Abstract
Elevated levels of circulating fibrinogen are associated with an increased risk of atherothrombotic diseases although a causative correlation between high levels of fibrinogen and cardiovascular complications has not been established. We hypothesized that a potential mechanism for an increased prothrombotic state is the post-translational modification of fibrinogen by tyrosine nitration. Mass spectrometry identified tyrosine residues 292 and 422 at the carboxyl terminus of the β-chain as the principal sites of fibrinogen nitration in vivo. Immunoelectron microscopy confirmed the incorporation of nitrated fibrinogen molecules in fibrin fibers. The nitration of fibrinogen in vivo resulted in four distinct functional consequences: increased initial velocity of fibrin clot formation, altered fibrin clot architecture, increased fibrin clot stiffness, and reduced rate of clot lysis. The rate of fibrin clot formation and clot architecture was restored upon depletion of the tyrosine-nitrated fibrinogen molecules. An enhanced response to the knob “B” mimetic peptides Gly-His-Arg-Proam and Ala-His-Arg-Proam suggests that incorporation of nitrated fibrinogen molecules accelerates fibrin lateral aggregation. The data provide a novel biochemical risk factor that could explain epidemiological associations of oxidative stress and inflammation with thrombotic complications.
Activation of the coagulation cascade converts soluble fibrinogen to insoluble fibrin, which polymerizes to produce, along with platelets, the hemostatic clot (1, 2). Whereas the normal activation of the coagulation cascade is essential for life, inappropriate activation may result in thrombosis and complications that arise from the formation of fibrin clots. Indeed pathologically induced thrombogenesis is associated with adverse cardiovascular events (3, 4), thromboembolism in chronic obstructive pulmonary disease (5), and vascular complications in autoimmune diseases (6). Because fibrinogen is an acute phase reactant, epidemiological studies have documented elevated fibrinogen levels in subjects with these disorders (7–10). Moreover, increased levels of circulating fibrinogen (11, 12) and common polymorphisms (13, 14) have been associated with an increased risk for thromboembolic episodes in subjects with coronary artery disease and in some cases of premature death from cardiovascular disease. Despite these established associations, a causative correlation between high levels of fibrinogen or polymorphisms and cardiovascular disease has not been firmly documented.
Recently it was shown that the levels of proteins modified on tyrosine residues to form 3-nitrotyrosine, a protein marker of nitric oxide-derived reactive nitrogen species, are enhanced in the plasma of coronary artery disease patients and independently predict risk for coronary artery disease (15). Using affinity capture of fibrinogen followed by stable isotope, liquid chromatography (LC)3/tandem mass spectrometry (LC/MS/MS) quantification we reported increased levels of nitrated fibrinogen in coronary artery disease subjects (16). Furthermore, biochemical and biophysical experiments indicated that nitration of fibrinogen in vitro accelerated the rate of fibrin formation, and altered the architecture and viscoelastic properties of the fibrin clot (16). In contrast to the effects of nitration, oxidation of fibrinogen in vitro decreased the rate of fibrin formation (16–18). Although these studies have suggested a potentially unrecognized link between enhanced oxidative/nitrative stress and pro-thrombogenic events, it remains unclear if nitration of fibrinogen will exhibit the same alterations in kinetics and architecture in vivo. Herein we provide evidence that nitration of fibrinogen in vivo results in changes in the kinetics, architecture, stiffness of fibrin clots, and fibrinolysis suggesting an increased risk for thombrotic complications.
EXPERIMENTAL PROCEDURES
Sources of Human Fibrinogen—Subjects (smokers and non-smokers) were between 18 and 45 years of age, with a sex and race distribution reflective of the United States population. They were in good health; in particular, they had no risk factors for cardiovascular disease other than smoking. Smokers had a history of 4–20 years of smoking 11–20 cigarettes/day. Non-smokers had a lifetime consumption of less than 20 cigarettes and lived and worked in a smoke-free environment. Subjects (smokers and non-smokers) were not on chronic medications including aspirin, NSAIDs, multivitamins, or herbal preparations. However, the oral contraceptive pill, anti-histamines, and acid suppressants were permitted. Smokers followed a smoking schedule typical of the number of cigarettes (11–20) for at least 14 months and over a 12-h monitored period in the General Clinical Research Center prior to the collection of blood. Blood samples were collected 1 h after the last cigarette in citrate tubes. Plasma was rapidly collected and stored at –80 °C. Compliance was confirmed biochemically by measurement of urinary cotinine. The protocols for all clinical studies were scrutinized and approved by the Institutional Review Board.
Quantification of Fibrinogen Nitration—An enzyme-linked immunosorbent assay was used to quantify the extent of fibrinogen nitration in plasma, using a polyclonal anti-nitrotyrosine antibody to coat the plate and a polyclonal anti-human fibrinogen antibody conjugated with horseradish peroxidase to detect bound fibrinogen. The enzyme-linked immunosorbent assay was confirmed using 3-nitrotyrosine quantification in affinity purified fibrinogen from human plasma by stable isotope dilution ESI/LC/MS/MS (19). The data are expressed as the ratio of 3-nitrotyrosine/tyrosine to normalize for variations in fibrinogen plasma concentration.
Fibrinogen Isolation—Fibrinogen was isolated from human plasma by glycine precipitation as described previously (20) and under supplemental materials. The pellets were reconstituted with 200 μl of 50 mm Tris, 140 mm NaCl, pH 7.4, and fibrinogen concentration was determined by BCA protein assay (Pierce) using fibrinogen as the standard (American Diagnostica, Stamford, CT).
Fibrinogen Polymerization and Fibrinolysis Assays—These assays were performed as described previously (16) with minor modifications described in detail under the supplemental data.
