Abstract
Iron is an essential nutrient but in excess may damage cells by generating reactive oxygen species due to Fenton reaction or by substituting for other transition metals in essential proteins. The budding yeast Saccharomyces cerevisiae detoxifies cytosolic iron by storage in the vacuole. Deletion of CCC1, which encodes the vacuolar iron importer, results in high iron sensitivity due to increased cytosolic iron. We selected mutants that permitted Δccc1 cells to grow under high iron conditions by UV mutagenesis. We identified a mutation (N44I) in the vacuolar zinc transporter ZRC1 that changed the substrate specificity of the transporter from zinc to iron. COT1, a vacuolar zinc and cobalt transporter, is a homologue of ZRC1 and both are members of the cation diffusion facilitator family. Mutation of the homologous amino acid (N45I) in COT1 results in an increased ability to transport iron and decreased ability to transport cobalt. These mutations are within the second hydrophobic domain of the transporters and show the essential nature of this domain in the specificity of metal transport.
The yeast vacuole plays an important role in transition metal storage and detoxification. In conditions of metal scarcity, metals stored in the vacuole can be mobilized by specific transporters and utilized for metabolic purposes. Conversely, export of metals from cytosol to vacuole is thought to prevent metal toxicity. Yeast mutants that are unable to store iron in the vacuole, either due to a lack of vacuolar structures (1), an inability to acidify vacuoles due to mutation in the V-ATPase (2, 3), or deletion of vacuole metal transporters (4–6) show sensitivity to high concentrations of metals.
Specific transporters mediate the transport of different transition metals from cytosol to vacuole. Among the best studied of the vacuolar transition metal transporters are the zinc transporters Zrc1 and Cot1 (4, 5, 7–9). These homologous proteins, which are involved in the transport of zinc and cobalt are members of the cation diffusion facilitator (CDF)3 family (for review see Ref. 10). Although CDF members show differences in size, cellular localization, and substrate metals transported, they share some common features. The majority of CDF family members have six putative transmembrane domains (TMD) and a highly conserved amino acid sequence extending from TMD II to III, which is a signature motif for the family. Based on the alignment of multiple sequences, highly conserved charged residues in TMD II and V are implicated in metal binding and transport. This finding is supported by structural studies on an Escherichia coli CDF family member Fief (also known as YiiP), a putative Zn2+ and Fe2+ transporter (11, 12).
Transport of iron from cytosol to vacuole is mediated in yeast by Ccc1 (6) and in plants by the CCC1 homologue VIT1 (13). These proteins define a unique family that appears to be restricted to fungi and plants. Little is known of the mechanism of transport, although it is clear that CCC1 is regulated by iron at transcriptional and post-transcriptional levels (14, 15). Deletion of CCC1 results in poor growth in high iron medium indicating that increased cytosolic iron may be toxic (6). The mechanism(s) leading to high iron toxicity is unknown. High iron toxicity occurs in cells deleted for CCC1 even in the absence of respiratory capacity (e.g. rho° cells) or anaerobically (16). These results call into question the assumption that high iron toxicity is due to the generation of iron-mediated reactive oxygen radicals. In an effort to define the mechanism of iron toxicity we initiated a genetic screen in which we identified mutant strains of Δccc1 that grew on high iron. Herein, we identify a missense mutation in the vacuolar zinc transporter Zrc1, which completely changes the substrate specificity of this transporter from Zn2+ to Fe2+. We show that a similar amino acid change in the homologous Cot1, a Co2+ and Zn2+ transporter, also results in a change in metal specificity.
EXPERIMENTAL PROCEDURES
Yeast Strains—The following yeast strains (W303 background) were used: DY150 (Mata ade2-1 his3-11 leu2-3,112 trp1-1 ura3-52 can1-100(oc)) and DY1457 (Matα ade6 his3-11 leu2-3,112 trp1-1 ura3-52 can1-100(oc)). Deletions of CCC1 and ZRC1 were generated by double fusion polymerase chain reaction using the HIS3 gene as a selectable marker (17). Primers for disruption of CCC1 were described (6). Primers for disruption of ZRC1 were Pri20–78 (5′-TCT CTT TTG ACC TTA GAC ACG-3′), Pri20–79 (5′-GTC GTG ACT GGG AAA ACC CTG GCG ATC GCT GCC ATG ATC GTG GAA-3′), Pri20–80 (TCC TGT GTG AAA TTG TTA TCC GCT GCT GAT CAG ATT CAA AGA GAG-3′), and Pri20–81 (GCA GTT TAC AGC GTC ATC TAC-3′). The COT1 gene was disrupted using URA3 as described (4). Strains with a FET3-lacZ reporter integrated at the HO locus were constructed as described (18). Wild type BY4743 (Mat a/α his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 lys2Δ0/+ met15Δ0/+ ura3Δ0/ura3Δ0), Δcot1::KanMX and Δpmr1::KanMX strains in the BY4743 background were obtained from Research Genetics.
Growth Media—Yeast strains were grown in YPD medium (1% yeast extract, 2% peptone, 2% dextrose) or in CM medium (0.67% yeast nitrogen base without amino acids, 2% dextrose, and 0.13% amino acid drop-out mixture). Low iron growth medium was made by adding 40 μm bathophenanthroline disulfonate (BPS), an iron chelator, to CM and then the addition of specified amounts of FeSO4. To make high iron plates, ferrous ammonium sulfate (250 mm stock in water) was added into media to give the indicated iron concentrations. FeSO4 (50 mm stock in 0.1 m HCl) was used in liquid culture as indicated together with a final concentration of 1 mm ascorbic acid.
Isolation of Mutants—DY150 Δccc1 or DY1457 Δccc1 cells were plated on CM agar plates at a density of 500 cells/plate and exposed to UV light to give a 50% survival. Mutagenized cells were grown for 2 days and then replicated to CM plates containing 5 mm ferrous ammonium sulfate. Cells were grown for another 3 days. Colonies able to grow on high iron were selected for further study.
Identification of ZRC1(N44I)—The mutated gene in dominant mutant R1 was identified by constructing a library from the R1 genomic DNA using standard protocols (19). Briefly, Sau3A partially digested genomic DNA was fractionated on a 10–40% sucrose gradient, and centrifuged at 25,000 × g for 22 h. The 8–10-kb genomic fraction was ligated to pRS416-zero, a centromeric plasmid, digested with BamHI and dephosphorylated with calf intestinal phosphatase (New England BioLabs). Ligation products were transformed into Electromax™ DH10B™ E. coli (Invitrogen) (20). Plasmids were extracted from transformed bacteria and pooled. The pooled library was transformed into Δccc1 cells and plasmids that conferred resistance to high iron (3 mm) were recovered. The genomic fragments in these plasmids were identified by sequencing.
