Abstract
Brain edema and the consequent increase in intracranial pressure and brain herniation are major complications of acute liver failure (fulminant hepatic failure) and a major cause of death in this condition. Ammonia has been strongly implicated as an important factor, and astrocyte swelling appears to be primarily responsible for the edema. Ammonia is known to cause cell swelling in cultured astrocytes, although the means by which this occurs has not been fully elucidated. A disturbance in one or more of these systems may result in loss of ion homeostasis and cell swelling. In particular, activation of the Na-K-Cl cotransporter (NKCC1) has been shown to be involved in cell swelling in several neurological disorders. We therefore examined the effect of ammonia on NKCC activity and its potential role in the swelling of astrocytes. Cultured astrocytes were exposed to ammonia (NH4Cl; 5 mm), and NKCC activity was measured. Ammonia increased NKCC activity at 24 h. Inhibition of this activity by bumetanide diminished ammonia-induced astrocyte swelling. Ammonia also increased total as well as phosphorylated NKCC1. Treatment with cyclohexamide, a potent inhibitor of protein synthesis, diminished NKCC1 protein expression and NKCC activity. Since ammonia is known to induce oxidative/nitrosative stress, and antioxidants and nitric-oxide synthase inhibition diminish astrocyte swelling, we also examined whether ammonia caused oxidation and/or nitration of NKCC1. Cultures exposed to ammonia increased the state of oxidation and nitration of NKCC1, whereas the antioxidants N-nitro-l-arginine methyl ester and uric acid all significantly diminished NKCC activity. These agents also reduced phosphorylated NKCC1 expression. These results suggest that activation of NKCC1 is an important factor in the mediation of astrocyte swelling by ammonia and that such activation appears to be mediated by NKCC1 abundance as well as by its oxidation/nitration and phosphorylation.
Hepatic encephalopathy is a neurological disorder that occurs in patients with severe liver failure. The acute form (fulminant hepatic failure) is associated with cerebral edema, increased intracranial pressure, herniation, and death (1). Although the pathogenetic mechanisms for brain edema are still not clear, ammonia has been strongly implicated as an important etiological factor (for reviews, see Refs. 2 and 3), and astrocyte swelling has been implicated as a major process responsible for the edema (cytotoxic edema) (4–7). Mechanisms mediating the astrocyte swelling and the subsequent brain edema in fulminant hepatic failure remain poorly understood.
Ion channels, exchangers, and transporters represent important factors in the mechanism of cell volume regulation (for reviews, see Refs. 8–11). Structural and functional changes in these systems may result in the loss of ion homeostasis (for a review, see Ref. 12) and the subsequent accumulation of intracellular water. These ion transporters and exchangers include the Na-K-Cl cotransporter (NKCC),3 volume-sensitive osmolyte anion channels, Na+/Ca2+ exchanger, the Na+/H+ exchanger, and the nonselective cation channel (NCCa-ATP) (11). The Na-K-Cl cotransporter-1 (NKCC1), in particular, has been shown to play an important role in the mediation of cell swelling/brain edema (for a review, see Ref. 13).
NKCC cotransporters are a class of membrane proteins that transport Na+, K+, and Cl– ions into and out of a wide variety of epithelial and nonepithelial cells and are part of a superfamily of cation-chloride cotransporters (14). Two isoforms of NKCC have been identified (NKCC1 and NKCC2) (15, 16). NKCC1 is present in many cell types, including astrocytes (17–22). The NKCC2 isoform is localized exclusively to the kidney (23, 24). NKCC1 activation was shown to contribute to astrocyte swelling/brain edema in ischemia and to brain edema after traumatic brain injury (25–30). Its role in astrocyte swelling after ammonia treatment and in the brain edema in fulminant hepatic failure, however, are not known.
This study investigated whether NKCC activation occurs in ammonia-treated astrocyte cultures and whether such activation contributes to astrocyte swelling. Our findings demonstrate that ammonia increased NKCC activity, which was associated with an increase in total and phosphorylated NKCC1 protein expression, as well as in its oxidation and nitration. Blocking NKCC activity with bumetanide attenuated ammonia-induced astrocyte swelling. Altogether, our findings suggest that activation of NKCC plays an important role in the mediation of ammonia-induced astrocyte swelling and possibly in the brain edema associated with fulminant hepatic failure.
EXPERIMENTAL PROCEDURES
Astrocyte Cultures—Astrocyte cultures were prepared from brains of 1–2-day-old rat pups by the method of Ducis et al. (31). Briefly, cerebral cortices were freed of meninges, minced, dissociated by trituration and vortexing, passed through sterile nylon sieves, placed in Dulbecco's modified Eagle's medium containing penicillin, streptomycin, and fetal bovine serum, and incubated at 37 °C in a humidified chamber provided with 5% CO2 and 95% air. After 10 days in culture, bovine serum was replaced with 10% horse serum. After 14 days, cultures were treated and maintained with dibutyryl-cAMP (Sigma) so as to enhance cell differentiation (32). Cultures consisted of at least 98% astrocytes, as determined by glial fibrillary acidic protein and glutamine synthetase immunocytochemistry. The remaining cells consisted of microglia. Experiments were carried out in 3–4-week-old cells. Procedures followed guidelines established by the National Institutes of Health Guide for the Care and use of Laboratory Animals and were approved by the local animal care committee (Institutional Animal Care and Use Committee).
NKCC Activity—NKCC activity was measured as the bumetanide-sensitive K+ influx, using 86Rb as a tracer for K+ by a modification of the method of Sun and Murali (33). Briefly, primary astrocyte cultures were treated once with a pathophysiological concentration of ammonia (NH4Cl; 5 mm); such a value is found in the brains of animals with acute liver failure (34). The ammonia concentration in the medium rapidly declines to undetectable levels by 30–60 min.4 At the end of the treatment (24 h), cultures were equilibrated and preincubated with or without 50 μm bumetanide for 15–30 min at 37 °C in an isotonic HEPES-minimal essential medium (140 mm NaCl, 5.36 mm KCl, 0.81 mm MgSO4, 1.27 mm CaCl2, 0.44 mm KH2PO4, 0.33 mm Na2HPO4, 5.55 mm glucose, and 20 mm HEPES (300 mosm)). Cultures were then exposed to 1 μCi/ml 86Rb for 3 min and subsequently rinsed with ice-cold 0.1 m MgCl2. Cells were then extracted in 1% SDS, and the radioactivity was analyzed by liquid scintillation. The 86Rb influx rate was calculated by subtracting the influx (with bumetanide) from total influx (without bumetanide) and expressed as nmol of 86Rb/mg of protein/min. Quadruplicate determinations were obtained throughout the study, and the protein content was determined employing the BCA assay (Pierce).
Measurement of Intracellular Na+ Concentration [Na+]i—As another measure of NKCC activity, [Na+]i level was determined by modifications of the methods of Rose and Ransom (35) and Su et al. (26), employing the fluorescent dye sodium-binding benzofuran isophthalate acetoxy methyl esters-AM. Culture medium was removed, and the cells were washed two times with 2 ml of prewarmed (37 °C) HEPES-minimal essential medium. Cells were incubated for 30 min at 37 °C for 1 h in 2 ml of fresh HEPES-minimal essential medium containing 0.05% pluronic acid and 10 μm sodium-binding benzofuran isophthalate acetoxy methyl esters-AM. At the end of the incubation period, the medium was aspirated, and the cells were washed twice with ice-cold phosphate-buffered saline. Cells were scraped into 500 μl of 0.2% Triton X-100 and sonicated for 5 s. A small aliquot of the cell extract was removed for protein estimation, and fluorescence intensity was measured in the remaining solution with a spectrofluorophotometer (Spectrophotofluorome, RF-1501; Shimadzu Scientific Instruments Inc., Norcross, GA) at dual excitation wavelength of 340 nm/385 nm, and emission fluorescence at 510 nm. Protein content was determined by the BCA method. The results were expressed as fluorescence intensity units/mg of protein.
