Abstract
Model class A amphipathic helical peptides mimic several properties of apolipoprotein A-I (apoA-I), the major protein component of high density lipoproteins. Previously, we reported the NMR structures of Ac-18A-NH2 (renamed as 2F because of two phenylalanines), the base-line model class A amphipathic helical peptide in the presence of lipid (Mishra, V. K., Anantharamaiah, G. M., Segrest, J. P., Palgunachari, M. N., Chaddha, M., Simon Sham, S. W., and Krishna, N. R. (2006) J Biol. Chem. 281 ,6511 -6519). Substitution of two Leu residues on the nonpolar face (Leu3 and Leu14) with Phe residues produced the peptide 4F (so named because of four phenylalanines), which has been extensively studied for its anti-inflammatory and antiatherogenic properties. Like 2F, 4F also forms discoidal nascent high density lipoprotein-like particles with 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC). Since subtle structural changes in the peptide-lipid complexes have been shown to be responsible for their antiatherogenic properties, we undertook high resolution NMR studies to deduce detailed structure of 4F in 4F·DMPC discs. Like 2F, 4F adopts a well defined amphipathic α-helical structure in association with the lipid at a 1:1 peptide/lipid weight ratio. Nuclear Overhauser effect (NOE) spectroscopy revealed a number of intermolecular close contacts between the aromatic residues in the hydrophobic face of the helix and the lipid acyl chain protons. Similar to 2F, the pattern of observed peptide-lipid NOEs is consistent with a parallel orientation of the amphipathic α helix, with respect to the plane of the lipid bilayer, on the edge of the disc (the belt model). However, in contrast to 2F in 2F·DMPC, 4F in the 4F·DMPC complex is located closer to the lipid headgroup as evidenced by a number of NOEs between 4F and DMPC headgroup protons. These NOEs are absent in the 2F·DMPC complex. In addition, the conformation of the DMPC sn-3 chain in 4F·DMPC complex is different than in the 2F·DMPC complex as evidenced by the NOE between lipid 2.CH and βCH2 protons in 4F·DMPC, but not in 2F·DMPC, complex. Based on the results of this study, we infer that the antiatherogenic properties of 4F may result from its preferential interaction with lipid headgroups.
Class A amphipathic helical peptides have been shown to mimic apolipoprotein A-I (apoA-I),3 the major protein accounting for 70% of the total protein present in high density lipoproteins (HDL). Epidemiological studies have established an inverse correlation between the plasma levels of HDL cholesterol and the risk of coronary artery disease. Reconstituted HDL of human apoA-I in combination with phospholipid has been shown to inhibit atherosclerosis in animal models of atherosclerosis (1-6) as well as in humans (7).
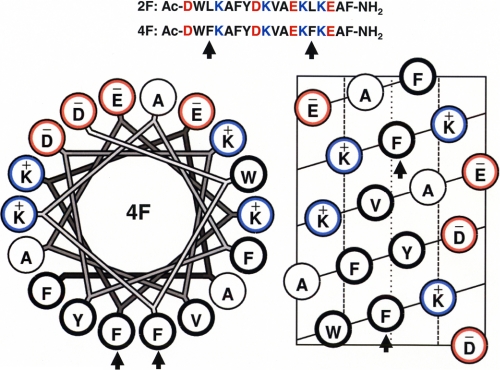
ApoA-I mimetic peptides may represent an alternative to apoA-I for large scale production of synthetic HDL as a therapeutic agent. The motif that is responsible for the association of apoA-I with lipid was determined as tandem repeating amphipathic α-helical domains that are present throughout the sequence of apoA-I. The observation that the amphipathic α-helical domains in exchangeable apolipoproteins possess a class A amphipathic motif with basic amino acid residues at the polar-nonpolar interface and negatively charged residues at the center of the polar face (8-10) led us to design the first model class A amphipathic helical peptide 18A with an amino acid sequence DWLKAFYDKVAEKLKEAF (11). This sequence does not possess any sequence homology with any of the exchangeable apolipoproteins, but the synthetic peptide 18A was able to form peptide-lipid complexes similar to apoA-I·lipid complexes (11, 12). Adding an acyl group to the amino terminus of this peptide and an amide to the C-terminal end resulted in Ac-18A-NH2 (also known as 2F, since the nonpolar face possesses two Phe residues) with increased α helicity and affinity for lipids (13). Recently, we determined the structure of this peptide in 50% (v/v) trifluoroethanol (14) and complexed to lipid (15) using high resolution solution NMR methods. A number of variations of 2F peptide were synthesized by replacing existing nonpolar amino acids with Phe residues on the nonpolar face. Thus, the peptides synthesized were 3F3(Ac-F318A-NH2), 3F14(Ac-F1418A-NH2), 4F(Ac-F3,1418A-NH2), 5F(Ac-F11,14,1718A-NH2), 6F(Ac-F10,11,14,1718A-NH2), and 7F(Ac-F3,10,11,14,1718A-NH2) (16). All of the peptides were able to solubilize multilamellar vesicles of egg yolk phosphatidylcholine and thus possessed increased lipid affinity (16). However, peptides 4F, 5F, and 6F were significantly more effective than the homologues 2F and 7F in their ability to inhibit low density lipoprotein-induced monocyte chemotaxis (16). Since these observations, apoA-I mimetic peptide 4F has been studied extensively as an anti-inflammatory agent in inhibiting atherosclerosis in various animal models of atherosclerosis (17-20), improving vascular function in streptozotokine-induced diabetes (21-23), influenza A-mediated inflammation (24, 25), and lipopolysaccharide-mediated inflammation (26) and even in human patients in the Phase I clinical trial demonstrating an improvement in HDL quality (27). Although the lipid-associated structure of 2F has been determined (15), the lipid-associated structure of 4F is unknown. In this paper, we describe the lipid-associated structure of 4F in an effort to understand unique properties of this peptide that may be responsible for its anti-inflammatory properties. The helical wheel and helical net representations of 4F are shown in Fig. 1. The location of Phe residues in 4F that were substituted for Leu in 2F are indicated by arrows in Fig. 1.
FIGURE 1.
Helical wheel (left) and helical net (right) diagrams of 4F. The two Phe residues that were substituted in 4F for Leu residues in 2F are indicated by arrows. The primary amino acid sequences of 2F and 4F are shown above the helical wheel and helical net diagrams. Red, acidic; blue, basic; thick black, hydrophobic; thin black, Ala.
