Abstract
In Schwann cells (SCs), cyclic adenosine monophosphate (cAMP) enhances the action of neuregulin, the most potent known mitogen for SCs, by synergistically increasing the activation of two crucial signaling pathways: ERK and Akt. However, the underlying mechanism of cross-talk between neuregulin and cAMP signaling remains mostly undefined. Here, we report that the activation of protein kinase A (PKA), but not that of exchange protein activated by cAMP (EPAC), enhances S-phase entry of SCs by synergistically enhancing the ligand-dependent tyrosine phosphorylation/activation of the neuregulin co-receptor, ErbB2-ErbB3. The role of PKA in neuregulin-ErbB signaling was confirmed using PKA inhibitors, pathway-selective cAMP analogs, and natural ligands stimulating PKA activity in SCs, such as adenosine and epinephrine. Two basic observations defined the synergistic action of PKA as “gating” for neuregulin-ErbB signaling: 1) the activation of PKA was not sufficient to induce S-phase entry or the activation of either ErbB2 or ErbB3; and 2) the presence of neuregulin was strictly required to ignite ErbB activation and thereby ERK and Akt signaling. However, PKA directly phosphorylated ErbB2 on Thr-686, a highly conserved intracellular regulatory site that was required for the PKA-mediated synergistic enhancement of neuregulin-induced ErbB2-ErbB3 activation and proliferation in SCs. The gating action of PKA on neuregulin-induced ErbB2-ErbB3 activation has important biological significance, because it insures signal amplification into the ERK and Akt pathways without compromising either the neuregulin dependence or the high specificity of ErbB signaling pathways.
cAMP is a crucial regulator of many cellular processes, including cell proliferation and differentiation. SCs2 are unique in their capacity to respond to cAMP, because an accumulation of intracellular cAMP enhances polypeptide growth factor-dependent proliferation (1). In isolated SCs, cAMP-stimulating agents synergistically increase the potency of neuregulin, platelet-derived growth factor, and fibroblast growth factor as mitogenic signals (2–6). Our previous studies indicated that, in SCs, the synergistic effect of cAMP on S-phase entry relies on the ability of this second messenger to enhance the potency and duration of neuregulin-stimulated MEK-ERK and PI3K-Akt activation, which are both required for cell cycle progression. In the absence of neuregulin, increased intracellular cAMP failed to induce the activation of MEK-ERK or PI3K-Akt (7).
Neuregulins comprise an extensive family of growth factors (8), which are the specific ligands for ErbB/HER family of receptor tyrosine kinases (RTKs) (9, 10). A membrane-bound form of neuregulin is a major component of the axonal mitogen that regulates SC proliferation by axonal contact in peripheral nerves (11, 12). SCs express ErbB2 and ErbB3 isoforms that signal as a heterodimeric complex-activating multiple pathways, including Ras-Raf-MEK-ERK and PI3K-PDK-Akt (12, 13). ErbB2 and ErbB3 complement each other to create an effective signal transducer complex. The extracellular domain of ErbB3 is required for binding to neuregulin, and the tyrosine kinase activity of ErbB2 is required for receptor auto- and cross-phosphorylation, inasmuch ErbB2 lacks a binding domain for neuregulin and ErbB3 lacks a catalytically active intracellular kinase domain (14). Upon ligand binding, SH3-containing molecules, such as the adaptor protein c-Shc and the regulatory subunit of PI3K (p85), are recruited to specific phosphorylated tyrosine residues on each activated receptor leading to the activation of Ras-ERK and PI3K-Akt, respectively (9).
Intracellular cAMP directly activates two main effectors: protein kinase A (PKA) and the newly identified exchange protein activated by cAMP (EPAC), an exchange factor for the small GTPase Rap1 (15). Together, PKA and EPAC appear to account for most of the effects of cAMP in mammalian cells (15–17). Interestingly, cAMP can regulate the flow of signals through other pathways, a function that is referred to as “gating” by cAMP (18). In particular, cAMP has been shown to regulate the Ras-ERK pathway (19). For example, the activation of PKA by cAMP does not affect the proliferation of NIH3T3 cells, but it inhibits Ras-stimulated ERK activity and Ras-mediated transformation (20) by phosphorylating Raf1 and reducing its kinase activity (21). As mentioned above, the regulation of neuregulin-induced ERK and Akt signaling by cAMP in SCs can be also considered an example of cAMP-mediated gating; however, the underlying mechanism is unknown.
Therefore, the goal of this study was to investigate how signals from neuregulin and cAMP interact to regulate ERK and Akt activation and S-phase progression in SCs. Using a combination of pharmacological inhibitors of PKA and pathway-selective cAMP analogs, we found evidence supporting an involvement of PKA, but not EPAC, in increasing the activation of the ErbB2-ErbB3 co-receptor. PKA activity was sufficient to enhance the neuregulin-induced phosphorylation of specific activating tyrosine residues in both ErbB2 and ErbB3 and thereby enhance both MEK-ERK and PI3K-Akt signaling. PKA activity was not sufficient, however, to replace neuregulin to initiate ErbB2 auto- and trans-phosphorylating activity toward ErbB3 or the activation of downstream MEK or PI3K signaling. Yet, PKA directly phosphorylated ErbB2 on at least one highly conserved PKA phospho-acceptor site, Thr-686, a transmodulatory site with a previously suggested role in enhancing the activation of ligand-activated heterodimerizing ErbB2 subpopulations (22). In this study, we provide evidence indicating that PKA synergistically enhances neuregulin-dependent ErbB2-ErbB3 activation and DNA synthesis in SCs through a mechanism that requires the direct phosphorylation of ErbB2 on Thr-686. We propose a model of ErbB2-ErbB3 regulation by PKA-mediated gating, which guarantees effective signal amplification into key pathways required for proliferation, without compromising the high specificity of the neuregulin-ErbB interaction or downstream signaling events.
EXPERIMENTAL PROCEDURES
Reagents—Cell-permeable cAMP analogs (Biolog) were as follows: N6-benzoyladenosine-3′,5′-cyclic monophosphate (6-Bnz-cAMP), N6-phenyladenosine-3′,5′-cyclic monophosphate (6-PHE-cAMP), N6-monobutyryladenosine-3′,5′-cAMP, (6-MB-cAMP), 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (8-CPT-cAMP), 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (PME-cAMP), N6-2′-O-dibutyryladenosine-3′,5′-cyclic monophosphate (db-cAMP), 8-bromoadenosine-3′,5′-cyclic monophosphorothioate (8-Br-cAMP), and adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer. Recombinant heregulin-β1177–244 active peptide was from Genentech (South San Francisco, CA). Recombinant purified platelet-derived growth factor-BB and fibroblast growth factor-1 were from R&D Systems. Forskolin, adenosine, epinephrine, isoproterenol, and H-89 were from Sigma. Cell-permeable myristoylated protein kinase inhibitor peptide 14–22 (Myr-PKI) and l-α-phosphatidyl-inositol-3,4-bisphosphate (PI(3,4)P2) were from Biomol (Plymouth Meeting, PA). ErbB3 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Total and Phospho-Akt (Ser-473 and Thr-308), -GSK3β (Ser-9), -MEK, -ERK, -ErbB2 (Tyr-1221/22, Tyr-877, and Tyr-1248) polyclonal antibodies, phospho-ErbB3 (Tyr-1289), total phosphotyrosine and phospho-PKA substrate antibodies were from Cell Signaling Technologies (Beverly, MA). P-ErbB2 (Thr-686) was from GeneTex (San Antonio, TX). [3H]thymidine (6.7 Ci/mmol) and Solvable™ were from PerkinElmer Life Sciences (Boston, MA). The expression vectors encoding the full-length human ErbB2 and ErbB3 sequences, pcDNAIII-ErbB2 and pcDNAIII-ErbB3, respectively, were provided by Dr. Kermit Carraway III. The expression vector pcDNAIII-PKA catalytic subunit was provided by Dr. J. Silvio Gutkind.
Primary Cultures of Adult-derived Human Schwann Cells and Peripheral Nerve Fibroblasts—Human peripheral nerve tissue, consisting of nerve roots comprising the cauda equina, was provided by the Life Alliance Organ Recovery Agency at the University of Miami Miller School of Medicine. Nerve fragments were incubated 6–8 days at 37 °C in Dulbecco's Modified Eagle's Medium (DMEM, Invitrogen) containing 10% heat-inactivated fetal bovine serum (FBS, HyClone), and 2 μm forskolin and 10 nm neuregulin (D10/FN medium). For dissociation, connective tissue-free nerve fascicles were incubated in DMEM containing 10% FBS, 0.5 mg/ml collagenase type I (Worthington, Lakewood, NJ), and 2.5 mg/ml dispase II (Roche Applied Science) overnight at 37 °C. The resulting cell suspension was plated onto mouse laminin-coated tissue culture dishes in D10/FN medium and allowed to grow until confluency. SCs were resuspended and purified by immunopanning with monoclonal antibodies against primate NGFR p75 (hybridoma supernatant, ATCC). The purity of the cultures was 96–99% based on immunostaining with anti-S100 (Dako), a protein expressed specifically in SCs. Cells were used for experiments between 2 and 8 population doublings (1–3 passages).
Fibroblasts from cauda equina were established by cultivating dissociated cell preparations on non-coated plastic dishes in DMEM containing 10% FBS without mitogens. The absence of neuregulin in the culture medium and an inappropriate substrate strongly retards the proliferation and survival of SCs, whereas the absence of forskolin allows fibroblasts to proliferate rapidly and override the growth of remaining SCs. Cultures obtained were >98% fibroblasts.