Plasmids Construction and Site-directed Mutagenesis—ZRC1(N44I) was subcloned from the genomic library using KpnI and BglII and inserted into pRS416 (a yeast centromeric vector), which had been digested with BamHI and KpnI. Wild type ZRC1 with its own promoter and 3′ end was generated using PCR from genomic DNA using pri54 (5′-GAT ATG AAA GTA GTT GCA TT-3′) and pri60 (5′-TTG GTA CAG GAG GGA ACA AG-3′). The PCR fragment was digested with BglII and KpnI and inserted into pRS416 digested with BamHI and KpnI. To introduce the N44I mutation into wild type ZRC1, pri64 (5′-GGC CTT GAT TGC CGA TTC ATT TCA CAT GTT GAT TGA TAT CAT CTC TCT TTT AGT GGC-3′) and pri65 (5′-GCC ACT AAA AGA GAG ATG ATA TCA ATC AAC ATG TGA AAT GAA TCG GCA ATC AAG GCC-3′) were used. To introduce the D45A mutation into wild type ZRC1, pri102 (5′-CAC ATG TTG AAT GCT ATC ATC TCT CTT TTA GTG GCA C-3′) and pri103 (5′-GTG CCA CTA AAA GAG AGA TGA TAG CAT TCA ACA TGT G-3′) were used. COT1/YEp352 was obtained from Dr. Douglas S. Conklin (4). To introduce N45I into wild type COT1, pri66 (5′-CGC GGA CTC ATT CCA TAT GCT AAT CGA TAT AAT TTC TCT TGT GG-3′) and pri67 (5′-CCA CAA GAG AAA TTA TAT CGA TTA GCA TAT GGA ATG AGT CCG CG-3′) were used. Site-directed mutagenesis was performed with the QuikChange site-directed mutagenesis kit from Stratagene according to the manufacturer's instructions. ZRC1 and ZRC1(N44I) were digested with KpnI and XbaI and inserted into a yeast episomal vector pTF62 (LEU2 marker). To generate His6-tagged versions of CCC1, ZRC1, and ZRC1(N44I) under the control of the galactose inducible promoter (GAL1) the following primers were used: CCC1, pri78 (5′-CGC GGA TCC ATG TCC ATT GTA GCA CTA AAG-3′) and pri79 (5′-CCG GAA TTC ACC CAG TAA CTT AAC AAA GAA-3′), for ZRC1 and ZRC1(N44I), pri80 (5′-GGG CGA AGA TCT ATG ATC ACC GGT AAA GAA TTG-3′) and pri81 (5′-CCG GAA TTC CAG GCA ATT GGA AGT ATT GCA-3′). DNA was amplified by PCR to generate each open reading frame without the stop codon. The amplified products were inserted into BamHI- and EcoRI-digested pYES2/CT (Invitrogen).
Metal Analysis—For whole cell metal analysis, 20 OD cells (about 2 × 108 cells) at log phase were collected, washed three times with 50 mm Tris-Cl (pH 6.5), 10 mm EDTA and once with deionized water. Vacuoles were prepared using Ficoll gradients as described previously (6). Samples were digested in 200 μl of nitric acid at 80 °C for 1 h, then diluted to 1.0 ml with deionized water. Metals were analyzed in a Perkin-Elmer Inductively Coupled Plasma-Optical Emission Spectrometer (ICP) and calculated using a standard curve generated from mixed metal standards.
Western Blot, β-Galactosidase Activity, and Protein Concentration Assay—Cells were disrupted with glass beads in the presence of protease inhibitors (1.0 mm phenylmethylsulfonyl fluoride, 1.0 μm pepstatin A, and 1.0 μm leupeptin) (Sigma). Samples (20 μg) were run on a 12% SDS-PAGE, transferred to nitrocellulose, and probed with rabbit anti-His6 tag (1:2000 Abcam) or mouse anti-Vma1 antibody (1:4000, Molecular Probes), followed by peroxidase-conjugated goat anti-rabbit or goat anti-mouse antibody (1:10,000, Jackson ImmunoResearch). Membranes were developed using chemiluminescence reagents (PerkinElmer Life Sciences). The reporter constructs FET3-lacZ and CCC1-lacZ were described previously (15, 18). β-Galactosidase activity was performed in 96-well plates using ortho-nitrophenyl-β-galactoside as a substrate. The generation of ortho-nitrophenol was monitored at 420 nm and the data are presented as nanomole/min/mg of protein (18). Protein concentration was determined by the bicinchoninic acid method (Pierce) using bovine serum albumin as standard.
RESULTS
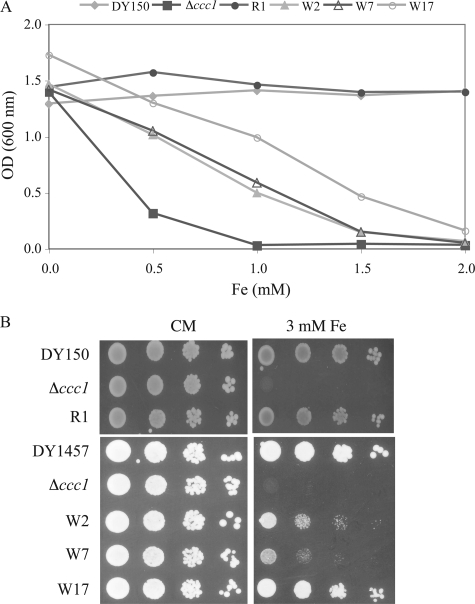
Identification of Yeast Mutants Resistant to High Iron—Deletion of CCC1 results in sensitivity to high iron (6, 15). We took advantage of this phenotype to identify UV-induced mutants of Δccc1 cells that were capable of growth on high iron medium. We screened 12,400 colonies and identified 15 mutants that would survive on high iron medium. The mutants were backcrossed to the parental strain (Δccc1) to determine whether they were single gene defects and whether the mutants were dominant or recessive. Seven of the mutants were single gene recessive mutations that fell into two complementation groups. Eight of the mutants were dominant. The mutants were characterized as having different sensitivity to high iron, as shown by liquid (Fig. 1A) and plate growth assays (Fig. 1B).
FIGURE 1.
Δccc1 cells mutagenized by UV are resistant to high iron. A, DY150 (wild type), Δccc1, and the Δccc1 mutants isolated after UV mutagenesis. R1, W2, W7, and W17 were grown to log phase in CM medium and then inoculated at a starting OD of 0.005 into CM medium with ferrous ammonium sulfate at the indicated concentrations. Cells were incubated at 30 °C overnight and the cell density was measured by absorbance at 600 nm. The experiment was performed multiple times and a representative experiment is presented. B, DY150 (wild type), DY150 Δccc1, R1 mutant, DY1457 (wild type), DY1457 Δccc1, and W2, W7, and W17 mutants were grown to log phase in CM medium and 104, 103, 102, and 101 cells spotted onto CM plates or CM plates with 3 mm ferrous ammonium sulfate. Plates were incubated at 30 °C for 2 days. The color difference of the colonies is due to different ade mutations (see “Experimental Procedures” for genotype).