MATERIALS AND METHODS
Peptide Synthesis, Purification, and NMR Sample Preparation—The peptide 4F (Ac-DWFKAFYDKVAEKFKEAFNH2) was synthesized and purified as described earlier (16). Two separate sets of samples were prepared for the NMR studies: (a) perdeuterated 1,2-dimyristoyl-d54-sn-glycero-3-phosphocholine-1,1,2,2-d4-N,N,N-trimethyl-d9 (DMPC) (Avanti Polar Lipids, Inc., Alabaster, AL) and peptide in 90% H2O, 10% D2O (100% deuterium oxide; Cambridge Isotope Laboratories, Inc.) and (b) protonated DMPC and peptide in 90% H2O, 10% D2O. To prepare these samples, lyophilized dry peptide was dissolved in methanol. DMPC (either protonated or perdeuterated) was dissolved separately in methanol. Appropriate amounts of peptide and DMPC solutions were mixed together to obtain a peptide/total lipid ratio of 1:1 (w/w). Organic solvent was evaporated under a stream of nitrogen gas, and the mixture was lyophilized to remove any residual solvent. To the dry peptide and lipid film, 0.6 ml of 5 mm KH2PO4 was added, and the sample was incubated at 37 °C overnight. The pH of the final solution was 5.5. The final peptide concentration in solution was ∼3 mm.
Size of the Discoidal Peptide-Lipid Complex—Apparent size of the discoidal peptide-lipid complex was determined using a fast protein liquid chromatography system (BioLogic, DuoFlow; Bio-Rad) and Superose 6 10/300 GL and Superdex 200 10/300 GL columns (Amersham Biosciences) linked in tandem and run at a flow rate of 0.4 ml/min in phosphate-buffered saline containing 0.02% sodium azide (pH 7.4). Complex elution was monitored using absorbance at 280 nm. The apparent Stokes diameter of the complex was estimated using high and low molecular weight calibration kits (Amersham Biosciences). The void and total volume of the columns were determined using blue dextran and K3(Fe(CN)6), respectively. The amounts of lipid and peptide in the eluted peak fraction were measured using an enzymatic colorimetric method (Phospholipids B; Wako Chemicals) and absorbance at 280 nm in the presence of 6 m guanidinium hydrochloride, respectively.
NMR Measurements and Structure Calculations—One- and two-dimensional NMR experiments were performed on a Bruker Avance-600 NMR system with a TCI CryoProbe and an Avance-500 NMR spectrometer at 37 °C. One-dimensional 31P experiments were run at 202.45 MHz on an Avance-500 spectrometer with sodium phosphate buffer as an external reference set at 0.0 ppm. 50-, 100-, 150-, and 200-ms mixing times were used in NOESY, and a 100-ms spin-lock time was used in total correlation spectroscopy (TOCSY) measurements. Two-dimensional data sets were collected with 2048 complex t2 points with 512 t1 increments. States-time-proportional phase incrementation was employed for frequency discrimination in the indirect dimension. Chemical shifts were referenced with respect to sodium 2,2-dimethyl-2-silapentane-5-sulfonate (0.0 ppm) used as an internal standard. To improve spectrum resolution, additional spectra were obtained at 37 °C on a Varian Inova 900 MHz spectrometer equipped with a cryogenic probe. Data were acquired using conventional sequences from the Varian library. In all experiments, the spectral width was 14 kHz, and a 3919 watergate scheme was used to suppress water in all experiments. The two-dimensional TOCSY data set was collected with a 60-ms DIPSI spin-lock of 10 kHz strength and an acquisition time of 400 ms in t2 and 28 ms in t1. The NOESY data set was collected with the same general parameters and a mixing time of 200 ms. These parameters provided a digital resolution in F2 of 2.5 Hz/point prior to zero filling.
The NMR data were transferred to a Silicon Graphics IRIS Indigo work station and processed using the program FELIX (version 2007) (Felix NMR Inc., San Diego, CA). NMR structures were calculated on a Silicon Graphics IRIS Indigo work station using X-PLOR (online) version 3.851. The structure calculation protocol involved (a) generation of a “template” coordinate set; (b) bound smoothing, full structure embedding, and regularization to produce a family of 100 distance geometry structures; (c) simulated annealing regularization and refinement for embedded distance geometry structures; and (d) simulated annealing refinement, as described previously (15). Average coordinates of the accepted structures were energyminimized using 200 cycles of conjugate gradient energy minimization. All of the NMR constraints were enforced during energy minimization.
Distance and Dihedral Angle Constraints—On the basis of cross-peak intensities in the NOESY spectra, recorded with mixing times of 100 and 200 ms, the NOE distance constraints were classified as follows. For NOEs involving intraresidue and sequential NH, αH, and βH protons, a distance constraint of 2.0-2.5 Å for strong, 2.0-3.0 Å for medium, and 2.0-4.0 Å for weak NOEs was used; for medium or long range NOEs involving NH and αH protons, a distance constraint of 2.0-4.0 Å was used; for NOEs involving medium or long range side chain protons, a distance constraint of 2.0-5.0 Å was used. Pseudoatom corrections, where appropriate, were added to the upper distance bounds, as described previously (28). Based on the difference (Δδ) in the observed and “coil” NH proton chemical shifts, hydrogen bond (H-O) distances were calculated as described earlier (29). These hydrogen bond distances were included as additional constraints (30) during structure calculation. Dihedral angles Φ and ψ were based on the difference in the observed and “coil” CαH proton chemical shifts (29); a minimum deviation of 40° was allowed in the dihedral constraints.
Molecular modeling was performed on a Silicon Graphics IRIS Indigo work station using the program SYBYL (version 7.2) (Tripos, Inc., St. Louis, MO).
RESULTS
The size exclusion profiles of 2F·DMPC (15) and 4F·DMPC complexes are compared in Fig. 2. The peptides elute predominantly as a lipid complex at the peptide/lipid ratio used (1:1, w/w) (Fig. 2). The apparent Stokes diameter of the complexes, estimated using high and low molecular weight calibration kits (Amersham Biosciences), are 69 Å (15) and 67 Å for 2F·DMPC and 4F·DMPC, respectively (Fig. 2). The lipid/peptide ratio in the eluted fractions was determined to be nearly identical.
FIGURE 2.
Size exclusion chromatography of 2F·DMPC (open circles) and 4F·DMPC (filled circles) complexes at a 1:1 (w/w) peptide/lipid ratio. Two columns, Superose 6 10/300 GL and Superdex 200 10/300 GL (Amersham Biosciences), were linked in tandem and run at a flow rate of 0.4 ml/min in phosphate-buffered saline containing 0.02% sodium azide (pH 7.4) using a fast protein liquid chromatography system (BioLogic, DuoFlow). Complex elution was monitored using absorbance at 280 nm. The apparent Stokes diameter of the complex was determined using high and low molecular weight calibration kits (Amersham Biosciences). Inset, calibration curve obtained using these standards.