[3H]Thymidine Incorporation Assays—Sub-confluent cultures of SCs growing on poly-l-Lysine (PLL)-laminin-coated 12-well plates (100,000 cells/well) or 24-well plates (50,000 cells/well) were deprived of mitogens for 2 days in DMEM-10% FBS and then for 1 day in HEPES-buffered DMEM containing 1% FBS. SCs return to quiescence following removal of the mitogenic stimulus for 3 days. The presence of a non-mitogenic concentration of FBS in the culture medium was essential to maintain cell attachment and prevent the loss of cells by apoptosis induced by serum removal. Cells were exposed to medium containing [3H]thymidine (0.25 μCi/ml) under the conditions described in the figure legends. As an ErbB/HER agonist, we used a neuregulin peptide consisting of the EGF homology domain of β1-heregulin (hereafter referred to as “neuregulin”) that is sufficient to bind and activate ErbB2-ErbB3 heterodimers (23, 24) and downstream signaling pathways in SCs (7). Unless otherwise noted, a concentration of 10 nm neuregulin and 2 μm forskolin was used for all stimulation experiments. 48 h after stimulation, cells were washed three times with phosphate-buffered saline, lysed with 300 μl of Solvable™ and incorporated tritium in the cells was determined by liquid scintillation counting. Cultures were assayed in triplicate samples in each condition. Before lysis and tritium counting, cells were co-labeled with Cell Tracker Green (5-chloromethylfluorescein diacetate, Molecular Probes) and the nuclear dye Hoechst 33342 (Sigma) and monitored live by fluorescence microscopy. No indication of reduced metabolic activity or induced apoptotic nuclei was observed for any of the treatments.
Cell Lines—HEK293T cells (ATCC) were routinely cultured in DMEM containing 10% FBS and penicillin-streptomycin-gentamycin (Invitrogen) on a PLL substrate.
Transient Transfections—Cultures of HEK293T cells were allowed to grow to sub-confluency in 24-well PLL-coated dishes and then transfected overnight with the FuGENE 6 Reagent in DMEM-HEPES without FBS (0.25 μg of total plasmid DNA/well), according to the protocol suggested by the manufacturer (Roche Applied Science). The total amount of transfected DNA was made equivalent by adding the control plasmid, pcDNAIII-β-gal, an expression vector for the enzyme β-galactosidase. At 24 h post-transfection, medium was replaced and cells were subjected to stimulation followed by cell lysis and Western blotting analysis, as described below.
Single cell suspensions (5 × 106 cells) of adult human SCs obtained from exponentially growing cultures were transfected using the Nucleofection method (4 μg of total plasmid DNA/transfection), according to the instructions suggested by the manufacturer (Oligodendrocyte Nucleofection Kit, program A-33, Amaxa Biosystems). After transfection, SCs were initially plated on PLL+laminin-coated six-well dishes in DMEM containing 10% FBS and then re-plated 4 h later to 24-well dishes (50,000 cells/well) for cell proliferation assays and Western blotting analysis. For experimentation, stimulation of transfected SCs was done 24-post transfection in HEPES-buffered DMEM containing 1% FBS. Transfection efficiency for SCs was 40–60% based on green fluorescent protein fluorescence from the expression vector pMAX-green fluorescent protein (Amaxa Biosystems), which was routinely used as a positive control to estimate transfection efficiency.
Western Blots—Protein samples from total cell lysates were prepared by resuspending the cells in lysis buffer (50 mm Tris, 150 mm NaCl, 1% SDS, 0.5 mm dithiothreitol) containing protease inhibitors (1 mm phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, and 20 μg/ml leupeptin) and phosphatase inhibitors (phosphatase cocktails I and II, Sigma). Lysates were combined with SDS sample buffer (400 mm Tris/HCl, pH 6.8, 10% SDS, 50% glycerol, 500 mm dithiothreitol, 2 μg/ml bromphenol blue), followed by 10 min boiling. Protein aliquots were resolved in denaturing polyacrylamide gels (SDS-PAGE), and fractionated proteins were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). Membranes were blocked with 5% bovine serum albumin in Tris-Buffer saline (TBS) containing 0.05% Tween-20 (TBS-T) and incubated overnight with the appropriate dilution of each primary antibody (1:1000 unless otherwise noted). The membranes were washed three times with TBS-T prior to incubation with horse-radish peroxidase-conjugated secondary antibodies, 1:10,000 (Promega, Madison, WI). Immunoreactive protein bands were detected by enhanced chemiluminescence using ECL Advanced or ECL Plus, depending on signal intensity, according to the manufacturer's instructions (Amersham Biosciences). For densitometric analysis, films were scanned at high resolution using a Bio-Rad Fluor-S MultiImager, and the relative optic density of the protein bands was estimated using Quantity One software (Bio-Rad).
Detection of PKA Activity—The kinase activity of PKA was assessed by immunodetecting the phosphorylation of cellular PKA substrates using an antibody that recognizes PKA-specific phospho-motifs ([RR]-X-[S*/T*]) in target proteins, either in fixed cells (immunostaining) or in cell lysates (Western blot). Curiously, phospho-PKA substrate antibodies detected only a limited set of substrates in cAMP-treated SCs, most likely due to the fact that only a proportion of the total cellular substrates phosphorylated by PKA, would be recognized by this specific antibody. Moreover, phosphorylated targets recognized by anti-PKA substrate antibodies were mostly cytoplasmic/membrane proteins based on immunostaining detection. For the immunostaining experiments, SCs were grown in 24-well plates coated sequentially with PLL and laminin and fixed with 4% paraformaldehyde (20 min, room temperature) followed by cold methanol (10 min, 4 °C). Cells were then washed three times with TBS and incubated with 5% normal goat serum in TBS (TBS-NGS) for 1 h at room temperature. Subsequently, the cells were incubated with anti-phospho-PKA substrate antibody (1:200 in TBS-NGS, overnight, 4 °C) and rinsed three times with TBS prior to incubation with anti-rabbit Alexa-594-conjugated secondary antibodies (1:200 in TBS-NGS, 1 h, room temperature). Cells were mounted with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA) and analyzed by conventional fluorescence microscopy. Digitalized images from immunofluorescence microscopy and from Western blot films were processed by using Adobe Photoshop version 7.0 and arranged for presentation using Adobe Illustrator CS3.
In Vitro Kinase Assays—The phosphorylation of ErbB2 by PKA was assayed in vitro by following a standard PKA kinase assay, using a purified preparation of PKA catalytic subunit (Promega) and including purified GST-HER/ErbB2 679–1255 (human, recombinant, Calbiochem) as a substrate in the reaction mixture (PKA assay kit, Promega). The reactions were initiated by the addition of 1 μl of purified PKA and allowed to proceed for 30 min at room temperature before denaturing the proteins (10 min, boiling bath) and assaying the products by Western blot using anti-phospho PKA substrate and anti-phospho-ErbB2 Thr-686 antibodies. Reactions were carried out in the absence and presence of a specific PKA inhibitor peptide (PKI-[6–22]-NH2, Upstate Biotechnology, Lake Placid, NY), which highly discriminates between PKA and protein kinase C.
ErbB/HER Sequence Analysis and Site-directed Mutagenesis— The available protein sequence of ErbB/HER2 from the different species analyzed was obtained from GenBank™ (NCBI), and the search for potential consensus PKA-specific motifs ([KR]-X-[T/S]) was done with the program PROSITE (free internet resource).
Individual point mutation of Thr-686 on pcDNAIII-ErbB2 was generated by site-directed mutagenesis (BPS Bioscience, mutagenesis service). Mutagenesis primers were designed to introduce an ACG to GCG mutation (Thr to Ala) to generate an alanine replacement of Thr-686 in the full-length ErbB2 coding sequence.
Rap1 Assays—To measure the nucleotide exchange activity of EPAC, we assayed the GTP-binding activity of its downstream effector Rap1, by using a standard method that involves the precipitation of GTP-bound Rap1 molecules by affinity binding to purified recombinant Ral-GDS-agarose beads (Rap1 activation assay, Roche Applied Science). SCs were allowed to grow up to 70–80% confluency in 10-cm plates coated with PLL-laminin (4 × 106 cells/dish), starved as described before and then stimulated with cAMP analogs for 30 min. The plates were washed with iced-cold TBS, and the cells were rapidly resuspended in lysis buffer. All subsequent steps were performed according to the manufacturer's recommendations. Purified samples containing GTP-bound Rap1 were denatured in SDS loading buffer, boiled, resolved by 15% SDS-PAGE, and analyzed by Western blotting using anti-total Rap1 antibodies (Santa Cruz Biotechnology, 1:1000). Samples from total cell lysates were analyzed in parallel as controls for total Rap1 expression.
RESULTS
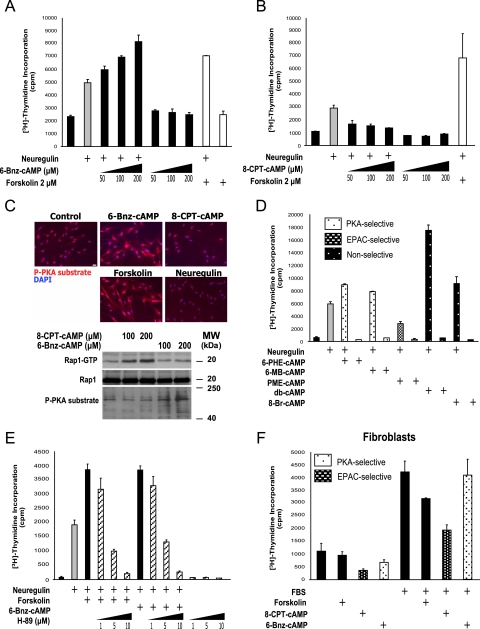
The Activation of PKA, but Not EPAC, Is Sufficient to Synergistically Enhance the Neuregulin-stimulated Proliferation of SCs—The mechanism underlying the action of cAMP in neuregulin-induced SC proliferation remains mostly undefined. As a first approach, we investigated the role of PKA or EPAC, by the use of pathway-selective cell-permeable cAMP analogs (25). We compared the effect of 6-Bnz-cAMP and 8-CPT-cAMP, two widely used PKA- and EPAC-selective cAMP analogs, respectively (26), for their ability to regulate neuregulin/HER-dependent SC proliferation. By measuring the incorporation of [3H]thymidine in adult-derived human SCs, we observed that the selective activation of PKA by 6-Bnz-cAMP was sufficient to synergistically and dose dependently enhance neuregulin-stimulated S-phase entry, resembling the effects of forskolin, a direct activator of adenylyl cyclases (Fig. 1A) and non-selective analogs of cAMP, such as db-cAMP and 8-Br-cAMP (Fig. 1D). In contrast, when SCs were stimulated with neuregulin in the presence of 8-CPT-cAMP, neuregulin-stimulated S-phase entry was significantly reduced (Fig. 1B), without diminishing the metabolic activity or the survival of the cells (not shown). In addition, the activation of EPAC by 8-CPT-cAMP was sufficient to antagonize the synergistic action of 6-Bnz-cAMP and forskolin on neuregulin-induced S-phase entry (not shown). Controls for the relative potency and specificity of the cAMP analogs used in the experiments are shown in Fig. 1C. Similarly, the PKA-selective analogs 6-PHE-cAMP and 6-MB-cAMP synergistically enhanced, whereas the EPAC-selective analog PME-cAMP reduced, neuregulin-induced S-phase entry (Fig. 1D), supporting opposite roles of PKA and EPAC in mediating the effects of cAMP in SC proliferation.