Cell Volume Determination—Cell volume was estimated by measuring the intracellular water space by the method of Kletzien et al. (36), as modified by Kimelberg (37) and Bender and Norenberg (38). Briefly, 1 mm 3-O-methylglucose and 0.5 μCi/ml 3H-labeled 3-O-methylglucose were added to the culture 6 h before the volume assay. At the end of the incubation period, culture medium was aspirated, and an aliquot was saved for radioactivity determination. Cells were rapidly washed six times with ice-cold buffer containing 229 mm sucrose, 1 mm Tris nitrate, 0.5 mm calcium nitrate, and 0.1 mm phloretin, pH 7.4. Cells were then harvested into 0.5 ml of 1 n sodium hydroxide. Radioactivity in the cell extracts and medium were determined, and an aliquot of the cell extract was used for protein estimation (BCA method). Values were normalized to protein level, and the cell volume was expressed as μl/mg of protein.
Western Blots—Astrocyte cultures were solubilized in lysis buffer (125 mm Tris-HCl, pH 6.8, 4% SDS, phosphatase inhibitors (Sigma), and a protease inhibitor mixture (Roche Applied Science)), and protein levels were measured by the BCA method. Equal amounts of protein were subjected to gel electrophoresis, as previously described (39), and transferred to nitrocellulose membranes. Following blocking with nonfat dry milk, membranes were incubated with respective antibodies. Total NKCC1 antibody was purchased from Chemicon International (Temecula, CA). Primary antibodies to detect phosphorylated (R5) and total NKCC1 were used at 1:1000. Anti-α-tubulin antibody was obtained from Oncogene (San Diego, CA). Anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies (Vector Laboratories) were used at 1:1000. Optical density of the bands were determined with the Chemi-Imager (Alpha Innotech, San Leandro, CA) digital imaging system, and the results were quantified with the Sigma Scan Pro (Jandell Scientific, San Jose, CA) program as a proportion of the signal of a housekeeping protein band (α-tubulin).
Determination of NKCC1 Oxidation and Nitration—NKCC1 oxidative adducts (an index of protein oxidation) were determined in NKCC1-immunoprecipitated samples by using the OxyBlot™ protein oxidation detection kit (S7150; Chemicon International). Lysates were immunoprecipitated with NKCC1 (T4) antibody (developed by Christian Lytle, University of California, Riverside; obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD, National Institutes of Health, and maintained by the University of Iowa Department of Biological Sciences (Iowa City, IA)) and subjected to gel electrophoresis and immunoblotting as described above. Anti-nitrotyrosine (mouse monoclonal antibody (1:1000; catalog number 487923; Calbiochem) was used to detect nitrated NKCC1 (T4).
Each experimental group consisted of 4–5 culture dishes/experiment for each time point studied in the cell swelling experiments. At least 2–4 plates were used for Western blot analysis. All experiments were performed from 4–7 separate seedings. Data were subjected to ANOVA, followed by Tukey's post hoc comparisons. At each time point, the experimental cultures were compared with their respective controls.
RESULTS
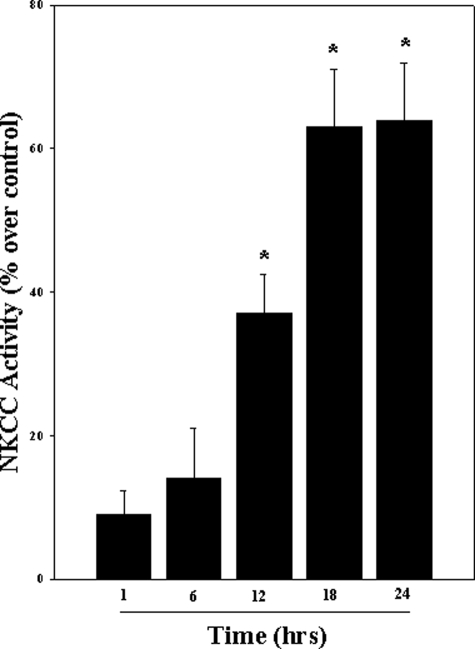
Activation of NKCC by Ammonia in Cultured Astrocytes—Ammonia significantly increased NKCC activity, as measured by the bumetanide-sensitive K+ influx, using 86Rb as a tracer for K+. The initial increase was observed at 12 h (37.5%; p < 0.05 versus control), which persisted up to 24 h, with the peak increase occurring at 18 and 24 h (61.4 and 63%, respectively; p < 0.05 versus control) (Fig. 1).
FIGURE 1.
Cultured astrocytes were exposed to 5 mm NH4Cl for different time periods (1–24 h), and the bumetanide-sensitive NKCC activity was measured. Ammonia significantly increased NKCC activity at 12, 18, and 24 h. Data were subjected to ANOVA (n = 5; *, p < 0.05 versus control). Error bars, mean ± S.E.
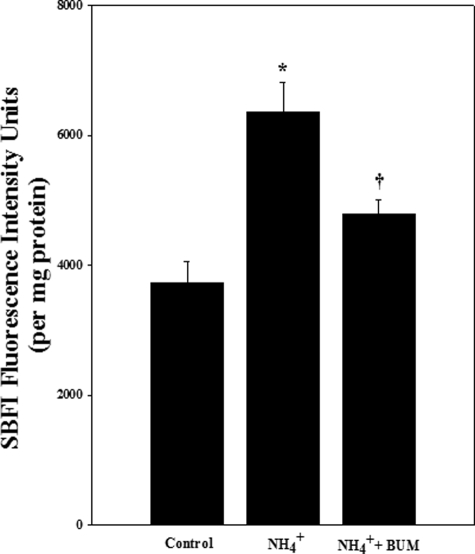
Ammonia Increased [Na+]i—As another measure of NKCC1 activity, we examined [Na+]i after exposure to ammonia for 24 h. [Na+]i was measured in the presence or absence of the NKCC inhibitor bumetanide. Cultures exposed to ammonia significantly increased [Na+]i (63.2%; p < 0.05 versus control) (Fig. 2). Such an increase was blocked by bumetanide (50 μm) (Fig. 2).
FIGURE 2.
Cultures were exposed to 5 mm NH4Cl for 24 h and [Na+]i was measured by using the Na+ fluorescent probe sodium-binding benzofuran isophthalate. Ammonia significantly increased [Na+]i. Treatment with bumetanide (BUM; 50 μm), an inhibitor of NKCC, significantly diminished this effect. Data were subjected to ANOVA (n = 3; *, p < 0.05 versus control; †, p < 0.05 versus NH4Cl). Error bars, mean ± S.E.