A combination of TOCSY and NOESY spectrum was used to make sequence-specific resonance assignments of the individual amino acids of 4F in the 4F·DMPC complex (31). Fig. 3, A and B, show the amide/aromatic proton region and the fingerprint region, respectively, of the NOESY spectrum of 4F·DMPC complex obtained using a mixing time of 200 ms. TOCSY cross-peaks in Fig. 3A are shown in red. Sequential dNN(i, i + 1) NOESY cross-peaks for all of the residues in 4F are identified in Fig. 3A. In addition, dNN(i, i + 2) cross-peaks were observed for Asp1/Phe3, Phe3/Ala5, Phe6/Asp8, and Lys13/Lys15 pairs and are indicated in Fig. 3A. In Fig. 3B, intraresidue HN/CαH NOEs for all of the residues in 4F are labeled. A number of other long range (i, i + 3, and i, i + 4) NOEs involving side chain protons of the nonpolar amino acid residues (Trp2/Ala5, Trp2/Phe6, Phe3/Phe6, Phe3/Tyr7, Phe6/Val10, Tyr7/Val10, Tyr7/Ala11, Val10/Phe14, Ala11/Phe14, Phe14/Ala17, and Phe14/Phe18) were observed (Fig. 3, A and B). The methyl protons of the N terminus acetyl protecting group showed NOEs to Asp1, Trp2, Phe3, and Lys4 backbone NH protons as well as to the Trp2 aromatic ring protons (Fig. 3B). In addition, NOEs were observed between the aromatic ring protons of Trp2/Phe3 and Phe3/Tyr7 (Fig. 3A). NOEs between Trp2/Phe6 and Phe6/Tyr7 ring protons, although probably present, could not be assigned unambiguously because of resonance overlap. The observed (i, i + 1), (i, i + 2), (i, i + 3), and (i, i + 4) NOEs are schematically shown in Fig. 4. For the sequential NOEs, thickness of the line is in proportion to their intensity (Fig. 4). Proton chemical shifts are related to the secondary structures of peptides and proteins (29, 32). The plots of the difference between the observed chemical shift and the “coil” chemical shift (29) (Δδ = δobs - δc) for the CαH and the NH protons in 4F are shown in Fig. 5, A and B, respectively. A periodic (3-4 residues) variation is evident in the case of NH proton chemical shifts (Δδ) (Fig. 5B).
FIGURE 3.
Amide/aromatic (A) and fingerprint (B) regions of the NOESY spectrum (mixing time 200 ms) of 4F·DMPC-d67 discs in 5 mm potassium phosphate, pH 5.5, at 310 K. The spectrum was obtained at 900 MHz. A, sequential dNN(i, i + 1) and the observed dNN(i, i + 2) (marked with an asterisk) NOEs are labeled. Also labeled (in brackets) are the observed NOEs between aromatic ring protons of Trp2/Phe3, Phe3/Tyr7, Phe6/Tyr7, and Phe14/Phe18. A, TOCSY spectrum (obtained at 900 MHz, using 60-ms mixing time) shown in red, is overlaid onto the NOESY spectrum. B, intraresidue HN/HA NOEs as well as observed NOEs between the N-terminal acetyl group (ACE) and other peptide protons and long range (i, i + 3 and i, i + 4) NOEs involving hydrophobic and aromatic side chains are labeled. The observed NOE between the Trp side chain and the N-terminal acetyl group is marked with an asterisk.
FIGURE 4.
Schematic representation of the observed sequential and long range NOEs. Line thickness for the sequential NOEs is proportional to their measured intensity. Medium and long range NOEs were not classified as strong, medium, or weak. Other observed long range NOEs, especially those observed between side chain protons of the nonpolar residues, are described under “Materials and Methods.”
FIGURE 5.
A, a plot of the ΔδCαH(δobserved - δcoil) chemical shift for all the residues in 4F. Note that the CαH protons show an upfield shift compared with the corresponding coil values. B, a plot of the ΔδNH (δobserved - δcoil) chemical shift for all of the residues in 4F. Note the 3-4-residue periodicity in the NH proton chemical shifts. Random coil chemical shifts used were those reported in Ref. 56.
The distance and dihedral constraints were used to calculate NMR structures of the peptide associated with DMPC disc. Starting from a set of 100 initial extended structures of 4F, 70 structures were accepted based on the following criteria: (a) no NOE violations >0.5 Å, (b) no dihedral angle violations >5°, (c) root mean square (r.m.s.) difference for bond deviations from ideality <0.01 Å, and (d) r.m.s. difference for angle deviations from ideality <2°. To obtain the average NMR structure, average coordinates of the 70 accepted structures were energy-minimized. All of the distance and the dihedral angle constraints were enforced during the energy minimization of the average coordinates. The structural statistics for the 70 accepted NMR structures and the energy-minimized average structure of 4F are summarized in Table 1. The 70 accepted structures were superimposed onto the energy-minimized average structure using MATCH (Sybyl, version 7.2). The program MATCH performs an automatic least squares fit of two molecules that differ only in the coordinates of their atoms. Fig. 6 shows superposition of the Cα atoms of all the 70 accepted structures on the energy-minimized average NMR structure of 4F. The energy-minimized average structure is shown as a ribbon/tube (Fig. 6). The side chains of energy-minimized average structure are also shown (Fig. 6).
TABLE 1.
Structural statistics for the 70 accepted (<SA>) and the energy-minimized average (<SA>r) NMR structures of 4F associated with DMPC disc
| Parameter | Value |
|---|---|
| Experimental restraints | |
| Intraresidue NOEs | 81 |
| Sequential NOEs | 34 |
| Medium and long range NOEs (i, i + 2; i, i + 3; and i, i + 4) | 35 |
| Interresidue side chain NOEs | 70 |
| Hydrogen bonds (H-O distance) | 15 |
| Φ angles | 17 |
| Ψ angles | 16 |
|
Total |
268 |
| Mean r.m.s. deviation from experimental restraints (<SA> ± S.D.) | |
| Distance restraints (Å) | 0.060 ± 0.003 |
|
Dihedral angle (degrees) |
0.098 ± 0.119 |
| Mean r.m.s. deviation from ideal geometry (<SA> ± S.D.) | |
| Bonds (Å) | 0.005 ± 0.000 |
| Angles (degrees) | 0.685 ± 0.044 |
|
Impropers (degrees) |
0.580 ± 0.043 |
| Ramachandran statistics from PROCHECK-NMRa | |
|
Residues in most favored α-helical region |
100% |
| r.m.s. deviation from the average structure (Å) | |
| Backbone N, Cα, C | 0.583 |
|
All heavy atoms |
1.744 |
| Pairwise r.m.s. deviation analysis | |
| Average backbone pairwise r.m.s. deviation (Å) | 0.826 ± 0.245 |
| Average nonhydrogen atom pairwise r.m.s. deviation (Å) | 2.542 ± 0.452 |
|
X-PLOR potential energies |
||
|---|---|---|
| <SA> ± S.D. | <SA>rb | |
| kcal/mol | ||
| Eoverall | 106.850 ± 10.905 | 96.671 |
| Ebond | 7.891 ± 0.725 | 7.334 |
| Eangle | 41.099 ± 4.989 | 36.417 |
| Eimpropers | 9.921 ± 1.453 | 9.213 |
| Evdw | 8.801 ± 2.674 | 8.175 |
| ENOE | 39.097 ± 3.931 | 35.531 |
| Ecdih | 0.039 ± 0.064 | 0.000 |
See Ref. 55.