FIGURE 1.
The activation of PKA is sufficient to synergistically increase human SC proliferation in response to neuregulin. A, synergistic effect of the PKA-selective cAMP analog, 6-Bnz-cAMP, on the stimulation of DNA synthesis in response to neuregulin. B, inhibitory effect of the EPAC-selective cAMP analog, 8-CPT-cAMP, on neuregulin-stimulated S-phase entry. In the experiments shown in A and B, mitogen-deprived human SCs were stimulated with neuregulin (10 nm) alone, or in combination with increasing concentrations (50–200 μm) of 6-Bnz-cAMP (A) or 8-CPT-cAMP (B) and allowed to incorporate [3H]thymidine for 48 h (“Experimental Procedures”). Forskolin (2 μm) was used as a positive control. In these and all subsequent graphs, bar heights are means of triplicate determinations; error bars represent standard deviations. Results are from one representative experiment out of at least three independent experiments performed. C, activity and specificity of pathway-selective cAMP analogs in SCs. Detection of PKA activity in living cells was performed by stimulating mitogen-deprived SCs with 6-Bnz-cAMP or with 8-CPT-cAMP for 30 min. Cells were fixed and immunostained (upper panel) or lysed and analyzed by Western blot (lower panel) using anti-phospho PKA substrate antibodies. Forskolin (2 μm) was used as positive control for PKA activity; cAMP analogs were used at 200 μm (above). Scale bar = 15 μm. EPAC activity was assayed by measuring the GTP binding activity of Rap1 under identical experimental conditions as for PKA activity (lower panel). D, effect of other non-selective and selective cAMP analogs on S-phase entry in SCs. Experimental conditions were identical to those described in A and B, above. Analogs were used at 200 μm. E, effect of pharmacological inhibition of PKA activity by H-89 on neuregulin-induced S-phase entry in SCs. Increasing concentrations of H-89 (indicated in the figure) were added to the culture medium 30 min before mitogenic stimulation and the incorporation of [3H]thymidine was evaluated 48 h after; H-89 was present throughout. F, effect of PKA- and EPAC-selective cAMP analogs on the proliferation of human fibroblasts from peripheral nerve. Sub-confluent cultures were subjected to serum deprivation for 3 days and then stimulated with 10% FBS alone or in combination with forskolin (2 μm), 8-CPT-cAMP, or 6-Bnz-cAMP (200 μm each) in media containing [3H]thymidine, and the incorporation of tritium by the cells was measured 48 h after.
Consistent with these results and with the previously proposed role of PKA in cell cycle progression in SCs (27), pretreatment with H-89, a potent pharmacological inhibitor of PKA kinase activity (28), dose dependently reduced the incorporation of [3H]thymidine in SCs stimulated with neuregulin in the presence of either forskolin or 6-Bnz-cAMP (Fig. 1E). Similarly, preincubation with either the inactive cAMP stereoisomer, Rp-cAMP, or with a cell-permeable active peptide from the heat-stable protein kinase inhibitor (myr-PKI), effectively antagonized the action of cAMP on SC proliferation (data not shown).
To rule out a possible contribution of fibroblasts to the observed results, we determined the effect of cAMP on the proliferation of human fibroblasts from adult peripheral nerve. These fibroblasts were unresponsive to neuregulin (not shown), but they were responsive to fetal bovine serum (Fig. 1F) and to platelet-derived growth factor and fibroblast growth factor1 (not shown). As opposed to SCs, forskolin, but not 6-Bnz-cAMP, decreased the incorporation of [3H]thymidine induced by serum (Fig. 1F), platelet-derived growth factor and fibroblast growth factor1 (not shown), confirming the cell type specificity of cAMP action in SC cultures. Interestingly, stimulation with 8-CPT-cAMP was sufficient to dramatically reduce both serum- (Fig. 1F) and growth factor-induced (not shown) proliferation, further supporting a role of EPAC in mediating the anti-proliferative effects of cAMP in both SCs and fibroblasts.
Overall, these results suggest that the regulation of S-phase progression by cAMP is not only cell type-specific but also highly dependent on the particular set of effectors activated by cAMP. Whereas EPAC activation converted cAMP from a proliferative to an anti-proliferative signal in SCs, the activation of PKA was by itself not only required but also sufficient to synergistically promote S-phase entry in the presence of neuregulin.
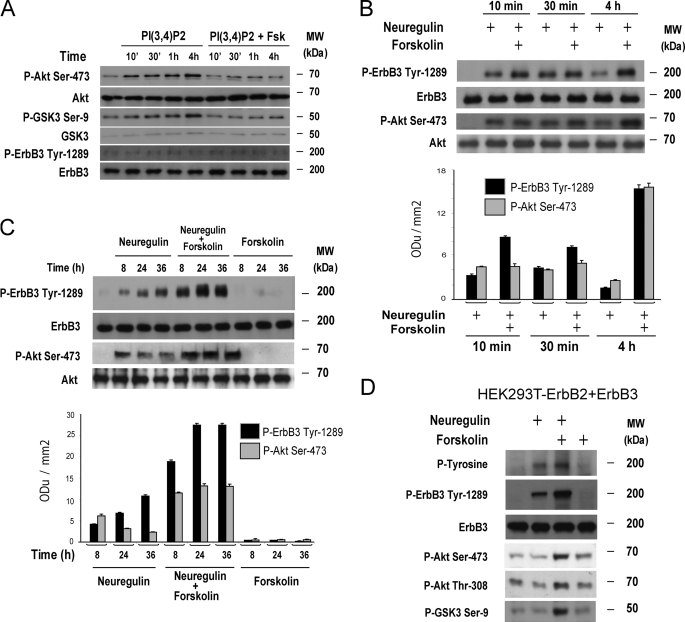
cAMP Synergistically Enhances the Neuregulin-stimulated Tyrosine Phosphorylation of ErbB3 and the Activation of PI3K/Akt Signaling— cAMP signaling potentially converges at multiple levels in the signaling axes Ras-Raf-MEK-ERK (19, 29) and PI3K-PDK-Akt (30, 31). Because our finding of a synergistic intensification of growth factor-stimulated Akt activation was unexpected, we first analyzed the effect of cAMP on Akt activation in the absence of neuregulin. We then used purified PI(3,4)P2, one of the known products of PI3K activity (32), to artificially increase Akt activity in SCs, in the absence or presence of forskolin as a cAMP-inducing agent. Time-course studies revealed that forskolin almost completely abolished the increase of Akt phosphorylation, and that of Akt's specific substrate GSK3, stimulated by PI(3,4)P2 (Fig. 2A). These observations suggest that: 1) cAMP exerts a negative regulation on the activation of intermediates downstream of PI3K activation and therefore, the presence of neuregulin is essential for the synergistic activation of Akt by cAMP, and 2) the synergistic action of cAMP on neuregulin-initiated Akt activation operates upstream or at the level of PI3K activation.
FIGURE 2.
cAMP synergistically enhances the activation of ErbB3 and PI3K-Akt in response to neuregulin. A, down-regulation of PI(3,4)P2-induced Akt and GSK3 phosphorylation by forskolin in human SCs. B and C, time course of forskolin's action on neuregulin-stimulated ErbB3 and Akt activation in SCs. In A–C, cells were subjected to mitogen- and serum-deprivation and then stimulated with 20 μm PI(3,4)P2 (A) or 10 nm neuregulin (B and C), with or without forskolin (2 μm) for the indicated time points. Total cell lysates were collected and analyzed by Western blotting using phospho- and total-ErbB3 and -Akt antibodies, as indicated in the figure. Bar graphics depict quantitative data from the Western blot profiles shown above. In A (lower panels), the unchanged phosphorylation of ErbB3 is shown as a control for the absence PI3K-ErbB3 cross-talk. D, effect of forskolin on neuregulin-stimulated ErbB3 activation, and Akt and GSK3 phosphorylation, in HEK293T cells transfected with expression vectors encoding for ErbB2 and ErbB3. Cells were transfected in starvation medium (DMEM without FBS) overnight and then stimulated for 1 h before collecting the cell lysates for Western blot analysis. No detectable levels of endogenous ErbB3 were seen in non-transfected HEK293T cells. Results shown in the figures are representative of at least three independent experiments performed.