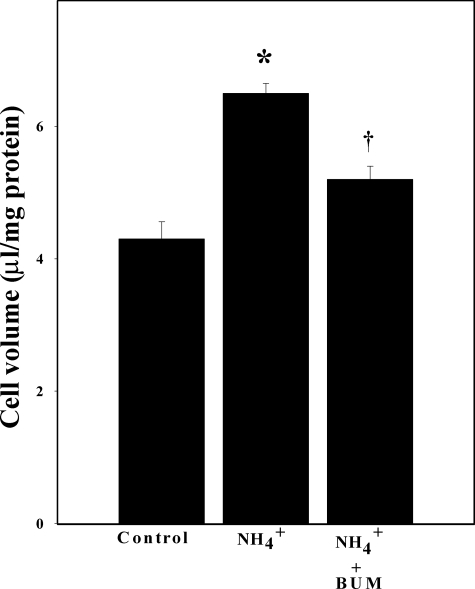
Inhibition of NKCC Activity Attenuated Ammonia-induced Astrocyte Swelling—Astrocyte cultures were exposed to ammonia for 24 h with or without bumetanide (50 μm), and cell volume was determined. The intracellular water space of control primary astrocytes culture was 4.2 ± 0.1 μl/mg of protein. Cultures exposed to ammonia showed a significant increase in cell volume (41% as compared with control; p < 0.05). Cell swelling was significantly attenuated by treatment with 50 μm bumetanide (64%; p < 0.05 versus control) (Fig. 3).
FIGURE 3.
Cultured astrocytes exposed to 5 mm NH4Cl significantly increased cell swelling (54%) at 24 h. Treatment with the NKCC inhibitor bumetanide (BUM; 50 μm) significantly diminished such swelling (65%). Data were subjected to ANOVA (n = 6; *, p < 0.05 versus control; †, p < 0.05 versus NH4Cl). Error bars, mean ± S.E.
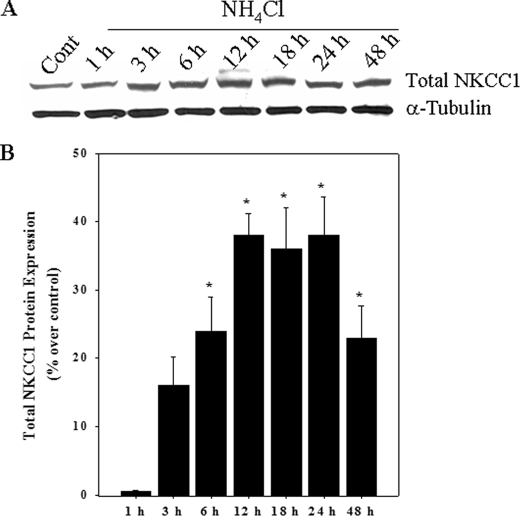
Ammonia Increased NKCC1 Protein Expression—Cultures exposed to ammonia significantly increased the total NKCC1 protein level as measured by Western blots. The initial increase was observed at 6 h and persisted for up to 24 h (Fig. 4), which was similar to the time course of NKCC activation (Fig. 1).
FIGURE 4.
A, Western blots show a significant increase in total NKCC1 protein level when cultures were exposed to 5 mm NH4Cl. B, quantification of NH4Cl-induced changes in NKCC1 protein expression. NKCC1 levels were normalized against α-tubulin. Data were subjected to ANOVA (n = 5; *, p < 0.05 versus control). Error bars, mean ± S.E.
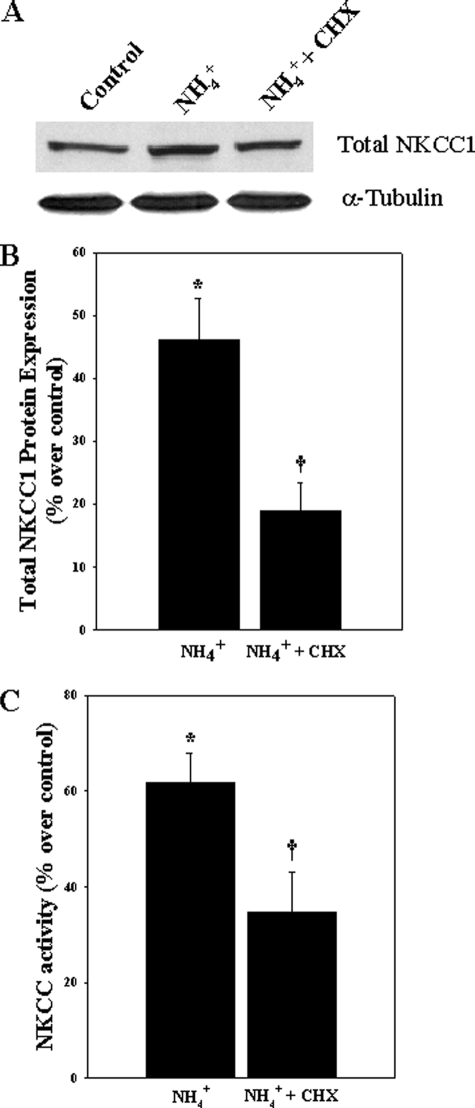
Inhibition of Protein Synthesis Diminished Ammonia-induced NKCC1 Protein Expression and Activity—To test whether the increase in NKCC activity was associated with an increase in NKCC1 protein synthesis, astrocyte cultures were exposed to 5 mm NH4Cl in the presence or absence of different concentrations of cyclohexamide (CHX) (100 nm, 500 nm, 1 μm, 2 μm, and 3 μm). NH4Cl significantly increased total NKCC1 protein level at 24 h (46.2% as compared with control; p < 0.05). This enhancement in protein abundance was significantly reduced by treatment with 1 μm CHX (61%; p < 0.05) (Fig. 5). Doses other than 1 μm CHX had no significant effect on ammonia-induced increase in total NKCC1 protein expression (data not shown). We then examined whether the increase in protein abundance contributed to changes in NKCC activity. Cultures exposed to NH4Cl significantly increased NKCC activity at 24 h (61.7%), whereas treatment with 1 μm CHX significantly diminished this activity (40.1%; p < 0.05) (Fig. 5), suggesting that an ammonia-induced increase in NKCC1 protein abundance contributes to its increased activity.
FIGURE 5.
A, Western blots show the effect of CHX on ammonia-induced total NKCC1 protein level. B, quantification of NKCC1 protein expression. NKCC1 levels were normalized against α-tubulin. C, effect of cyclohexamide on ammonia-induced NKCC activity. Data were subjected to ANOVA (n = 5; *, p < 0.05 versus control; †, p < 0.05 versus NH4Cl). Error bars, mean ± S.E.
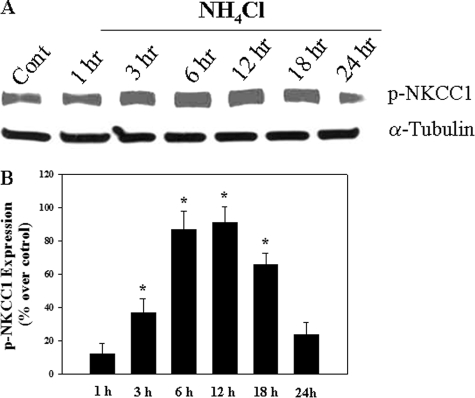
Ammonia Increased Phosphorylated (Activated) NKCC1 (p-NKCC1)—Cultures exposed to ammonia significantly increased p-NKCC1 protein expression. The initial increase was observed at 3 h and persisted for up to 18 h (Fig. 6). At 24 h, a slight increase (23.1%) in p-NKCC1 was observed, but it was not significant from control.
FIGURE 6.
A, Western blots show a significant increase in p-NKCC1 level when cultures were exposed to 5 mm NH4Cl. B, quantification of NH4Cl-induced phospho-NKCC1. Phospho-NKCC1 levels were normalized against α-tubulin. Data were subjected to ANOVA (n = 4; *, p < 0.05 versus control). Error bars, mean ± S.E.