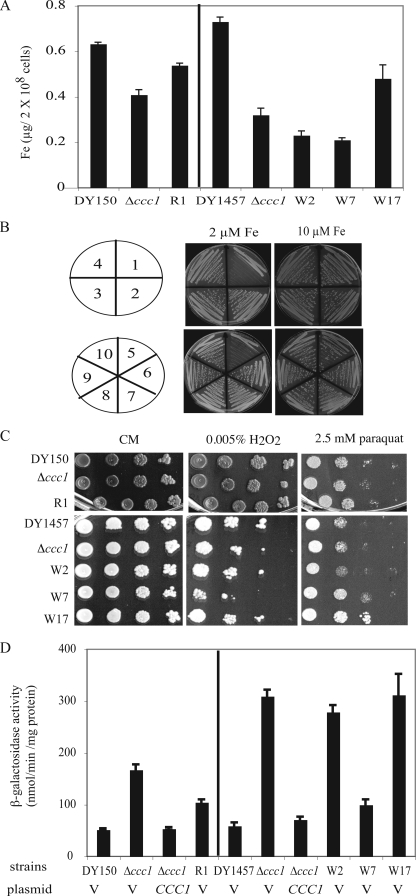
There are several potential mechanisms that might lead to high iron-resistant growth: decreased iron uptake, increased iron export, increased iron storage, or increased antioxidant defenses. To determine whether the mutant strains had decreased iron acquisition, cells were grown in CM medium overnight and cellular iron levels were measured by ICP. Mutants W2 and W7 accumulate less iron than control Δccc1 cells. R1 and W17 accumulated more iron than the parental Δccc1 strain (Fig. 2A). If resistance to high iron was due to increased iron export from cells, then the mutant strains might not grow on low iron. This situation was observed for a mutant PDR1 in which high iron resistance was correlated with low iron sensitivity (21). The Δccc1 mutant strains were able to grow on iron-limited medium, which requires a functional iron transport system (Fig. 2B). These results suggest that the resistance to high iron cannot be explained only by decreased iron acquisition or increased iron export. If iron toxicity was due to increased oxidative damage resulting from Fenton chemistry, then increased antioxidant defenses might reduce such damage. To test this possibility we examined the ability of mutant cells to resist the effect of the oxidants H2O2 and paraquat. One mutant (W17) showed a slight increase in paraquat resistance, but in general the mutants were as sensitive to oxidants as the parental strains (Fig. 2C).
FIGURE 2.
Characteristics of high iron-resistant Δccc1 mutants. A, DY150, DY150 Δccc1, R1 mutant, DY1457, DY1457 Δccc1 and W2, W7, and W17 mutants were grown in CM medium overnight. 20 OD cells (2 × 108) were collected, washed, and digested in nitric acid and iron contents were determined by ICP. B, strains were streaked onto CM plates containing 40 μm BPS with 2 and 10 μm FeSO4 added back. Plates were incubated at 30 °C for 2 days. 1, Δfet3; 2, DY150; 3, DY150Δccc1; 4, R1 mutant; 5, Δfet3; 6, DY1457; 7, DY1457Δccc1; 8, W2; 9, W7; 10, W17. C, cells as in A were grown in CM medium, spotted onto CM or CM with 0.005% H2O2 or 2.5 mm paraquat. Plates were incubated at 30 °C for 2 days. D, cells as in A were transformed with a reporter construct CCC1-lacZ and either a control vector (V) or alow copy CCC1 plasmid. The cells were grown in CM-Leu-Ura medium to log phase, harvested, and β-galactosidase activity determined. The data were normalized for protein concentration and error bars represent S.D. from three experiments.
We tested the possibility that the mutants were able to store or sequester cytosolic iron by measuring the expression of an iron-responsive reporter. Transcription of CCC1 or a CCC1-lacZ reporter construct is increased by iron through activation of the transcription factor Yap5 (15). Wild type, Δccc1, and Δccc1 mutants were transformed with a CCC1-lacZ construct and the effect of iron on induction of β-galactosidase activity was determined. Compared with wild type cells, Δccc1 cells showed a higher expression of CCC1-lacZ, indicating the presence of increased cytosolic iron. This induction of CCC1-lacZ was repressed when the CCC1 gene was introduced back to Δccc1 cells (Fig. 2D). Mutants W2 and W17 showed similar amounts of CCC1-lacZ activity compared with the parental Δccc1 strain. R1 and W7, however, showed lower levels of β-galactosidase compared with the parental strains. The R1 mutant was the most resistant to growth inhibition by high iron (cf. Fig. 1A). Mutant R1 had a slight increase in cellular iron compared with Δccc1 but less cytosolic iron as indicated by the CCC1-lacZ reporter assay. This implies that the resistance to high iron growth might be due to its ability to sequester cytosolic iron. Taken together these results showed that different mechanisms may contribute to high iron resistance in mutant strains.
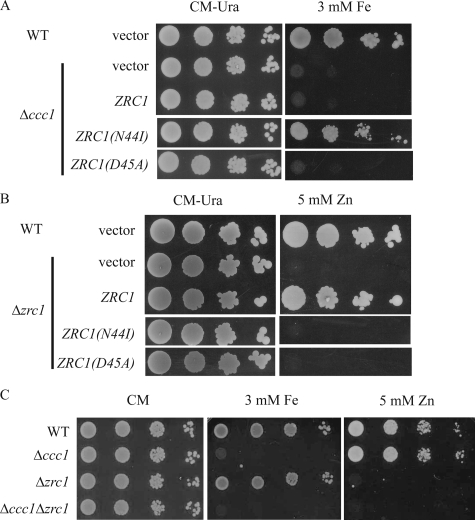
The High Iron Resistance of Mutant R1 Is Caused by a Mutation in ZRC1—To identify the gene responsible for the high iron resistance of R1 a low copy genomic library was generated from R1 cells. The library was transformed into the Δccc1 cells and transformants able to grow on high iron were identified. Plasmids conferring resistance to high iron were isolated and the genes on the insert were identified. Several plasmids containing overlapping segments of chromosome XIII were isolated. ZRC1, a gene that encodes a vacuolar zinc transporter (5, 22), was identified as a common gene on all rescued plasmids. Subcloning of the insert showed that the R1-specific ZRC1 plasmid conferred iron resistance when transformed into Δccc1 cells (data not shown). Sequence analysis of R1-ZRC1 revealed a mutation at position 131 of the coding sequence that altered an adenine to thymidine, which resulted in the substitution of isoleucine for asparagine at amino acid position 44 (referred to henceforth as ZRC1(N44I)). To confirm that this single amino acid change was responsible for high iron resistance, the asparagine at position 44 of the wild type Zrc1 was changed to an isoleucine by site-directed mutagenesis. When transformed into Δccc1 cells, ZRC1(N44I) was able to confer high iron resistance to Δccc1 cells (Fig. 3A).
FIGURE 3.
