGTT-3′, and 5′-CCAGGTGCTACAGCTCCTAGGAAAGAATGC-3′; and cPLA2α:5′-CCTTTGAGTTCATTTTGGATCCTAA-3′, 5′-TGTAGCTGTGCCTAGGGTTTCAT-3′, and 5′-AGGAAAATGTTTTGGAGATCACACTGATGGATG-3′. PCR, in triplicate, utilized the following conditions: 2 min at 50 °C and 10 min at 95 °C, followed by a total of 40 two-temperature cycles (15 s at 95 °C and 1 min at 60 °C). Relative mRNA levels were normalized to ribosome RNA as internal standard.
[3H]AA Release—Aortic SMCs at 70% confluency in 12-well plates were labeled with [3H]AA (1 μCi per dish, for 20 h) and washed with DMEM/F-12 medium containing 0.25% (2.5 mg/ml) fatty acid-free bovine serum albumin, followed by two more washes with DMEM/F-12 medium alone (with no fetal bovine serum and platelet-derived growth factor-BB). For the PLA2 inhibition experiments, cells were pretreated with either the iPLA2 inhibitor BEL or the pyrrolidine cPLA2α inhibitor Pyr. Cells were then incubated in culture medium (2 ml) without platelet-derived growth factor-BB that contained TG (1 μm), ionophore A23187 (10 μm), or vehicle (0.1%, v/v DMSO) alone. For some experiments, aortic SMCs were washed with media containing EGTA (final concentration, 3 mm) to chelate extracellular Ca2+ prior to agonist stimulation in EGTA-containing media. After 5 or 10 min, the medium (1 ml) was removed and lipids were extracted into 2 ml of chloroform/methanol/acetic acid (50/48/2, v/v). Lipids extracted into the CHCl3 layer were resolved by TLC using petroleum ether/ethyl ether/glacial acetic acid (70/30/1, v/v) with oleic acid as fatty acid standard as previously described (32). Regions containing fatty acids were identified with iodine vapor and scraped into vials, and the [3H]AA content was determined by liquid scintillation spectrometry. The cells were scraped into lysis buffer (50 mm Tris, pH 7.4, containing 150 mm NaCl, 1 mm EDTA, 0.25% sodium deoxycholate, 1% Nonidet P-40, 0.1% SDS, 1 mm phenylmethanesulfonyl fluoride, 2 μg/ml aprotinin, and 1 μg/ml leupeptin), and smooth muscle α-actin (∼43 kDa) was determined by ECL immunoblotting following SDS-PAGE to normalize [3H]AA release relative to the intensity of the immunoreactive α-actin band determined utilizing a Kodak Image Station 440 CF. For determination of COX-2, total cPLA2α and phosphorylated (Ser-505) cPLA2α protein expression levels in wild-type and iPLA2β–/– aortic SMCs, aliquots of cell lysates were loaded and resolved by SDS-PAGE gel (100 μg of protein/lane) and transferred to polyvinylidene difluoride membranes that were probed with either a monoclonal IgG1 COX-2 antibody, a monoclonal IgG2b cPLA2α antibody (directed against residues 1–216), or a polyclonal antibody to cPLA2α phosphoserine-505. The polyvinylidene difluoride blots were then incubated with peroxidase-conjugated anti-mouse IgG or protein A, detected by ECL, and quantified using a Kodak Image Station.
Measurement of Intracellular Ca2+ Concentration—Isolated vascular SMCs were grown in optical dishes (Bioptechs, Inc.) to ∼70% confluency. After washing twice with buffer (130 mm NaCl, 4.8 mm KCl, 1.2 mm MgCl2 6H2O, 17 mm HEPES, 11 mm glucose, 20 mm NaHCO3, 1.8 mm CaCl2, pH 7.3), the cells were incubated in the presence or absence of AACOCF3 (25 μm) for 1 h, or BEL or cPLA2α inhibitor Pyr at the indicated concentrations for 10 min at 37 °C after preincubation (90 min, in the dark) of the cells in buffer containing Fura-2/AM (final concentration, 5 μm) dissolved in DMSO containing Pluronic F-127 (final concentration, 0.02%). Cells were rinsed with Ca2+-free buffer that contained 200 μm EGTA to remove Ca2+ and residual Fura-2/AM. Fresh Ca2+-free buffer was placed in the dishes and allowed to equilibrate with the cells for 10 min prior to taking fluorescence measurements. The dishes were mounted on the stage of a Zeiss inverted digital microscope (40× air objective) equipped with Intelligent Imaging Innovations software and illuminated alternately at excitation wavelengths of 340 nm and 380 nm, followed by emission measurements at 510 nm. Thapsigargin (1 μm) was added to the cells at the times indicated in the figures, and intracellular [Ca2+] was presented as the ratio (F340/F380) of light emitted at 510 nm after excitation at 340 nm and 380 nm, respectively. Ca2+ was re-introduced into the medium of TG-treated cells at the indicated time to achieve a final concentration of 1.8 mm.
PGE2 Immunoassay—First passage, subcultured, confluent aortic SMCs obtained from wild-type mice were washed with DMEM alone and incubated with culture medium (10% fetal bovine serum) containing TG (1 μm), ionophore A23187 (10 μm), or vehicle alone for 5 or 10 min. The medium was collected and diluted 10-fold in assay buffer, and the quantity of PGE2 was determined by immunoassay according to the manufacturer's instructions.
Statistical Analyses—Values are expressed as means ± S.E. The significance of experimental observations was determined using a Student's t test for unpaired data. Results were considered significant at the p < 0.05 level.
RESULTS
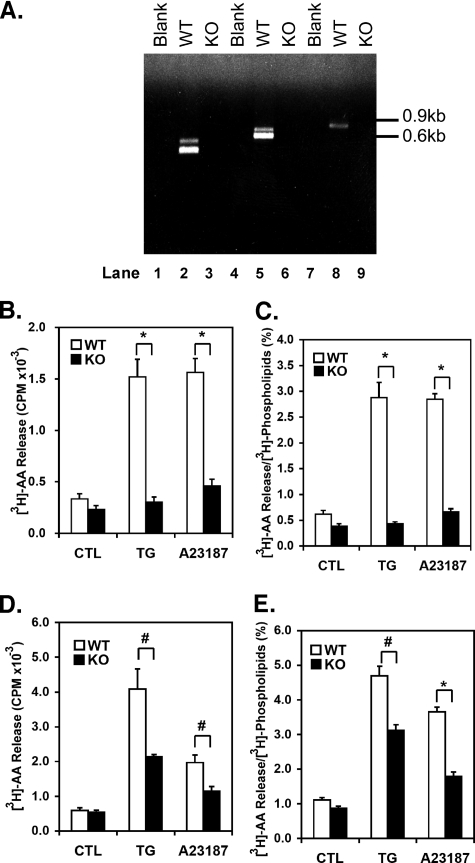
AA Release from Mouse Vascular SMCs Induced by TG or Ionophore (A23187) Is Markedly Attenuated in iPLA2β–/– Mice—To ensure that the tissues used in the experiments using wild-type and iPLA2β–/– mice contained and lacked iPLA2β, respectively, we performed quantitative PCR analyses on vascular SMC mRNA corresponding to three distinct regions of the iPLA2β coding sequence. As anticipated, each primer set yielded products of the expected sizes with RNA from wild-type aortic SMCs (lanes 2, 5, and 8), but not with iPLA2β-null aortic SMC RNA as template for reverse transcription-PCR (lanes 3, 6, and 9) (Fig. 1A). These results demonstrate the presence of iPLA2β in wild-type SMCs and substantiate the genetic knockout of iPLA2β in the animals used in this study that is consistent with previous reports using this mouse line (31, 36, 37).
FIGURE 1.
Arachidonic acid release from mouse aortic SMCs induced by depletion of internal Ca2+ stores with TG or ionophore A23187. A, total RNA was extracted from either wild-type (WT) or iPLA2β-null (KO) first passage aortic SMCs for the preparation of cDNA using reverse transcriptase. Three pairs of primers, each amplifying a different region of the iPLA2β cDNA sequence, were used to examine expression of iPLA2β mRNA. PCR conditions utilized a 30-cycle reaction with steps at 53 °C for 30 s, 72 °C for 2 min, and 94 °C for 30 s per cycle. PCR products were analyzed by gel electrophoresis (1% agarose). The following primers were used to amplify fragments of iPLA2β cDNA: forward primer F1, 5′-CTGCAGAATTCCATGTCGAAAGATAACATGGAG-3′; reverse primer R1, 5′-CCGAAGCGGCCGCTCCTTCATACGGAAGTACAC-3′; forward primer F2, 5′-ATGATTATCAGCATGGACAGCA-3′; and reverse primer R2, 5′-ACACAGGTTACAGGCACTTGAGG-3′. The primer pair F1 and R1 amplified a 431-bp product (lanes 1–3); the primer pair F2 and R1 amplified a 683-bp product (lanes 4–6); and the primer pair F1 and R2 amplified an 898-bp product (lanes 7–9). Aortic SMCs from wild-type (open bars) or iPLA2β-null (filled bars) mice were radiolabeled with [3H]AA (1 μCi/well) for 20 h. Unincorporated [3H]AA was removed by washing the cells with medium containing fatty acid-free bovine serum albumin followed by incubation with either 1 μm TG (TG), ionophore A23187 (10 μm), or 0.1% DMSO vehicle alone (CTL) for either 5 min (B and C) or 10 min (D and E). The medium (1 ml) was then removed, and lipids were extracted with chloroform/methanol/acetic acid (50/48/2, v/v, 2 ml). Free [3H]AA present in the chloroform layer was resolved from that present in glycerophospholipids by TLC and quantified by liquid scintillation spectrometry. The cell monolayer was resuspended in lysis buffer, and the relative smooth muscle α-actin content of each sample was determined by Western blotting analyses with densitometric measurement of band intensities. The quantity of released [3H]AA was normalized to smooth muscle α-actin content as described under “Experimental Procedures.” Results are expressed as the amount of [3H]AA released relative to the amount of smooth muscle α-actin (B and D) or as the fractional percentage of [3H]AA released relative to that present in endogenous radiolabeled phospholipid pools (C and E). Data represent results from triplicate determinations from each of three separate animals in each group and are presented as the means ± S.E. *, p < 0.01; #, p < 0.05.