Immunodepletion of Nitrated Fibrinogen—Nitrated fibrinogen was isolated using the polyclonal anti-nitrotyrosine antibodies described elsewhere (21). The antibodies were covalently coupled to aminolink plus-agarose beads (Pierce). Isolated fibrinogen (200 μg of total protein for nitrotyrosine depletion experiments and 500 μg for mass spectrometry) dissolved in 400 μl of 50 mm Tris, 140 mm NaCl, pH 7.4, was incubated overnight with the beads at 4 °C. The samples were then centrifuged at 3,000 × g for 3 min and the flow-through, which contained the nitrotyrosine-depleted fibrinogen, was used for polymerization assays. The beads were washed with 10 column volumes of Tris-buffered saline. The nitrated fibrinogen was eluted with 0.1 m glycine, pH 2.7, concentrated to a small volume using YM-10 microcon filters (Millipore, Billerica, MA) and used for Western blot experiments or trypsinized for mass spectrometry. The average depletion for nitrated fibrinogen molecules was 65 ± 11%, n = 4. Because the nitrotyrosine immunodepletion required handling that could alter the fibrinogen polymerization properties, aliquots of isolated fibrinogen were also processed through the same procedure in beads linked to nonspecific rabbit immunoglobulin.
Peptide Capture—Fibrinogen (500 μg) was digested as described under supplemental materials and elsewhere (22). Anti-nitrotyrosine antibodies were added to the peptide mixture (antibody to protein ratio 1:25) and incubated overnight at 4 °C. The mixture was transferred into a 10-kDa MWCO filter, washed with 5 volumes of phosphate-buffered saline, 5 volumes of 0.5 m NaCl, and finally 3 volumes of water. The bound peptides were eluted with 1 m formic acid, 10% acetonitrile, concentrated, and analyzed by LC/ESI/MS/MS.
Gels and Western Blotting—Samples were separated in 10% Tris glycine or 4–12% bis-tris gradient gels (Invitrogen) and stained with colloidal blue (Invitrogen) or transferred to a polyvinylidiene difluoride membrane and probed with anti-nitrotyrosine or polyclonal anti-human fibrinogen antibodies (DAKO, Carpinteria, CA). Band intensities where quantified using the Odyssey software version 1.2 (LI-COR, Lincoln, NE).
Mass Spectrometry and MS/MS Spectra Evaluation—Samples were analyzed with a LTQ linear ion trap instrument (Thermo Electron, San Jose, CA) as described under the supplemental materials and elsewhere (23, 24). High resolution LC/MS/MS of the nitrated peptides were analyzed with an LTQ-Orbitrap hybrid instrument (Thermo Fisher, Bremen, Germany) equipped with an Eksigent 1Dplus nanoLC and autosampler (Eksigent, Dublin, CA). One full MS scan from 400 to 2,000 m/z at a resolution of 60,000 was acquired followed by data-dependent acquisition of MS/MS scans as previously described (23, 24). In separate experiments, the one full MS scan from 400 to 2,000 m/z at a resolution of 60,000 was followed by acquisition of MS/MS in a targeted fashion collecting MS/MS spectra for the specific m/z values 864.86, 842.86, 842.37, 850.36, 857.35, 865.35, 893.38, 900.89, and 915.89. MS/MS spectra were collected using an isolation width of 2 m/z, an activation time of 30 ms, and activation Q of 0.250 and 35% normalized collision energy using 1 microscan and maximum injection time of 100 for each scan. The mass spectrometer was tuned prior to analysis using the synthetic peptide TpepK (AVAGKAGAR). Typical tune parameters were as follows: spray voltage of between 1.8 KV, a capillary temperature of 150 °C, a capillary voltage of 50 V, and tube lens 100 V. Peptide sequences matched to MS/MS spectra by Sequest were accepted based on the selection criteria described previously (23, 24).
Immunoelectron Microscopy, Scanning Electron Microscopy, and Viscoelastic Properties of Clots—These experiments were performed as described under supplemental materials and previously (21, 25, 26).
RESULTS
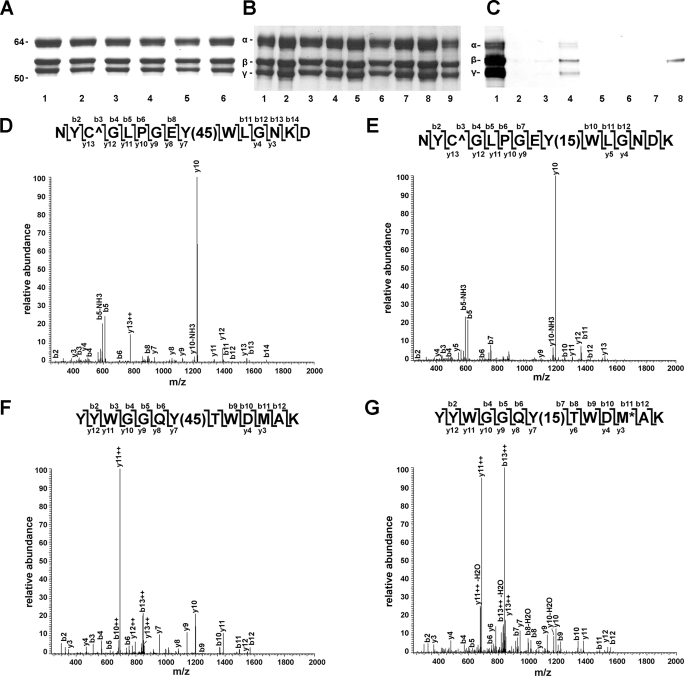
To identify the tyrosine residues targeted by reactive nitrogen species in vivo plasma from smokers and non-smokers was used to isolate fibrinogen (Fig. 1A). Consistent with previous reports that smoking increases oxidant stress (17, 18, 27) the circulating levels of nitrated fibrinogen were significantly higher in smokers compared with non-smokers (51.0 ± 5.5 versus 36.0 ± 3.2 μmol of 3-nitrotyrosine/mol of tyrosine, mean ± S.E., p = 0.04, n = 31). Because a fibrinogen molecule has 134 tyrosine residues two complementary approaches were employed to enrich for nitrated fibrinogen or nitrated peptides. Affinity capturing with anti-nitrotyrosine antibodies was used to enrich for nitrated fibrinogen molecules from the glycine-isolated fibrinogen (Fig. 1B). The enriched fractions were probed with anti-nitrotyrosine antibodies, which revealed positive reactivity primarily with the β-chain of fibrinogen (Fig. 1C). The enriched fractions were digested with trypsin and analyzed by mass spectrometry. Alternatively, the isolated fibrinogen was first digested with trypsin followed by immunoaffinity enrichment for nitrated peptides. A modified method for peptide capture that employed incubation of peptides with antibodies not linked to agarose beads in 10-kDa cutoff filters was applied to reduce nonspecific interactions.