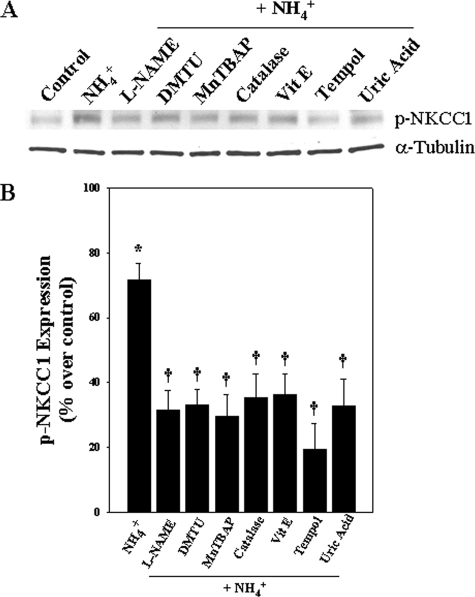
Inhibition of the Ammonia-induced Increase in p-NKCC1 by Antioxidants, N-Nitro-l-arginine Methyl Ester (l-NAME), and Uric Acid—Pretreatment (15 min) of cultures with antioxidants (dimethylthiourea (100 μm), Mn(III) tetrakis (4-benzoic acid) porphyrin (10 μm), catalase (250 units/ml), α-tocopherol (100 μm), and tempol (10 μm)) significantly decreased ammonia-induced increase in p-NKCC1 levels (53.7, 58.6, 50.5, 49.1, and 72.7%, respectively; p < 0.05) (Fig. 7). Cultures treated with the nitric-oxide synthase (NOS) inhibitor l-NAME (250 μm) and the peroxynitrite scavenger uric acid (500 μm) also significantly diminished the ammonia-induced increase in p-NKCC1 levels (56.1 and 53.8%, respectively; p < 0.05) (Fig. 7). Altogether, these findings strongly suggest that oxidative/nitrosative stress contributes to the ammonia-induced NKCC1 phosphorylation.
FIGURE 7.
Effect of antioxidants, l-NAME, and uric acid on p-NKCC1 expression. Astrocytes were pretreated (15 min) with dimethylthiourea (DMTU; 100 μm), Mn(III) tetrakis (4-benzoic acid) porphyrin (MnTBAP; 10 μm), catalase (250 units/ml), α-tocopherol (100 μm), tempol (10 μm), l-NAME (250 μm), and uric acid (500 μm) and exposed to ammonia for 24 h, and p-NKCC1 levels were measured. Antioxidants, l-NAME, and uric acid significantly inhibited p-NKCC1 expression. Data were subjected to ANOVA (n = 4; *, p < 0.05 versus control; †, p < 0.05 versus NH4Cl). Error bars, mean ± S.E.
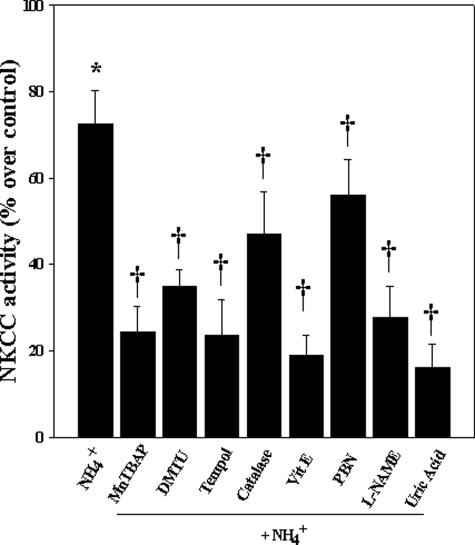
Inhibition of Ammonia-induced NKCC Activity by Antioxidants, l-NAME, and Uric Acid—Cultures exposed to NH4Cl significantly increased NKCC activity at 24 h (74% as compared with control). Pretreatment (15 min) of cultures with antioxidants Mn(III) tetrakis (4-benzoic acid) porphyrin (10 μm), dimethylthiourea (100 μm), tempol (10 μm), catalase (250 units/ml), and α-tocopherol (100 μm) significantly decreased ammonia-induced NKCC activity (68.7, 52, 69.3, 48.5, and 71.6%, respectively; p < 0.05) (Fig. 8). Although N-tert-butyl-α-phenylnitrone diminished NKCC1 activity (by 23.6%; p < 0.05 versus NH4Cl), this decrease was not significant (Fig. 8). Cultures treated with l-NAME (250 μm) and uric acid (500 μm) also significantly diminished ammonia-induced NKCC activity (63 and 76.5%, respectively; p < 0.05) (Fig. 8).
FIGURE 8.
Effect of antioxidants, l-NAME, and uric acid on NKCC1 activity. Astrocytes were treated with Mn(III) tetrakis (4-benzoic acid) porphyrin (MnT-BAP; 10 μm), dimethylthiourea (DMTU; 100 μm), tempol (10 μm), catalase (250 units/ml), α-tocopherol (100 μm), l-NAME (250 μm), and uric acid (500 μm) for 24 h, and NKCC1 activity was measured. Antioxidants, l-NAME, and uric acid significantly inhibited NKCC1 activity. Data were subjected to ANOVA (n = 5; *, p < 0.05 versus control; †, p < 0.05 versus NH4Cl). Error bars, mean ± S.E.
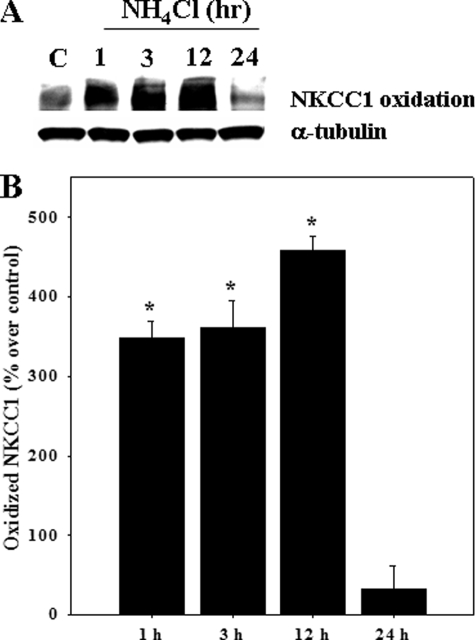
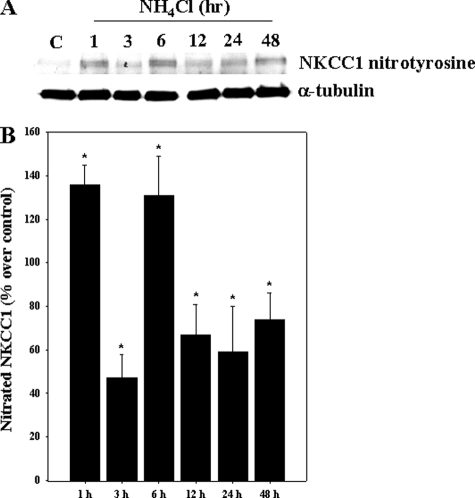
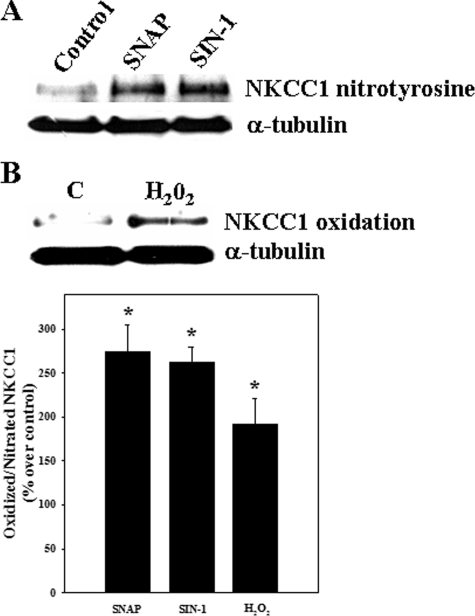
Oxidation and Nitration of NKCC1 by Ammonia—Astrocyte cultures exposed to ammonia displayed an increase in both oxidized (carbonylated) and nitrated (nitrotyrosinated) NKCC1. Increased oxidation of NKCC1 was detected at 1 h (348% as compared with control; p < 0.05), which persisted for up to 12 h (Fig. 9). Ammonia also increased the nitration of NKCC1 (1–48 h). The initial increase in nitrated NKCC1 was observed at 1 h (136.4% versus control), after which there was a decline at 3 h, followed by an increase at 6–48 h (Fig. 10). As positive controls, cells were exposed to hydrogen peroxide (H2O2; 250 μm) and the nitric oxide (NO) donors S-nitroso-N-acetyl penicillamine (SNAP; 200 μm) and 3-morpholinosydnoimine HCl (SIN-1; 500 μm). Such treatment exhibited a significant increase in oxidized and nitrated NKCC1 levels at 3 h (Fig. 11).