Treatment of wild-type vascular aortic SMCs with either TG or calcium ionophore A23187 induces the robust hydrolysis of esterified AA from cellular phospholipids. However, the relative contributions of different PLA2s to this process and their distinct roles in vascular SMC biology remain poorly understood. Accordingly, we examined the effect of genetic ablation of iPLA2β on [3H]AA release from aortic SMCs prelabeled with [3H]AA. Treatment of wild-type (WT) aortic SMCs with TG or A23187 for 5 min resulted in the robust release of [3H]AA in comparison to vehicle alone (4.6 ± 0.5-fold and 4.7 ± 0.3-fold increases, respectively; Fig. 1, B and C). In marked contrast, TG and ionophore-induced [3H]AA release from iPLA2β-null (KO) cells was nearly abolished regardless of which parameter was used for comparison (i.e. the amount of [3H]AA release normalized to α-actin or the fractional percentage of [3H]AA release to radiolabeled phospholipids; Fig. 1, B and C). Specifically, essentially no [3H]AA above control levels was released from iPLA2β-null vascular SMCs treated with TG and only minimal release (<20%) occurred after ionophore treatment (A23187) for 5 min (Fig. 1, B and C). There was no significant difference in the amount of [3H]AA incorporated into wild-type aortic SMCs in comparison to that incorporated into cells from iPLA2β–/– animals (mean incorporation of [3H]AA into wild-type and iPLA2β-null aortic SMCs after 20-h incubation with 1 μCi of [3H]AA was ∼0.089 μCi and ∼0.085 μCi per dish, respectively). In similar experiments performed with wild-type and iPLA2β-null aortic SMCs radiolabeled with [3H]oleic acid, neither TG nor ionophore induced significant oleic acid release despite robust labeling of phospholipids with [3H]oleic acid (data not shown). Statistical analyses demonstrated that the profound reduction in AA release was highly significant using either TG (∼90% reduction, p < 0.01) or ionophore (∼80% reduction, p < 0.01) as stimulant when compared with wild-type cells following a 5-min treatment interval.
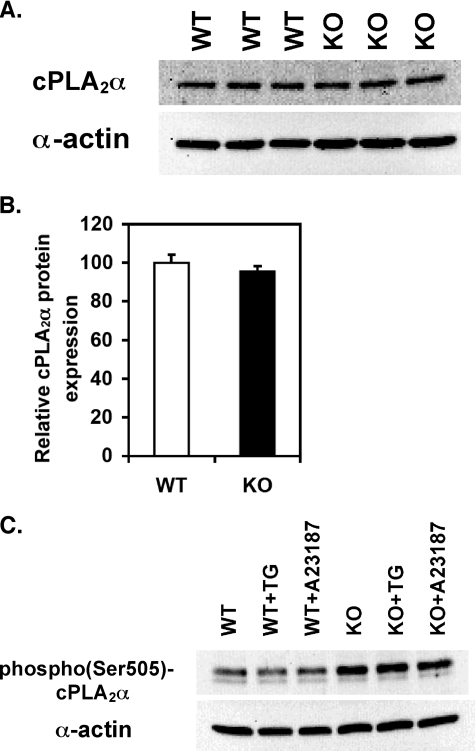
We next sought to determine whether a longer stimulation time (10 min) with TG or ionophore would result in increased [3H]AA release from iPLA2β–/– cells. As shown in Fig. 1 (D and E), iPLA2β-null cells were capable of releasing 54 ± 3% and 40 ± 7% of the [3H]AA in comparison to control wild-type cells following TG and A23187 treatment, respectively, for 10 min. To address whether this delayed [3H]AA release from iPLA2β–/– SMCs was potentially a result of cPLA2α, we quantified the expression level of total cPLA2α protein and that of cPLA2α phosphorylated at Ser-505 (indicative of cPLA2α activation). Importantly, total cPLA2α protein was not significantly altered in iPLA2β–/– aortic SMCs as determined by immunoblot analysis (Fig. 2, A and B), ruling out decreased cPLA2α expression as a possible explanation for the significantly diminished ability of iPLA2β-null cells to release [3H]AA. Surprisingly, the amount of phospho-cPLA2α protein (pS505) in iPLA2β-null SMCs was higher than that in wild-type cells (Fig. 2C). However, this increase in cPLA2α phosphorylation was not sufficient to reconstitute normal amounts of [3H]AA release from iPLA2β-null cells following stimulation with either TG or ionophore.
FIGURE 2.
Relative protein expression levels of total and phosphorylated (Ser-505) cytosolic PLA2α in wild-type (WT) and iPLA2β-null (KO) aortic SMCs. Expression of cPLA2α protein in aortic SMCs obtained from three separate explant preparations from wild-type (WT) and iPLA2β-null (KO) mice was determined by Western blotting. Proteins from cell homogenates of WT (lanes 1–3) and KO (lanes 4–6) were resolved by SDS-PAGE (100 μg of protein/lane) and transferred to polyvinylidene difluoride membranes that were probed with a monoclonal IgG2b antibody directed against cPLA2α residues 1–216 (A). The relative cPLA2α protein expression levels in WT and KO were densitometrically determined from immunoblot intensities after normalization to smooth muscle α-actin protein levels (B). First passage, subcultured aortic SMCs from wild-type (WT) and iPLA2β-null (KO) animals were incubated with either 1 μm TG, 10 μm A23187, or vehicle alone for 5 min. Proteins from cell homogenates resolved by SDS-PAGE were probed with a polyclonal antibody directed against phosphorylated Ser-505 of cPLA2α (C).
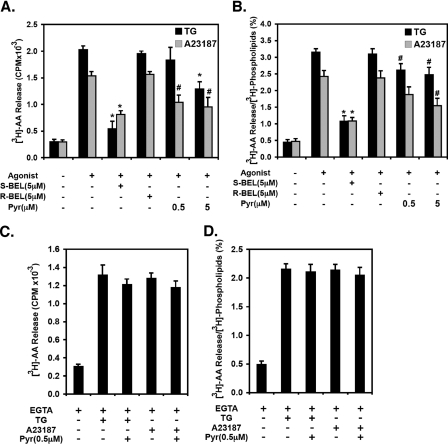
Next, we investigated the contribution of cPLA2α and iPLA2 activities to TG- and A23187-induced AA release utilizing wild-type aortic SMCs with highly selective inhibitors to the iPLA2 or cPLA2 families of phospholipases to confirm the results of the genetic experiments using iPLA2β–/– cells. Previously, we have demonstrated that iPLA2β is more potently inhibited by the S-enantiomer of the mechanism-based iPLA2 inhibitor BEL (S-BEL) than the R-enantiomer (R-BEL), while the inverse was found for iPLA2γ (32). For the specific pharmacologic inhibition of iPLA2β, iPLA2γ, and cPLA2α activities, first passage aortic SMCs were preincubated for 10 min with either S-BEL, R-BEL, or a highly selective cPLA2α inhibitor, Pyr, possessing an IC50 of ∼5 nm in vitro as previously measured (38) and confirmed in this study (data not shown). In cells treated with TG for 5 min, 0.5 μm and 5 μm Pyr inhibited only 16 ± 11% and 34 ± 9% of the [3H]AA release, respectively. In contrast, 82 ± 7% of the [3H]AA released into the media during this time was inhibited by the iPLA2β-selective inhibitor S-BEL at 5 μm concentration (Fig. 3, A and B). Similarly, pretreatment of wild-type cells with either 0.5 μm or 5 μm Pyr inhibited 34 ± 12% and 47 ± 13% (respectively) of the [3H]AA release induced by ionophore. In contrast, 64 ± 6% of ionophore stimulated [3H]AA release was inhibited by 5 μm S-BEL (Fig. 3, A and B). Pretreatment of wild-type cells with 0.5 μm Pyr inhibited 92 ± 1% of cPLA2α activity as assessed by in vitro assays using cell homogenates in the presence of calcium ion. These results were confirmed by the lack of additional inhibition by either pretreatment with 5 μm Pyr or inclusion of 0.5 μm Pyr just prior to the in vitro assay (data not shown). In addition, we found that 5 μm Pyr inhibited ∼75% of recombinant iPLA2β and iPLA2γ activities as was previously reported (39). Thus, at doses of Pyr where specific inhibition of cPLA2α was present (0.5 μm), TG-induced AA release was not inhibited. In the case of ionophore-mediated stimulation, 34% of AA release in wild-type cells was inhibited by Pyr, which is consistent with the demonstrated presence of cPLA2α in iPLA2β–/– cells and the rapid supraphysiologic amounts of calcium introduced into the cell after ionophore treatment.