FIGURE 1.
Fibrinogen β-chain carboxyl terminus tyrosine residues are nitrated in vivo. A, isolated fibrinogen analyzed on 10% SDS-PAGE gels and stained with colloidal blue. Lanes 1–6, isolated fibrinogen, 2.5 μg/lane with 3-nitrotyrosine/tyrosine ratio <25 μmol/mol (lanes 1 and 2), 25–50 μmol/mol (lanes 3 and 4), >50 μmol/mol (lanes 5 and 6). B, affinity enrichment for nitrated fibrinogen. Lanes 1, 4, and 7, input isolated fibrinogen; lanes 2, 5, and 8, unbound fibrinogen; lanes 3, 6, and 9, fibrinogen bound to anti-nitrotyrosine antibodies. Fibrinogen was analyzed on 10% SDS-PAGE gels and stained with colloidal blue. C, a representative sample after immunoprecipitation probed with a polyclonal anti-human fibrinogen antibody (lanes 1–4) or a polyclonal anti-nitrotyrosine antibody (lanes 5–8). Lane 1, input fibrinogen; lanes 2 and 3, washes; lane 4, bound fraction. D and E, representative MS/MS spectra of peptides 284NYCGLPGEY+45WLGNDK298 and 416YYWGGQY+45TWDMAK428, respectively. F and G, representative MS/MS spectra of peptides 284NYCGLPGEY+15WLGNDK298 and 416YYWGGQY+15TWDMAK428 after reduction.
Four separate samples were analyzed with the first approach. Each sample consisted of pooled fibrinogen isolated from four individuals. Five putative fibrinogen β-chain tryptic peptides were detected with +45 atomic mass unit increases that mapped to tyrosine residues. The peptide capture approach was then applied to 9 different samples of individual fibrinogen preparations derived from smokers and non-smokers, which were analyzed with an LTQ-Orbitrap hybrid MS instrument. This analysis confirmed with high mass accuracy the presence of two peptides with the expected +45 atomic mass unit increase. The peptides 284NYCGLPGEY+45WLGNDK298 and 416YYWGGQY+45TWDMAK428 were identified in 6 and 7 of the 9 samples, respectively. The inability to detect these peptides in all samples reflects the low abundance of these peptides as well as technical limitations with the acquisition of quality spectra for nitrated peptides by mass spectrometry. To further confirm these identifications, the immunoaffinity enriched peptide mixtures were treated with dithionite, which reduces 3-nitrotyrosine to 3-aminotyrosine. Following reduction, the peptide mixture was analyzed by selectively monitoring for the monoisotopic masses of the unmodified, 3-nitrotyrosine, and 3-aminotyrosine containing peptides of the two dominant modified sequences (peptides 284–298 and 416–428). This analysis revealed the presence of unmodified peptides, and peptides with increased masses of +15 atomic mass units (284NYCGLPGEY+15WLGNDK298 and 416YYWGGQY+15TWDMAK428) indicating the presence of 3-aminotyrosine derived from the reduction of 3-nitrotyrosine. Typical spectra of these two peptides before and after reduction with dithionite are shown in Fig. 1, D–G. Together the data indicate that the predominant sites of fibrinogen nitration in vivo are residues 292 and 422 in the carboxyl terminus of β-chain.
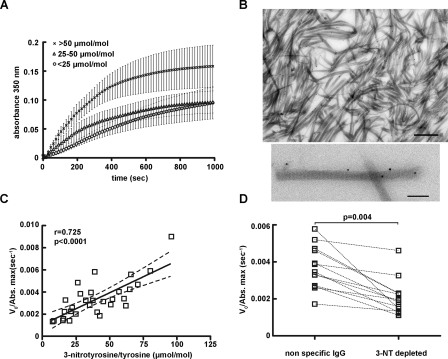
To explore the functional consequences of nitration, fibrinogen was isolated from 30 individuals, both smokers and non-smokers to represent the entire spectrum of 3-nitrotyrosine levels (ranging from 0 to 108 μmol of 3-nitrotyrosine/mol of tyrosine). The quality of the preparation was assessed by SDS-PAGE (Fig. 1A). The samples were then divided into 3 groups based on the levels of fibrinogen nitration: levels <25 μmol of 3-nitrotyrosine/mol of tyrosine, levels between 25 and 50 μmol of 3-nitrotyrosine/mol of tyrosine, and levels >50 μmol of 3-nitrotyrosine/mol of tyrosine. Ex vivo polymerization was initiated by the addition of 1 NIH unit/ml of human α-thrombin. As shown in Fig. 2A, polymerization curves of fibrinogen samples with levels >50 μmol of 3-nitrotyrosine/mol of tyrosine exhibited a steeper increase in absorbance and a higher final turbidity as compared with samples with moderate, 25–50 μmol of 3-nitrotyrosine/mol of tyrosine and low <25 μmol of 3-nitrotyrosine/mol of tyrosine levels (p < 0.05, n = 10). Using previously characterized affinity purified anti-nitrotyrosine antibodies and immunoelectron microscopy we confirmed that indeed nitrated fibrinogen molecules were incorporated in the fibrin fibers (Fig. 2B).