ZRC1(N44I) confers resistance to high iron. A, wild type (WT) cells transformed with empty vector and Δccc1 transformed with empty vector, ZRC1, ZRC1(N44I), or ZRC1(D45A) were spotted onto CM-Ura plates with or without 3 mm ferrous ammonium sulfate. Plates were incubated at 30 °C for 2 days. B, wild type and Δzrc1 cells transformed with empty vector ZRC1, ZRC1(N44I), or ZRC1(D45A) were spotted on CM-Ura plates with or without 5 mm ZnSO4. Plates were incubated at 30 °C for 2 days. C, WT, Δccc1, Δzrc1, or Δccc1Δzrc1 cells were spotted on CM with or without 3 mm ferrous ammonium sulfate or 5 mm ZnSO4. Plates were incubated at 30 °C for 2 days.
Zrc1 was identified initially as a high copy suppressor of zinc-sensitive growth (5) and deletion of ZRC1 increases zinc sensitivity (7). Cells with a deletion in ZRC1 showed zinc-sensitive growth that was suppressed by expression of plasmid containing ZRC1 but not by a plasmid containing ZRC1(N44I) (Fig. 3B). This result demonstrates that Zrc1(N44I) has lost its intrinsic zinc transport activity, and may explain why the R1 mutant is sensitive to high zinc concentration (data not shown).
It is possible that a loss of function of ZRC1 protects Δccc1 from high iron toxicity by altering zinc homeostasis and modulating the entire yeast transcriptome. To test this we generated a mutant ZRC1 based on the sequence alignment of Zrc1 with the E. coli FieF, another member of the cation diffusion facilitator family (23). The crystal structure of FieF suggests that Asp49 is involved in binding the zinc substrate. The homologous residue in Zrc1 is Asp45 and site-specific mutagenesis of ZRC1(D45A) resulted in an expressed protein that was unable able to protect Δzrc1 cells from high zinc toxicity (Fig. 3B). This construct was unable to permit Δccc1 cells to grow on high iron medium (Fig. 3A). Furthermore, deletion of ZRC1 in Δccc1 cells did not protect Δccc1 from high concentrations of iron (Fig. 3C). Together, these data suggest that loss of zinc transport activity is not sufficient to protect Δccc1 from high iron toxicity and also explains why mutant R1 shows dominant characteristics.
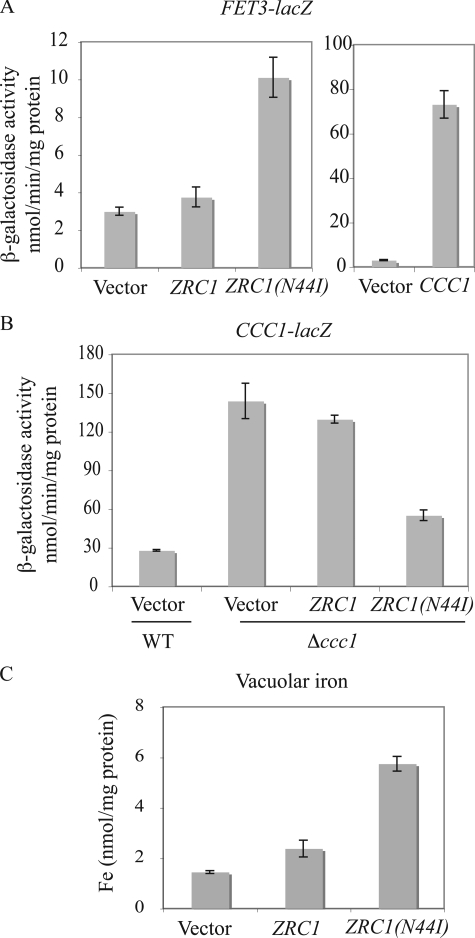
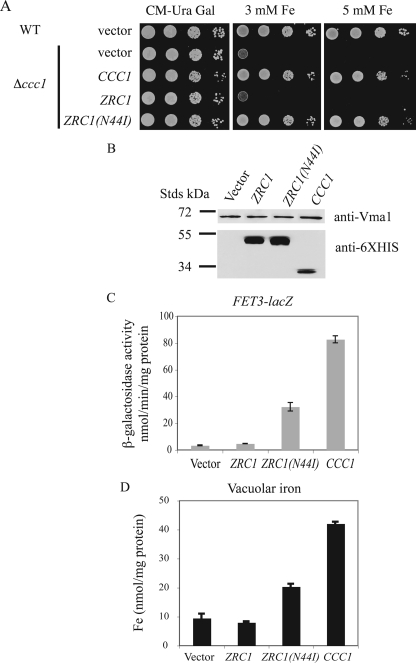
Zrc1(N44I) Increased Vacuolar Iron—Because Zrc1 localizes to the vacuolar membrane (9), it is possible that Zrc1(N44I) transports iron into the vacuole, which would decrease cytosolic iron. We tested this possibility by assaying the expression of a FET3-lacZ reporter. This reporter construct is regulated by the low iron sensing transcription factor Aft1 (24). Overexpression of CCC1, by transporting iron into vacuoles, leads to lower cytosolic iron inducing the expression of a FET3-lacZ reporter (6) (Fig. 4A). Expression of ZRC1(N44I) was also able to induce FET3-lacZ activity compared with cells transformed with either a vector or wild type ZRC1. Conversely, expression of ZRC1(N44I) but not wild type ZRC1 was able to reduce the activity of a CCC1-lacZ reporter construct (Fig. 4B). These results confirm that expression of ZRC1(N44I) lowers cytosolic iron. To prove that Zrc1(N44I) transports iron into vacuoles, iron was measured in vacuoles isolated from Δccc1 cells transformed with empty vector or plasmids expressing ZRC1 or ZRC1(N44I). Overexpression of wild type ZRC1 showed a slight increase in vacuolar iron relative to vector-transformed cells (Fig. 4C). Overexpression of ZRC1(N44I), however, resulted in a 3–4-fold increase in vacuolar iron. Together, these results show that ZRC1(N44I) is a gain of function allele that can confer iron-resistant growth by exporting iron from cytosol to vacuole.
FIGURE 4.
ZRC1(N44I) alters cytosolic iron by transporting iron into the vacuole. A, wild type (WT) cells with integrated FET3-lacZ were transformed with empty vector, ZRC1, ZRC1(N44I), or CCC1 under their own promoters. Cells were grown to log phase and β-galactosidase activity determined. B, WT cells with empty vector, Δccc1 cells with empty vector, ZRC1, or ZRC1(N44I) were transformed with a CCC1-lacZ reporter construct. Cells were grown in CM medium to log phase and β-galactosidase activity determined. C, Δccc1 cells transformed with a high copy empty vector, ZRC1, or ZRC1(N44I) under the ZRC1 promoter were grown in CM medium with 50 μm FeSO4 overnight. Vacuoles were isolated and iron content was determined by ICP. All data were normalized for protein concentrations and error bars represent S.D. from three experiments.