FIGURE 3.
Effects of phospholipase A2 inhibitors on AA release from wild-type mouse aortic SMCs induced by TG or ionophore A23187. First passage, subcultured, subconfluent aortic SMCs from wild-type mice were radiolabeled with [3H]AA (1 μCi/well) for 20 h. Unincorporated [3H]AA was removed by washing the cells with medium containing fatty acid-free bovine serum albumin followed by preincubation with either iPLA2 inhibitors (5 μm S-BEL or R-BEL), a cPLA2α inhibitor (0.5 or 5 μm Pyr), or vehicle alone for 10 min. Cells were then incubated with 1 μm TG (TG), ionophore A23187 (10 μm), or 0.1% DMSO vehicle alone (CTL) for 5 min in the presence of 2 mm calcium (A and B) or 3 mm EGTA (C and D). The released [3H]AA in the medium was extracted into chloroform, resolved by TLC, measured by liquid scintillation spectrometry, and normalized to smooth muscle α-actin content as described under “Experimental Procedures.” Results are expressed as the amount of [3H]AA released relative to the amount of smooth muscle α-actin (A and C) or as the fractional percentage of [3H]AA released relative to that present in cellular radiolabeled phospholipid pools (B and D). Data represent results from each of three separate wild-type animals and are presented as the means ± S.E. *, p < 0.01; #, p < 0.05.
To determine the amount of AA release that is mediated by the entry of calcium into the cell, additional experiments with wild-type cells in media without calcium containing 3 mm EGTA were performed. Importantly, prevention of calcium influx in wild-type SMCs only modestly inhibited the release of AA (∼30%) (Fig. 3, compare A and B to C and D). This modest reduction in AA release is consistent with the amount of AA release induced by A23187 in calcium-containing media (presumably due to cPLA2α) in SMCs from iPLA2β–/– mice. Moreover, the release of [3H]AA from wild-type SMCs incubated in EGTA-containing media was not inhibited by Pyr. Collectively, these findings are consistent with iPLA2β mediating the majority of AA release from aortic SMCs at early time points with other contributions arising from cPLA2α at longer time points.
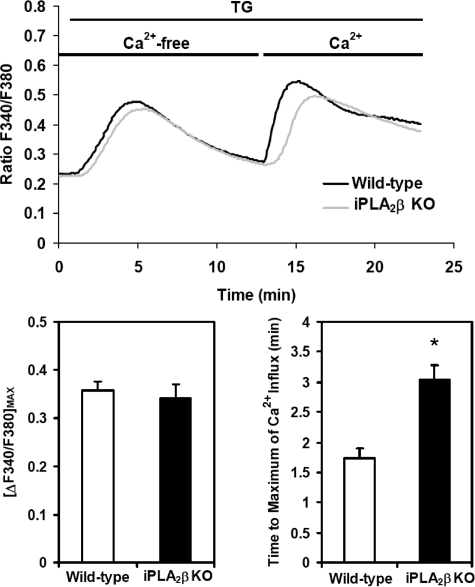
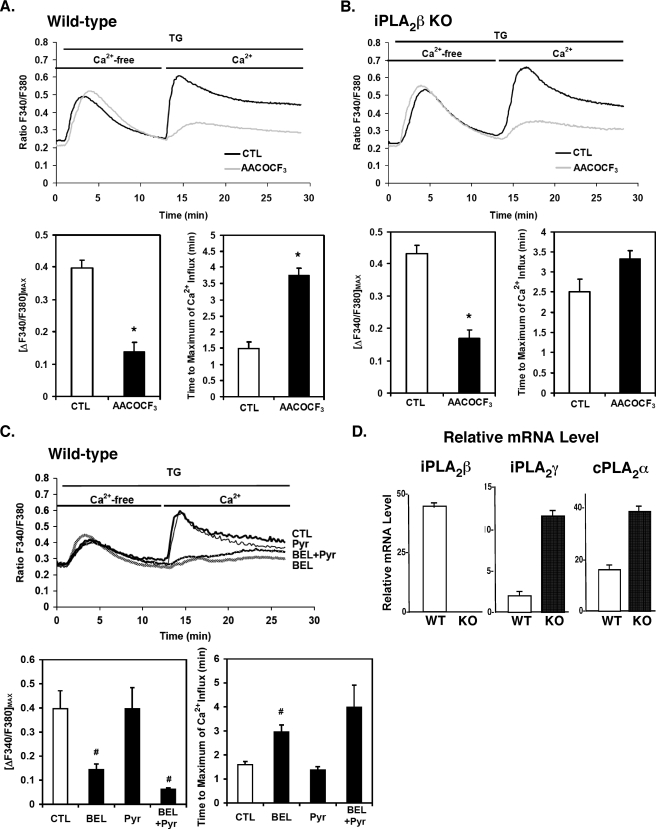
Genetic Ablation of iPLA2β Decreases the Rate of Ca2+ Entry into Vascular SMCs Induced by Ca2+ Store Depletion—In many cell types, including vascular SMCs, Ca2+ store depletion induced by sarco/endoplasmic reticulum Ca2+-ATPase inhibitors such as TG results in Ca2+ influx through activation of plasma membrane cation channels designated store-operated channels. To further investigate the role of iPLA2β in this process, we compared the responses of vascular SMCs from wild-type and iPLA2β–/– mice loaded with the cell-permeable Ca2+-sensitive indicator Fura-2/AM (40). After loading, wild-type and iPLA2β-null aortic SMCs were placed in Ca2+-free buffer that contained the Ca2+-chelator EGTA (200 μm) and then treated with TG (1 μm). The intracellular Ca2+ concentration was monitored by changes in Fura-2 fluorescence (Fig. 4). The rise in intracellular [Ca2+] observed after the addition of TG in Ca2+-free medium reflects a combination of the decreased entry into the endoplasmic reticulum as well as potential release from the endoplasmic reticulum mediated by Ca2+ pool depletion or altered microenvironments in localized endoplasmic reticulum pools (41, 42). The magnitude and time course of this response after TG addition did not differ significantly between wild-type and iPLA2β-null cells. After 12 min of incubation with TG in Ca2+-free medium, Ca2+ was added to the medium to achieve a final concentration of 1.8 mm. The subsequent rise in cellular [Ca2+] observed after adding Ca2+ to the medium of TG-treated cells reflects Ca2+ influx from the extracellular space that is likely mediated by multiple ion channels (see below). The rate of Ca2+ store depletion-induced Ca2+ entry in wild-type cells was more rapid than that in iPLA2β-null cells, although the maximal amplitude of the rise in [Ca2+] was similar in these two cell types (Fig. 4). Comparison of the times required to reach the maximum of intracellular [Ca2+] revealed the response of iPLA2β-null cells was clearly delayed in comparison to wild-type cells (p < 0.01) (Fig. 4). These results are consistent with the notion that iPLA2β regulates the rate-determining step in Ca2+-store depletion-induced Ca2+ entry. Furthermore, these results suggest that alternate, iPLA2β-independent mechanisms for Ca2+ entry are also present that, although not rate-determining for calcium entry in wild-type SMCs, they become rate-determining in iPLA2β–/– SMCs. Thus, the early stages of the spatiotemporal dispersion of Ca2+ signals in vascular SMCs are influenced by iPLA2β activity, and contributions from other enzymes (and/or mechanisms) become apparent in iPLA2β–/– cells.
FIGURE 4.
TG-induced changes in intracellular [Ca2+] of wild-type and iPLA2β-null mouse aortic SMCs incubated in Ca2+-free or Ca2+-replete medium. First passage aortic SMCs from wild-type (WT) and iPLA2β–/– (KO) mice were grown to ∼70% confluency and then incubated in the dark at room temperature with 5 μm Fura-2/AM in a buffered solution (pH 7.3). The Fura-2/AM-containing buffer was then removed and replaced with Ca2+-free buffer containing 200 μm EGTA, and the cells were incubated for 10 min at room temperature to permit equilibration. Representative fluorescence tracings of intracellular [Ca2+] following addition of TG (1 μm) at t = 1 min in the presence of Ca2+-free buffer (t = 0–13 min) followed by continued incubation in the presence of fresh calcium- and TG-containing buffer added after 13 min. The magnitude of [Ca2+] entry was determined by ratiometric comparisons of the fluorescence intensities as a function of time after calcium re-addition. Intracellular [Ca2+] is expressed as the ratio of the fluorescence emission intensities at 510 nm achieved at excitation wavelengths of 340 nm and 380 nm (F340/F380) as described under “Experimental Procedures.” The maximal amplitudes of calcium entry (lower left panel) and the mean times required to achieve maximal Ca2+ amplitude (lower right panel) after adding Ca2+ to the medium of TG-treated cells are shown. Results presented are triplicate determinations obtained from each of three separate animals in each group and are expressed as the means ± S.E. *, p < 0.01.