FIGURE 9.
Cultured astrocytes were treated with 5 mm NH4Cl for different time periods (1–24 h), and oxidized NKCC1 protein was determined by using “OxyBlot.” A, Western blots show a significant increase in oxidized NKCC1 at 1, 3, and 12 h after ammonia treatment. Lane C, control. B, quantification of ammonia-induced NKCC1 oxidative adduct formation. Oxidized NKCC1 levels are normalized against α-tubulin. Data were subjected to ANOVA (n = 4; *, p < 0.05 versus control). Error bars, mean ± S.E.
FIGURE 10.
Cultured astrocytes were treated with 5 mm NH4Cl for different time periods (1–48 h), and nitrated NKCC1 was measured. A, Western blots show a significant increase in nitrated NKCC1 from 1–48 h after ammonia treatment. Lane C, control. B, quantification of ammonia-induced NKCC1 nitration. Nitrated NKCC1 levels are normalized against α-tubulin. Data were subjected to ANOVA (n = 5; *, p < 0.05 versus control). Error bars, mean ± S.E.
FIGURE 11.
Astrocyte cultures were treated with hydrogen peroxide (H2O2; 250 μm) and nitric oxide donors (SNAP (200 μm) and SIN-1 (500 μm)), and nitrated NKCC1 was measured at 3 h. A, both SNAP and SIN-1 increased protein tyrosine nitration. B, H2O2 increased oxidized NKCC1. C, quantification of ammonia-induced NKCC1 oxidation/nitration. Nitrated NKCC1 levels are normalized against α-tubulin. Data were subjected to ANOVA (n = 5; *, p < 0.05 versus control). Error bars, mean ± S.E.
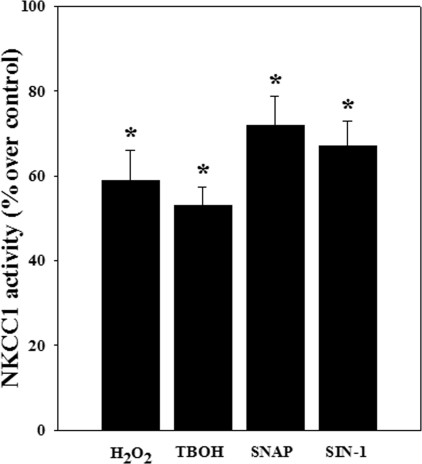
Activation of NKCC by Oxidants and NO Donors—Astrocytes were treated with hydrogen peroxide (H2O2; 25 μm), tert-butylhydroperoxide (25 μm), SNAP (50 μm), and SIN-1 (100 μm) for 24 h, and NKCC activity was determined. Oxidants and NO donors significantly increased NKCC activity as compared with the control group (H2O2, 58%; tert-butylhydroperoxide, 53%; SNAP, 71%; and SIN-1, 64%; p < 0.05) (Fig. 12).
FIGURE 12.
NKCC1 activity after exposure of astrocyte cultures to oxidants and NO donors. Astrocytes were treated with H2O2 (25 μm), tert-butylhydroperoxide (TBOH) (25 μm), SNAP (50 μm), and SIN-1 (100 μm) for 24 h, and NKCC1 activity was measured. Both oxidants (H2O2 (58%) and tert-butylhydroperoxide (53%)) and NO donors (SNAP (71%) and SIN-1 (64%)) significantly increased NKCC1 activity. Data were subjected to ANOVA (n = 4; *, p < 0.05 versus control). Error bars, mean ± S.E.
DISCUSSION
This study demonstrates for the first time that the ion transporter protein NKCC1 is involved in the mechanism of ammonia-induced astrocyte swelling. Cultured astrocytes exposed to ammonia increased the bumetanide-sensitive NKCC activity, which was associated with an increase in total and phosphorylated (activated) NKCC1 protein. Inhibition of protein synthesis with cyclohexamide significantly diminished NKCC1 protein expression and NKCC activity. The time course of NKCC activation paralleled the previously established time course of ammonia-induced astrocyte swelling (40). Blocking NKCC activation with bumetanide diminished ammonia-induced cell swelling. Additionally, cultures exposed to ammonia increased the state of oxidation, nitration, and phosphorylation of NKCC1, and such protein modifications led to the increase in NKCC activity. This increase in activity was diminished by antioxidants, an NOS inhibitor (l-NAME), and a peroxynitrite scavenger (uric acid). Taken together, these findings suggest that ammonia-induced activation of NKCC is an important factor in the mediation of cell swelling in cultured astrocytes and that such activation appears to be mediated by increased NKCC1 protein abundance as well as by oxidation, nitration, and phosphorylation of NKCC1.
The use of ammonia-treated cultured astrocytes as a model for hepatic encephalopathy is highly appropriate. First, substantial evidence invokes the role of ammonia in the pathogenesis of hepatic encephalopathy (2, 3), and astrocytes are the principal cell affected in this condition (6, 41–43). Moreover, many of the findings occurring in hepatic encephalopathy are also observed in these cultures, including characteristic morphologic changes (44, 45), cell swelling (46), defects in glutamate transport (47–49), up-regulation of the peripheral benzodiazepine receptor (50) (recently renamed the 18-kDa translocator protein (51)), reduction in levels of glial fibrillary acidic protein and myo-inositol (52, 53), disturbance in energy metabolism (54), and evidence of oxidative/nitrosative stress (55, 56). However, although astrocyte cultures have been a useful model for the study of astrocyte properties and function (57–59), a recent report noted differences between cultured astrocytes and astrocytes isolated from adult mice brains (ex vivo) with regard to their gene expression profile (60). To what extent these ex vivo astrocytes reflect their in vivo counterparts, however, remains to be determined.
The reason for the predisposition of astrocytes to be principally affected by ammonia is unclear. It is probably a consequence of glutamine, resulting from the action of glutamine synthetase, an enzyme that in the central nervous system is predominantly, if not exclusively, located in astrocytes (61). Emerging evidence suggests that glutamine is the agent responsible for the neurotoxicity caused by ammonia (62).
