Abstract
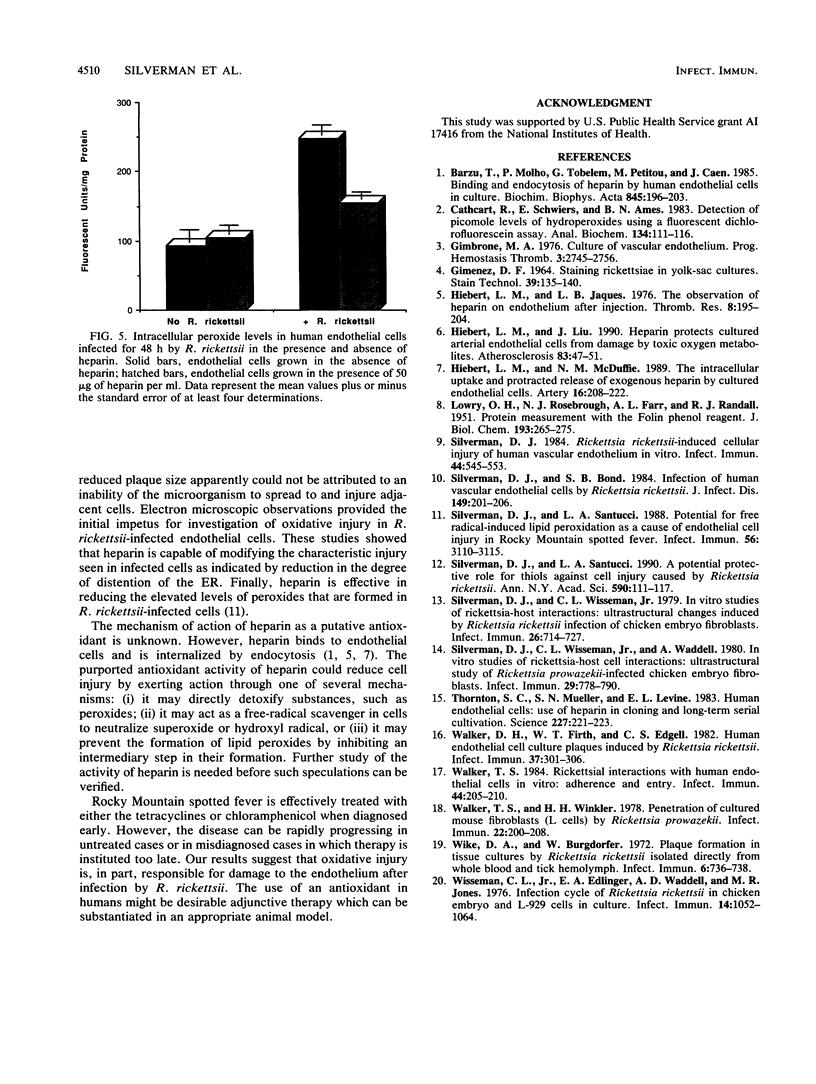
Routine culture of endothelial cells currently includes the use of heparin, which significantly reduces cell doubling time and increases cell population size. Heparin protects cultured arterial endothelial cells from damage by toxic oxygen metabolites produced by the action of xanthine and xanthine oxidase. Because of our hypothesis implicating free radicals in cell injury caused by Rickettsia rickettsii, we have carried out a series of experiments to examine the effects of heparin on injury to endothelial cells infected by this microorganism. These studies showed that heparin does not inhibit replication of R. rickettsii in the cytoplasm of endothelial cells. Furthermore, heparin appears to exhibit a protective effect on the infected host cell as measured by (i) reduced plaque size, (ii) increased longevity of the cell monolayer, (iii) reduction in the amount of lactic dehydrogenase released from infected cells, and (iv) reduction in the levels of intracellular peroxides formed in infected cells. Electron microscopic studies also show a significant reduction in dilatation of the rough-surfaced endoplasmic reticulum of the infected cells in the presence of heparin. These observations appear to lend additional support to involvement of an oxidative mechanism in human endothelial cell injury caused by R. rickettsii.