To identify the contributions of other phospholipases in this process, we treated both wild-type and iPLA2β-null cells with the nonselective PLA2 inhibitor AACOCF3. Pretreatment with AACOCF3 dramatically attenuated both the rate and the amplitude of transmembrane calcium flux and decreased the rate of intracellular [Ca2+] rise in both TG-stimulated wild-type and iPLA2β-null cells, suggesting the contribution of other PLA2s to calcium signaling in SMCs (Fig. 5, A and B). Next, we examined the effects of pre-treatment of wild-type SMCs with either racemic BEL or Pyr inhibitors on TG-induced Ca2+ influx. Treatment of cells with the cPLA2α-specific inhibitor (Pyr, 0.5 μm) did not inhibit the rise in intracellular calcium after reintroduction of calcium to the media. In sharp contrast, the iPLA2 inhibitor, BEL, suppressed transmembrane calcium entry (Fig. 5C). The inhibition of calcium entry by BEL in SMCs was similar to that previously reported by Smani et al. (43, 44).
FIGURE 5.
Effects of the PLA2 inhibitors AACOCF3, bromoenol lactone (BEL), and pyrrolidine (Pyr) on TG-induced changes in intracellular [Ca2+] and quantitative PCR of PLA2 message levels in wild-type and iPLA2β-null mouse aortic smooth muscle. First passage aortic SMCs from wild-type (WT) and iPLA2β-null (KO) mice were incubated with Fura-2/AM and then pretreated with either AACOCF3 (25 μm), or ethanol vehicle alone for 1 h at 37 °C. The ratio of fluorescence (F340/F380) in TG-treated cells was monitored as described under “Experimental Procedures.” The maximal amplitudes of Ca2+ entry and the mean time required to achieve maximal amplitude after re-addition of Ca2+ to the medium of TG-treated cells are displayed in the lower two (A and B). Aortic SMCs from wild-type mice were incubated with Fura-2/AM and then were pretreated with either racemic BEL (10 μm), Pyr (0.5 μm), or vehicle alone for 10 min at 37 °C. The F340/F380 ratio was then monitored as described in A and B. The maximal amplitudes of Ca2+ entry and the mean time required to achieve maximal amplitude after re-addition of Ca2+ are displayed in lower two panels (C). Results are independent determinations obtained from three separate animals in each group and are reported as the means ± S.E. *, p < 0.01; #, p < 0.05. Total RNA from wild-type (WT) and iPLA2β-null (KO) first passage aortic SMCs was extracted, and cDNA was prepared with reverse transcriptase. Relative levels of the indicated PLA2 mRNA were quantified by real-time quantitative PCR using specific primers for iPLA2β, iPLA2γ, and cPLA2α as described under “Experimental Procedures.” Each PCR amplification was performed in triplicate, utilizing the following cycling conditions: 2 min at 50 °C and 10 min at 95 °C, followed by a total of 40 two-temperature cycles (15 s at 95 °C and 1 min at 60 °C). Standard curves were generated with serial dilutions of each cDNA sample, and relative mRNA levels were compared at various time points utilizing concurrently amplified ribosomal RNA as an internal standard. Representative results from three independent isolations are presented with standard error bars generated from triplicate determinations (D).
To determine the potential induction/suppression of other PLA2 enzymes in the iPLA2β–/– SMCs, we measured the levels of iPLA2β, cPLA2α, and iPLA2γ mRNAs by quantitative PCR. As expected, iPLA2β message levels were undetectable while those of cPLA2α and iPLA2γ were increased by ∼2- and ∼5-fold, respectively, in iPLA2β–/– SMCs (Fig. 5D). The expression level of cPLA2α protein was not significantly altered (Fig. 2). However, because there are >15 known members of the cPLA2 and iPLA2 families, increases in these message levels do not establish which of the remaining family members (or other enzymes) are responsible for the observed TG-induced increases in calcium ion concentration in the iPLA2β–/– aortic SMCs. Regardless of the other enzymes involved, the results clearly indicate that iPLA2β catalyzes the rate-determining step in Ca2+ entry and that other AACOCF3- and BEL-sensitive pathways can partially compensate for the loss of iPLA2β, albeit at a lower kinetic rate. Collectively, these results demonstrate the parallel processing of signals mediating the entry of Ca2+ after calcium pool depletion by multiple distinct enzymatic mechanisms.
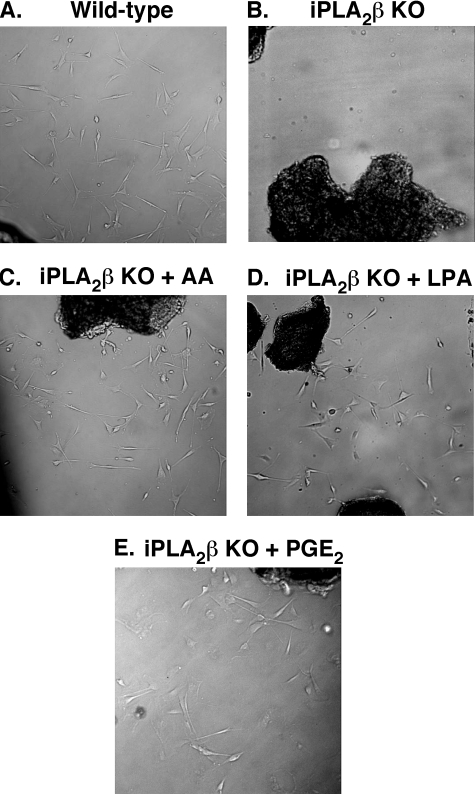
Migration and Proliferation of SMCs Is Blocked in Mesenteric Arterial Explants from iPLA2β-null Mice—When cross-sections of mesenteric arteries excised from wild-type mice are placed in cell culture dishes, SMCs migrate from the vascular explant onto the dish surface and proliferate, as illustrated in Fig. 6A. In stark contrast, virtually no observable migration or proliferation occurred with SMCs from explants of mesenteric arteries from iPLA2β-null mice under identical conditions (Fig. 6B). To determine if AA could rescue proliferation and migration in iPLA2β–/– cells, additional experiments were performed. Intriguingly, treatment of vascular explants with 2 μm AA acid restored SMC migration and proliferation (Fig. 6C). Next, we examined if SMCs null for iPLA2β demonstrated compromised cell growth when stimulated with LPA, a ligand for the EDG family (endothelial differentiation genes 2, 4, and 7) and the non-EDG family (LPA-4 and -5) of LPA receptors (45, 46). Addition of 2 μm LPA restored cell migration and proliferation (Fig. 6D) thereby demonstrating the downstream functional integrity of a known G-protein-coupled SMC growth pathway in the iPLA2β–/– mouse. The role of lysolipid-mediated signaling in cell proliferation in wild-type SMCs was addressed by pharmacologic inhibition of the LPA pathway. Specifically, the addition of 10 μm 1-bromo-3(S)-hydroxy-4-(palmitoyloxy)butyl phosphonate (BrP-LPA) inhibited 94% of the cell proliferation observed with control wild-type cells. Supplementation with exogenous LPA did not result in additional cell growth. This is in complete agreement with the highly potent antagonism of the LPA receptor previously demonstrated to be mediated by this compound. Next, to address the possible role of cyclooxygenase-derived eicosanoids in SMC migration and differentiation, extracellular provision of PGE2 (5 μm) was found to significantly rescue iPLA2β-null SMC migration and proliferation (Fig. 6E). Thus, both products of the phospholipase reaction, fatty acids (e.g. AA and/or oxygenated metabolites derived from AA) and lysolipids, could rescue defective SMC migration and proliferation present in iPLA2β –/– cells. Collectively, these results demonstrate the roles of integrated growth programs initiated by the dual products of the phospholipase reaction and their downstream metabolites.
FIGURE 6.
AA, LPA, and PGE2 stimulate the migration and proliferation of iPLA2β-null SMCs from mouse mesenteric arterial explants. Mesenteric arterial explants were excised from wild-type (WT) and iPLA2β-null (KO) mice, covered with poly-l-lysine-coated glass coverslips, and incubated in the absence (A and B) or presence of AA (2 μm)(C), LPA (2 μm)(D), or PGE2 (5 μm) (E) in the cell culture medium (replaced at 2-day intervals). Images were obtained using a digital microscope (10× air objective) at the time of initial cell migration from the tissue explants. Results are representative of three independent explant isolations.