NKCC is involved in the regulation of several cellular functions, including ion transport across secretory and absorptive epithelia (for a review, see Ref. 63), NH+4 transport (64), and the maintenance and regulation of cell volume and ion gradients (for a review, see Ref. 65). The electroneutral NKCC protein transports Na+, K+, and Cl– into cells under both physiological and pathophysiological conditions with a stoichiometry of 1 Na+:1 K+:2 Cl– (for a review, see Ref. 14). NKCC can be inhibited by either bumetanide or furosemide (66, 67).
The cotransporter activity in astrocytes is significantly stimulated in response to high K+ in a Ca2+-dependent manner (21). Stimulation of NKCC1 by high extracellular concentration of K+ results in cell swelling through a net increase of intracellular Na+, K+, Cl–, and accompanying water. Su et al. (26) showed that inhibition of NKCC by bumetanide abolishes high extracellular K+-induced swelling and intracellular Cl– accumulation in rat cortical astrocytes. Additionally, it has been shown that astrocytes from NKCC1 cotransporter-null mice exhibit an absence of swelling when exposed to high extracellular K+ (26). An increase in [Na+]i concentration due to increased NKCC1 activity was observed in rat cortical astrocytes after chemical hypoxia, in rat spinal cord astrocytes after glucose deprivation (68), and in mouse cortical astrocytes after ischemia (69). Such NKCC1-mediated intracellular accumulation of Na+, K+, and Cl– was shown to cause cellular swelling (70), and bumetanide, an inhibitor of NKCC, diminished both cell swelling and the intracellular Na+, K+, and Cl– accumulation in cultured astrocytes after oxygen and glucose deprivation (71). Our findings are consistent with these reports, in that we found increased NKCC activity when astrocyte cultures exposed to ammonia and that blocking such activity significantly reduced ammonia-induced astrocyte swelling. These findings are also concordant with in vivo studies showing the involvement of NKCC in the brain edema associated with ischemia and trauma (22, 28–30).
In addition to increased NKCC activity, we also found a significant increase in total NKCC1 protein level after ammonia treatment. Inhibition of such protein abundance significantly blocked ammonia-induced NKCC1 protein expression and NKCC activity, suggesting that the ammonia-induced increase in NKCC1 protein abundance contributes to its increased activity. Consistent with this view, Su et al. (26) reported that NKCC1 protein expression and activity are elevated in dibutyryl cAMP-treated astrocyte cultures and that such increases were blocked by the protein synthesis inhibitor CHX. Conversely, studies have also shown that reduced NKCC1 protein expression diminished NKCC activity in bovine brain endothelial cells after exposure to hypoxia/aglycemia (72).
Phosphorylation of proteins generally results in conformational changes that may affect their activity. Phosphorylation of NKCC1 has been shown to activate its activity (67, 73), and such phosphorylation correlated well with NKCC activation in epithelial cells (74) and in cultured cortical neurons (75). Our study demonstrates that cultured astrocytes exposed to ammonia results in the phosphorylation (activation) of NKCC1, thereby implicating its involvement in the ammonia-induced increase in NKCC activity.
Modification of proteins by free radicals can result in a gain or loss of function. Thus, the superoxide anion has been shown to enhance Na+ entry into the thick ascending limb of the rat kidney by increasing NKCC activity (76). Similarly, the exposure of cultured rabbit ciliary epithelial cells to H2O2 results in increased NKCC activity along with increased intracellular Na+ levels (77). On the other hand, Elliott and Schilling (78) found inhibition of NKCC activity when endothelial cells were exposed to the oxidant tert-butylhydroperoxide. Although oxidation may increase or decrease NKCC activity, the present study found that astrocytes exposed to ammonia caused the oxidation of NKCC1 protein and that antioxidants diminished the ammonia-induced NKCC1 activation. Additionally, astrocyte cultures exposed to H2O2 resulted in the oxidation of NKCC1, and H2O2 as well as tert-butylhydroperoxide significantly increased NKCC activity, suggesting that oxidation of NKCC1 after ammonia contributes to the stimulation of NKCC activity.
The involvement of oxidative stress in the activation NKCC corresponds well with our previous findings showing that ammonia stimulates the production of free radicals (79) and induces oxidative stress (55, 56), which contributes to the cell swelling in cultured astrocytes (80). Since oxidants can increase astrocyte swelling and antioxidants and NOS inhibition reduces ammonia-induced astrocyte swelling (39, 81), it is likely that structural and functional changes in NKCC1, resulting from its oxidation, represent mechanisms contributing to cell swelling. Although these findings strongly suggest that oxidative stress affects NKCC activity, it is possible that some of the activation could be an indirect effect, since oxidation may also affect ancillary proteins (e.g. Na+/Ca2+ exchanger) (82) that are known to regulate NKCC activity.
Like oxidative stress, protein tyrosine nitration may affect protein structure and function. Nitration of protein tyrosine residues was shown to facilitate protein phosphorylation, and such phosphorylation resulted in increased activation of that protein (e.g. Lyn, a tyrosine kinase of the Src family) (83, 84). In the present study, we found nitration of NKCC1 after ammonia treatment and that the NOS inhibitor l-NAME and peroxynitrite scavenger uric acid significantly diminished ammonia-induced NKCC1 activity. Additionally, cultures exposed to the NO donors SIN-1 and SNAP showed an increase in nitrated NKCC1, a process that was associated with an increase in its activity. Since ammonia was shown to increase protein tyrosine nitration and NOS inhibitors reduced ammonia-induced astrocyte swelling (39, 81), it is likely that nitration of NKCC1 plays an important role in the increase in NKCC activity and in cell swelling.
Since ammonia-induced oxidation/nitration of NKCC1 contributes to the stimulation of NKCC activity and since phosphorylation is well known to activate NKCC, we considered the possibility that oxidation/nitration of NKCC may facilitate the ammonia-induced phosphorylation of NKCC1. Astrocytes exposed to ammonia increased p-NKCC1 and such increase was significantly diminished by treatment of cultures with antioxidants and l-NAME, suggesting that ammonia-induced oxidative/nitrosative stress may also contribute to the phosphorylation and thus to the activation of NKCC1.
In summary, this report shows that activation of NKCC is involved in the mediation of ammonia-induced astrocyte swelling and that increased NKCC1 protein abundance, phosphorylation, oxidation, and nitration of NKCC1 all contribute to such activation although each with a different time course. Targeting NKCC1 may provide a useful strategy for the treatment of the brain edema associated with fulminant hepatic failure and other hyperammonemic states.
Acknowledgments
The helpful suggestions by Dr. K. V. Rama Rao are greatly appreciated. We thank Alina Fernandez-Revuelta for the preparation of cell cultures.
This work was supported, in whole or in part, by National Institutes of Health Grant DK063311. This work was also supported by a Merit Review from the Department of Veterans Affairs. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: NKCC, Na-K-Cl cotransporter; ANOVA, analysis of variance; p-NKCC1, phosphorylated NKCC1; l-NAME, N-nitro-l-arginine methyl ester; NOS, nitric-oxide synthase; SNAP, S-nitroso-N-acetyl penicillamine; SIN-1, 3-morpholinosydnoimine HCl; CHX, cyclohexamide.