Because stimulation with neuregulin impacts directly on ErbB tyrosine phosphorylation, and because PI3K activation is primarily linked to the activation of ErbB3, we investigated the effect of cAMP on ErbB3 phosphorylation on Tyr-1289, an activating residue that recruits p85 and thereby mediates Akt activation (33). The short term (Fig. 2B) and long term (Fig. 2C) kinetics of the effect of forskolin on Tyr-1289 phosphorylation showed that forskolin synergistically enhanced ErbB3 activation by neuregulin, even long after the initial stimulation (namely, 24–36 h post-stimulation). However, only the long term synergistic enhancement of neuregulin-induced activation of ErbB3 by cAMP (longer than 30 min post-stimulation) correlated with an enhancement of Akt phosphorylation (Fig. 2B), confirming our previous observations (7). At earlier time points (namely, 10 min following the onset of stimulation), the increased phosphorylation of ErbB3 induced by neuregulin and forskolin co-treatment did not translate into an increased Akt phosphorylation signal (Fig. 2B), possibly due to the counteracting inhibitory action that cAMP exerts concurrently on PI3K activated Akt downstream of ErbB3 activation. Importantly, in the absence of added neuregulin, increased levels of cAMP failed to promote the phosphorylation of ErbB3 on Tyr-1289 (Fig. 2C), which is also consistent with the unchanged activation of Akt by cAMP treatment (7).
A similar synergistic effect of forskolin on neuregulin-stimulated ErbB3 tyrosine phosphorylation, as assessed by using anti-phospho-ErbB3 Tyr-1289 and confirmed by using anti-phospho tyrosine antibodies (Fig. 2D, upper panels), was observed in HEK293T cells transiently transfected to co-overexpress ErbB2 and ErbB3. HEK293T cells express negligible levels of ErbB2 and no detectable ErbB3 under basal conditions. As shown in the same figure (lower panels) the enhanced tyrosine phosphorylation of ErbB3 by neuregulin and forskolin correlated with similarly enhanced levels of Akt (Ser-473 and Thr-308) and GSK3 phosphorylation. This result extends the relevance of the regulatory role of cAMP on ErbB3 activation to other cellular systems expressing ErbB2-ErbB3 hetero-dimers.
Cumulatively, these results indicate that in SCs the synergistic enhancement of neuregulin-dependent ErbB3 activation by cAMP 1) underlies the synergistic activation of Akt previously observed and 2) overrides the down-regulation of PI3K-activated Akt signaling mediated by cAMP downstream of PI3K activation.
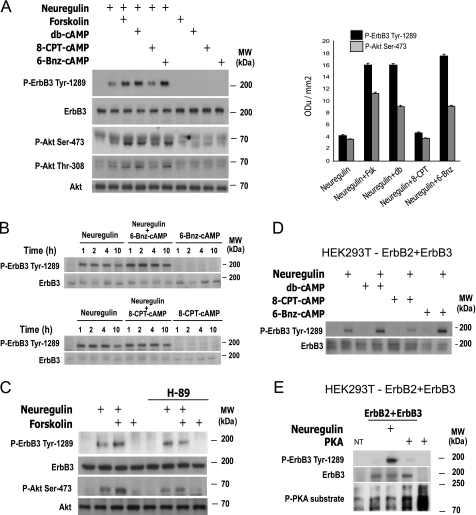
The Activation of PKA, but Not EPAC, Is Sufficient to Synergistically Increase the Neuregulin-stimulated Activation of ErbB3 and PI3K-Akt Signaling—To further define the role of PKA and EPAC in the regulation of neuregulin-dependent ErbB3 and Akt activation by cAMP, we used 6-Bnz-cAMP and 8-CPT-cAMP to discriminate between PKA- and EPAC-mediated effects. The results indicate that PKA activation by 6-Bnz-cAMP was sufficient to elicit a synergistic intensification of the neuregulin-initiated phosphorylation of ErbB3 on Tyr-1289 and of Akt on both Ser-473 and Thr-308, resembling the action of forskolin and db-cAMP (Fig. 3, A and B). In contrast, EPAC activation had no apparent effect on basal levels or on neuregulin-stimulated phosphorylation of ErbB3 or Akt (Fig. 3, A and B). Likewise, 6-Bnz-cAMP, as well as forskolin and db-cAMP, elicited a synergistic enhancement of neuregulin-stimulated tyrosine phosphorylation of ErbB3 in ErbB2-ErbB3-transfected HEK293T cells (Fig. 3D). Moreover, inhibition of PKA by preincubating SCs with H-89 abrogated the synergistic enhancement of neuregulin-initiated ErbB3 and Akt phosphorylation by forskolin, without interfering with neuregulin-induced phosphorylation of ErbB3 or Akt (Fig. 3C).
FIGURE 3.
PKA activation, but not EPAC activation, by cAMP is required and sufficient to enhance the neuregulin-stimulated phosphorylation of ErbB3 and downstream PI3K-Akt signaling. A, synergistic effect of PKA activation on neuregulin-induced ErbB3 Tyr-1289 phosphorylation and Akt activation by the use of non-selective and PKA-selective cAMP-inducing agents in human SCs. B, temporal changes of neuregulin-dependent ErbB3 phosphorylation induced by the PKA-selective analog, 6-Bnz-cAMP, and the EPAC-selective analog, 8-CPT-cAMP. C, antagonistic effect of H-89 on the synergistic increase of neuregulin-initiated ErbB3 and Akt phosphorylation in response to forskolin in SCs. D, effect of PKA- and EPAC-selective analogs on neuregulin-induced ErbB3 phosphorylation in transfected HEK293T cells overexpressing ErbB2 and ErbB3. E, effect of PKA catalytic subunit overexpression on ErbB3 phosphorylation in HEK293T cells expressing ErbB2+ErbB3. Experiments were performed essentially as described in Fig. 2 (legend). Cells were stimulated for 4 h (A–C), 1 h (D–E), or the indicated time points (B) and analyzed by Western blotting using the antibodies indicated in the figure. Analogs of cAMP were used at 200 μm each. In C, cells were preincubated with H-89 (5 μm) for 30 min before hormonal stimulation. In E, the phosphorylation of cellular PKA substrates (lower panel) is shown as a control for the effect of PKA catalytic subunit (PKA) overexpression. Note that neuregulin triggers ErbB3 phosphorylation but no detectable PKA activity in transfected HEK293T cells. NT, non-transfected cells.
A notable feature of the action of cAMP on ErbB3 activation was that stimulating PKA activity was not sufficient to directly enhance ErbB3 tyrosine phosphorylation in SCs (Fig. 3, A–C) or transfected HEK293T cells (Fig. 3D). Similarly, overexpression of the active catalytic subunit of PKA, which induces a persistent and strong phosphorylation of intracellular PKA substrates, along with ErbB2+ErbB3, did not substitute for neuregulin to trigger tyrosine phosphorylation of ErbB3 (Fig. 3E), further supporting the idea that PKA acts to specifically enhance the activation of neuregulin-bound ErbB3 hetero-complexes. In summary, these results indicate that activation of PKA is sufficient to mediate the synergistic enhancement of neuregulin-dependent ErbB3 activation by cAMP, thereby triggering downstream signaling into the PI3K-Akt pathway.
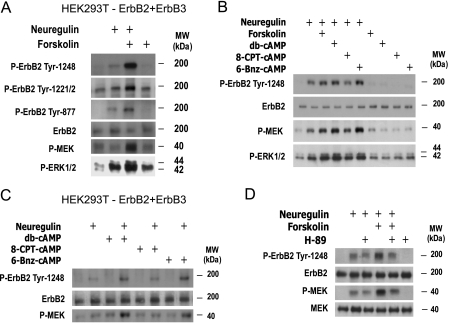
cAMP Synergistically Enhances Neuregulin-stimulated Tyrosine Phosphorylation of ErbB2 and MEK-ERK Signaling: Role of PKA but Not EPAC in cAMP Action—Our previous studies indicated that the enhancement of neuregulin-stimulated MEK-ERK and PI3K-Akt activation by cAMP followed similar activation kinetics, suggesting a common underlying regulatory mechanism (7). The enhanced neuregulin-dependent tyrosine phosphorylation of ErbB3 by cAMP-PKA reported above could result from either a reduced de-phosphorylation or an increased cross-phosphorylation by activated ErbB2, as ErbB3 lacks intrinsic tyrosine kinase capacity (34). We therefore investigated the regulation of ErbB2 activation by cAMP and PKA, using phosphorylation state-specific antibodies of kinase active ErbB2. Using HEK293T cells ectopically expressing ErbB2 and ErbB3, we observed that increasing cAMP levels with forskolin synergistically enhanced the neuregulin-dependent phosphorylation of at least three activating tyrosine sites in the intracellular domain of ErbB2, Tyr-1248, Tyr-1221/1222, and Tyr-877 (Fig. 4A). These tyrosine residues are consensus binding sites for Shc proteins and mediate signal transduction from activated ErbB2 into the Ras-ERK pathway. We observed that enhanced tyrosine phosphorylation of neuregulin-stimulated ErbB2 by forskolin treatment coincided with enhanced phosphorylation of ErbB3 (compare Fig. 2D and Fig. 4A), and highly correlated with enhanced activation of MEK-ERK both in SCs (Fig. 4B) and in transfected HEK293T cells (Fig. 4, A–C).
FIGURE 4.
PKA activation, but not EPAC activation, by cAMP is required and sufficient to enhance the neuregulin-stimulated phosphorylation of ErbB2 and downstream MEK-ERK signaling. A, effect of forskolin on the neuregulin-stimulated tyrosine auto-phosphorylation of ErbB2 in HEK293T cells overexpressing ErbB2+ErbB3. The phosphorylation of ErbB2 on Tyr-1248, Tyr-1221/1222, and Tyr-877 (upper panels) was assessed in cells stimulated for 1 h with neuregulin alone or in combination with forskolin, as stated in the figure. The phosphorylation of MEK and ERK was determined in paralleled samples (lower panels). B and C, synergistic enhancement of neuregulin-induced ErbB2 tyrosine phosphorylation and MEK-ERK activation by non-selective and pathway-selective cAMP-stimulating agents in human SCs (B) and HEK293T cells overexpressing ErbB2 and ErbB3 (C). D, involvement of PKA in the synergistic enhancement of ErbB2 phosphorylation in human SCs. All experimental conditions were identical to those described in Fig. 3.