FIGURE 2.
Fibrinogen nitration accelerates fibrin clot formation. A, polymerization curves represent the mean ± S.E., n = 10 subjects per group. B, representative immuno-EM image of fibrin fibers labeled with anti-nitrotyrosine antibodies and 10 nm protein gold; bar, 500 nm (top). Higher magnification of single fibers (bottom) showing the distinctive fibrin band pattern and labeling with anti-nitrotyrosine antibodies; bar, 200 nm. C, the initial velocity V0 of each separate curve, normalized for the final turbidity (V0/Abs. max) was plotted against the 3-nitrotyrosine/tyrosine ratio. Spearman analysis reveals a positive correlation between V0/Abs max and 3-nitrotyrosine/tyrosine ratio (r = 0.73, p < 0.0001, n = 30). D, polymerization assays after immunodepletion. Paired t test statistical analysis showed a marked reduction of the V0/Abs. max after immunodepletion with the anti-nitrotyrosine (NT) immunoglobulin (p = 0.0004, n = 14).
The initial velocity V0 of fibrin clot formation normalized for the maximum absorbance (V0/Abs max) showed a positive correlation with the 3-nitrotyrosine levels (Spearman's r = 0.73, p < 0.0001, Fig. 2C). Nitration levels were also positively associated with the initial velocity V0 (Spearman's r = 0.35, p = 0.05), but not with the final turbidity (Spearman's r = 0.24, p = 0.25). The inclusion of 2.5 mm calcium exhibited the same accelerated kinetics and increased final clot turbidity in samples of purified fibrinogen with >50 μmol of 3-nitrotyrosine/mol of tyrosine compared with samples with low nitration levels (data not shown). The same effect was also evident when fibrin clots were made in whole plasma in place of isolated fibrinogen (Spearman's r = 0.43, p = 0.03, n = 23).
Two different approaches were applied to evaluate the contribution of 3-nitrotyrosine to the altered kinetics of fibrin clot formation. Fibrinogen samples were depleted of nitrated fibrinogen molecules by affinity capturing. Aliquots of the same fibrinogen preparations underwent the same procedure using nonspecific immunoglobulin to eliminate any interference from sample handling. Depletion of nitrated fibrinogen molecules decreased the initial velocity of clot formation as compared with the nonspecific immunoglobulin for identical fibrinogen concentrations (Fig. 2D). Alternatively, the clotting assay was performed using either isolated fibrinogen or plasma in the presence of the polyclonal anti-nitrotyrosine antibodies or nonspecific rabbit immunoglobulin. Inclusion of the specific anti-nitrotyrosine antibody reduced the initial rate of fibrin clot formation compared with the nonspecific IgG in both isolated fibrinogen (p = 0.001, n = 12) and whole plasma (p = 0.016, n = 22).
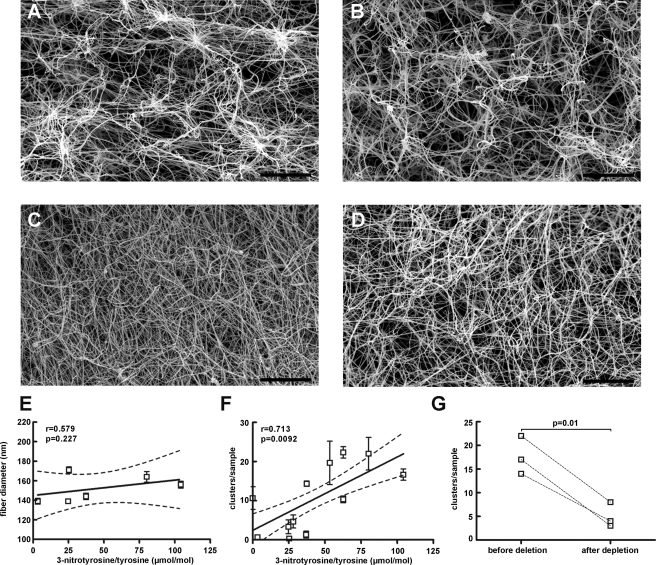
The effect of tyrosine nitration on clot morphology was investigated by scanning electron microscopy. A distinct feature in the fibrin clots made from fibrinogen with levels >50 μmol of 3-nitrotyrosine/mol of tyrosine was the presence of clusters of fibrin. This feature is not apparent in fibrin clots with low 3-nitrotyrosine levels, which appear dense and homogenous (Fig. 3C). Clusters were counted in fibrin clots from 12 different samples with fibrinogen nitration values ranging from 0 to 104 μmol of 3-nitrotyrosine/mol of tyrosine. We defined a cluster the structure formed by more than 8 single fibers crossing at a single point. The fibers depart the cluster as single fibers and continue in separate directions, hereby forming a well defined cluster, which appears as a bright spot in the image (Fig. 3A). Six images per sample under ×1000 magnification were selected randomly and clusters were manually counted. Each measurement was repeated for 3 consecutive days and the average number of clusters per sample was plotted against fibrinogen nitration levels. A significant correlation between the number of clusters per sample and fibrinogen nitration level (Spearman's r = 0.70, p = 0.01) was revealed (Fig. 3F). Because final turbidity directly relates to the thickness of the fibrin fibers, the thickness of individual fibers was also quantified. Fiber diameters of ∼150 fibers per image were measured from 5 images per clot in 6 randomly selected clots. The diameter of individual fibrin fibers did not vary as a function of 3-nitrotyrosine content (r = 0.58, p = 0.23) (Fig. 3E). Scanning electron micrographs of fibrin clots made from isolated fibrinogen after depletion of nitrated fibrinogen molecules were examined to ascertain the role of fibrinogen nitration on the architecture of the fibrin clots. Removal of nitrated fibrinogen molecules resulted in formation of fibrin clots with significantly reduced fiber clustering (Fig. 3G).