These data show that Zrc1(N44I) can transport iron but it is hard to compare the intrinsic iron transport activity of Zrc1(N44I) with that of Ccc1, as expression of each transporter is regulated differently. CCC1 transcription and mRNA stability are increased by iron, whereas ZRC1 (and by implication ZRC1(N44I)) shows increased transcription due to low zinc (7). To determine the intrinsic iron transport activity of Ccc1, Zrc1, and Zrc1(N44I), each was cloned into a GAL1-regulated vector. We placed a His6 epitope at the carboxyl terminus of each gene, which permitted us to determine protein levels. GAL1 regulated CCC1-HIS6 and ZRC1(N44I)-HIS6 were able to suppress the high iron growth defect of Δccc1 cells, whereas as a GAL1 regulated ZRC1-HIS6 was not (Fig. 5A). Under similar growth conditions, Zrc1-HIS6 and Zrc1(N44I)-HIS6 were expressed to higher levels than Ccc1-HIS6 (Fig. 5B). Measurement of iron transport activity based on FET3-lacZ expression showed that Zrc1-HIS6 had no measurable iron transport activity, whereas both Zrc1(N44I)-HIS6 or Ccc1-HIS6 induced expression of FET3-lacZ (Fig. 5C). Based on the expression levels of the proteins Zrc1(N44I)-HIS6 has ∼10–15% of the iron transport activity of Ccc1-HIS6. Δccc1 cells transformed with empty vector or galactose-regulated constructs were grown in galactose medium with 100 μm FeSO4. Vacuoles were isolated and iron content determined. Overexpression of CCC1-HIS6 and ZRC1(N44I)-HIS6, but not ZRC1-HIS6 increased vacuolar iron compared with vector-transformed control cells (Fig. 5D). There was more iron in Ccc1-HIS6 vacuoles than in Zrc1(N44I)-HIS6 vacuoles, again indicating that Ccc1 was more efficient in transporting iron into vacuoles.
FIGURE 5.
Iron transport efficiency of Zrc1(N44I). A, wild type (WT) cells transformed with empty vector (pYES2 GAL1 promoter), Δccc1 cells transformed with pYES2, ZRC1, ZRC1(N44I), or CCC1 tagged with His6 at the carboxyl terminus were grown in CM with 2% raffinose at 30 °C overnight. Cells were washed and spotted onto CM-Ura medium containing 2% galactose with or without 3 or 5 mm ferrous ammonium sulfate. Plates were incubated at 30 °C for 3 days. B, Δccc1 cells as in A were grown in CM medium with 2% raffinose then inoculated into CM medium with 2% galactose for 12 h. Cells were harvested, disrupted by glass beads, and protein levels measured by Western blot using a rabbit anti-His6 antibody or a mouse anti-Vma1 antibody followed by peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG. The vacuolar protein Vma1 was used as a loading control. C, Δccc1 cells with an integrated FET3-lacZ at the HO locus were transformed with empty vector pYES2, ZRC1, ZRC1(N44I), or CCC1 as in A. Cells were grown in CM medium with 2% raffinose and inoculated into CM with 2% galactose for 12 h. Cells were harvested and β-galactosidase activity determined. The data are normalized for protein concentrations and error bars represent S.D. from three experiments. D, cells as in A were grown in CM-Ura medium containing 2% galactose with 100 μm FeSO4 and 1 mm ascorbate overnight. Vacuoles were isolated and iron content was determined by ICP. All data were normalized for protein concentrations and error bars represent S.D. from three experiments.
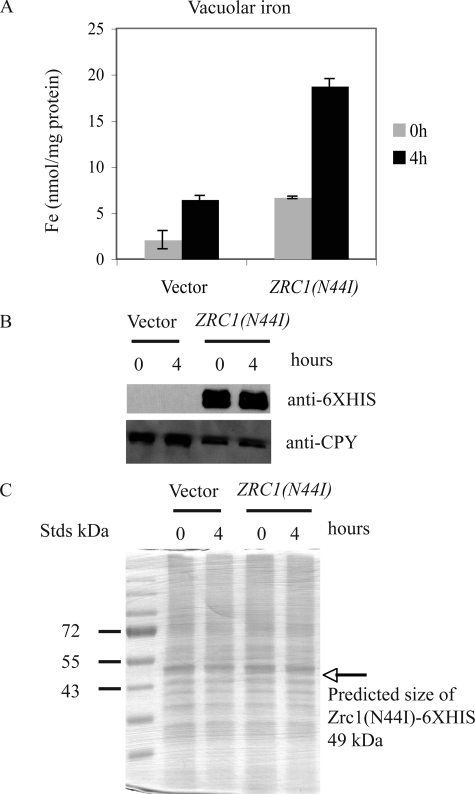
It might be possible that iron is not transported into the vacuole but rather the iron is tightly bound to the cytosolic facing surface of Zrc1(N44I). The difference in vacuolar iron between Δccc1 cells overexpressing ZRC1(N44I) and control cells (empty vector) was about 10–12 nmol/mg of vacuolar protein. The crystal structure of the E. coli CDF family member FieF suggests that it has 4 Zn binding sites (11). Assuming one molecule of Zrc1(N44I) binds 4 atoms of iron, then based on the molecular mass of Zrc1 (48,344 daltons), would require ∼0.12–0.15 mg of Zrc1 protein/mg of vacuole protein to bind the measured iron. This means that Zrc1(N44I) would have to constitute 12–15% of vacuolar proteins. To test this prediction we grew Δccc1 cells, transformed with either a control vector or GAL-regulated ZRC1(N44I) in galactose medium overnight and then in galactose medium containing 100 μm FeSO4 for 4 h. Cells were harvested, vacuolar iron determined, and vacuolar proteins applied to SDS-PAGE and the gel analyzed by Western blot and silver stain. Vacuoles isolated from control cells only showed a slight increase in iron over the time course of the experiment, probably due to endocytosis of iron from the culture media. Vacuoles isolated from Zrc1(N44I) showed a much greater increase in vacuolar iron (Fig. 6A). Western blot showed that during this time course, the Zrc1(N44I)-HIS6 protein was expressed and remained constant (Fig. 6B). Silver staining of vacuolar proteins from both control and Zrc1(N44I)-HIS6 expressing cells had a similar protein distribution in which the band corresponding to Zrc1(N44I)-HIS6 was not notable (Fig. 6C), suggesting that the protein abundance would be insufficient to bind vacuolar iron.
FIGURE 6.
Measurement of vacuolar iron content and Zrc1(N44I) protein abundance. A, Δccc1 cells were transformed with a control vector (pYES2) or a ZRC1(N44I)-HIS6 containing plasmid. Cells were grown in galactose CM overnight and then incubated in the same medium containing 100 μm FeSO4. Vacuoles were isolated from control and ZRC1(N44I) expressing cells at time 0 and 4 h after incubation in iron-rich medium. The iron content of isolated vacuoles was determined by ICP and the data normalized to protein content. B, isolated vacuoles were solubilized and analyzed by SDS-PAGE and Western blot using a rabbit anti-His6 antibody or a mouse anti-carboxypeptidase Y antibody followed by peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG. C, the same samples as in B were also analyzed by silver staining. The arrow represents the predicted mass of Zrc1(N44I)-HIS6.