200 cycles of conjugate gradient minimization were used. All of the NMR constraints were enforced during the energy minimization of the average coordinates.
FIGURE 6.
A relaxed eye stereo view of the 70 accepted NMR structures superimposed on the energy-minimized average structure of 4F. The N terminus is shown toward the top, and the C terminus is toward the bottom. Note the distinct segregation of nonpolar and polar side chains along the long axis of the α-helix.
Fig. 7 shows a DMPC molecule with different protons labeled. To observe intermolecular NOEs between peptide and lipid protons, NOESY spectra were obtained with the peptide and protonated lipid and peptide and perdeuterated lipid in 90% H2O and 10% D2O. Fig. 8 shows an overlay view of the two NOESY spectra obtained using a mixing time of 200 ms. The spectrum obtained with protonated DMPC is shown in black, and that with perdeuterated DMPC is shown in red. The assignments of the observed lipid protons are marked beside the observed NOESY cross-peaks (Fig. 8). Fig. 9A shows the one-dimensional horizontal slices of the NOESY spectrum of the 4F·DMPC complex in the presence of protonated DMPC. The corresponding lipid protons are indicated beside the horizontal slices (Fig. 9A). Fig. 9B shows the one-dimensional horizontal slice of the NOESY spectrum corresponding to the (CH2)n protons of the DMPC alkyl chain and the observed peptide aromatic proton signals. To compare intermolecular peptide-lipid NOEs, NOESY spectra of 2F·DMPC and 4F·DMPC complexes were recorded under identical conditions. Fingerprint regions of the spectra obtained using a 200-ms mixing time and processed using identical window functions are shown in Fig. 10, A and B, for 2F·DMPC and 4F·DMPC, respectively. It is evident that, compared with 2F·DMPC, stronger peptide-lipid NOEs are observed in the 4F·DMPC complex (Fig. 10). This is further shown in Fig. 11, which compares one-dimensional horizontal slices of NOESY cross-peaks between 2F and 4F and methyl protons of the choline headgroup in 2F·DMPC and 4F·DMPC, respectively. It is also important to note that intensities of the NOE cross-peaks between DMPC protons are also different between 2F·DMPC and 4F·DMPC complexes (Fig. 10). For example, although there is a relatively strong NOE cross-peak between DMPC 2.CH and βCH2 protons in 4F·DMPC complex, this NOE is not seen in the case of the 2F·DMPC complex (Fig. 10).
FIGURE 7.
Structure of a DMPC molecule. The various protons in the molecule are labeled. Various atoms are color-coded as follows. Blue, nitrogen; orange, phosphorus; red, oxygen; green, carbon.
FIGURE 8.
An overlay view of the NOESY spectrum (mixing time 200 ms) of 4F·DMPC-d67 (shown in red) and 4F·DMPC (shown in black). The NOESY cross-peaks of DMPC protons are labeled.
FIGURE 9.
A, horizontal one-dimensional slices extracted from the NOESY spectrum (mixing time 200 ms) of 4F in the presence of protonated DMPC. The corresponding lipid resonances are shown. B, horizontal one-dimensional slice of the NOESY spectrum (mixing time 200 ms) of 4F in the presence of protonated DMPC, showing intermolecular NOEs between lipid (CH2)n protons and the peptide aromatic ring (Trp2, Phe3, Phe6, Tyr7, Phe14, and Ph18) protons. Peak assignments are shown in the inset. The intramolecular NOE between lipid (CH2)n protons and the 2.CH proton is at 5.222 ppm.
FIGURE 10.
A, fingerprint region of the NOESY spectrum (mixing time 200 ms) of 2F·DMPC. B, fingerprint region of the NOESY spectrum (mixing time 200 ms) of 4F·DMPC. Both of the spectra were processed using an identical window function. Note that compared with 2F·DMPC (A), stronger peptide-lipid NOEs are observed in the 4F·DMPC (B) complex. It is also important to note that intensities of the NOE cross-peaks of DMPC protons are different between 2F·DMPC (A) and 4F·DMPC (B) complexes. For example, although there is a relatively strong NOE cross-peak between DMPC 2.CH and βCH2 protons in the 4F·DMPC complex (B), this NOE is not seen in the case of the 2F·DMPC complex (A).
FIGURE 11.
Comparison of one-dimensional horizontal slices of NOESY cross-peaks between 2F and 4F and methyl protons of choline headgroup in 2F·DMPC and 4F·DMPC, respectively. NOESY peaks between methyl protons of the choline headgroup and Trp2 indole ring proton (HE1) are indicated by arrows.
DISCUSSION
We have shown that in a set of class A amphipathic helical peptides, despite similar physical-chemical properties, only some of the peptide-lipid complexes are antiatherogenic (33). Such complexes are also involved in improving the antiatherogenic properties of HDL in atherosclerosis-sensitive animal models (33). We undertook the present studies to understand subtle differences in the peptide-lipid complex properties that lead to differences in the biological properties.
Peptide 4F Forms a Well Defined Class A Amphipathic α-Helix—The NMR structure of 2F has been reported by us previously (15). It has been shown previously that the end group blockage increases the helical content of the unblocked peptide 18A by removing the destabilizing interactions of the helix macrodipole with the charged termini (13). It is worthy of note that in an α-helix the N terminus acetyl and the C terminus amide blocking groups also provide for the formation of two additional hydrogen bonds (one at each end) between residues i (acceptor) and i + 4 (donor), compared with the unblocked peptide. The N-terminal acetyl group shows NOEs to Asp1, Trp2, Phe3, and Lys4 backbone NH protons as well as to Trp2 ring protons (Fig. 3B), suggesting its involvement in helix stabilization.
The observation of NOEs between hydrophobic side chains spaced at (i, i + 3) and (i, i + 4) positions in the linear sequence (Trp2/Phe6, Trp2/Ala5, Phe3/Tyr7, Phe6/Val10, Tyr7/Ala11, and Phe14/Phe18) (Fig. 3, A and B) is also consistent with an α-helical structure of the peptide. In addition, the aromatic rings of Trp2, Phe3, Phe6, and Tyr7 are sufficiently close in space to give rise to the observed NOEs between their ring protons (Fig. 3A).