M. D. Norenberg, unpublished observation.
References
- 1.Vaquero, J., Chung, C., and Blei, A. T. (2003) Ann. Hepatol. 2 12–22 [PubMed] [Google Scholar]
- 2.Albrecht, J., and Jones, E. A. (1999) J. Neurol. Sci. 170 138–146 [DOI] [PubMed] [Google Scholar]
- 3.Hazell, A. S., and Butterworth, R. F. (1999) Proc. Soc. Exp. Biol. Med. 222 99–112 [DOI] [PubMed] [Google Scholar]
- 4.Martinez, A. J. (1968) Acta Neuropathol. (Berl.) 11 82–86 [DOI] [PubMed] [Google Scholar]
- 5.Norenberg, M. D. (1977) Lab. Invest. 36 618–627 [PubMed] [Google Scholar]
- 6.Norenberg, M. D. (2001) in Astrocytes in the Aging Brain (de Vellis J, ed) pp. 477–496, Humana Press, Totowa, NJ
- 7.Traber, P., DalCanto, M., Ganger, D., and Blei, A. T. (1989) Gastroenterology 96 885–891 [PubMed] [Google Scholar]
- 8.Hoffmann, E. K., and Dunham, P. B. (1995) Int. Rev. Cytol. 161 173–262 [DOI] [PubMed] [Google Scholar]
- 9.Mobasheri, A., Mobasheri, R., Francis, M. J., Trujillo, E., Alvarez de la Rosa, D., and Martín-Vasallo, P. (1998) Histol Histopathol. 13 893–910 [DOI] [PubMed] [Google Scholar]
- 10.O'Neill, W. C. (1999) Am. J. Physiol. 276, C995–C1011 [DOI] [PubMed] [Google Scholar]
- 11.Liang, D., Bhatta, S., Gerzanich, V., and Simard, J. M. (2007) Neurosurg. Focus 22 E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kintner, D. B., Wang, Y., and Sun, D. (2007) Front. Biosci. 12 762–770 [DOI] [PubMed] [Google Scholar]
- 13.Chen, H., and Sun, D. (2005) Neurol. Res. 27 280–286 [DOI] [PubMed] [Google Scholar]
- 14.Haas, M., and Forbush, B., III (1998) J. Bioenerg. Biomembr. 30 161–172 [DOI] [PubMed] [Google Scholar]
- 15.Xu, J. C., Lytle, C., Zhu, T. T., Payne, J. A., Benz, E., Jr., and Forbush, B., III (1994) Proc. Natl. Acad. Sci. U. S. A 91 2201–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas, M., and Forbush, B., III (2000) Annu. Rev. Physiol. 62 515–534 [DOI] [PubMed] [Google Scholar]
- 17.Tas, P. W., Massa, P. T., and Koschel, K. (1986) Neurosci. Lett. 70 369–373 [DOI] [PubMed] [Google Scholar]
- 18.Tas, P. W., Massa, P. T., Kress, H. G., and Koschel, K. (1987) Biochim. Biophys. Acta 903 411–416 [DOI] [PubMed] [Google Scholar]
- 19.Walz, W. (1992) Can. J. Physiol. Pharmacol. 70 (suppl.) 260–262 [Google Scholar]
- 20.Plotkin, M. D., Kaplan, M. R., Peterson, L. N., Gullans, S. R., Hebert, S. C., and Delpire, E. (1997) Am. J. Physiol. 272 C173–C183 [DOI] [PubMed] [Google Scholar]
- 21.Su, G., Haworth, R. A., Dempsey, R. J., and Sun, D. (2000) Am. J. Physiol. 279 C1710–C1721 [DOI] [PubMed] [Google Scholar]
- 22.Yan, Y., Dempsey, R. J., and Sun, D. (2001) Brain Res. 911 43–55 [DOI] [PubMed] [Google Scholar]
- 23.Ecelbarger, C. A., Terris, J., Hoyer, J. R., Nielsen, S., Wade, J. B., and Knepper, M. A. (1996) Am. J. Physiol. 271, F619–F628 [DOI] [PubMed] [Google Scholar]
- 24.Payne, J. A., and Forbush, B., III (1994) Proc. Natl. Acad. Sci. U. S. A 91 4544–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacVicar, B. A., Feighan, D., Brown, A., and Ransom, B. (2002) Glia 37 114–123 [DOI] [PubMed] [Google Scholar]
- 26.Su, G., Kintner, D. B., and Sun, D. (2002) Am. J. Physiol. 282 C1136–C1146 [DOI] [PubMed] [Google Scholar]
- 27.Su, G., Kintner, D. B., Flagella, M., Shull, G. E., and Sun, D. (2002) Am. J. Physiol. 282 C1147–C1160 [DOI] [PubMed] [Google Scholar]
- 28.Staub, F., Stoffel, M., Berger, S., Eriskat, J., and Baethmann, A. (1994) J. Neurotrauma 11 679–690 [DOI] [PubMed] [Google Scholar]
- 29.Lu, K. T., Wu, C. Y., Cheng, N. C., Wo, Y. Y., Yang, J. T., Yen, H. H., and Yang, Y. L. (2006) Eur. J. Pharmacol. 548 99–105 [DOI] [PubMed] [Google Scholar]
- 30.Lu, K. T., Wu, C. Y., Yen, H. H., Peng, J. H., Wang, C. L., and Yang, Y. L. (2007) Neurol. Res. 29 404–409 [DOI] [PubMed] [Google Scholar]
- 31.Ducis, I., Norenberg, L. O. B., and Norenberg, M. D. (1990) Brain Res. 531 318–321 [DOI] [PubMed] [Google Scholar]
- 32.Juurlink, B. H., and Hertz, L. (1985) Dev. Neurosci. 7 263–277 [DOI] [PubMed] [Google Scholar]
- 33.Sun, D., and Murali, S. G. (1999) J. Neurophysiol. 81 1939–1948 [DOI] [PubMed] [Google Scholar]
- 34.Swain, M., Butterworth, R. F., and Blei, A. T. (1992) Hepatology 15 449–453 [DOI] [PubMed] [Google Scholar]
- 35.Rose, C. R., and Ransom, B. R. (1996) J. Physiol. 491 291–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kletzein, R. F., Pariza, M. W., Becker, J. E., and Potter, V. R. (1975) Ann. Biochem. 68 537–544 [DOI] [PubMed] [Google Scholar]
- 37.Kimelberg, H. K. (1987) J. Physiol. (Paris) 82 294–303 [PubMed] [Google Scholar]
- 38.Bender, A. S., and Norenberg, M. D. (1998) J. Neurosci. Res. 54 673–680 [DOI] [PubMed] [Google Scholar]
- 39.Jayakumar, A. R., Panickar, K. S., Murthy, C. R. K., and Norenberg, M. D. (2006) J. Neurosci. 26 4774–4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rama Rao, K. V., Chen, M., Simard, J. M., and Norenberg, M. D. (2003) J. Neurosci. Res. 74 891–897 [DOI] [PubMed] [Google Scholar]
- 41.Norenberg, M. D. (1987) Neurochem. Pathol. 6 13–33 [DOI] [PubMed] [Google Scholar]
- 42.Norenberg, M. D. (1996) Semin. Liver Dis. 16 245–253 [DOI] [PubMed] [Google Scholar]
- 43.Norenberg, M. D. (1998) Metab. Brain Dis. 13 319–335 [DOI] [PubMed] [Google Scholar]