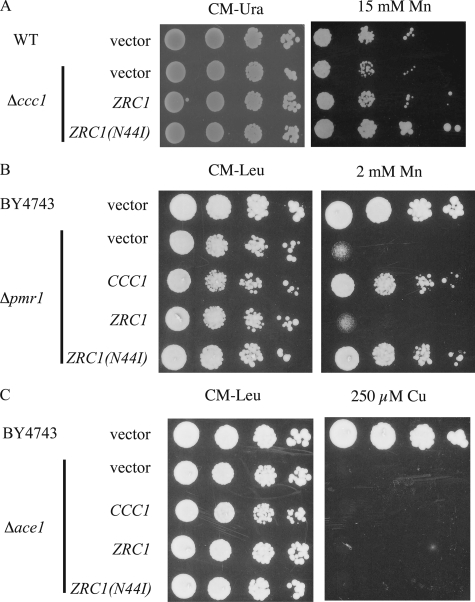
Zrc1(N44I) Transports Manganese—Many transporters that transport iron also transport Mn2+ (25, 26). Deletion of CCC1 results in sensitivity to high Mn2+ (27), although with the concentrations and strain used here the sensitivity was slight at best. Expression of ZRC1(N44I) suppressed Mn2+ toxicity in Δccc1 cells to a greater extent than ZRC1 (Fig. 7A). Pmr1 is a Golgi membrane P-type ATPase involved in transporting Ca2+ and Mn2+ into the Golgi (28). Deletion of PMR1 leads to accumulation of Mn2+ in the cytosol, increasing the sensitivity of cells to high concentrations of Mn2+. Indeed, CCC1 was identified as a high copy suppressor of Δpmr1 Mn2+ toxicity phenotype, indicating it also transports Mn2+ into vacuoles (27). Overexpression of CCC1 or ZRC1(N44I), but not wild type ZRC1, protected Δpmr1 cells from Mn2+ toxicity (Fig. 7B). These results indicate that Zrc(N44I) also has the ability to transport Mn2+. To examine whether ZRC1(N44I) can transport copper into vacuoles, we studied the effect of overexpression of ZRC1(N44I) in copper-sensitive cells. ACE1 encodes a transcription factor that regulates the expression of metallothioneins, small intracellular proteins that can bind and detoxify copper (29). Deletion of ACE1 renders cells copper sensitive but overexpression of ZRC1 or ZRC1(N44I) did not suppress or enhance the copper sensitivity of Δace1 cells (Fig. 7C).
FIGURE 7.
ZRC1(N44I) shows an altered metal specificity. A, wild type (WT) cells (DY150) transformed with empty vector or Δccc1 cells transformed with empty vector, ZRC1, or ZRC1(N44I) were spotted onto CM-Ura plates, CM-Ura plates with 15 mm MnCl2. Plates were incubated at 30 °C for 3 days. B, wild type cells (BY4743) or Δpmr1 cells were transformed with empty vector, CCC1, ZRC1, or ZRC1(N44I) in a high copy vector with their native promoter. Cells were spotted onto CM-Leu plates with or without 2 mm MnCl2. Plates were incubated at 30 °C for 2 days. C, wild type cells (BY4743) or Δace1 cells were transformed with empty vector, CCC1, ZRC1, or ZRC1(N44I) in a high copy vector with their native promoter. Cells were spotted onto CM-Leu plates with or without 250 μm CuSO4. Plates were incubated at 30 °C for 2 days.
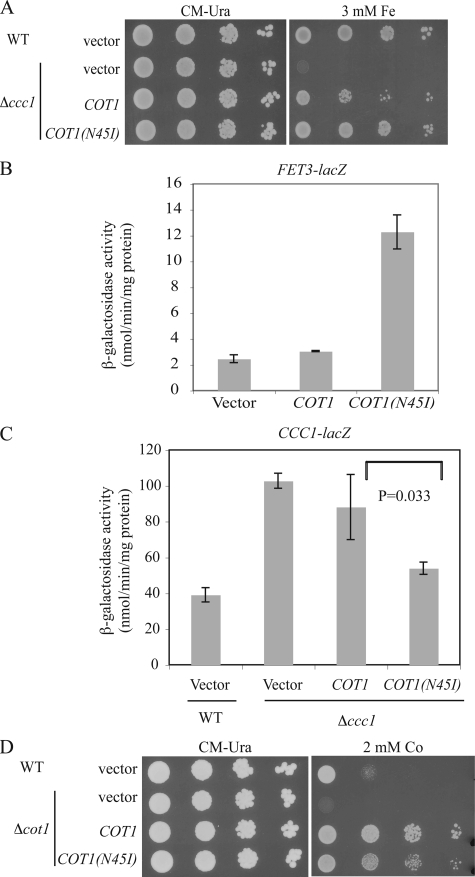
Cot1(N45I) Shows Increased Iron Transport and Decreased Cobalt Transport—The N44I mutation in Zrc1 is in the second hydrophobic domain. The sequence of this domain is highly conserved in homologous CDF proteins involved in Zn2+ transport, which are found throughout the biological kingdoms (Fig. 8). This sequence is highly conserved in the Zrc1 homologue Cot1, which shows 78% amino acids similarity to Zrc1. Cot1 was initially identified as a gene that conferred cobalt resistance when overexpressed (4). Cot1 has Zn2+ transport activity, as overexpression of COT1 confers zinc resistance and deletion of COT1 increases zinc sensitivity in Δzrc1 cells (30). The corresponding asparagine at position 45 of Cot1 was changed to isoleucine by site-directed mutagenesis to examine the effect on the substrate specificity of Cot1. Wild type COT1 or COT1(N45I) were transformed into Δccc1 cells and growth on high iron medium was examined. High copy expression of COT1 in Δccc1 cells was able to confer high iron resistance (Fig. 9A). Expression of COT1(N45I) increased the resistance of Δccc1 cells to high iron. Overexpressed COT1(N45I) was able to induce FET3-lacZ reporter expression (Fig. 9B) and reduce expression of the CCC1-lacZ reporter (Fig. 9C) indicating an alteration of iron homeostasis. The N45I mutation, however, decreased the ability of COT1 to confer cobalt resistance on Δcot1 cells (Fig. 9D).
FIGURE 8.
Alignment of Zrc1 homologues. The Zrc1 protein sequence was used to BLAST search its homologues in other species. Sequences of selected homologues were retrieved and aligned with ClustalW2. E. coli FieF, a CDF member, whose structure has already been determined, was also included in the alignment. The position of the mutated amino acids (N) in Zrc1 and Cot1 is indicated in italic. The two aspartate residues involved in zinc binding in E. coli FieF are bolded. The predicated transmembrane domains are indicated. The abbreviations are: Sc, S. cerevisiae; Hs, Homo sapiens; Mm, Mus musculus; Ec, E. coli K-12. Asterisk (*) denotes identity; colon (:) denotes similarity.