The pattern of stronger dNN(i, i + 1) and weaker dαN(i, i + 1) NOEs as well as the observation of several well resolved dαN(i, i + 4) and dαβ(i, i + 3) NOEs (Fig. 4) confirms that 4F adopts an α-helical structure in DMPC discs. A few of the dαN(i, i + 4) and dαβ (i, i + 3) NOEs could not be confirmed due to resonance overlap but are likely to be present as well (Fig. 4). The 1H NMR chemical shifts in peptides and proteins are dependent on the secondary structure (29, 32) (e.g. CαH protons move upfield (relative to their random coil value) upon helix formation). An inspection of Fig. 5A reveals that all of the CαH protons in 4F experience an upfield chemical shift relative to their “coil” values (29). Thus, the chemical shifts of CαH protons are consistent with an α-helical structure of 4F.
The NH protons in 4F show a periodic 3-4-residue variation in the (Δδ) chemical shifts (Fig. 5B). The periodicity in the NH proton chemical shifts has been related to the amphipathicity of the helix (15, 32). The periodicity in the NH proton chemical shift is thought to result from a shortening (strengthening) of the hydrogen bonds on the hydrophobic side (a medium of low dielectric) and a lengthening (weakening) of the hydrogen bonds on the hydrophilic side (a medium of high dielectric) of the helix. The NMR structures obtained based on the distance and dihedral angle constraints show that the peptide 4F forms a well defined class A amphipathic α helix with hydrophobic residues clustered on one side and hydrophilic residues clustered on the opposite side of the long axis of the helix (Fig. 6).
Peptide 4F Interacts with the Lipid Acyl Chains and Oriented Parallel to the Plane of the Bilayer—To determine intermolecular peptide/lipid NOEs, NOESY spectra of the peptide 4F obtained with protonated DMPC and deuterated DMPC in H2O were compared. As in the case of the 2F·DMPC complex reported by us previously (15), a number of intermolecular peptide-lipid NOEs were identified in the case of protonated DMPC (Fig. 8). These NOEs were further identified by extracting one-dimensional horizontal slices of the NOESY spectrum obtained with protonated DMPC. Like 2F (15), a number of NOEs were seen between lipid alkyl chain methylene protons, (CH2)n, and the peptide aromatic protons (Fig. 9A). It is important to note that these intermolecular NOEs were seen with aromatic ring protons of the residues that are distributed throughout in the linear sequence (e.g. Trp2, Phe3, Phe6, Tyr7, Phe14, and Phe18) (Fig. 9B). Similar patterns of intermolecular NOEs between DMPC methylene protons, (CH2)n, and 4F aromatic ring protons were seen at different mixing times (50, 100, 150, and 200 ms) used in the NOESY experiments (supplemental Figs. 1 and 2). This observation rules out the possibility of spin diffusion and strongly suggests that, similar to 2F (15), 4F is oriented parallel to the plane of the membrane (and, therefore, perpendicular to the lipid acyl chains; “belt orientation”) in the DMPC discoidal particles.
To probe and compare the microenvironments of Lys residues in 2F·DMPC (15, 34) and 4F·DMPC complexes, labeling of the Lys residues of 2F and 4F with 13CH3 groups was performed by the reductive methylation procedure of Jentoft and Dearborn (35), as described earlier for apoA-I discoidal complexes (36). The chemical shifts of 13C-labeled Lys residues in 2F·DMPC and 4F·DMPC complexes were almost identical, indicating that the microenvironments of Lys residues in the two complexes are similar (13C NMR spectra not shown). A similar pattern of bis(sulfosuccinimidyl) suberate cross-linked products resolved on the SDS-PAGE (results not shown) further indicates that Lys side chains are oriented similarly in 2F·DMPC and 4F·DMPC discs.
Peptide 4F Is Located Closer to the DMPC Headgroup in 4F·DMPC Complex than 2F in 2F·DMPC Complex—Compared with 2F·DMPC, the observation of stronger NOEs between 4F and DMPC methyl protons of the choline (-N-(CH3)3) headgroup (Fig. 11) as well as βCH2 protons (Fig. 10B), indicates that 4F is located closer to the DMPC headgroup than 2F in DMPC discoidal particles. Both 2F and 4F contain a single Trp residue as the second amino acid residue in the primary sequence (Fig. 1). It is interesting to note that methyl protons of the DMPC choline headgroup show a much stronger NOE to Trp indole ring proton in the 4F·DMPC complex than in the 2F·DMPC complex (Fig. 11). Based on the steady-state fluorescence quenching experiments with water-soluble quencher acrylamide, we observed that Trp residues in both 2F and 4F DMPC discoidal particles are equally inaccessible (data not shown) to the quencher compared with peptide free in solution. The Trp indole ring possesses hydrogen bonding ability, with the NH group as hydrogen donor. In addition, the aromatic rings of Trp, Phe, and Tyr can participate in cation-π interactions (37). It is important to note that, compared with Leu, Phe has an about 12% larger hydrophobic surface area (38). In addition, compared with Leu, Phe shows a preference for the water-lipid interfacial region (39). Since, compared with 2F, 4F has two additional Phe residues located on the nonpolar face of the helix, we propose that compared with 2F·DMPC, additional cation-π interactions between choline headgroup and Phe residues of 4F in the 4F·DMPC complex, facilitate the location of 4F closer to the headgroup region. Such a preferential interaction of 4F with the lipid headgroup also results in changes in the lipid headgroup as evidenced by the NOE between lipid 2.CH and βCH2 protons in 4F·DMPC but not in the case of 2F·DMPC (Fig. 10).
It has been shown earlier that 4F exerts a greater degree of penetration into vesicles of pure phosphatidylcholine in the absence of cholesterol than into vesicles of phosphatidylcholine and cholesterol (40). 4F promotes the separation of cholesterol from the phospholipid, resulting in the formation of cholesterol crystallites, even at mol fractions of cholesterol as low as 0.3 (40). Preferential interaction of 4F with the lipid headgroup in the discoidal complexes observed in this study helps explain these earlier observations in lipid vesicles (40).
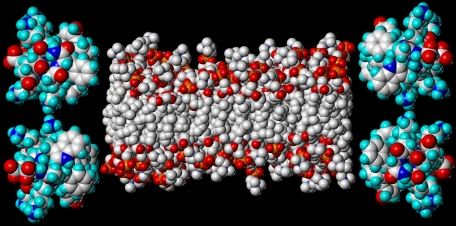
Proposed Model of 4F·DMPC Discoidal Structure—Based on the similar size and similar lipid/peptide ratio in 2F·DMPC and 4F·DMPC discoidal particles, the overall structure of the two particles is likely to be quite similar. The discoidal morphology of both 2F·DMPC and 4F·DMPC complexes is confirmed by negative stain transmission electron microscopy (results not shown). However, as discussed above, 4F is located closer to the DMPC headgroup than 2F in the peptide-DMPC discs. Also, the conformation of the DMPC headgroup is different in 4F·DMPC than in 2F·DMPC, as evidenced by the presence of stronger NOE between 2.CH and βCH2 protons seen in the case of 4F·DMPC complex and the absence of this NOE in the case of 2F·DMPC complex (Fig. 10). This is presumably because of overall reduction in the thickness of the DMPC bilayer in the case of 4F·DMPC discoidal complex compared with 2F·DMPC complex (41). It is important to note that the interfacial region contributes equally to the overall thickness of a lipid bilayer as the hydrocarbon region (41). Our proposed model for the 4F·DMPC discoidal particles is shown in Fig. 12.