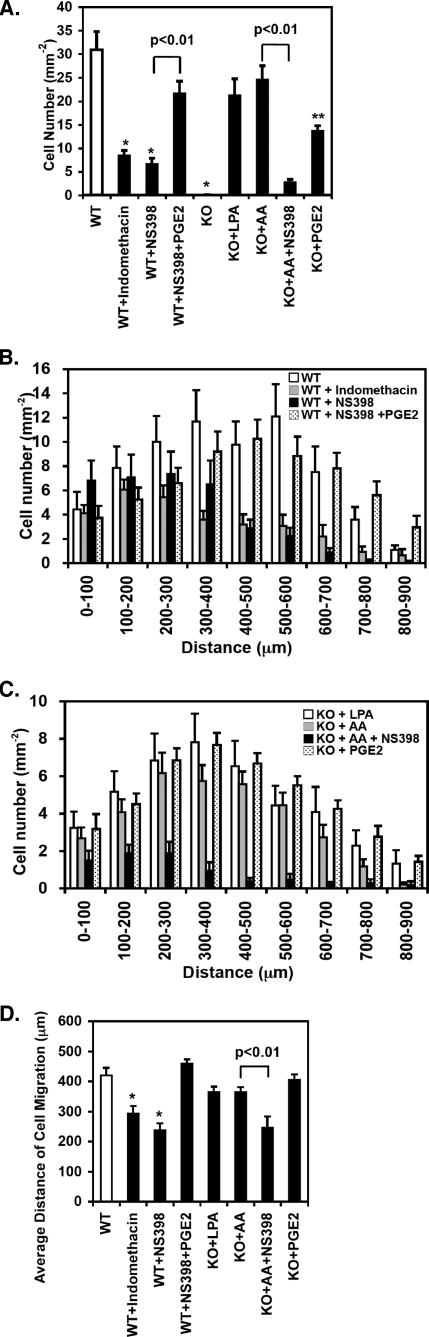
Identification of the Mechanisms Contributing to Vascular SMC Growth and Mobility in Wild-type and iPLA2β–/– Mesenteric Arterial SMCs—Because both products of the phospholipase reaction possessed potent effects on SMC proliferation and migration, we next sought to discriminate the roles of AA and lysolipids in wild-type vessels using pharmacologic inhibition in this surrogate model of vascular injury. To this end, we isolated and incubated mesenteric arterial SMCs from wild-type mice and treated them with either the non-selective COX inhibitor indomethacin or the COX-2-specific inhibitor NS-398. In the presence of indomethacin (20 μm), the number of wild-type cells obtained from the explants of wild-type vessels was decreased by 73 ± 3% (p < 0.01) (Fig. 7A). Moreover, the COX-2-specific inhibitor NS-398 (10 μm) demonstrated similar amounts of inhibition (79 ± 4%) substantiating the central role of COX-2 in this process. Collectively, these results demonstrate that iPLA2β-mediated production of AA and its downstream COX-2-generated eicosanoid products are crucial factors for SMC migration and cell growth (Fig. 7, B and C). Notably, the majority of COX inhibitor-treated migratory wild-type cells were present within an average distance of 200–300 μm from the edge of the nearest vessel explant (at 21 days post-isolation), whereas non-treated cells migrated an average of 400–500 μm from the explant edge (Fig. 7D). Importantly, the AA-mediated rescue of migration and proliferation in iPLA2β–/– cells was nearly completely ablated in the presence of the COX-2-selective inhibitor, NS-398 (10 μm). Moreover, defective migration and proliferation in iPLA2β-null cells could be rescued by provision of PGE2 to mesenteric arterial explants (Figs. 6 and 7).
FIGURE 7.
COX-2-specific inhibition prevents AA-mediated rescue of the migration and proliferation of iPLA2β-null SMCs obtained from mouse mesenteric arterial explants. Mesenteric arterial explants were excised from wild-type (WT) and iPLA2β-null (KO) mice, covered with poly-l-lysinecoated glass coverslip, and incubated in the absence or presence of LPA (2 μm), AA (2 μm), or PGE2 (5 μm) with or without the COX-2-specific inhibitor NS-398 (10 μm) or the non-selective COX inhibitor indomethacin (20 μm) in the cell culture medium (replaced at 2-day intervals). Images of tissue fragments with the migrating cells were obtained using a digital microscope (10× air objective) (at 21 days post-isolation), after which migrating and proliferating cells were counted and averaged for each group. The number of cells per tissue fragment was normalized by multiplication by the fraction of dishes containing migrating cells per group (A). The number of cells within the indicated path-independent distance of each cell from the nearest explant edge with standard error bars were measured for each group of wild-type (WT)(B) and iPLA2β-null (KO) cells (C). A summary of the average distance of migration using the data (results) given in B and C ± S.E. is presented in D. Results were obtained from independent preparations from at least three separate animals for each group. *, p < 0.01 when compared with WT control. **, p < 0.01 when compared with KO control.
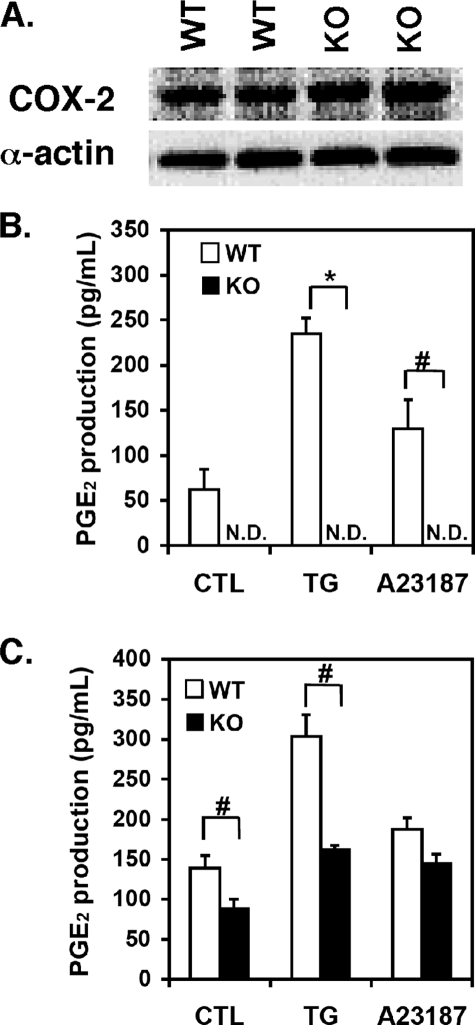
Basal and Stimulus-provoked Production of PGE2 Is Markedly Attenuated in SMCs from iPLA2β–/– Mice—To determine the role of iPLA2β in the production of the bioactive metabolite PGE2 in vascular SMCs we used an enzyme-linked immunoassay to measure PGE2 in the media of SMC cultures from wild-type or iPLA2β-null mice. First, we determined that SMCs obtained from wild-type and iPLA2β-null mouse aortae express similar amounts of COX-2 protein by Western blot analysis (Fig. 8A). Next, confluent cells were incubated in culture medium containing either TG (1 μm), ionophore A23187 (10 μm), or vehicle alone for 5 min, and PGE2 released into the medium was measured. Wild-type cells growing in culture produced PGE2 that was present in the medium at a level of 62 ± 22 pg/ml. In stark contrast, constitutive PGE2 production was not detectable in the media of cultures of iPLA2β-null cells (lower limits of detection were 20 pg/ml). Treatment of cultures of wild-type SMCs with TG resulted in the release of 235 ± 18 pg/ml PGE2 into the media after 5 min representing a 3.8 ± 0.3-fold increase in PGE2 production (Fig. 8B). Remarkably, treatment of resting cells from iPLA2β–/– SMCs with TG did not result in measurable amounts of PGE2 production (i.e. <20 pg/ml). Similarly, treatment of wild-type cells with ionophore resulted in a 2-fold increase in PGE2 production while ionophore treatment of cells from iPLA2β–/– mice did not produce detectable amounts of PGE2 (Fig. 8B). PGE2 production from iPLA2β-null cells after TG stimulation for 10 min was still markedly impaired (∼50% of wild-type levels). Ionophore-stimulated production of PGE2 at 10 min in iPLA2β-null cells was 78% of that produced from wild-type cells (Fig. 8C). These decreases in PGE2 production at the 5- and 10-min time points closely parallel those observed for the liberation of radiolabeled AA in iPLA2β-null SMCs. Taken together, these results demonstrate that PGE2 produced via the COX-2 pathway is an important participant in SMC migration and proliferation and that PGE2 production in SMCs is downstream of iPLA2β.
FIGURE 8.
COX-2 protein expression and PGE2 production in wild-type and iPLA2β KO aortic SMCs. Expression of COX-2 protein in aortic SMCs obtained fromtwoseparateexplantpreparationsfromwild-type (WT) and iPLA2β-null (KO) mice was determined by Western blotting as described under “Experimental Procedures.” Proteins from cell homogenates of WT and KO cells were resolved by SDS-PAGE (100 μg of protein/lane) and transferred to polyvinylidene difluoride membranes that were probed with a monoclonal IgG1 antibody directed against COX-2 (A). First passage, subcultured, confluent aortic SMCs obtained from wild-type (WT) and iPLA2β-null (KO) mice were incubated in cell culture medium (10% fetal bovine serum) containing 1 μm TG, ionophore A23187 (10 μm), or DMSO vehicle alone (CTL) for 5 (B) or 10 min (C). Culture medium was collected, and PGE2 present in the medium was measured by immunoassay. PGE2 production was not detectable in the medium of iPLA2β KO cells under either basal conditions or after TG or ionophore stimulation. Results from four independent determinations are presented as the means ± S.E. *, p < 0.01; #, p < 0.05; N.D., not detectable (lower limits of detection of the assay were < 20 pg/ml).