FIGURE 3.
Scanning electron microscopy of fibrin clots. A–C, fibrinogen isolated from three different subjects with 3-nitrotyrosine/tyrosine ratios of 80.2, 37.0, 3.3 μmol/mol, respectively, was used to generate the fibrin clots. D, image from the same sample as in A after immunoaffinity depletion of nitrated molecules. Based on the efficiency of immunoaffinity depletion (65 ± 11%, n = 4) the level of 3-nitrotyrosine/tyrosine is ∼28 μmol/mol in this sample. E, fiber diameter is independent of levels of fibrinogen nitration. F, correlation between fibrin clot cluster formation and fibrinogen nitration levels. Spearman's r = 0.71, p = 0.0092, n = 12. G, removal of tyrosine-nitrated molecules by immunoaffinity depletion results in fibrin clots with a significantly decreased number of clusters (p = 0.01, n = 3). Bar = 20 μm.
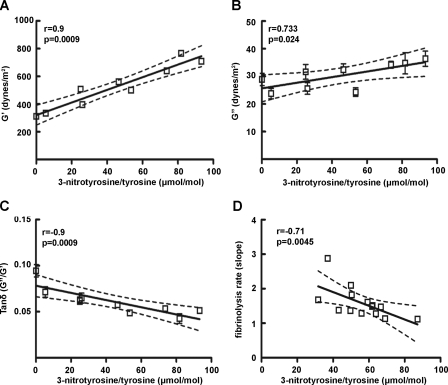
The physical properties of fibrin clots were determined by measurement of both the elastic and inelastic parameters in nine different clots. A positive correlation between changes in the storage modulus G′, which reflects clot stiffness, with the 3-nitrotyrosine levels of fibrinogen (r = 0.90, p = 0.0009) were observed (Fig. 4A). Similarly changes in the loss module G″, which indicates clot plasticity were positively correlated with the 3-nitrotyrosine levels of fibrinogen (r = 0.73, p = 0.024) (Fig. 4B). The loss tangent tanδ calculated from the ratio G″/G′, which indicates the ratio of energy lost to energy stored in a cyclic deformation declined exponentially when plotted against the levels of 3-nitrotyrosine in fibrinogen. This association is primarily derived by the relatively large increase in the G′ values as a function of fibrinogen nitration (Fig. 4C). Moreover, higher nitration levels were associated with lower plasmin-induced fibrinolysis rates (Fig. 4D), whereas fibrinogen degradation products were similar among samples with different nitration levels (supplemental Fig. 1).
FIGURE 4.
Viscoelastic properties of fibrin clots. A, correlation between the G′ value (clot stiffness) and 3-nitrotyrosine levels (Spearman r = 0.95, p < 0.0001, n = 9). B, correlation between the storage module G″ (clot plasticity) with the 3-nitrotyrosine burden (Spearman r = 0.73, p = 0.02, n = 9). C, tanδ (G″/G′) association with 3-nitrotyrosine/tyrosine ratio (Spearman r =–0.9, p = 0.0009). The G′ and G″ values of each separate clot were measured in triplicate. D, fibrinolytic rate is negatively associated with 3-nitrotyrosine levels in fibrin clots (Spearman's r =–0.71, p = 0.0045, n = 14).
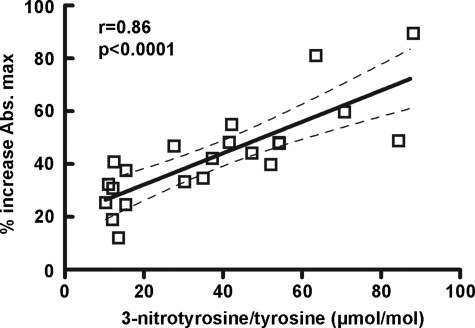
The mass spectrometric analysis revealed that the modified tyrosine moieties reside on the carboxyl-terminal of fibrinogen β-chain, in close proximity to the backbone ridge of the hole “b” (28). The amino acid sequence Gly-His-Arg on the N-terminal of the β-chain interacts with a pocket on the C-terminal of the β-chain (29) and appears to enhance lateral aggregation (30). The observed effect of 3-nitrotyrosine formation on fibrinogen lateral aggregation, which features the effect of knob “B” mimetic peptides, along with the localization of the modified residues suggested that tyrosine nitration might accelerate fibrinogen clotting by means of enhancement of knob B:hole b interactions. To explore this hypothesis, fibrinogen was isolated from 21 plasma samples and polymerization was initiated with the addition of 0.1 unit/ml α-thrombin to 3 μm fibrinogen in the absence or presence of 250 μm of the knob B mimetic peptide Gly-His-Arg-Pro-amide (Gly-His-Arg-Proam). The presence of the peptide accelerated the lateral aggregation in all samples. However, the response to the mimetic peptide, assessed by the percent increase in the final turbidity, exhibited a significant correlation with the 3-nitrotyrosine levels (Spearman's r = 0.86, p < 0.0001) (Fig. 5). Similar results were obtained when polymerization assays were performed with the knob B-specific (28) peptide Ala-His-Arg-Pro-amide (Ala-His-Arg-Proam) (Spearman's r = 0.56, p = 0.035). The data suggest that the presence of tyrosine nitration might induce conformational changes that may facilitate the knob B/hole b interactions.