To investigate the role of PKA and EPAC in ErbB2 tyrosine phosphorylation we compared the effect of forskolin and db-cAMP with that of 6-Bnz-cAMP and 8-CPT-cAMP, in the absence and presence of neuregulin. The results indicated that treatment with 6-Bnz-cAMP mimicked the effect of forskolin and db-cAMP in enhancing neuregulin-stimulated phosphorylation of ErbB2, without changing the extent of ErbB2 phosphorylation when administered alone, as shown in Figs. 4B (SCs) and 4C (transfected HEK293T cells). Interestingly, stimulation of HEK293T cells, but not SCs, with cAMP-stimulating agents, including forskolin (Fig. 4A) and the PKA-selective analog 6-Bnz-cAMP (not shown) elicited a modest but significant increase in the levels of ERK phosphorylation without changing the levels of MEK activation (Fig. 4, A and C). In contrast, treatment with 8-CPT-cAMP had no apparent effect on the basal or the neuregulin-stimulated tyrosine phosphorylation of ErbB2 or MEK-ERK activation (Fig. 4, B and C). Time-course studies further revealed that 8-CPT-cAMP treatment had no effect on MEK-ERK activation at any time between 10 min and 24 h after stimulation (not shown) (26), despite its ability to effectively activate Rap1 in SCs (Fig. 1C). The synergistic enhancement of neuregulin-initiated ErbB2 activation by cAMP was strictly dependent on the kinase activity of PKA, as judged by the ability of H-89 to inhibit the enhancement of neuregulin-initiated ErbB2 and MEK phosphorylation by forskolin, without interfering with ErbB2 or MEK activation by neuregulin (Fig. 4D).
Overall, these results suggest a novel role of PKA in enhancing the ligand-dependent auto- and trans-phosphorylating activity of ErbB2. The gating effect of PKA on ErbB2 activation by neuregulin explains the dual synergistic action of PKA in increasing both MEK-ERK activation and the activation of ErbB3.
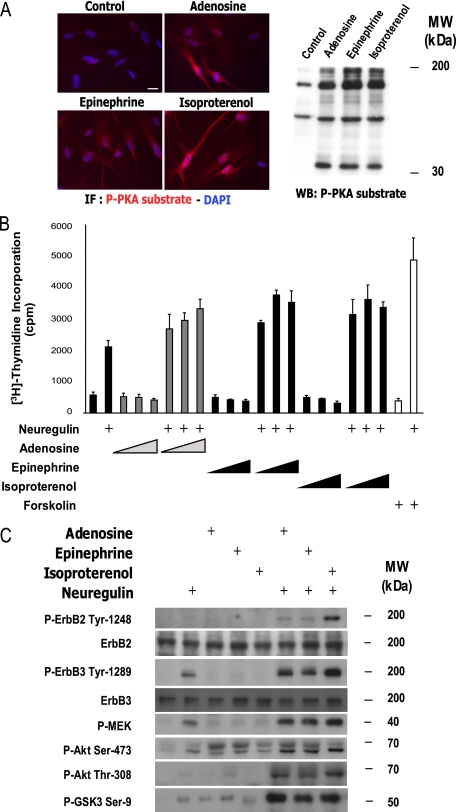
Adenosine and Epinephrine Synergistically Enhance SC Proliferation, ErbB2-ErbB3 Activation, and Signaling through MEK-ERK and PI3K-Akt— cAMP is one key modulator of SC proliferation, and it has been hypothesized that a component of the mitogenic activity of axons (37) involves the induction of cAMP in SCs (38). However, the only known axonal mitogen for SCs belongs to the neuregulin family, and our results show that neuregulin stimulation does not directly trigger PKA activity in human SCs (Fig. 1C). So far, adenosine has been the only described axon-derived molecule inducing an increase of intracellular cAMP in SCs contacting axons (39). Other known potential inducers of cAMP in SCs are calcitonin gene-related peptide (40) and epinephrine (41). We have now tested these three candidates and only adenosine and epinephrine, as well as the adrenergic analog isoproterenol, promoted PKA activity in human SCs (Fig. 5A) and synergistically enhanced neuregulin-stimulated S-phase entry in a dose-dependent (Fig. 5B) and in a PKA-dependent manner, as assessed by using the PKA inhibitor H-89 (not shown), resembling the action of agents that artificially promote cAMP accumulation. Moreover, adenosine and epinephrine promoted a synergistic increase in the extent of neuregulin-stimulated ErbB2 and ErbB3 tyrosine phosphorylation, and in the activation of both MEK- and PI3K-mediated signaling (Fig. 5C).
FIGURE 5.
Adenosine and epinephrine synergistically enhance neuregulin-stimulated proliferation, ErbB2-ErbB3 activation, and MEK-ERK/PI3K-Akt signaling in human SCs. A, stimulation of PKA activity by adenosine and epinephrine in human SCs. Cells were treated with adenosine (200 μm), epinephrine and isoproterenol (10 μm each) for 30 min, fixed and immunostained (IF, left panel) or analyzed by Western blot (WB, right panel) using phospho-PKA substrate antibodies. Scale bar = 15 μm. DAPI, 4′,6-diamidino-2-phenylindole. B, effect of adenosine and epinephrine on DNA synthesis. Cells were stimulated with increasing concentrations of adenosine (10, 100, and 200 μm), epinephrine and isoproterenol (1, 10, and 20 μm each) in the absence and presence of neuregulin and the incorporation of [3H]thymidine was measured 48 h post-stimulation. Forskolin (2 μm) was used as a positive control. C, effect of adenosine and epinephrine on signaling pathways downstream of ErbB2-ErbB3 activation. Cells were stimulated with adenosine, epinephrine, and isoproterenol (as indicated in A), in the absence or presence of neuregulin (10 nm) for 4 h. Cells were lysed and analyzed by Western blot using the antibodies indicated in the figure.
In summary, these results are consistent with the idea that, in SCs, PKA activity induced by natural ligands activating G-protein-coupled receptors linked to Gαs stimulation and the production of cAMP, enhances the mitogenic activity of neuregulin by enhancing the activation of ErbB2-ErbB3, and thereby MEK-ERK and PI3K-Akt signaling. This gives biological relevance to the convergence of Gαs-coupled-G-protein-coupled receptor and ErbB signals in maximizing the efficiency of G1-S progression in SCs.
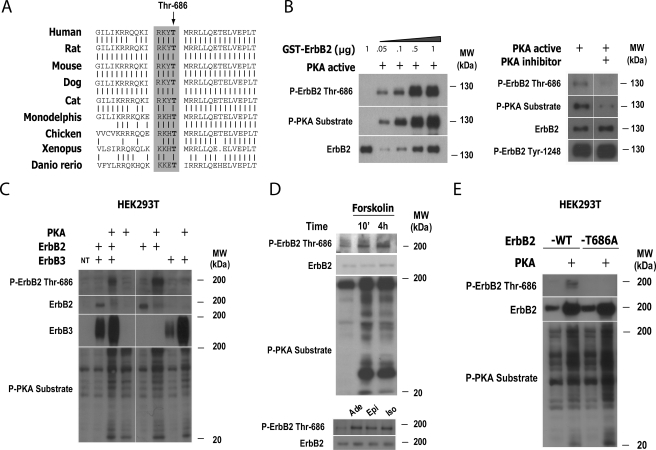
PKA Directly Phosphorylates ErbB2 on the Conserved Regulatory Site Thr-686—The mechanism by which PKA enhances the activation of ErbB2 remains elusive, basically because PKA, a Thr-/Ser kinase, does not trigger the phosphorylation of tyrosine residues. In the absence of EGF, PKA has been shown to increase EGF receptor transactivation and ERK1/2 signaling (35, 36). However, the observed effects of PKA on ErbB2 activation are strictly ligand-dependent and therefore also dependent on the capacity of ErbB2 to hetero-dimerize with neuregulin-bound ErbB3, as ErbB2 lacks the ability to directly bind to and be activated by specific ligands (14). The possibility still exists that PKA may phosphorylate ErbB2 at either single or multiple sites regulating the ligand-induced tyrosine kinase activity and/or the stability of heterodimerizing ErbB2. By performing a search of candidate PKA-specific phosphorylation motifs in the ErbB2 sequence, we identified two intracellular sites that have been highly conserved from fish to humans, i.e. the homologous residues of Thr-686 and Thr-900 of the human ErbB2 sequence (Fig. 6A, shown only for Thr-686). Indeed, the phosphorylation of ErbB2 on Thr-686, a juxtamembrane site located N-terminal to the ErbB2 tyrosine kinase domain, has been suggested to enhance the kinase activity of ligand-activated hetero-dimerizing ErbB2 in response to agonists stimulating protein kinase C (22). Noteworthy, we have observed that Thr-686 lies within a motif that clearly matches PKA, but not protein kinase C, specific consensus motif. To investigate whether PKA was able to phosphorylate ErbB2 directly, we performed in vitro kinase assays using purified GST-ErbB2 intracellular domain as a potential substrate for purified PKA catalytic subunit (Fig. 6B, left). To confirm the specificity of the kinase reaction, in vitro assays were performed in the absence and presence of a specific PKA inhibitory peptide (Fig. 6B, right). We then analyzed the reaction products by using phospho-specific antibodies recognizing either general phosphorylated PKA substrates or the specific ErbB2 residue Thr-686. The results indicated that ErbB2 can serve as an in vitro substrate for the kinase activity of PKA and that PKA specifically phosphorylates ErbB2 on at least one candidate site, Thr-686. As a control, we show that the inclusion of PKA inhibitors in the in vitro kinase reaction reduced the extent of ErbB2 phosphorylation on Thr-686 without changing the intrinsic tyrosine kinase activity of the intracellular domain of ErbB2, as assessed by detecting the specific auto-phosphorylated site Tyr-1248 (Fig. 6B, right).