DISCUSSION
Previous studies involving pharmacologic inhibition of iPLA2β have suggested that this enzyme is involved in modulating vasomotor tone and AA release from vascular cells in various settings (27, 32, 43, 44, 47). These include vascular relaxation induced by acetylcholine and nitric oxide (47), AA release from vascular endothelial and SMCs (27, 32), and activation of SMC store-operated Ca2+ channels (43, 44). Because all pharmacologic agents, including BEL (29, 48–50), have effects on molecules other than their intended targets, conclusions based on evidence using chemical inhibitors requires genetic confirmation. This is particularly the case with BEL, because all characterized members of the iPLA2 family are inhibited by low concentrations of this inhibitor (<10 μm). Thus, it is neither possible to determine the type of iPLA2 responsible for an observed physiologic effect from the use of BEL alone, nor is it possible to exclude other potential enzymatic reactions catalyzed by other enzymes, with certainty. Similarly, although the use of antisense DNA and small interference RNA has proven useful in many settings, it is becoming increasingly clear that off-target effects from the use of these approaches are often confounding factors in the interpretation of experimental results. Indeed, it has been stated that, because all antisense DNA and small interference RNA are potential pharmacologic agents in their own right, off-target effects can be anticipated in some cases (51–55). Accordingly, the most definitive way to unambiguously demonstrate the role of a specific enzyme for a biologic function is to genetically ablate the enzyme of interest and identify the resultant effects on the phenotypic characteristics of interest in comparison with pharmacologic treatment. In the present study, we used vascular SMCs from iPLA2β–/– mice to unambiguously demonstrate that: 1) the initial phase of TG- or ionophore A23187-induced AA release is catalyzed by iPLA2β, which was confirmed by utilizing pharmacologic inhibitors and extracellular calcium chelation by EGTA; 2) Ca2+-store depletion-induced Ca2+ entry occurs more slowly in iPLA2β-null aortic SMCs than in wild-type cells; 3) migration and proliferation of mesenteric arterial SMCs in vascular explants from iPLA2β-null mice are essentially absent in comparison to wild-type cells; 4) exogenous provision of AA, PGE2, or LPA rescues iPLA2β-null mesenteric arterial SMC migration and proliferation to nearly wild-type levels; and 5) in wild-type explants, SMC migration and proliferation require the production of PGE2 and/or eicosanoids downstream of COX-2 as demonstrated by the use of a COX-2-specific inhibitor NS-398 and rescue of the genetic iPLA2β knockout phenotype by PGE2. The demonstration that TG or A23187 treatment fails to induce early AA release from iPLA2β-null vascular SMCs is consistent with our previous observations that the iPLA2 inhibitor BEL suppresses Ca2+-store depletion-induced AA release from A-10 vascular SMCs (27). The results of the present experiments using animals with genetic ablation of iPLA2β clearly support results obtained through enantioselective mechanism-based inhibition of AA release by BEL (32). The increased inhibition of AA release by S-BEL in TG-stimulated versus ionophore-stimulated cells is in accordance with the hypothesis that TG-mediated calcium depletion of internal stores results in rapid iPLA2β activation upstream of store-operated calcium influx. In contrast, the non-physiologic calcium ion carrier A23187 likely simultaneously activates SMC cPLA2α by non-channel (i.e. ionophore)-mediated transmembrane calcium influx (as demonstrated by the enhanced release of AA in the presence of extracellular calcium) and iPLA2β through depletion of internal calcium ion stores (as demonstrated by the Pyr-insensitive release of AA in the absence of extracellular calcium). At short time intervals (i.e. 5 min), participation of iPLA2β is predominant, whereas at longer time intervals cPLA2α increasingly participates in AA release.
Previously, lysolipids generated by iPLA2β activated during store depletion were shown to promote extracellular calcium entry through both cation-nonselective store-operated channels as well as calcium-release activated Ca2+ channels (43, 44). The present results are consistent with dual roles for iPLA2β where it serves both as a direct mediator of AA release through hydrolysis of AA-containing phospholipids that simultaneously generates lysolipids that amplify eicosanoid release through activation of capacitative calcium influx. Importantly, capacitative calcium influx has been demonstrated to be required for the robust physiologic activation of cPLA2α-mediated AA release (56). Thus, the generation of eicosanoids in SMCs is accomplished through parallel processing using both the direct iPLA2β-mediated release of AA as well as the iPLA2β-mediated activation of capacitative calcium influx and the resultant activation of cPLA2α. Accordingly, the majority of TG- or ionophore-induced AA release either in the presence or absence of extracellular calcium is mediated by iPLA2β activity early after stimulation, whereas at later time points contributions from cPLA2α activity are mediated by capacitative calcium influx and its downstream sequelae.
In support of these results, we demonstrated that the rate of TG-induced Ca2+ influx is kinetically attenuated in iPLA2β–/– mice. However, treatment with the generic PLA2 inhibitor AACOCF3 resulted in the nearly complete ablation of the maximal amplitude and sustained calcium entry in both wild-type and iPLA2β-null SMCs, indicating the likely involvement of other iPLA2s or cPLA2s in modulating this process (Fig. 5).
We point out that other compensatory differences likely exist between the present experiments with iPLA2β-null and wild-type SMCs. In this regard, we have measured levels of cPLA2α and iPLA2γ mRNA species by quantitative reverse transcription-PCR and have found that both are increased in iPLA2β-null versus wild-type cells. Remarkably, the level of cPLA2α phosphorylated at Ser-505 is higher in iPLA2β–/– aortic SMCs than their wild-type counterparts, although the total cPLA2α protein expression level is not significantly altered. Increased expression of other types of PLA2s may also influence the results in iPLA2β–/– cells that likely compensate to facilitate the slower, but partially restored Ca2+ entry (albeit at a lower rate) after calcium addition. However, the results obtained with iPLA2β–/– and BEL-treated wild-type SMCs with respect to AA release and TG-stimulated Ca2+ signaling supports a predominant role for iPLA2β in the rate-determining step for the entry of calcium ion after calcium pool depletion.
Because cPLA2α has been shown to require a critical time delay for proper phosphorylation and/or translocation/activation from the cytosol to the perinuclear membrane as induced by increases in intracellular [Ca2+] (13, 57, 58), relatively slow activation of cPLA2α may directly contribute to a sustained calcium plateau through further liberation of AA and lysophospholipids. Although the time required to achieve the maximal amplitude in the Ca2+ response is different in iPLA2β-null and wild-type SMCs, iPLA2β-null cells contain significantly elevated levels of phosphorylated cPLA2α, which would be expected to facilitate activation. However, it is clear that many additional factors are involved in a cell type- and context-dependent fashion. Moreover, the majority of initial phase AA released from wild-type cells was unaffected by chelation of extracellular Ca2+ or inhibition by the selective cPLA2α inhibitor Pyr, further supporting the dual roles of iPLA2β in eicosanoid release and lysolipid generation.
Mesenteric arterial SMCs from wild-type mice readily migrate from vascular explants and proliferate in culture. In contrast, both SMC migration and proliferation are greatly reduced in explants from iPLA2β-null mice. Interestingly, incubation of iPLA2β-null explants with either micromolar concentrations of AA or lysophosphatidic acid restores SMC migration to nearly wild-type levels (Fig. 7). Addition of exogenous AA (2 μm) increased the number of cells migrating from iPLA2β–/– mesenteric arteries to levels essentially identical to those of wild-type controls. Although the correlation between cell migration and growth is not completely understood, both processes can be stimulated via multiple signaling pathways (e.g. transduction by AA (or AA-derived eicosanoids, i.e. PGE2) (59–62) and LPA (63–65)). Smooth muscle cells use spatiotemporally coordinated programs for vessel repair and remodeling, which are critical in the maintenance of vascular patency, tone, and physiologic responsivity. The short half-life of many eicosanoids suggests that iPLA2β is central in modulating acute responses to pathophysiologic perturbations, whereas longer term responses are mediated by the integrated actions of iPLA2β and cPLA2α.