FIGURE 5.
Positive association between the response to the knob B mimetic peptide Gly-His-Arg-Proam and fibrinogen nitration levels (Spearman's r = 0.86, p < 0.0001, n = 21). The response to the mimetic peptide was assessed by the percentage increase in final turbidity compared with the baseline (final turbidity in the absence of the peptide).
DISCUSSION
Epidemiological studies have indicated that increased levels of circulating fibrinogen are an independent predictor of coronary heart disease and in some cases premature death from cardiovascular disease, although a causative correlation between high levels of fibrinogen and cardiovascular disease has not been firmly established (7, 8, 11, 12). Cigarette smoking has also been shown to increase the circulating levels of fibrinogen and smokers have a higher risk for cardiovascular disease and thrombotic events (31, 32). Oxidative stress has been considered as one of the mechanisms responsible for the development and progression of cardiovascular and pulmonary disorders and is increased, as reflected by indices of lipid peroxidation, in otherwise apparently healthy smokers (33, 34). Moreover, protein tyrosine nitration has been considered one of the post-translational modifications derived from the reaction of nitric oxide-derived oxidants with proteins. Notwithstanding its recognized value as a marker of oxidant burden in human disease, it remains unclear whether protein nitration is responsible for alteration in protein function that imparts an increased risk and therefore represents a disease modifier. Attributing tyrosine nitration a causative pathophysiological function has been hindered by concerns relating to the low yield of nitration quantified on specific proteins and the co-existence of other oxidative modifications on the protein.
The current study provides direct experimental evidence to indicate that tyrosine nitration of fibrinogen is a specific functional modification and a risk factor for increased thrombotic tendency observed under conditions of oxidative stress. Using enrichment strategies and mass spectrometry we localized the sites of in vivo nitration at 2 specific tyrosine residues, Tyr292 and Tyr422 on the β-chain among the 134 tyrosine residues in a fibrinogen molecule. This selectivity of tyrosine nitration (35) could explain the consistent effect of tyrosine nitration on fibrin clot formation. If nitration of fibrinogen occurred at random, then one would observe variable effects, if any, on the function of the protein. However, in this case the effect of tyrosine nitration is consistent and is characterized by the acceleration of lateral aggregation of fibrin molecules. Ex vivo studies revealed significant correlation between the levels of fibrinogen nitration and the velocity of fibrin clot formation, whereas specific elimination of the nitrated fibrinogen molecules restored the kinetics of fibrin clot formation. These results support a functional consequence of tyrosine nitration on fibrin polymerization in the form of “gain of function” rather than “loss of function,” which is typically observed in the oxidatively damaged proteins, including oxidized fibrinogen (16–18). Indeed exposure of fibrinogen to different oxidative conditions in vitro (singlet oxygen, metal, and myeloperoxidase-derived oxidants) was found to reduce the velocity of fibrin clot formation and to result in dense fibrin clots (16–18). However, the in vitro studies do not always reproduce faithfully the in vivo conditions such as the sites and magnitude of modifications. Whereas the increase in the velocity of fibrin clot formation and altered fibrin clot architecture observed ex vivo are entirely consistent with in vitro exposures (16), the ex vivo changes in fibrin clot stiffness and the closely related rate of clot lysis differ from in vitro exposures to nitrating oxidants. Therefore the current work used exclusively isolated fibrinogen from human subjects to investigate fibrinogen nitration as a risk factor that influences normal homeostasis.
The profound effects on fibrin clot structure and biophysical properties were produced by a relative small fraction of fibrinogen molecules modified by nitration, which based on two primary sites of nitration represent 0.6–6% of the β-chain per molecule of fibrinogen. Kinetic analysis of fibrin formation revealed that nitration influences the lateral aggregation, an event that follows the initial fibrinopeptide cleavage, oligomerization, and the formation of half-staggered, double-stranded protofibrils. We hypothesize that in the organized assembly of fibrin molecules addition of a molecule with nitrated tyrosine residues accelerates the association of the knob B:hole b interactions, because the two sites of nitration are within the hole b of the β-chain. The knobs B are exposed after B fibrinopeptide release and engage the hole b in the carboxyl terminus of the β-chain during lateral aggregation. The location of the tyrosine-modified residues suggested that 3-nitrotyrosine formation might accelerate lateral aggregation by enhancing the knob B:hole b interactions. To explore this hypothesis, we performed clotting assays in the presence or absence of knob B mimetic peptides Gly-His-Arg-Proam and Ala-His-Arg-Proam. The former, which is considered the prototypical knob B mimetic, has been shown to interact to a minor extent with the hole “γ” as well (36), whereas the latter has been shown to be hole b specific (28). Interestingly, the response of fibrinogen polymerization to the knob B mimetic peptides, assessed by the percent increase in the final turbidity, exhibited a positive correlation with the 3-nitrotyrosine levels. The data suggest that the presence of tyrosine nitration might induce conformational changes that facilitate the knob B:hole b interactions. An alternative plausible explanation for the effect of nitration relates to the location of Tyr422. This residue is located in close proximity to the coiled-coil connecting the middle and ends of the fibrinogen molecule. Nitration of Tyr422 could affect the stability of the interaction between BbAsp398 and AaLys157 that is thought to be responsible for anchoring the COOH-terminal β-chain to the coiled-coil in fibrinogen. This bond is broken and there is a conformational change of the C-terminal β-chain upon binding of Gly-His-Arg-Proam peptide in hole b. Thus, there are potentially direct links between nitration of these tyrosine residues and the function of this region of fibrinogen in lateral aggregation, although detailed structural effects of nitration of these residues require further investigation.