FIGURE 6.
PKA phosphorylates ErbB2 on the highly conserved PKA consensus site Thr-686. A, sequence alignment of known ErbB/HER2 protein sequences from diverse species showing high conservation of the potential PKA phosphorylation site homologous to Thr-686 from the human ErbB2 sequence (arrow). Gray area: conserved motif ([RK/KK]-X-[T]) in the ErbB2 sequence, which matches a predicted consensus PKA phosphorylation site. B, phosphorylation of ErbB2 by PKA on Thr-686 in vitro using purified active PKA catalytic subunit (PKA active) and different concentrations of a bacterially expressed purified preparation of GST-ErbB2 intracellular domain as a substrate in the kinase reaction (left panel). Reactions were also performed in the absence and presence of a specific PKA inhibitory peptide (“Experimental Procedures”) to confirm the specificity of the kinase reaction (right panel). Reaction products were analyzed by Western blot using the antibodies indicated in the figure. C, phosphorylation of ErbB2 on Thr-686 by PKA in vivo. Where indicated, HEK293T cells were transfected with pcDNAIII-ErbB2 alone or in combination with expression vectors encoding ErbB3 and/or the active PKA catalytic subunit (PKA), as described under “Experimental Procedures.” Total lysates were analyzed by Western blot using anti-phospho ErbB2 Thr-686 antibody (upper panel) and total ErbB2 (center) and ErbB3 (lower panel) antibodies, as a control for ErbB2 and ErbB3 expression. D, stimulation of ErbB2 phosphorylation on Thr-686 by PKA-stimulating agents in SCs. Mitogen-starved SCs were treated with forskolin (2 μm) for 10 min and 4 h, or alternatively with adenosine (200 μm), epinephrine and isoproterenol (10 μm each) for 30 min. Cells were lysed and analyzed by Western blot using anti-phospho ErbB2 Thr-686 and total ErbB2 (control) antibodies. E, point mutation of Thr-686 abolishes PKA-mediated phosphorylation of ErbB2. HEK293T cells were transfected with pcDNAIII-ErbB2 wild type (-WT) or with a mutated construct carrying an alanine replacement of Thr-686 (-T686A). ErbB2 constructs were transfected alone or in combination with an expression vector encoding the catalytic subunit of PKA (PKA). Experiments were performed essentially as described in C. In C–E, lower panels show the expression of phosphorylated cellular PKA substrates as a control for the effect of the PKA-inducing treatments. Note that the induction of high levels of PKA activity by forskolin is sustained with time in SCs and clearly coincides with the phosphorylation of ErbB2 on Thr-686. NT, non-transfected cells.
Aligned with these observations, the phosphorylation of overexpressed full-length ErbB2 on Thr-686 was also seen in vivo when the active PKA catalytic subunit was co-expressed along with either ErbB2+ErbB3 (Fig. 6C, left) or ErbB2 alone in HEK293T cells (Fig. 6C, right). As a control for the specific detection of phosphorylated Thr-686 ErbB2, we show that anti-phospho-ErbB2 Thr-686 antibodies did not display cross-reactivity with overexpressed ErbB3 proteins, even in the presence of activated PKA (Fig. 6C, right). Phosphorylation of ErbB2 on Thr-686 was also elicited by cAMP-stimulating agents, such as forskolin in transfected HEK293T cells (not shown). Most importantly, Thr-686 phosphorylation of endogenously expressed ErbB2 was also observed in SCs treated with forskolin (Fig. 6D, above) and with the PKA-stimulating hormones, adenosine, epinephrine, and isoproterenol (Fig. 6D, below). It is worth noting that treatment with neuregulin alone did not elicit the phosphorylation of ErbB2 on Thr-686 (not shown), which parallels the inability of neuregulin to stimulate PKA activity in SCs.
To further confirm the PKA-mediated phosphorylation of ErbB2 on Thr-686, we performed site-directed mutagenesis on the pcDNAIII-ErbB2 expression vector to introduce a non-phosphorylatable alanine replacement of Thr-686 in the full-length human ErbB2 coding sequence. Results shown in Fig. 6E indicated that, when expressed at comparable levels in HEK293T cells, ErbB2 wild type was effectively phosphorylated by overexpressed PKA, whereas the Thr-686-defective mutant protein, ErbB2-T686A, was not. This result indicates a requirement of Thr-686 for the PKA-mediated phosphorylation of ErbB2 in vivo and confirms the specific action of the antibodies used for the detection of this particular phosphorylated site of ErbB2. Collectively, these results further suggest that one key event in the synergistic enhancement of ligand-activated ErbB2-ErbB3 heterodimers by cAMP is the direct phosphorylation of ErbB2 by PKA on the regulatory site Thr-686.
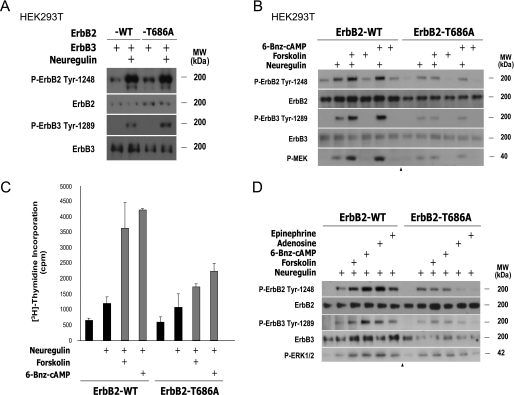
Synergism between Neuregulin and cAMP in the Control of ErbB2-ErbB3 Signaling and Cell Proliferation Requires ErbB2 Phosphorylation on Thr-686—The availability of a non-phosphorylatable mutant form of ErbB2 on Thr-686, provided us with an opportunity to examine the requirement of PKA-mediated phosphorylation of ErbB2 on the regulation of neuregulin-dependent ErbB2-ErbB3 signaling and the overall effect on SC proliferation. We first compared the ability of the mutant protein ErbB2-T686A, with that of the wild-type ErbB2, to be activated by the combined administration of neuregulin and PKA-stimulating agents in HEK293T cells co-overexpressing ErbB3. In this heterologous system, we observed that mutation of ErbB2 on Thr-686 was sufficient to abolish the synergistic action of both non-selective (forskolin) and PKA-selective (6-Bnz-cAMP) activating agents on the neuregulin-dependent auto- and trans-phosphorylating tyrosine kinase activity of ErbB2, as assessed by using phospho-specific ErbB2 (Tyr-1248) and ErbB3 (Tyr-1289) antibodies, respectively (Fig. 7B). Consistent with these observations, the synergistic action of PKA on the neuregulin-dependent activation of signaling events downstream of ErbB2-ErbB3 activation was also eliminated in cells expressing ErbB2-T686A (shown only for MEK activation), indicating a requirement of ErbB2 phosphorylation on Thr-686 for the gating action of PKA on the ligand-dependent activation of ErbB2-ErbB3 heterodimers. As a control, we confirmed that mutation of Thr-686 did not apparently change the cellular localization of ErbB2 (not shown) or the extent of ErbB2 tyrosine kinase activity under either basal conditions (control non-stimulated cells) or in response to neuregulin in HEK293T cells co-transfected with ErbB3 (Fig. 7A).
FIGURE 7.
PKA enhances neuregulin-induced ErbB2 and ErbB3 activation and DNA synthesis through the phosphorylation of ErbB2 on Thr-686. A and B, phosphorylation of ErbB2 on Thr-686 is required for the increased neuregulin-dependent ErbB2-ErbB3 activation by PKA but not for neuregulin-induced tyrosine kinase activity of ErbB2, in transfected HEK293T cells. HEK293T cells were transfected with expression vectors encoding either ErbB2-WT or ErbB2-T686A, together with ErbB3, and stimulated for 10 min with neuregulin alone (A) or for 1 h with neuregulin, in the absence and presence of forskolin or 6-Bnz-cAMP (B). Total cell lysates were analyzed by Western blot using the activation state and total antibodies indicated in the figure. C and D, phosphorylation of ErbB2 on Thr-686 is required for the increased neuregulin-dependent S-phase entry (C) and ErbB2-ErbB3 activation (D) by PKA in SCs. SCs were transfected (“Experimental Procedures”) with either ErbB2-WT or ErbB2-T686A expression vectors and stimulated for 4 h for Western blot analysis (D) or for 48 h in the presence of [3H]thymidine for proliferation assays (C). Experimental conditions were identical to those described in previous figures. An arrowhead is shown where Western blot images from the same gel were cut and consolidated into one single image.
To analyze the contribution of ErbB2 phosphorylation on Thr-686 in the regulation of neuregulin-initiated proliferation by PKA, we transiently transfected primary cultures of human SCs with either the wild type or the Thr-686 mutant form of ErbB2 and examined the incorporation of [3H]thymidine in response to neuregulin in the absence and presence of PKA-stimulating agents. The results shown in Fig. 7C indicated that SCs expressing ErbB2-T686A, in comparison to those expressing ErbB2 wild type, displayed a reduced mitogenic response to the combined action of neuregulin and forskolin or neuregulin and 6-Bnz-cAMP, without altering the response to neuregulin alone. Furthermore, we found evidence indicating that ErbB2-T686A-expressing SCs displayed an impaired ability, compared with SCs expressing wild-type ErbB2, to synergistically increase ErbB2 and ErbB3 activation, as well as the activation of downstream signaling intermediates (shown only for ERK phosphorylation), in the combined presence of neuregulin- and cAMP/PKA-stimulating agents, including not only forskolin and 6-Bnz-cAMP but also the natural ligands, adenosine and epinephrine (Fig. 7D).
Taken as a whole, these results suggest that cAMP, through the activation of PKA, mediates the synergistic enhancement of neuregulin-initiated ErbB2-ErbB3 activation and proliferation in SCs at least in part, through the direct phosphorylation of ErbB2 on a key regulatory residue, Thr-686.