FIGURE 9.
COT1(N45I) protects Δccc1 cells from high iron toxicity. A, wild type (WT) cells transformed with a high copy empty vector and Δccc1 cells transformed with empty vector, COT1, or COT1(N45I) under the COT1 promoter were spotted onto CM-Ura plates with or without 3 mm ferrous ammonium sulfate. Plates were incubated at 30 °C for 2 days. B, WT cells containing an integrated FET3-lacZ at the HO locus were transformed with a high copy empty vector, COT1, or COT1(N45I) under the COT1 promoter. Cells were grown to log phase and β-galactosidase activity determined. C, WT cells transformed with a high copy empty vector, Δccc1 cells transformed with empty vector, COT1, or COT1(N45I) were transformed with a CCC1-lacZ reporter construct. Cells were grown to log phase and β-galactosidase activity determined. The data are normalized for protein concentrations and error bars represent S.D. from three experiments. D, wild type cells (BY4743) transformed with a high copy empty vector and Δcot1 cells transformed with empty vector, COT1, or COT1(N45I) under the COT1 promoter were spotted onto CM-Ura with or without 2 mm CoCl2. Plates were incubated at 30 °C for 2 days.
Overexpression of COT1 can partially suppress the high iron growth deficit of Δccc1 cells (Fig. 9A), suggesting that Cot1 may be a low affinity iron transporter. In CM medium, however, expression of COT1 has little effect on Fet3-lacZ activity. COT1 is a target of the transcription factors Aft1 and Aft2 (31) and shows a modest induction under low iron conditions. Low iron conditions prevent accumulation of the vacuolar iron transporter Ccc1, as CCC1 transcription is activated under high iron conditions (15) and CCC1 mRNA is destabilized under low iron conditions by the Aft1-regulated gene CTH2 (14). We considered the possibility that expression of a low affinity vacuolar iron transporter might protect cells against iron shock. Compelling evidence shows that expression of Zrc1 by low zinc conditions protects cells against sudden increases in cytosolic zinc, termed zinc shock (7). We tested the possibility that Cot1 might play an analogous role and protect cells from “iron shock.” Cells grown in low iron were transferred to high iron medium and growth assayed. Wild type cells showed no obvious growth defect when incubated in high iron (Fig. 10). In contrast, Δccc1 cells showed a severe growth deficiency. Deletion of COT1 by itself did not affect growth in high iron nor did it exacerbate the growth defect of a CCC1 deletion. These results do not support a role for Cot1 in either iron shock conditions or in protecting cells from high iron.
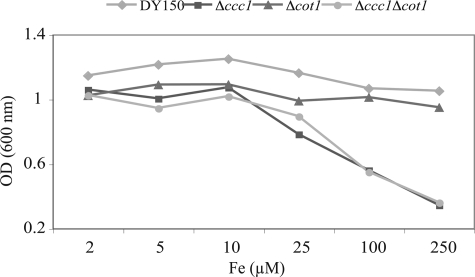
FIGURE 10.
COT1 is not involved in iron shock. WT cells, Δccc1, Δcot1, and Δccc1Δcot1 were grown in CM medium with 40 μm BPS overnight. Cells were washed and inoculated into CM medium with 40 μm BPS and specified concentrations of FeSO4. Cell density was determined 12 h later. The experiment was performed multiple times and a representative experiment is presented.
DISCUSSION
Deletion of the vacuolar iron transporter Ccc1 results in growth inhibition on high iron medium. We initiated this study to identify genes that suppressed the high iron growth defect. Our study identified both recessive and dominant mutations that permitted Δccc1 cells to grow on high iron medium. The recessive genes fell into two complementation groups. We also identified eight dominant mutants, which were due to mutations in single genes, although genetic analysis did not permit us to determine whether the dominant mutants were in the same or different genes. Phenotypic analysis suggested that the dominant genes show differences in iron sensitivity and in their ability to modulate cytosolic iron levels (Figs. 1 and 2). Only one of the mutants showed notable resistance to oxidants suggesting that modulation of iron-dependent oxygen toxicity is not a prominent mechanism for high iron resistance. This result confirms our previous observation that high iron toxicity does not necessarily result from increased Fenton chemistry, as high iron growth deficit occurs anaerobically.
We identified the molecular basis of one of the dominant iron-resistant mutants to be a single amino acid mutation in the vacuolar Zn2+ transporter Zrc1 (22, 30). Targeted site-specific mutagenesis is the most common approach to examining the substrate specificity of enzymes or transporters. In combination with either structural studies or genome wide informatics, targeted mutations have revealed much about the importance of specific domains or amino acids in determining substrate specificity. The role of amino acids in determining the substrate selectivity or transporters has been reported previously for such transporters, as the plant potassium transporter HKT1 (32), H+-sucrose symporter AtSUC1 from Arabidopsis thaliana (33), plasma membrane H+-ATPase PMA2 from Nicotiana plumbaginifolia (34), the plant A. thaliana ZIP family member metal transporter IRT1 (35), and yeast Saccharomyces cerevisiae Mn2+/Ca2+ transporter Pmr1 (36). Amino acid substitutions generally abolish transporter activity or increase the transport activity of one of its substrates relative to another. Here we show that a single amino acid substitution in Zrc1 or Cot1 dramatically changed the substrate specificity. Genetic and biochemical studies have shown that Zrc1 is able to transport Zn2+, Ni2+, Cd2+, but not Fe2+ or Mn2+ (9). We confirmed this conclusion by measuring resistance to high iron growth conditions and by reporter constructs that assay cytosolic iron levels. Substitution of an asparagine at position 44 to an isoleucine abolished the ability of Zrc1 to transport Zn2+ but conferred the ability to transport Fe2+ and Mn2+. A similar change in Cot1 also altered its substrate specificity to iron while reducing its ability to transport Co2+.
Zrc1 and Cot1 belong to the CDF family of transition metal transporters. Members of this family are found in all biological kingdoms and most usually transport metals out of the cytosol either into organelles or out of cells. CDF transporters usually have six transmembrane regions in which there is a high degree of sequence conservation in the charged residues of transmembrane domains II and V, which is thought to bind metals and form a transmembrane pore. The structure of the E. coli CDF member FieF, which transports iron, has been determined with Zn2+ in the metal binding site (23). Structural and mutagenesis studies using E. coli FieF have focused on the importance of the Asp45 and Asp49 in transmembrane TMD II, and His153 and Asp157 in TMD V as residues that coordinate Zn2+ in the crystal structure, and by implication iron in the native transporter. These charged residues are conserved in CDF family members although each family member shows different substrate specificities. The mutated asparagine is adjacent to the conserved aspartic acid in TMD II of Zrc1 and Cot1. The substitution of a hydrophilic residue with a hydrophobic residue may alter the conformation of the metal binding site, changing the metal specificity of transport. Our study suggests that the microenviroment of the amino acids adjacent to the metal binding aspartate determines the substrate selectivity. The mutated ZRC1 was identified by a screen using random UV mutagenesis to select for cells showing high iron resistance. The approach of using a strong selection system in conjunction with mutagenesis of genes encoding transporters offers the possibility of facile identification of other residues critical for determining the substrate specificity of CDF transporters.