The mechanical properties of fibrin clots are different in individuals with premature thrombotic events compared with healthy controls (37–41). It has also been suggested that these differences might influence the occurrence of thrombotic events. For example, fibrin clot stiffness, which was associated with reduced fibrinolytic rates, was found to be an independent predictor of premature atherothrombotic events (39). Despite these intriguing associations, the biochemical reasons for the altered mechanical properties remain unknown. In this work we demonstrate that fibrin clot properties are strongly affected by the presence of tyrosine-nitrated molecules. We report a positive association between the levels of fibrinogen nitration-accelerated kinetics and clot stiffness as well as a negative association between levels of fibrinogen nitration and the rate of clot lysis. We also demonstrated altered fibrin clot architecture, which were restored upon removal of tyrosine-nitrated molecules. This is compatible with a role for fibrinogen tyrosine nitration as a risk factor for the increased thrombotic tendency during inflammation and oxidant stress.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants P50 HL70128 (to G. F. and H. I.), RO1 HL54926 (to H. I.), and ES013508 from the NIEHS Center of Excellence in Environmental Toxicology (to H. I.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and additional methods.
Footnotes
The abbreviations used are: LC, liquid chromatography; MS, masss pectrometry; bis-tris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
References
- 1.Lord, S. T. (2007) Curr. Opin. Hematol. 14 236–241 [DOI] [PubMed] [Google Scholar]
- 2.Mosesson, M. W. (2005) J. Thromb. Haemostasis 3 1894–1904 [DOI] [PubMed] [Google Scholar]
- 3.Prandoni, P., Bilora, F., Marchiori, A., Bernardi, E., Petrobelli, F., Lensing, A. W., Prins, M. H., and Girolami, A. (2003) N. Engl. J. Med. 348 1435–1441 [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg, R. D., and Aird, W. C. (1999) N. Engl. J. Med. 340 1555–1564 [DOI] [PubMed] [Google Scholar]
- 5.Alpert, J. S., Smith, R., Carlson, J., Ockene, I. S., Dexter, L., and Dalen, J. E. (1976) J. Am. Med. Assoc. 236 1477–1480 [PubMed] [Google Scholar]
- 6.Ferro, D., Pittoni, V., Quintarelli, C., Basili, S., Saliola, M., Caroselli, C., Valesini, G., and Violi, F. (1997) Circulation 95 1425–1432 [DOI] [PubMed] [Google Scholar]
- 7.Thompson, S. G., Kienast, J., Pyke, S. D., Haverkate, F., and van de Loo, J. C. (1995) N. Engl. J. Med. 332 635–641 [DOI] [PubMed] [Google Scholar]
- 8.Kannel, W. B., Wolf, P. A., Castelli, W. P., and D'Agostino, R. B. (1987) J. Am. Med. Assoc. 258 1183–1186 [PubMed] [Google Scholar]
- 9.Wedzicha, J. A., Seemungal, T. A., MacCallum, P. K., Paul, E. A., Donaldson, G. C., Bhowmik, A., Jeffries, D. J., and Meade, T. W. (2000) Thromb. Haemostasis 84 210–215 [PubMed] [Google Scholar]
- 10.Afeltra, A., Vadacca, M., Conti, L., Galluzzo, S., Mitterhofer, A. P., Ferri, G. M., Del Porto, F., Caccavo, D., Gandolfo, G. M., and Amoroso, A. (2005) Arthritis Rheum. 53 452–459 [DOI] [PubMed] [Google Scholar]
- 11.Wilhelmsen, L., Svardsudd, K., Korsan-Bengtsen, K., Larsson, B., Welin, L., and Tibblin, G. (1984) N. Engl. J. Med. 311 501–505 [DOI] [PubMed] [Google Scholar]
- 12.Meade, T. W., Mellows, S., Brozovic, M., Miller, G. J., Chakrabarti, R. R., North, W. R., Haines, A. P., Stirling, Y., Imeson, J. D., and Thompson, S. G. (1986) Lancet 2 533–537 [DOI] [PubMed] [Google Scholar]
- 13.Blake, G. J., Schmitz, C., Lindpaintner, K., and Ridker, P. M. (2001) Eur. Heart J. 22 2262–2266 [DOI] [PubMed] [Google Scholar]
- 14.Carter, A. M., Catto, A. J., Kohler, H. P., Ariens, R. A., Stickland, M. H., and Grant, P. J. (2000) Blood 96 1177–1179 [PubMed] [Google Scholar]
- 15.Shishehbor, M. H., Aviles, R. J., Brennan, M. L., Fu, X., Goormastic, M., Pearce, G. L., Gokce, N., Keaney, J. F., Jr., Penn, M. S., Sprecher, D. L., Vita, J. A., and Hazen, S. L. (2003) J. Am. Med. Assoc. 289 1675–1680 [DOI] [PubMed] [Google Scholar]
- 16.Vadseth, C., Souza, J. M., Thomson, L., Seagraves, A., Nagaswami, C., Scheiner, T., Torbet, J., Vilaire, G., Bennett, J. S., Murciano, J. C., Muzykantov, V., Penn, M. S., Hazen, S. L., Weisel, J. W., and Ischiropoulos, H. (2004) J. Biol. Chem. 279 8820–8826 [DOI] [PubMed] [Google Scholar]