Full text
PDF

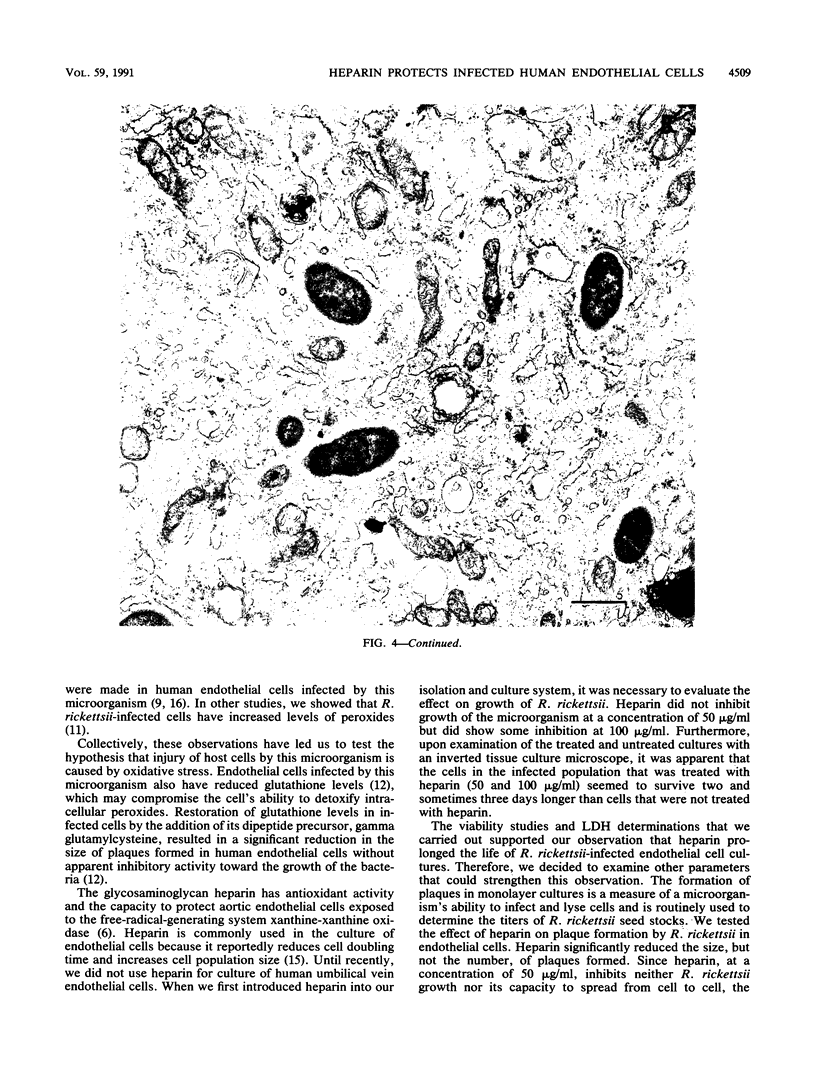
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bârzu T., Molho P., Tobelem G., Petitou M., Caen J. Binding and endocytosis of heparin by human endothelial cells in culture. Biochim Biophys Acta. 1985 May 30;845(2):196–203. doi: 10.1016/0167-4889(85)90177-6. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Ames B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983 Oct 1;134(1):111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Hiebert L. M., Jaques L. B. The observation of heparin on endothelium after injection. Thromb Res. 1976 Feb;8(2):195–204. doi: 10.1016/0049-3848(76)90262-0. [DOI] [PubMed] [Google Scholar]
- Hiebert L. M., Liu J. M. Heparin protects cultured arterial endothelial cells from damage by toxic oxygen metabolites. Atherosclerosis. 1990 Jul;83(1):47–51. doi: 10.1016/0021-9150(90)90129-7. [DOI] [PubMed] [Google Scholar]
- Hiebert L. M., McDuffie N. M. The intracellular uptake and protracted release of exogenous heparins by cultured endothelial cells. Artery. 1989;16(4):208–222. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Silverman D. J., Bond S. B. Infection of human vascular endothelial cells by Rickettsia rickettsii. J Infect Dis. 1984 Feb;149(2):201–206. doi: 10.1093/infdis/149.2.201. [DOI] [PubMed] [Google Scholar]
- Silverman D. J. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect Immun. 1984 Jun;44(3):545–553. doi: 10.1128/iai.44.3.545-553.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Santucci L. A. A potential protective role for thiols against cell injury caused by Rickettsia rickettsii. Ann N Y Acad Sci. 1990;590:111–117. doi: 10.1111/j.1749-6632.1990.tb42213.x. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Santucci L. A. Potential for free radical-induced lipid peroxidation as a cause of endothelial cell injury in Rocky Mountain spotted fever. Infect Immun. 1988 Dec;56(12):3110–3115. doi: 10.1128/iai.56.12.3110-3115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr In vitro studies of rickettsia-host cell interactions: ultrastructural changes induced by Rickettsia rickettsii infection of chicken embryo fibroblasts. Infect Immun. 1979 Nov;26(2):714–727. doi: 10.1128/iai.26.2.714-727.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. In vitro studies of Rickettsia-host cell interactions: ultrastructural study of Rickettsia prowazekii-infected chicken embryo fibroblasts. Infect Immun. 1980 Aug;29(2):778–790. doi: 10.1128/iai.29.2.778-790.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Firth W. T., Edgell C. J. Human endothelial cell culture plaques induced by Rickettsia rickettsii. Infect Immun. 1982 Jul;37(1):301–306. doi: 10.1128/iai.37.1.301-306.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S. Rickettsial interactions with human endothelial cells in vitro: adherence and entry. Infect Immun. 1984 May;44(2):205–210. doi: 10.1128/iai.44.2.205-210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Burgdorfer W. Plaque formation in tissue cultures by Rickettsia rickettsi isolated directly from whole blood and tick hemolymph. Infect Immun. 1972 Nov;6(5):736–738. doi: 10.1128/iai.6.5.736-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Edlinger E. A., Waddell A. D., Jones M. R. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect Immun. 1976 Oct;14(4):1052–1064. doi: 10.1128/iai.14.4.1052-1064.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]