Acknowledgments
We express our appreciation to members of the Kaplan laboratory for critically reading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant DK30534 (to J. K.), support for use of the Core Facilities was provided through NCI, National Institutes of Health Grant NCI-CCSG P30CA 42014 and NIDDK Center of Excellence Award 5P30KD72437. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: CDF, cation diffusion facilitator family; BPS, bathophenanthroline disulfonate; CM, complete medium; ICP, inductively coupled plasma-optical emission spectrometer; TMD, transmembrane domain; YPD, yeast extract peptone dextrose medium.
References
- 1.Ramsay, L. M., and Gadd, G. M. (1997) FEMS Microbiol. Lett. 152 293–298 [DOI] [PubMed] [Google Scholar]
- 2.Eide, D. J., Bridgham, J. T., Zhao, Z., and Mattoon, J. R. (1993) Mol. Gen. Genet. 241 447–456 [DOI] [PubMed] [Google Scholar]
- 3.Szczypka, M. S., Zhu, Z., Silar, P., and Thiele, D. J. (1997) Yeast 13 1423–1435 [DOI] [PubMed] [Google Scholar]
- 4.Conklin, D. S., McMaster, J. A., Culbertson, M. R., and Kung, C. (1992) Mol. Cell. Biol. 12 3678–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamizono, A., Nishizawa, M., Teranishi, Y., Murata, K., and Kimura, A. (1989) Mol. Gen. Genet. 219 161–167 [DOI] [PubMed] [Google Scholar]
- 6.Li, L., Chen, O. S., McVey Ward, D., and Kaplan, J. (2001) J. Biol. Chem. 276 29515–29519 [DOI] [PubMed] [Google Scholar]
- 7.MacDiarmid, C. W., Milanick, M. A., and Eide, D. J. (2003) J. Biol. Chem. 278 15065–15072 [DOI] [PubMed] [Google Scholar]
- 8.Simm, C., Lahner, B., Salt, D., LeFurgey, A., Ingram, P., Yandell, B., and Eide, D. J. (2007) Eukaryot. Cell 6 1166–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDiarmid, C. W., Milanick, M. A., and Eide, D. J. (2002) J. Biol. Chem. 277 39187–39194 [DOI] [PubMed] [Google Scholar]
- 10.Montanini, B., Blaudez, D., Jeandroz, S., Sanders, D., and Chalot, M. (2007) BMC Genomics 8 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei, Y., Li, H., and Fu, D. (2004) J. Biol. Chem. 279 39251–39259 [DOI] [PubMed] [Google Scholar]
- 12.Grass, G., Otto, M., Fricke, B., Haney, C. J., Rensing, C., Nies, D. H., and Munkelt, D. (2005) Arch. Microbiol. 183 9–18 [DOI] [PubMed] [Google Scholar]
- 13.Kim, S. A., Punshon, T., Lanzirotti, A., Li, L., Alonso, J. M., Ecker, J. R., Kaplan, J., and Guerinot, M. L. (2006) Science 314 1295–1298 [DOI] [PubMed] [Google Scholar]
- 14.Puig, S., Askeland, E., and Thiele, D. J. (2005) Cell 120 99–110 [DOI] [PubMed] [Google Scholar]
- 15.Li, L., Bagley, D., Ward, D. M., and Kaplan, J. (2008) Mol. Cell. Biol. 28 1326–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, L., and Kaplan, J. (2004) J. Biol. Chem. 279 33653–33661 [DOI] [PubMed] [Google Scholar]
- 17.Amberg, D. C., Botstein, D., and Beasley, E. M. (1995) Yeast 11 1275–1280 [DOI] [PubMed] [Google Scholar]
- 18.Crisp, R. J., Pollington, A., Galea, C., Jaron, S., Yamaguchi-Iwai, Y., and Kaplan, J. (2003) J. Biol. Chem. 278 45499–45506 [DOI] [PubMed] [Google Scholar]
- 19.Ausubel, F. M. (1995) Current Protocols in Molecular Biology, Wiley, New York
- 20.Calvin, N. M., and Hanawalt, P. C. (1988) J. Bacteriol. 170 2796–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuttle, M. S., Radisky, D., Li, L., and Kaplan, J. (2003) J. Biol. Chem. 278 1273–1280 [DOI] [PubMed] [Google Scholar]
- 22.MacDiarmid, C. W., Gaither, L. A., and Eide, D. (2000) EMBO J. 19 2845–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu, M., and Fu, D. (2007) Science 317 1746–1748 [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi-Iwai, Y., Dancis, A., and Klausner, R. D. (1995) EMBO J. 14 1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dix, D. R., Bridgham, J. T., Broderius, M. A., Byersdorfer, C. A., and Eide, D. J. (1994) J. Biol. Chem. 269 26092–26099 [PubMed] [Google Scholar]
- 26.Forbes, J. R., and Gros, P. (2003) Blood 102 1884–1892 [DOI] [PubMed] [Google Scholar]
- 27.Lapinskas, P. J., Lin, S. J., and Culotta, V. C. (1996) Mol. Microbiol. 21 519–528 [DOI] [PubMed] [Google Scholar]
- 28.Lapinskas, P. J., Cunningham, K. W., Liu, X. F., Fink, G. R., and Culotta, V. C. (1995) Mol. Cell. Biol. 15 1382–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winge, D. R. (1998) Prog. Nucleic Acids Res. Mol. Biol. 58 165–195 [DOI] [PubMed] [Google Scholar]
- 30.Conklin, D. S., Culbertson, M. R., and Kung, C. (1994) Mol. Gen. Genet. 244 303–311 [DOI] [PubMed] [Google Scholar]
- 31.Rutherford, J. C., Jaron, S., and Winge, D. R. (2003) J. Biol. Chem. 278 27636–27643 [DOI] [PubMed] [Google Scholar]
- 32.Rubio, F., Gassmann, W., and Schroeder, J. I. (1995) Science 270 1660–1663 [DOI] [PubMed] [Google Scholar]
- 33.Lu, J. M., and Bush, D. R. (1998) Proc. Natl. Acad. Sci. U. S. A 95 9025–9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morsomme, P., de Kerchove d'Exaerde, A., De Meester, S., Thines, D., Goffeau, A., and Boutry, M. (1996) EMBO J. 15 5513–5526 [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers, E. E., Eide, D. J., and Guerinot, M. L. (2000) Proc. Natl. Acad. Sci. U. S. A 97 12356–12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandal, D., Woolf, T. B., and Rao, R. (2000) J. Biol. Chem. 275 23933–23938 [DOI] [PubMed] [Google Scholar]