- 44.Gregorios, J. B., Mozes, L. W., Norenberg, L. O. B., and Norenberg, M. D. (1985) J. Neuropathol. Exp. Neurol. 44 397–403 [DOI] [PubMed] [Google Scholar]
- 45.Gregorios, J. B., Mozes, L. W., and Norenberg, M. D. (1985) J. Neuropathol. Exp. Neurol. 44 404–414 [DOI] [PubMed] [Google Scholar]
- 46.Norenberg, M. D., Baker, L., Norenberg, L. O. B., Blicharska, J., Bruce-Gregorios, J. H., and Neary, J. T. (1991) Neurochem. Res. 16 833–836 [DOI] [PubMed] [Google Scholar]
- 47.Bender, A. S., and Norenberg, M. D. (1996) Neurochem. Res. 21 567–573 [DOI] [PubMed] [Google Scholar]
- 48.Norenberg, M. D., Huo, Z., Neary, J. T., and Roig-Cantesano, A. (1997) Glia 21 124–133 [PubMed] [Google Scholar]
- 49.Zhou, B., and Norenberg, M. D. (1999) Neurosci. Lett. 276 145–148 [DOI] [PubMed] [Google Scholar]
- 50.Itzhak, Y., and Norenberg, M. D. (1994) Neurosci. Lett. 177 35–38 [DOI] [PubMed] [Google Scholar]
- 51.Papadopoulos, V., Baraldi, M., Guidotti, A., Knudsen, T. B., Lacapère, J.-J., Lindemann, P., Norenberg, M. D., Nutt, D., Weizman, A., Zhang, M.-R., and Gavish, M. (2006) Trends Pharm. Sci. 27 402–409 [DOI] [PubMed] [Google Scholar]
- 52.Norenberg, M. D., Neary, J. T., Norenberg, L. O. B., and McCarthy, M. J. (1990) J. Neuropath Exp. Neurol. 49 399–405 [DOI] [PubMed] [Google Scholar]
- 53.Isaacks, R. E., Bender, A. S., Kim, C. Y., Shi, Y. F., and Norenberg, M. D. (1999) Neurochem. Res. 24 51–59 [DOI] [PubMed] [Google Scholar]
- 54.Pichili, V. B. R., Rama Rao, K. V., Jayakumar, A. R., and Norenberg, M. D. (2007) Glia 55 801–809 [DOI] [PubMed] [Google Scholar]
- 55.Norenberg, M. D., Jayakumar, A. R., and Rama Rao, K. V. (2004) Metab. Brain Dis. 19 313–329 [DOI] [PubMed] [Google Scholar]
- 56.Norenberg, M. D. (2003) Hepatology 37 245–248 [DOI] [PubMed] [Google Scholar]
- 57.Hansson, E. (1986) Neurochem. Res. 11 759–767 [DOI] [PubMed] [Google Scholar]
- 58.Hertz, L., Juurlink, B. H. J., and Szuchet, S. (1994) in Handbook of Neurochemistry (Lajtha, A., ed) pp. 603–661, Plenum Press, New York
- 59.Kimelberg, H. (1983) Cell Mol. Neurobiol. 3 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cahoy, J. D., Emery, B., Kaushal, A., Foo, L. C., Zamanian, J. L., Christopherson, K. S., Xing, Y., Lubischer, J. L., Krieg, P. A., Krupenko, S. A., Thompson, W. J., and Barres, B. A. (2008) J. Neurosci. 28 264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Norenberg, M. D., and Martinez-Hernandez, A. (1979) Brain Res. 161 303–310 [DOI] [PubMed] [Google Scholar]
- 62.Albrecht, J., and Norenberg, M. D. (2006) Hepatology 44 788–794 [DOI] [PubMed] [Google Scholar]
- 63.Haas, M. (1994) Am. J. Physiol. 267 C869–C885 [DOI] [PubMed] [Google Scholar]
- 64.Nagaraja, T. N., and Brookes, N. (1998) Am. J. Physiol. 274 C883–C891 [DOI] [PubMed] [Google Scholar]
- 65.Kaplan, M. R., Mount, D. B., and Delpire, E. (1996) Annu. Rev. Physiol. 58 649–668 [DOI] [PubMed] [Google Scholar]
- 66.Kimelberg, H. K., and Frangakis, M. V. (1985) Brain Res. 361 125–134 [DOI] [PubMed] [Google Scholar]
- 67.Russell, J. M. (2000) Physiol. Rev. 80 211–276 [DOI] [PubMed] [Google Scholar]
- 68.Rose, C. R., Waxman, S. G., and Ransom, B. R. (1998) J. Neurosci. 18 3554–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silver, I. A., Deas, J., and Erecinska, M. (1997) Neuroscience 78 589–601 [DOI] [PubMed] [Google Scholar]
- 70.Xie, Y., Dengler, K., Zacharias, E., Wilffert, B., and Tegtmeier, F. (1994) Brain Res. 652 216–224 [DOI] [PubMed] [Google Scholar]
- 71.Lenart, B., Kintner, D. B., Shull, G. E., and Sun, D. (2004) J. Neurosci. 24 9585–9597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abbruscato, T. J., Lopez. S. P., Roder, K., and Paulson, J. R. (2004) J. Pharmacol. Exp. Ther. 310 459–468 [DOI] [PubMed] [Google Scholar]
- 73.Lytle, C., and Forbush, B. (1992) J. Biol. Chem. 267 25438–25443 [PubMed] [Google Scholar]
- 74.Darman, R. B., and Forbush, B. (2002) J. Biol. Chem. 277 37542–37550 [DOI] [PubMed] [Google Scholar]
- 75.Schomberg, S. L., Bauer, J., Kintner, D. B., Su, G., Flemmer, A., Forbush, B., and Sun, D. (2003) J. Neurophysiol. 89 159–167 [DOI] [PubMed] [Google Scholar]
- 76.Juncos, R., and Garvin, J. L. (2005) Am. J. Physiol. 288 F982–F987 [DOI] [PubMed] [Google Scholar]
- 77.Chin, S., and Delamere, N. A. (1999) Curr. Eye. Res. 18 254–260 [DOI] [PubMed] [Google Scholar]
- 78.Elliott, S. J., and Schilling, W. P. (1992) Am. J. Physiol. 263 H96–H102 [DOI] [PubMed] [Google Scholar]
- 79.Murthy, C. R., Rama Rao, K. V., Bai, G., and Norenberg, M. D. (2001) J. Neurosci. Res. 66 282–288 [DOI] [PubMed] [Google Scholar]
- 80.Norenberg, M. D., Jayakumar, A. R., Rama Rao, K. V., and Panickar, K. S. (2007) Metab. Brain Dis. 22 219–234 [DOI] [PubMed] [Google Scholar]
- 81.Sinke, A. P., Jayakumar, A. R., Panickar, K. S., Moriyama, M., Reddy, P. V. B., and Norenberg, M. D. (2006) J. Neurochem. 106 2302–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kintner, D. B., Luo, J., Gerdts, J., Ballard, A. J., Shull, G. E., and Sun, D. (2007) Am. J. Physiol. 292 C1113–C1122 [DOI] [PubMed] [Google Scholar]
- 83.MacMillan-Crow, L. A., Greendorfer, J. S., Vickers, S. M., and Thompson, J. A. (2000) Arch. Biochem. Biophys. 377 350–356 [DOI] [PubMed] [Google Scholar]
- 84.Mallozzi, C., Di Stasi, A. M., and Minetti, M. (2001) FEBS Lett. 503 189–195 [DOI] [PubMed] [Google Scholar]