DISCUSSION
Here, we have presented evidence that cAMP enhances neuregulin-mediated effects, because it serves as a “gate” to increase the flow of signaling through the ligand-activated ErbB receptors. Specifically, the activation of PKA, but not EPAC, was sufficient to synergistically enhance the neuregulin-mediated tyrosine phosphorylation/activation of both receptor partners, ErbB2 and ErbB3, which act as an effective heterodimeric signal transduction unit in the regulation of G1/S transition in SCs. Our results strongly support the concept that PKA exerts its effects, at least in part, through the direct phosphorylation of ErbB2 on a regulatory residue, Thr-686. The gating action of PKA on neuregulin-ErbB signaling was supported by at least two key observations: 1) the effect of PKA on ErbB2-ErbB3 activation was synergistic, and it was only manifested in the presence of neuregulin, and 2) the stimulation of PKA activity by itself had no effect. This mechanism of action could explain the synergistic cross-talk that cAMP exerts on neuregulin-inducedMEK-ERKandPI3K-Aktactivation(7), and on neuregulin-dependent SC proliferation. These novel findings have been summarized in the schematic presented in Fig. 8.
FIGURE 8.
Cross-talk between PKA and ErbB signaling in the regulation of SC proliferation. We propose a model that explains how cAMP synergistically enhances cell proliferation in SCs. The direct phosphorylation of the intracellular domain of ErbB2 on Thr-686 is required for the cooperative action of PKA and neuregulin in the regulation of ErbB2 tyrosine kinase activity and thereby on the phosphorylation of key tyrosine sites on ErbB2 and ErbB3 linked to Ras- and PI3K-mediated signaling, respectively. We have identified adenosine and epinephrine as two candidate physiological inducers of PKA activity in SCs exerting cross-talk with neuregulin signaling at the ErbB2-ErbB3 level.
The role of PKA on the regulation of neuregulin-induced ErbB2-ErbB3 activation was confirmed by showing that: 1) both general and pathway-selective PKA agonists, including synthetic analogs of cAMP and ligands activating Gαs-coupled G-protein-coupled receptors, such as adenosine and epinephrine, effectively reproduced the effects of cAMP, 2) pharmacological antagonists of PKA activity effectively attenuated the effects of cAMP, and 3) mutation of a candidate PKA-specific phosphorylation site on ErbB2 effectively antagonized the synergistic action PKA-stimulating agents on ErbB2-ErbB3 activation. Moreover, we validated our results by confirming that PKA activation synergistically increases neuregulin-induced ErbB2-ErbB3 signaling when both receptors are ectopically and transiently overexpressed in a reconstituted system in HEK293T cells. This observation extends the biological significance of the PKA-neuregulin cross-talk at the ErbB2-ErbB3 receptor level to other systems expressing this receptor heterodimer, such as cancer cells, where the uncontrolled expression or activity of ErbB2-ErbB3 has long been linked to tumorigenesis (42, 43).
A significant consequence of the gating action of PKA at the neuregulin receptor level is that the flow of information through the ligand-activated ErbB receptors is greatly amplified, without compromising the high specificity of ErbB-mediated pathways or the cell's dependence on neuregulin for the response in cell division. Moreover, the presence of neuregulin was strictly required to ignite ErbB activation, because PKA activation could not mimic the effect of neuregulin in triggering the phosphorylation of tyrosine residues on either ErbB2 or ErbB3. Much recent work has indicated, however, that cAMP signaling cross-talks at different levels along RTK-induced pathways exerting either a positive or a negative effect on RTK downstream signaling (19, 29, 44). Because different RTKs use many common effectors, such as Ras-Raf-MEK-ERK and PI3K-PDK-Akt, an action of cAMP on any of these possible intermediates might subvert the requirement for a specific growth factor or change important properties of the transduction mechanism. Our results indicate that, in the absence of neuregulin, for example, cAMP inhibits Akt activation through an as yet undefined mechanism, suggesting that another level of signal integration, though negative, operates between cAMP and Akt pathways in SCs. Our results suggest that, only in the presence of neuregulin, cAMP acts as an enhancer of Akt signaling in SCs. Therefore, the high specificity and amplification potential of the action of PKA at the receptor level might contribute to override or compensate for the negative regulation that cAMP exerts simultaneously on the PI3K-mediated activation of Akt. However, further experimentation is required to analyze this interesting possibility.
It is well recognized that cAMP might use the same intracellular mediators to either directly transduce signals in the cytoplasm and nucleus or to act as a gate for other pathways (18). Even though PKA has been suggested to mimic EGF action and directly trigger tyrosine phosphorylation of EGF receptor (36, 45), our results clearly indicate a particular case of PKA-mediated gating in the synergistic enhancement of neuregulin-dependent ErbB2-ErbB3 activation by PKA. To the best of our knowledge, evidence for RTK gating by cAMP has only been reported for TrkB receptors in hippocampal neurons. In that case, cAMP increases BDNF-induced TrkB tyrosine phosphorylation and dendritic spine formation, without inducing an effect when administered alone (46). However, the mechanism underlying the regulation of TrkB by cAMP was not defined. Our findings indicate a pivotal role of PKA in enhancing both the auto- and trans-phosphorylating activity of ligand-activated ErbB2. We also identified Thr-686 as a highly conserved regulatory PKA phospho-acceptor site in the intracellular domain of ErbB2, which serves as an in vitro and in vivo substrate for PKA-mediated phosphorylation. Most importantly, our results indicate that the phosphorylation of this threonine residue on ErbB2 plays an essential role in the synergistic action that PKA exerts on neuregulin-initiated ErbB2 tyrosine kinase activity, and therefore on the enhanced activation of ErbB3, MEK-ERK, and PI3K-Akt signaling. Indeed, replacement of the PKA target site on ErbB2 did not impair either the basal or the neuregulin-dependent tyrosine kinase activity of ErbB2, but dramatically diminished the cooperating activity of neuregulin and PKA-stimulating agents on ErbB2 activation, thus suggesting that phosphorylation of Thr-686 is only required for the ligand-dependent gating of ErbB2 in response to PKA activation. In a previous study, it was proposed that the phosphorylation of Thr-686 contributes to amplify ErbB2 signaling by enhancing the stability of ligand-bound ErbB2 heterodimers (22). In fact, the prolonged activation of neuregulin-dependent ErbB2-ErbB3 activation that we have observed in the presence of PKA activation supports the concept of enhanced receptor stability. However, understanding the details of the molecular mechanism of PKA-mediated gating of activated ErbB2-ErbB3 dimers requires further investigation.
cAMP has the ability to either stimulate or inhibit cell proliferation in a cell type-specific manner (19); however the differential contribution of PKA and EPAC is not completely understood. In this study we show that stimulation of SCs with PKA-selective cAMP analogs enhanced neuregulin-induced S-phase entry. In contrast, the selective activation of EPAC had an overall antiproliferative effect. In peripheral nerve fibroblasts, increases in intracellular cAMP, as well as EPAC-selective activation, reduced growth factor-induced proliferation, confirming the cell type specificity of the cellular response to cAMP. The mechanism by which EPAC exerts negative effects on cell cycle progression is unknown, because the activation of EPAC-Rap1 by cAMP appears not to be linked to the activation of either ERK, as previously reported (26), or Akt (our results), in SCs. Because EPAC activation significantly reduces neuregulin-dependent SC proliferation without reducing either the basal or the neuregulin-initiated activation of MEK, ERK, or Akt, we believe that EPAC-Rap1 reduces proliferation by targeting an event that lies downstream or is independent of ERK or Akt signaling. Indeed, further studies are required to address the question of the specific involvement of Rap1 in the inhibition of cell proliferation by 8-CPT-cAMP. A differential role of PKA and EPAC on cell proliferation was described in PC12 cells. In this neuronal cell line, the activation of PKA promoted cell proliferation, whereas the activation of EPAC not only reduced proliferation but also enhanced neurite outgrowth (45). In human SCs, however, EPAC inhibits proliferation without inducing differentiation, because we did not detect an increase in the expression of differentiation markers in SCs stimulated with the EPAC-selective analog 8-CPT-cAMP (data not shown). In addition, caution should be taken when interpreting results coming from the use of 8-CPT-cAMP as an EPAC activator. Even though 8-CPT-cAMP is an excellent pharmacological tool to discriminate between EPAC- and PKA-mediated pathways it might not exactly mimic EPAC-mediated cellular responses evoked by drugs or hormones stimulating cAMP. On one hand, this EPAC-selective analog induces a strong and a likely non-physiological activation of EPAC; on the other hand, it is able to do so without inducing PKA activation, a condition that might never reproduce in a physiological context, given the higher affinity of PKA compared with EPAC for the binding of cAMP (16).
Overall, the present evidence suggests that the biological outcome of an elevated concentration of cAMP in SCs is highly dependent on the specific set of effectors activated. In fact, it is highly likely that the counterbalancing action of the pro-mitogenic effects of PKA and the anti-proliferative action of EPAC might underlie the overall response in cell proliferation to agents increasing cAMP. This also suggests the interesting possibility that a change in the spectrum or the balance of expressed PKA versus EPAC isoforms could allow SCs to respond differently to signals using the cAMP second messenger system. SCs are above all exquisite cells in their relationship to axons, because key features of SC function such as migration, survival, proliferation, and myelination are strictly dependent on active axonal signals, including axonal neuregulins (47). However, how neuregulins control so many cellular behaviors that are so diverse in SCs is poorly understood. The present findings clearly indicate that ErbB signaling is feasible to be gated, and therefore modulated, by PKA, at the ErbB2-ErbB3 receptor level. We have also identified adenosine and epinephrine as direct PKA activators and candidate physiological regulators of neuregulin-dependent ErbB activation in the control of SC proliferation. In this sense, PKA might exert a biologically relevant level of cell signaling integration for the transition through different developmental stages in SCs subjected to a continuous stimulation by axonal neuregulins.