Bioactive signaling eicosanoids (e.g. prostaglandins) are well known regulators of cell motility, proliferation, angiogenesis, and apoptosis (59–61, 66, 67). Eicosanoids as well as LPA also increase the expression and activity of matrix metalloproteinases, which regulate cell adhesion and facilitate cell migration (68–70). The present results demonstrate by genetic knockout and pharmacologic rescue that iPLA2β plays a critical proximal role in the cyclooxygenase-mediated generation of AA metabolites that are necessary for SMC migration and proliferation. This conclusion is substantiated by the demonstration that the migration distance and the number of proliferating wild-type cells were dramatically attenuated by treatment with a non-selective COX inhibitor. Moreover, the COX-2-selective inhibitor NS-398 was equipotent as indomethacin demonstrating that the majority of the observed effects were mediated by COX-2 under the conditions employed. In mice null for iPLA2β, the COX-2-specific inhibitor NS-398 prevented the AA-mediated rescue of SMC proliferation and migration demonstrating that one or more COX-2 products, and not AA itself, modulate cell migration and proliferation. Supporting these results, we demonstrated that provision of a COX-2 product, PGE2, could mimic the majority of AA-mediated rescue of the defects in iPLA2β-null SMC migration and proliferation (Fig. 7). Furthermore, we found that PGE2 production in iPLA2β–/– SMCs was below the level of detection even in the presence of potent agonists that produced robust amounts of PGE2 in wild-type SMCs at early time points. These results suggest the attenuated cell migration and proliferation manifest in iPLA2β-null cells are caused by defects in both iPLA2β-mediated AA and PGE2 production (Fig. 8). Collectively, the present study demonstrates the dual roles of iPLA2β in both catalyzing AA release at early time points as well as facilitating store-operated calcium entry necessary for the activation of cPLA2α. Thus, through the integrated iPLA2β-mediated initiation and cPLA2α amplification of cellular signals from calcium store depletion, multiple pathways for the generation of bioactive metabolites can be recruited. The acute and chronic contributions of iPLA2 and cPLA2 family members to eicosanoid and lysolipid production are almost certainly cell type-specific and likely reflect alterations in the nature, duration, and context of the stimulus.
In summary, these results demonstrate the importance of iPLA2β in the TG- and ionophore-induced release of AA during the initial stages of stimulation, its prominent role in calcium ion homeostasis, and the essential role of iPLA2β in SMC migration and proliferation through coupling of AA release to COX-mediated prostaglandin production. The importance of eicosanoids in modulating vascular tone, the development and stability of atherosclerotic plaques, and vascular remodeling are well known. Accordingly, future experiments aimed at identifying the roles of iPLA2β-dependent signaling pathways in regulation of vascular tone, the initiation and progression of atherosclerotic lesions, and the stability of atherosclerotic plaques should provide additional mechanistic insights into the contributions of iPLA2β to multiple aspects of vascular biology.
This work was supported, in whole or in part, by National Institutes of Health Grants 5PO1HL57278-10 and 2RO1HL41250-13. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PLA2, phospholipases A2; AA, arachidonic acid; AACOCF3, arachidonyl trifluoromethylketone; BEL, (E)-6-(bromomethylene)-3-(1-naphthalenyl)-2H-tetrahydropyran-2-one; BrP-LPA, 1-bromo-3(S)-hydroxy-4-(palmitoyloxy)butyl phosphonate; CaM, calmodulin; PGE2, prostaglandin E2; Fura-2/AM, Fura-2/acetoxymethylester; cPLA2, cytosoli cPLA2; iPLA2, calcium-independent PLA2; sPLA2, secretory PLA2; LPA, lysophosphatidic acid; Pyr, N-{(2S,4R)-4-(biphenyl-2-ylmethylisobutylamino)-1-[2-(2,4-difluorobenzoyl)benzoyl]pyrrolidin-2-ylmethyl}-3-[4-(2,4-dioxothiazolidin-5-ylidenemethyl)phenyl]acrylamide; SMC, smooth muscle cell; TG, thapsigargin; WT, wild type; DMEM, Dulbecco's modified Eagle's medium; EDG, endothelial differentiation gene.
References
- 1.Ghosh, M., Tucker, D. E., Burchett, S. A., and Leslie, C. C. (2006) Prog. Lipid Res. 45 487–510 [DOI] [PubMed] [Google Scholar]
- 2.Ma, Z., and Turk, J. (2001) Prog. Nucleic Acids Res. Mol. Biol. 67 1–33 [DOI] [PubMed] [Google Scholar]
- 3.Khanapure, S. P., Garvey, D. S., Janero, D. R., and Letts, L. G. (2007) Curr. Top. Med. Chem. 7 311–340 [DOI] [PubMed] [Google Scholar]
- 4.Yedgar, S., Cohen, Y., and Shoseyov, D. (2006) Biochim. Biophys. Acta 1761 1373–1382 [DOI] [PubMed] [Google Scholar]
- 5.Fink, K. L., and Gross, R. W. (1984) Circ. Res. 55 585–594 [DOI] [PubMed] [Google Scholar]
- 6.Yan, W., Jenkins, C. M., Han, X., Mancuso, D. J., Sims, H. F., Yang, K., and Gross, R. W. (2005) J. Biol. Chem. 280 26669–26679 [DOI] [PubMed] [Google Scholar]
- 7.Ulven, T., and Kostenis, E. (2006) Curr. Top. Med. Chem. 6 1427–1444 [DOI] [PubMed] [Google Scholar]
- 8.Thompson, M. D., Takasaki, J., Capra, V., Rovati, G. E., Siminovitch, K. A., Burnham, W. M., Hudson, T. J., Bosse, Y., and Cole, D. E. (2006) Mol. Diagn. Ther. 10 353–366 [DOI] [PubMed] [Google Scholar]
- 9.Tomura, H., Mogi, C., Sato, K., and Okajima, F. (2005) Cell Signal. 17 1466–1476 [DOI] [PubMed] [Google Scholar]
- 10.Schaloske, R. H., and Dennis, E. A. (2006) Biochim. Biophys. Acta 1761 1246–1259 [DOI] [PubMed] [Google Scholar]
- 11.Loeb, L. A., and Gross, R. W. (1986) J. Biol. Chem. 261 10467–10470 [PubMed] [Google Scholar]
- 12.Alonso, F., Henson, P. M., and Leslie, C. C. (1986) Biochim. Biophys. Acta 878 273–280 [DOI] [PubMed] [Google Scholar]
- 13.Glover, S., de Carvalho, M. S., Bayburt, T., Jonas, M., Chi, E., Leslie, C. C., and Gelb, M. H. (1995) J. Biol. Chem. 270 15359–15367 [DOI] [PubMed] [Google Scholar]
- 14.Hefner, Y., Borsch-Haubold, A. G., Murakami, M., Wilde, J. I., Pasquet, S., Schieltz, D., Ghomashchi, F., Yates, J. R., 3rd, Armstrong, C. G., Paterson, A., Cohen, P., Fukunaga, R., Hunter, T., Kudo, I., Watson, S. P., and Gelb, M. H. (2000) J. Biol. Chem. 275 37542–37551 [DOI] [PubMed] [Google Scholar]
- 15.Kita, Y., Ohto, T., Uozumi, N., and Shimizu, T. (2006) Biochim. Biophys. Acta 1761 1317–1322 [DOI] [PubMed] [Google Scholar]
- 16.Wolf, R. A., and Gross, R. W. (1985) J. Biol. Chem. 260 7295–7303 [PubMed] [Google Scholar]
- 17.Hazen, S. L., Stuppy, R. J., and Gross, R. W. (1990) J. Biol. Chem. 265 10622–10630 [PubMed] [Google Scholar]
- 18.Wolf, M. J., and Gross, R. W. (1996) J. Biol. Chem. 271 30879–30885 [DOI] [PubMed] [Google Scholar]
- 19.Tang, J., Kriz, R. W., Wolfman, N., Shaffer, M., Seehra, J., and Jones, S. S. (1997) J. Biol. Chem. 272 8567–8575 [DOI] [PubMed] [Google Scholar]
- 20.Hazen, S. L., Zupan, L. A., Weiss, R. H., Getman, D. P., and Gross, R. W. (1991) J. Biol. Chem. 266 7227–7232 [PubMed] [Google Scholar]
- 21.Zupan, L. A., Weiss, R. H., Hazen, S. L., Parnas, B. L., Aston, K. W., Lennon, P. J., Getman, D. P., and Gross, R. W. (1993) J. Med. Chem. 36 95–100 [DOI] [PubMed] [Google Scholar]