FIGURE 12.
A molecular model showing four average energy-minimized molecules of 4F oriented parallel to the plane of the membrane in a head-to-tail fashion (15). Molecular models were generated using the program SYBYL. DMPC molecules were extracted from a lipid bilayer containing 120 DMPC molecules after a molecular dynamic simulation of 200 ns (54). Note that nonpolar faces of 4F molecules are located close to the lipid headgroup region. This location is consistent with the observed NOEs between aromatic ring protons of 4F and DMPC headgroup protons.
Relevance to Biological Activities of 4F—Dyslipidemia is associated with oxidative stress and the generation of biologically active oxidized lipids. Biologically active oxidized phospholipids can initiate and modulate many of the cellular events attributed to the pathogenesis of atherosclerosis (42). A novel family of atherogenic oxidized choline glycerophospholipids (oxPCCD36) that are formed during the oxidization of low density lipoprotein by multiple pathways and are present in vivo at sites of enhanced oxidative stress have been isolated and structurally characterized (43, 44). oxPCCD36 serve as high affinity ligands for the macrophage scavenger receptor CD36 (43) and facilitate macrophage foam cell formation through recognition and uptake of oxidized low density lipoprotein by CD36 (45). It has been suggested that the selectively blocking the interaction between oxPCCD36 and CD36 might be an useful therapeutic approach against atherothrombotic diseases (46). The conformation of a prototypic high affinity CD36 ligand, 1-palmitoyl-2-(5-keto-6-octene-dioyl)phosphatidylcholine (KOdiA-PC), near the hydrophobic-hydrophilic interface within membrane bilayers was probed by determining multiple critical internuclear distances using NOESY (47). It was shown that the truncated oxidized sn-2 fatty acid chain of KOdiA-PC within membranes protrudes into the aqueous phase (47). It was hypothesized that this allows a direct physical access of the KOdiA-PC to the cell surface macrophage CD36 receptor (47). It has been shown recently that this unusual conformation for an oxidized phospholipid within a membrane may not be unique to KOdiA-PC but rather might represent a more global phenomenon of oxidized phospholipids, enabling direct physical contact between pattern recognition receptor and molecular pattern ligand (48, 49).
We have shown previously that the two homologous apoA-I mimetic peptides, 3F-2 and 3F14, differ in their in vitro antiatherogenic properties (50), and the peptide 3F-2 inhibits atherosclerosis in female apoE null mice, whereas 3F14 does not (51). Using several NMR methods, we have shown that these two peptides insert to different extents into lipid vesicles (51). 3F-2 with aromatic residues at the center of the nonpolar face partitions closer to the phospholipid headgroup in lipid vesicles compared with 3F14 (51). We have shown that compared with 2F, 4F exhibits more potent in vitro antiatherogenic properties (16). The present study provides further support to our hypothesis that peptides located closer to the lipid headgroup exhibit potent antiatherogenic properties.
It is likely that a potential mechanism of antiatherogenic property of 4F is due to its preferential association with the lipid headgroups, as demonstrated in the present study for the first time. This may allow the shielding of the oxidized phospholipids at the cell surface by 4F, thereby preventing interaction of oxidized phospholipids with their receptor (e.g. CD36).
It has been shown recently using surface plasmon resonance that 4F bound oxidized lipids with much higher affinity than human apoA-I (52). The extraordinary ability of 4F to bind proinflammatory oxidized lipids is suggested to account for its remarkable anti-inflammatory properties (52). It is likely that 2F and 4F bind to membranes containing oxidized lipids differently (53). It is interesting to note that the peptide 3F-2, which has been shown to inhibit atherosclerosis in apoE null mice (51), also bound oxidized phospholipids with very high affinity similar to 4F (52). 3F-2 has been shown to associate with the lipid headgroup (51). The results obtained in the present study further support our hypothesis that antiatherogenic peptides associate with the lipid headgroup and bind oxidized lipids with high affinity.
Supplementary Material
Acknowledgments
We thank Professor Axel T. Brünger for the access to online version 3.851 of X-PLOR. Professor James H. Prestegard is acknowledged for access to the Southeast Collaboratory for Biomolecular NMR facility at the University of Georgia. We acknowledge S. W. Simon Sham for the NMR experiments at 600 MHz. We thank Dr. Christine Angela Curcio and Jeffrey Messinger for negative stain transmission electron microscopy. We thank Martin K. Jones for help during the computational work and Tamara Keenum for expert technical assistance.
The atomic coordinates of NMR structures of 4F in 4F-DMPC disc, 1H chemical shifts, and distance and dihedral constraints (accession number 20034), have been deposited in the Biological Magnetic Resonance Data Bank (http://www.bmrb.wisc.edu).
This work was supported, in whole or in part, by National Institutes of Health Grants RO1HL089328, PO1 HL34343 (NHLBI) and CA-13148 (NCI). The 600-MHz CryoProbe was funded by 1S10RR021064-01A1 (NCRR, National Institutes of Health). This research benefited from activities at the SE Collaboratory for High-Field Biomolecular NMR, a research resource at the University of Georgia, funded by NIGMS, National Institutes of Health (NIG MSEC Grant GM66340) and the Georgia Research Alliance. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
Footnotes
The abbreviations used are: ApoA-I, apolipoprotein A-I; DMPC, 1,2-dimyristoyl-sn-glycero-3-phosphocholine; KOdiA-PC, 1-palmitoyl-2-(5-keto-6-octene-dioyl)phosphatidylcholine; NOE, nuclear Overhauser effect; NOESY, NOE spectroscopy; TOCSY, total correlation spectroscopy; r.m.s., root mean square; HDL, high density lipoprotein.