- 17.Inada, Y., Hessel, B., and Blomback, B. (1978) Biochim. Biophys. Acta 532 161–170 [DOI] [PubMed] [Google Scholar]
- 18.Shacter, E., Williams, J. A., and Levine, R. L. (1995) Free Radic. Biol. Med. 18 815–821 [DOI] [PubMed] [Google Scholar]
- 19.Brennan, M. L., Wu, W., Fu, X., Shen, Z., Song, W., Frost, H., Vadseth, C., Narine, L., Lenkiewicz, E., Borchers, M. T., Lusis, A. J., Lee, J. J., Lee, N. A., Abu-Soud, H. M., Ischiropoulos, H., and Hazen, S. L. (2002) J. Biol. Chem. 277 17415–17427 [DOI] [PubMed] [Google Scholar]
- 20.Kazal, L. A., Amsel, S., Miller, O. P., and Tocantins, L. M. (1963) Proc. Soc. Exp. Biol. Med. 113 989–994 [DOI] [PubMed] [Google Scholar]
- 21.Heijnen, H. F., van Donselaar, E., Slot, J. W., Fries, D. M., Blachard-Fillion, B., Hodara, R., Lightfoot, R., Polydoro, M., Spielberg, D., Thomson, L., Regan, E. A., Crapo, J., and Ischiropoulos, H. (2006) Free Radic. Biol. Med. 40 1903–1913 [DOI] [PubMed] [Google Scholar]
- 22.Manza, L. L., Stamer, S. L., Ham, A. J., Codreanu, S. G., and Liebler, D. C. (2005) Proteomics 5 1742–1745 [DOI] [PubMed] [Google Scholar]
- 23.Greco, T. M., Hodara, R., Parastatidis, I., Heijnen, H. F., Dennehy, M. K., Liebler, D. C., and Ischiropoulos, H. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 7420–7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parastatidis, I., Thomson, L., Fries, D. M., Moore, R. E., Tohyama, J., Fu, X., Hazen, S. L., Heijnen, H. F., Dennehy, M. K., Liebler, D. C., Rader, D. J., and Ischiropoulos, H. (2007) Circ. Res. 101 368–376 [DOI] [PubMed] [Google Scholar]
- 25.Weisel, J. W., and Nagaswami, C. (1992) Biophys. J. 63 111–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan, E. A., Mockros, L. F., Weisel, J. W., and Lorand, L. (1999) Biophys. J. 77 2813–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pignatelli, B., Li, C. Q., Boffetta, P., Chen, Q., Ahrens, W., Nyberg, F., Mukeria, A., Bruske-Hohlfeld, I., Fortes, C., Constantinescu, V., Ischiropoulos, H., and Ohshima, H. (2001) Cancer Res. 61 778–784 [PubMed] [Google Scholar]
- 28.Doolittle, R. F., Chen, A., and Pandi, L. (2006) Biochemistry 45 13962–13969 [DOI] [PubMed] [Google Scholar]
- 29.Litvinov, R. I., Gorkun, O. V., Galanakis, D. K., Yakovlev, S., Medved, L., Shuman, H., and Weisel, J. W. (2007) Blood 109 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisel, J. W., Veklich, Y., and Gorkun, O. (1993) J. Mol. Biol. 232 285–297 [DOI] [PubMed] [Google Scholar]
- 31.Kannel, W. B., D'Agostino, R. B., and Belanger, A. J. (1987) Am. Heart J. 113 1006–1010 [DOI] [PubMed] [Google Scholar]
- 32.Dotevall, A., Kutti, J., Teger-Nilsson, A. C., Wadenvik, H., and Wilhelmsen, L. (1987) Eur. J. Haematol. 38 55–59 [DOI] [PubMed] [Google Scholar]
- 33.Reilly, M., Delanty, N., Lawson, J. A., and FitzGerald, G. A. (1996) Circulation 94 19–25 [DOI] [PubMed] [Google Scholar]
- 34.Morrow, J. D., Frei, B., Longmire, A. W., Gaziano, J. M., Lynch, S. M., Shyr, Y., Strauss, W. E., Oates, J. A., and Roberts, L. J., 2nd (1995) N. Engl. J. Med. 332 1198–1203 [DOI] [PubMed] [Google Scholar]
- 35.Gow, A. J., Farkouh, C. R., Munson, D. A., Posencheg, M. A., and Ischiropoulos, H. (2004) Am. J. Physiol. 287 L262–L268 [DOI] [PubMed] [Google Scholar]
- 36.Everse, S. J., Spraggon, G., Veerapandian, L., and Doolittle, R. F. (1999) Biochemistry 38 2941–2946 [DOI] [PubMed] [Google Scholar]
- 37.Fatah, K., Hamsten, A., Blomback, B., and Blomback, M. (1992) Thromb. Haemostasis 68 130–135 [PubMed] [Google Scholar]
- 38.Fatah, K., Silveira, A., Tornvall, P., Karpe, F., Blomback, M., and Hamsten, A. (1996) Thromb. Haemostasis 76 535–540 [PubMed] [Google Scholar]
- 39.Collet, J. P., Allali, Y., Lesty, C., Tanguy, M. L., Silvain, J., Ankri, A., Blanchet, B., Dumaine, R., Gianetti, J., Payot, L., Weisel, J. W., and Montalescot, G. (2006) Arterioscler. Thromb. Vasc. Biol. 26 2567–2573 [DOI] [PubMed] [Google Scholar]
- 40.Gurbel, P. A., Bliden, K. P., Guyer, K., Cho, P. W., Zaman, K. A., Kreutz, R. P., Bassi, A. K., and Tantry, U. S. (2005) J. Am. Coll. Cardiol. 46 1820–1826 [DOI] [PubMed] [Google Scholar]
- 41.Mills, J. D., Ariens, R. A., Mansfield, M. W., and Grant, P. J. (2002) Circulation 106 1938–1942 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.