Supplementary Material
Acknowledgments
We thank S. Rendon, T. J. Painter, L. White, and A. Kanaya for technical assistance and Dr. F. Schwede for advice on the use of cAMP analogs. We also thank Drs. Carraway III and Gutkind for DNA constructs and Genentech, Inc. for β1-heregulin.
This work was supported, in whole or in part, by National Institutes of Health Grant NS009923 from NINDS. This work was also supported by The Miami Project to Cure Paralysis. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental S1.
Footnotes
The abbreviations used are: SC, Schwann cell; MEK, mitogen-activated protein kinase/ERK kinase; ERK, extracellular signal-regulated kinase; PI3K, phosphatidylinositol 3-kinase; RTK, receptor tyrosine kinase; PKA, protein kinase A; EPAC, exchange protein activated by cAMP; 6-Bnz-cAMP, N6-benzoyladenosine-3′,5′-cyclic monophosphate; 6-PHE-cAMP, N6-phenyladenosine-3′,5′-cyclic monophosphate; 8-CPT-cAMP, 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate; db-cAMP, N6-2′-O-dibutyryladenosine-3′,5′-cyclic monophosphate; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; EGF, epidermal growth factor; GST, glutathione S-transferase; PI(3,4)P2, l-α-phosphatidylinositol 3,4-bisphosphate; PLL, poly-l-lysine; PDK, 3′-phosphoinositde-dependent kinase; 6-MB-cAMP, N6-monobutyryladenosine-3′,5′-cAMP; PME-cAMP, 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate; 8-Br-cAMP, 8-bromoadenosine-3′,5′-cyclic monophosphorothioate.
References
- 1.Raff, M. C., Abney, E., Brockes, J. P., and Hornby-Smith, A. (1978) Cell 15 813-822 [DOI] [PubMed] [Google Scholar]
- 2.Sobue, G., Shuman, S., and Pleasure, D. (1986) Brain Res. 362 23-32 [DOI] [PubMed] [Google Scholar]
- 3.Salzer, J. L., and Bunge, R. P. (1980) J. Cell Biol. 84 739-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis, J. B., and Stroobant, P. (1990) J. Cell Biol. 110 1353-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahmatullah, M., Schroering, A., Rothblum, K., Stahl, R. C., Urban, B., and Carey, D. J. (1998) Mol. Cell. Biol. 18 6245-6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim, H. A., Ratner, N., Roberts, T. M., and Stiles, C. D. (2001) J. Neurosci. 21 1110-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monje, P. V., Bartlett Bunge, M., and Wood, P. M. (2006) Glia 53 649-659 [DOI] [PubMed] [Google Scholar]
- 8.Falls, D. L. (2003) Exp. Cell Res. 284 14-30 [DOI] [PubMed] [Google Scholar]
- 9.Alroy, I., and Yarden, Y. (1997) FEBS Lett. 410 83-86 [DOI] [PubMed] [Google Scholar]
- 10.Buonanno, A., and Fischbach, G. D. (2001) Curr. Opin. Neurobiol. 11 287-296 [DOI] [PubMed] [Google Scholar]
- 11.Morrissey, T. K., Levi, A. D., Nuijens, A., Sliwkowski, M. X., and Bunge, R. P. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 1431-1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garratt, A. N., Britsch, S., and Birchmeier, C. (2000) BioEssays 22 987-996 [DOI] [PubMed] [Google Scholar]
- 13.Vartanian, T., Goodearl, A., Viehover, A., and Fischbach, G. (1997) J. Cell Biol. 137 211-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Citri, A., Skaria, K. B., and Yarden, Y. (2003) Exp. Cell Res. 284 54-65 [DOI] [PubMed] [Google Scholar]
- 15.de Rooij, J., Zwartkruis, F. J., Verheijen, M. H., Cool, R. H., Nijman, S. M., Wittinghofer, A., and Bos, J. L. (1998) Nature 396 474-477 [DOI] [PubMed] [Google Scholar]
- 16.Bos, J. L. (2003) Nat. Rev. Mol. Cell. Biol. 4 733-738 [DOI] [PubMed] [Google Scholar]
- 17.Kopperud, R., Krakstad, C., Selheim, F., and Doskeland, S. O. (2003) FEBS Lett. 546 121-126 [DOI] [PubMed] [Google Scholar]
- 18.Iyengar, R. (1996) Science 271 461-463 [DOI] [PubMed] [Google Scholar]
- 19.Stork, P. J., and Schmitt, J. M. (2002) Trends Cell Biol. 12 258-266 [DOI] [PubMed] [Google Scholar]
- 20.Wu, J., Dent, P., Jelinek, T., Wolfman, A., Weber, M. J., and Sturgill, T. W. (1993) Science 262 1065-1069 [DOI] [PubMed] [Google Scholar]
- 21.Hafner, S., Adler, H. S., Mischak, H., Janosch, P., Heidecker, G., Wolfman, A., Pippig, S., Lohse, M., Ueffing, M., and Kolch, W. (1994) Mol. Cell. Biol. 14 6696-6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulliford, T., Ouyang, X., and Epstein, R. J. (1999) Cell Signal. 11 245-252 [DOI] [PubMed] [Google Scholar]
- 23.Barbacci, E. G., Guarino, B. C., Stroh, J. G., Singleton, D. H., Rosnack, K. J., Moyer, J. D., and Andrews, G. C. (1995) J. Biol. Chem. 270 9585-9589 [DOI] [PubMed] [Google Scholar]
- 24.Carraway, K. L., 3rd, Soltoff, S. P., Diamonti, A. J., and Cantley, L. C. (1995) J. Biol. Chem. 270 7111-7116 [DOI] [PubMed] [Google Scholar]
- 25.Rehmann, H. (2005) “Experimental Procedures” Enzymol. 407 159-173 [Google Scholar]
- 26.Enserink, J. M., Christensen, A. E., de Rooij, J., van Triest, M., Schwede, F., Genieser, H. G., Doskeland, S. O., Blank, J. L., and Bos, J. L. (2002) Nat. Cell Biol. 4 901-906 [DOI] [PubMed] [Google Scholar]
- 27.Kim, H. A., DeClue, J. E., and Ratner, N. (1997) J. Neurosci. Res. 49 236-247 [PubMed] [Google Scholar]
- 28.Lochner, A., and Moolman, J. A. (2006) Cardiovasc. Drug Rev. 24 261-274 [DOI] [PubMed] [Google Scholar]
- 29.Dumaz, N., and Marais, R. (2005) FEBS J. 272 3491-3504 [DOI] [PubMed] [Google Scholar]
- 30.Kim, S., Jee, K., Kim, D., Koh, H., and Chung, J. (2001) J. Biol. Chem. 276 12864-12870 [DOI] [PubMed] [Google Scholar]
- 31.Mei, F. C., Qiao, J., Tsygankova, O. M., Meinkoth, J. L., Quilliam, L. A., and Cheng, X. (2002) J. Biol. Chem. 277 11497-11504 [DOI] [PubMed] [Google Scholar]
- 32.Franke, T. F., Kaplan, D. R., Cantley, L. C., and Toker, A. (1997) Science 275 665-668 [DOI] [PubMed] [Google Scholar]
- 33.Hellyer, N. J., Kim, M. S., and Koland, J. G. (2001) J. Biol. Chem. 276 42153-42161 [DOI] [PubMed] [Google Scholar]
- 34.Sierke, S. L., Cheng, K., Kim, H. H., and Koland, J. G. (1997) Biochem. J. 322 757-763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piiper, A., Dikic, I., Lutz, M. P., Leser, J., Kronenberger, B., Elez, R., Cramer, H., Muller-Esterl, W., and Zeuzem, S. (2002) J. Biol. Chem. 277 43623-43630 [DOI] [PubMed] [Google Scholar]
- 36.Piiper, A., Lutz, M. P., Cramer, H., Elez, R., Kronenberger, B., Dikic, I., Muller-Esterl, W., and Zeuzem, S. (2003) Biochem. Biophys. Res. Commun. 301 848-854 [DOI] [PubMed] [Google Scholar]
- 37.Wood, P. M., and Bunge, R. P. (1975) Nature 256 662-664 [DOI] [PubMed] [Google Scholar]
- 38.Jessen, K. R., Mirsky, R., and Morgan, L. (1991) Ann. N. Y. Acad. Sci. 633 78-89 [DOI] [PubMed] [Google Scholar]
- 39.Stevens, B., Ishibashi, T., Chen, J. F., and Fields, R. D. (2004) Neuron Glia Biol. 1 23-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng, L., Khan, M., and Mudge, A. W. (1995) J. Cell Biol. 129 789-796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fink, T., Davey, D. F., and Ansselin, A. D. (1999) Can. J. Physiol. Pharmacol. 77 204-210 [PubMed] [Google Scholar]
- 42.Holbro, T., Beerli, R. R., Maurer, F., Koziczak, M., Barbas, C. F., 3rd, and Hynes, N. E. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 8933-8938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alimandi, M., Romano, A., Curia, M. C., Muraro, R., Fedi, P., Aaronson, S. A., Di Fiore, P. P., and Kraus, M. H. (1995) Oncogene 10 1813-1821 [PubMed] [Google Scholar]
- 44.Piiper, A., and Zeuzem, S. (2004) Curr. Pharm. Des. 10 3539-3545 [DOI] [PubMed] [Google Scholar]
- 45.Kiermayer, S., Biondi, R. M., Imig, J., Plotz, G., Haupenthal, J., Zeuzem, S., and Piiper, A. (2005) Mol. Biol. Cell 16 5639-5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji, Y., Pang, P. T., Feng, L., and Lu, B. (2005) Nat. Neurosci. 8 164-172 [DOI] [PubMed] [Google Scholar]
- 47.Jessen, K. R., and Mirsky, R. (2005) Nat. Rev. Neurosci. 6 671-682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.