References
- 1.Duverger, N., Viglietta, C., Berthou, L., Emmanuel, F., Tailleux, A., Parmentier-Nihoul, L., Laine, B., Fievet, C., Castro, G., Fruchart, J. C., Houbebine, L. M., and Denefle, P. (1996) Arterioscler. Thromb. Vasc. Biol. 161424 -1429 [DOI] [PubMed] [Google Scholar]
- 2.Plump, A. S., Scott, C. J., and Breslow, J. L. (1994) Proc. Natl. Acad. Sci. U. S. A. 919607 -9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin, E. M., Ishida, B. Y., Clift, S. M., and Krauss, R. M. (1991) Proc. Natl. Acad. Sci. 88 434-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin, E. M., Krauss, R. M., Spangler, E. A., Verstuyft, J. G., and Clift, S. M. (1991) Nature 353265 -267 [DOI] [PubMed] [Google Scholar]
- 5.Schultz, J. R., Gong, E. L., McCall, M. R., Nichols, A. V., Clift, S. M., and Rubin, E. M. (1992) J. Biol. Chem. 26721630 -21636 [PubMed] [Google Scholar]
- 6.Tangirala, R. K., Tsukamoto, K., Chun, S. H., Usher, D., Pure, E., and Rader, D. J. (1999) Circulation 1001816 -1822 [DOI] [PubMed] [Google Scholar]
- 7.Tardif, J. C., Gregoire, J., L'Allier, P. L., Ibrahim, R., Lesperance, J., Heinonen, T. M., Kouz, S., Berry, C., Basser, R., Lavoie, M. A., Guertin, M. C., Rodes-Cabau, J., for The Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators (2007) JAMA 2971675 -1682 [DOI] [PubMed] [Google Scholar]
- 8.Segrest, J. P., De, L. H., Dohlman, J. G., Brouillette, C. G., and Anantharamaiah, G. M. (1990) Proteins 8 103-117 [DOI] [PubMed] [Google Scholar]
- 9.Segrest, J. P., Jones, M. K., De Loof, H., Brouillette, C. G., Venkatachalapathi, Y. V., and Anantharamaiah, G. M. (1992) J. Lipid Res. 33141 -166 [PubMed] [Google Scholar]
- 10.Segrest, J. P., Garber, D. W., Brouillette, C. G., Harvey, S. C., and Anantharamaiah, G. M. (1994) Adv. Protein Chem. 45303 -369 [DOI] [PubMed] [Google Scholar]
- 11.Anantharamaiah, G. M., Jones, J. L., Brouillette, C. G., Schmidt, C. F., Chung, B. H., Hughes, T. A., Bhown, A. S., and Segrest, J. P. (1985) J. Biol. Chem. 26010248 -10255 [PubMed] [Google Scholar]
- 12.Chung, B. H., Anatharamaiah, G. M., Brouillette, C. G., Nishida, T., and Segrest, J. P. (1985) J. Biol. Chem. 26010256 -10262 [PubMed] [Google Scholar]
- 13.Venkatachalapathi, Y. V., Phillips, M. C., Epand, R. M., Epand, R. F., Tytler, E. M., Segrest, J. P., and Anantharamaiah, G. M. (1993) Proteins 15 349-359 [DOI] [PubMed] [Google Scholar]
- 14.Mishra, V. K., Palgunachari, M. N., Anantharamaiah, G. M., Jones, M. K., Segrest, J. P., and Krishna, N. R. (2001) Peptides 22567 -573 [DOI] [PubMed] [Google Scholar]
- 15.Mishra, V. K., Anantharamaiah, G. M., Segrest, J. P., Palgunachari, M. N., Chaddha, M., Sham, S. W. S., and Krishna, N. R. (2006) J. Biol. Chem. 2816511 -6519 [DOI] [PubMed] [Google Scholar]
- 16.Datta, G., Chaddha, M., Hama, S., Navab, M., Fogelman, A. M., Garber, D. W., Mishra, V. K., Epand, R. M., Epand, R. F., Lund-Katz, S., Phillips, M. C., Segrest, J. P., and Anantharamaiah, G. M. (2001) J. Lipid Res. 421096 -1104 [PubMed] [Google Scholar]
- 17.Navab, M., Anantharamaiah, G. M., Reddy, S. T., Hama, S., Hough, G., Grijalva, V. R., Wagner, A. C., Frank, J. S., Datta, G., Garber, D., and Fogelman, A. M. (2004) Circulation 1093215 -3220 [DOI] [PubMed] [Google Scholar]
- 18.Navab, M., Anantharamaiah, G. M., Hama, S., Hough, G., Reddy, S. T., Frank, J. S., Garber, D. W., Handattu, S., and Fogelman, A. M. (2005) Arterioscler. Thromb. Vasc. Biol. 251426 -1432 [DOI] [PubMed] [Google Scholar]
- 19.Ou, J., Ou, Z., Jones, D. W., Holzhauer, S., Hatoum, O. A., Ackerman, A. W., Weihrauch, D. W., Gutterman, D. D., Guice, K., Oldham, K. T., Hillery, C. A., and Pritchard, K. A., Jr. (2003) Circulation 1072337 -2341 [DOI] [PubMed] [Google Scholar]
- 20.Ou, Z., Ou, J., Ackerman, A. W., Oldham, K. T., and Pritchard, K. A., Jr. (2003) Circulation 1071520 -1524 [DOI] [PubMed] [Google Scholar]
- 21.Kruger, A. L., Peterson, S., Turkseven, S., Kaminski, P. M., Zhang, F. F., Quan, S., Wolin, M. S., and Abraham, N. G. (2005) Circulation 1113126 -3134 [DOI] [PubMed] [Google Scholar]
- 22.Peterson, S. J., Husney, D., Kruger, A. L., Olszanecki, R., Ricci, F., Rodella, L. F., Stacchiotti, A., Rezzani, R., McClung, J. A., Aronow, W. S., Ikehara, S., and Abraham, N. G. (2007) J. Pharmacol. Exp. Ther. 322514 -520 [DOI] [PubMed] [Google Scholar]
- 23.Peterson, S. J., Drummond, G., Kim, D. H., Li, M., Kruger, A. L., Ikehara, S., and Abraham, N. G. (2008) J. Lipid Res. 491658 -1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Lenten, B. J., Wagner, A. C., Anantharamaiah, G. M., Garber, D. W., Fishbein, M. C., Adhikary, L., Nayak, D. P., Hama, S., Navab, M., and Fogelman, A. M. (2002) Circulation 1061127 -1132 [DOI] [PubMed] [Google Scholar]
- 25.Van Lenten, B. J., Wagner, A. C., Navab, M., Anantharamaiah, G. M., Hui, E. K.-W., Nayak, D. P., and Fogelman, A. M. (2004) Circulation 1103252 -3258 [DOI] [PubMed] [Google Scholar]