- 22.Schneider, G., Neuberger, G., Wildpaner, M., Tian, S., Berezovsky, I., and Eisenhaber, F. (2006) BMC Bioinformatics 7 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazen, S. L., and Gross, R. W. (1991) J. Biol. Chem. 266 14526–14534 [PubMed] [Google Scholar]
- 24.Jenkins, C. M., Yan, W., Mancuso, D. J., and Gross, R. W. (2006) J. Biol. Chem. 281 15615–15624 [DOI] [PubMed] [Google Scholar]
- 25.Wolf, M. J., and Gross, R. W. (1996) J. Biol. Chem. 271 20989–20992 [DOI] [PubMed] [Google Scholar]
- 26.Jenkins, C. M., Wolf, M. J., Mancuso, D. J., and Gross, R. W. (2001) J. Biol. Chem. 276 7129–7135 [DOI] [PubMed] [Google Scholar]
- 27.Wolf, M. J., Wang, J., Turk, J., and Gross, R. W. (1997) J. Biol. Chem. 272 1522–1526 [DOI] [PubMed] [Google Scholar]
- 28.Mancuso, D. J., Jenkins, C. M., and Gross, R. W. (2000) J. Biol. Chem. 275 9937–9945 [DOI] [PubMed] [Google Scholar]
- 29.Jenkins, C. M., Mancuso, D. J., Yan, W., Sims, H. F., Gibson, B., and Gross, R. W. (2004) J. Biol. Chem. 279 48968–48975 [DOI] [PubMed] [Google Scholar]
- 30.Chakravarty, P. K., Krafft, G. A., and Katzenellenbogen, J. A. (1982) J. Biol. Chem. 257 610–612 [PubMed] [Google Scholar]
- 31.Bao, S., Miller, D. J., Ma, Z., Wohltmann, M., Eng, G., Ramanadham, S., Moley, K., and Turk, J. (2004) J. Biol. Chem. 279 38194–38200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins, C. M., Han, X., Mancuso, D. J., and Gross, R. W. (2002) J. Biol. Chem. 277 32807–32814 [DOI] [PubMed] [Google Scholar]
- 33.Dubey, R. K., and Overbeck, H. W. (1994) Cell Tissue Res. 275 133–141 [DOI] [PubMed] [Google Scholar]
- 34.Sun, X., Kaltenbronn, K. M., Steinberg, T. H., and Blumer, K. J. (2005) Mol. Pharmacol. 67 631–639 [DOI] [PubMed] [Google Scholar]
- 35.Mancuso, D. J., Jenkins, C. M., Sims, H. F., Cohen, J. M., Yang, J., and Gross, R. W. (2004) Eur. J. Biochem. 271 4709–4724 [DOI] [PubMed] [Google Scholar]
- 36.Moran, J. M., Buller, R. M., McHowat, J., Turk, J., Wohltmann, M., Gross, R. W., and Corbett, J. A. (2005) J. Biol. Chem. 280 28162–28168 [DOI] [PubMed] [Google Scholar]
- 37.Bao, S., Song, H., Wohltmann, M., Ramanadham, S., Jin, W., Bohrer, A., and Turk, J. (2006) J. Biol. Chem. 281 20958–20973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seno, K., Okuno, T., Nishi, K., Murakami, Y., Watanabe, F., Matsuura, T., Wada, M., Fujii, Y., Yamada, M., Ogawa, T., Okada, T., Hashizume, H., Kii, M., Hara, S., Hagishita, S., Nakamoto, S., Yamada, K., Chikazawa, Y., Ueno, M., Teshirogi, I., Ono, T., and Ohtani, M. (2000) J. Med. Chem. 43 1041–1044 [DOI] [PubMed] [Google Scholar]
- 39.Ghomashchi, F., Stewart, A., Hefner, Y., Ramanadham, S., Turk, J., Leslie, C. C., and Gelb, M. H. (2001) Biochim. Biophys. Acta 1513 160–166 [DOI] [PubMed] [Google Scholar]
- 40.Grynkiewicz, G., Poenie, M., and Tsien, R. Y. (1985) J. Biol. Chem. 260 3440–3450 [PubMed] [Google Scholar]
- 41.Thastrup, O., Cullen, P. J., Drobak, B. K., Hanley, M. R., and Dawson, A. P. (1990) Proc. Natl. Acad. Sci. U. S. A. 87 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lytton, J., Westlin, M., and Hanley, M. R. (1991) J. Biol. Chem. 266 17067–17071 [PubMed] [Google Scholar]
- 43.Smani, T., Zakharov, S. I., Leno, E., Csutora, P., Trepakova, E. S., and Bolotina, V. M. (2003) J. Biol. Chem. 278 11909–11915 [DOI] [PubMed] [Google Scholar]
- 44.Smani, T., Zakharov, S. I., Csutora, P., Leno, E., Trepakova, E. S., and Bolotina, V. M. (2004) Nat. Cell Biol. 6 113–120 [DOI] [PubMed] [Google Scholar]
- 45.Rivera, R., and Chun, J. (2008) Rev. Physiol. Biochem. Pharmacol. 160 25–46 [DOI] [PubMed] [Google Scholar]
- 46.Lee, C. W., Rivera, R., Gardell, S., Dubin, A. E., and Chun, J. (2006) J. Biol. Chem. 281 23589–23597 [DOI] [PubMed] [Google Scholar]
- 47.Seegers, H. C., Gross, R. W., and Boyle, W. A. (2002) J. Pharmacol. Exp. Ther. 302 918–923 [DOI] [PubMed] [Google Scholar]
- 48.Daniels, S. B., Cooney, E., Sofia, M. J., Chakravarty, P. K., and Katzenellenbogen, J. A. (1983) J. Biol. Chem. 258 15046–15053 [PubMed] [Google Scholar]
- 49.Fuentes, L., Perez, R., Nieto, M. L., Balsinde, J., and Balboa, M. A. (2003) J. Biol. Chem. 278 44683–44690 [DOI] [PubMed] [Google Scholar]
- 50.van Tienhoven, M., Atkins, J., Li, Y., and Glynn, P. (2002) J. Biol. Chem. 277 20942–20948 [DOI] [PubMed] [Google Scholar]
- 51.Jackson, A. L., Burchard, J., Schelter, J., Chau, B. N., Cleary, M., Lim, L., and Linsley, P. S. (2006) RNA (N. Y.) 12 1179–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fedorov, Y., Anderson, E. M., Birmingham, A., Reynolds, A., Karpilow, J., Robinson, K., Leake, D., Marshall, W. S., and Khvorova, A. (2006) RNA (N. Y.) 12 1188–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin, X., Ruan, X., Anderson, M. G., McDowell, J. A., Kroeger, P. E., Fesik, S. W., and Shen, Y. (2005) Nucleic Acids Res. 33 4527–4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein, C. A. (1995) Nat. Med. 1 1119–1121 [DOI] [PubMed] [Google Scholar]
- 55.Benimetskaya, L., Loike, J. D., Khaled, Z., Loike, G., Silverstein, S. C., Cao, L., el Khoury, J., Cai, T. Q., and Stein, C. A. (1997) Nat. Med. 3 414–420 [DOI] [PubMed] [Google Scholar]
- 56.Chang, W. C., Nelson, C., and Parekh, A. B. (2006) FASEB J. 20 2381–2383 [DOI] [PubMed] [Google Scholar]
- 57.Peters-Golden, M., and McNish, R. W. (1993) Biochem. Biophys. Res. Commun. 196 147–153 [DOI] [PubMed] [Google Scholar]
- 58.Hirabayashi, T., Kume, K., Hirose, K., Yokomizo, T., Iino, M., Itoh, H., and Shimizu, T. (1999) J. Biol. Chem. 274 5163–5169 [DOI] [PubMed] [Google Scholar]
- 59.Hu, Z. W., Kerb, R., Shi, X. Y., Wei-Lavery, T., and Hoffman, B. B. (2002) J. Pharmacol. Exp. Ther. 303 563–573 [DOI] [PubMed] [Google Scholar]
- 60.Rao, R., Redha, R., Macias-Perez, I., Su, Y., Hao, C., Zent, R., Breyer, M. D., and Pozzi, A. (2007) J. Biol. Chem. 282 16959–16968 [DOI] [PubMed] [Google Scholar]
- 61.Herbert, S. P., and Walker, J. H. (2006) J. Biol. Chem. 281 35709–35716 [DOI] [PubMed] [Google Scholar]
- 62.Zhao, X., Wang, D., Zhao, Z., Xiao, Y., Sengupta, S., Xiao, Y., Zhang, R., Lauber, K., Wesselborg, S., Feng, L., Rose, T. M., Shen, Y., Zhang, J., Prestwich, G., and Xu, Y. (2006) J. Biol. Chem. 281 29357–29368 [DOI] [PubMed] [Google Scholar]
- 63.Kim, J., Keys, J. R., and Eckhart, A. D. (2006) Cell Signal. 18 1695–1701 [DOI] [PubMed] [Google Scholar]
- 64.Kaneyuki, U., Ueda, S., Yamagishi, S., Kato, S., Fujimura, T., Shibata, R., Hayashida, A., Yoshimura, J., Kojiro, M., Oshima, K., and Okuda, S. (2007) Vasc. Pharmacol. 46 286–292 [DOI] [PubMed] [Google Scholar]
- 65.Song, Y., Wilkins, P., Hu, W., Murthy, K. S., Chen, J., Lee, Z., Oyesanya, R., Wu, J., Barbour, S. E., and Fang, X. (2007) Biochem. J. 406 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young, W., Mahboubi, K., Haider, A., Li, I., and Ferreri, N. R. (2000) Circ. Res. 86 906–914 [DOI] [PubMed] [Google Scholar]
- 67.Nieves, D., and Moreno, J. J. (2007) Apoptosis 12 1979–1988 [DOI] [PubMed] [Google Scholar]
- 68.Wu, W. T., Chen, C. N., Lin, C. I., Chen, J. H., and Lee, H. (2005) Endocrinology 146 3387–3400 [DOI] [PubMed] [Google Scholar]
- 69.Leppert, D., Hauser, S. L., Kishiyama, J. L., An, S., Zeng, L., and Goetzl, E. J. (1995) FASEB J. 9 1473–1481 [DOI] [PubMed] [Google Scholar]
- 70.Yen, J. H., Khayrullina, T., and Ganea, D. (2008) Blood 111 260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]