- 26.Gupta, H., Dai, L., Datta, G., Garber, D. W., Grenett, H., Li, Y., Mishra, V., Palgunachari, M. N., Handattu, S., Gianturco, S. H., Bradley, W. A., Anantharamaiah, G. M., and White, C. R. (2005) Circ. Res. 97236 -243 [DOI] [PubMed] [Google Scholar]
- 27.Bloedon, L. T., Dunbar, R., Duffy, D., Pinell-Salles, P., Norris, R., DeGroot, B. J., Movva, R., Navab, M., Fogelman, A. M., and Rader, D. J. (2008) J. Lipid Res. 491344 -1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wuthrich, K., Billeter, M., and Braun, W. (1983) J. Mol. Biol. 169949 -961 [DOI] [PubMed] [Google Scholar]
- 29.Wishart, D. S., Sykes, B. D., and Richards, F. M. (1991) J. Mol. Biol. 222311 -333 [DOI] [PubMed] [Google Scholar]
- 30.Gibbs, A. C., Kondejewski, L. H., Gronwald, W., Nip, A. M., Hodges, R. S., Sykes, B. D., and Wishart, D. S. (1998) Nat. Struct. Biol. 5284 -288 [DOI] [PubMed] [Google Scholar]
- 31.Basus, V. J. (1989) Methods Enzymol. 177132 -149 [DOI] [PubMed] [Google Scholar]
- 32.Wishart, D. S., and Sykes, B. D. (1994) Methods Enzymol. 239363 -392 [DOI] [PubMed] [Google Scholar]
- 33.Datta, G., Epand, R. F., Epand, R. M., Chaddha, M., Kirksey, M. A., Garber, D. W., Lund-Katz, S., Phillips, M. C., Hama, S., Navab, M., Fogelman, A. M., Palgunachari, M. N., Segrest, J. P., and Anantharamaiah, G. M. (2004) J. Biol. Chem. 27926509 -26517 [DOI] [PubMed] [Google Scholar]
- 34.Lund-Katz, S., Phillips, M. C., Mishra, V. K., Segrest, J. P., and Anantharamaiah, G. M. (1995) Biochemistry 349219 -9226 [DOI] [PubMed] [Google Scholar]
- 35.Jentoft, N., and Dearborn, D. G. (1979) J. Biol. Chem. 2544359 -4365 [PubMed] [Google Scholar]
- 36.Sparks, D. L., Phillips, M. C., and Lund-Katz, S. (1992) J. Biol. Chem. 26725830 -25838 [PubMed] [Google Scholar]
- 37.Dougherty, D. A. (1996) Science 271163 -168 [DOI] [PubMed] [Google Scholar]
- 38.Livingstone, J. R., Spolar, R. S., and Record, M. T., Jr. (1991) Biochemistry 304237 -4244 [DOI] [PubMed] [Google Scholar]
- 39.Wimley, W. C., and White, S. H. (1996) Nat. Struct. Mol. Biol. 3842 -848 [DOI] [PubMed] [Google Scholar]
- 40.Epand, R. M., Epand, R. F., Sayer, B. G., Melacini, G., Palgulachari, M. N., Segrest, J. P., and Anantharamaiah, G. M. (2004) Biochemistry 435073 -5083 [DOI] [PubMed] [Google Scholar]
- 41.Hristova, K., Wimley, W. C., Mishra, V. K., Anantharamiah, G. M., Segrest, J. P., and White, S. H. (1999) J. Mol. Biol. 29099 -117 [DOI] [PubMed] [Google Scholar]
- 42.Berliner, J. A., and Watson, A. D. (2005) N. Engl. J. Med. 3539 -11 [DOI] [PubMed] [Google Scholar]
- 43.Podrez, E. A., Poliakov, E., Shen, Z., Zhang, R., Deng, Y., Sun, M., Finton, P. J., Shan, L., Gugiu, B., Fox, P. L., Hoff, H. F., Salomon, R. G., and Hazen, S. L. (2002) J. Biol. Chem. 27738503 -38516 [DOI] [PubMed] [Google Scholar]
- 44.Podrez, E. A., Poliakov, E., Shen, Z., Zhang, R., Deng, Y., Sun, M., Finton, P. J., Shan, L., Febbraio, M., Hajjar, D. P., Silverstein, R. L., Hoff, H. F., Salomon, R. G., and Hazen, S. L. (2002) J. Biol. Chem. 27738517 -38523 [DOI] [PubMed] [Google Scholar]
- 45.Podrez, E. A., Febbraio, M., Sheibani, N., Schmitt, D., Silverstein, R. L., Hajjar, D. P., Cohen, P. A., Frazier, W. A., Hoff, H. F., and Hazen, S. L. (2000) J. Clin. Invest. 1051095 -1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson, S. P., and Calkin, A. C. (2007) Nat. Med. 131015 -1016 [DOI] [PubMed] [Google Scholar]
- 47.Li, X. M., Salomon, R. G., Qin, J., and Hazen, S. L. (2007) Biochemistry 465009 -5017 [DOI] [PubMed] [Google Scholar]
- 48.Greenberg, M. E., Li, X. M., Gugiu, B. G., Gu, X., Qin, J., Salomon, R. G., and Hazen, S. L. (2008) J. Biol. Chem. 2832385 -2396 [DOI] [PubMed] [Google Scholar]
- 49.Hazen, S. L. (2008) J. Biol. Chem. 28315527 -15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epand, R. M., Epand, R. F., Sayer, B. G., Datta, G., Chaddha, M., and Anantharamaiah, G. M. (2004) J. Biol. Chem. 27951404 -51414 [DOI] [PubMed] [Google Scholar]
- 51.Handattu, S. P., Garber, D. W., Horn, D. C., Hughes, D. W., Berno, B., Bain, A. D., Mishra, V. K., Palgunachari, M. N., Datta, G., Anantharamaiah, G. M., and Epand, R. M. (2007) J. Biol. Chem. 2821980 -1988 [DOI] [PubMed] [Google Scholar]
- 52.Van Lenten, B. J., Wagner, A. C., Jung, C. L., Ruchala, P., Waring, A. J., Lehrer, R. I., Watson, A. D., Hama, S., Navab, M., Anantharamaiah, G. M., and Fogelman, A. M. (2008) J. Lipid Res. 492302 -2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anantharamaiah, G. M., Mishra, V. K., Garber, D. W., Datta, G., Handattu, S. P., Palgunachari, M. N., Chaddha, M., Navab, M., Reddy, S. T., Segrest, J. P., and Fogelman, A. M. (2007) J. Lipid Res. 481915 -1923 [DOI] [PubMed] [Google Scholar]
- 54.Gurtovenko, A. A., Patra, M., Karttunen, M., and Vattulainen, I. (2004) Biophys. J. 86,3461 -3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laskowski, R. A., Rullmannn, J. A., MacArthur, M. W., Kaptein, R., and Thornton, J. M. (1996) J Biomol. NMR 8 477-486 [DOI] [PubMed] [Google Scholar]
- 56.Wuthrich, K. (1986) NMR of Proteins and Nucleic Acids, John Wiley & Sons, Inc., New York
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.