Abstract
Reactive oxygen species (ROS) have been implicated in vascular smooth muscle cell (VSMC) apoptosis, a hallmark of advanced atherosclerotic lesions. Transient oxidation and inactivation of protein-tyrosine phosphatases play a critical role in cellular response to ROS production. However, the function of leukocyte antigen-related (LAR) protein-tyrosine phosphatase in ROS signaling is not known. To determine the expression of LAR in ROS-induced apoptosis, we investigated hydrogen peroxide-induced cell death and signaling in aortic VSMCs from wild-type and LAR-/- mice. Histone-associated DNA fragmentation and caspase-3/7 activity were significantly enhanced, mitochondrial membrane integrity was compromised, and cell viability was significantly decreased following H2O2 treatment in LAR-/- VSMCs compared with wild-type cells. Stronger and sustained increase in autophosphorylation and activity of Fyn, an Src family tyrosine kinase, was observed in LAR-/- cells compared with wild-type cells following H2O2 treatment. LAR binds to activated Fyn in H2O2-treated VSMCs, and recombinant LAR dephosphorylates phosphorylated-Fyn in vitro. In addition, LAR deficiency enhanced H2O2-induced phosphorylation of Janus kinase 2 (JAK2), signal transducer and activator of transcription 3 (STAT3), and p38 mitogen-activated protein kinase (MAPK). PP2, a Fyn-specific inhibitor, blocked JAK2, STAT3, and p38 MAPK activation and significantly attenuated apoptosis induced by H2O2. AG490, a JAK2-specific inhibitor, significantly attenuated H2O2-induced apoptosis, and blocked H2O2-induced activation of STAT3, but not p38 MAPK in both wild-type and LAR-/- VSMCs. Attenuation of Fyn expression by short hairpin RNA significantly decreased H2O2-induced downstream signaling and apoptosis in VSMCs. Together, these data indicate that LAR regulates Fyn/JAK2/STAT3 and Fyn/p38 MAPK pathways involved in ROS-induced apoptosis.
Apoptosis is an essential component of normal development as well as of most developmental abnormalities and human diseases (1). Vascular smooth muscle cell (VSMC)2 apoptosis occurs after vessel injury, in remodeling, and in advanced atherosclerotic lesions (2-4). A significant increase in VSMC death rate was observed in unstable versus stable angina plaques, suggesting the involvement of VSMC apoptosis in plaque rupture (5). Indeed, direct stimulation of VSMC apoptosis induces destabilization and rupture of atherosclerotic plaques (6, 7). Plaque rupture often leads to thrombosis with clinical manifestations of myocardial infarction or stroke. Atherosclerotic lesions are a highly pro-oxidant environment and contain high levels of reactive oxygen species (ROS), which can induce VSMC apoptosis (8-11).
In addition to atherosclerosis, ROS have been implicated in hypertension and other vascular diseases, and intracellular signaling cascades stimulated by ROS play an important role in the pathogenesis of these diseases. ROS-induced stimulation of protein phosphorylation pathways modulates transcription factor activities and gene expression, which results in a variety of responses such as cell growth, differentiation, or apoptosis (12, 13). The particular response observed will depend on the cell type, and the concentration and duration of ROS production.
Hydrogen peroxide, the most stable form of ROS, inhibits protein-tyrosine phosphatases (PTPs) in vitro and reversibly in intact cells (14-17). PTPs contain a catalytically essential cysteine residue in their signature active site motif, HCXXGXXR(S/T) (where X is any amino acid), with an extremely low pKa (18). The low pKa promotes the function of the cysteine as a nucleophile in catalysis but renders it a target of oxidation with concomitant inhibition of PTP activity (14, 19). Recently, Groen et al. (20) reported that PTPs undergo differential oxidation at physiological pH and H2O2 concentrations, which indicates that cellular responses are fine-tuned to various oxidative stimuli. ROS generation is the proximal event in cell surface receptor activation by growth factors and cytokines. PTPs control protein-tyrosine phosphorylation, which is a key regulatory mechanism of many intracellular signaling pathways. Therefore, oxidation and inhibition of PTPs by ROS may be an initial and critical step for ROS and growth factor-induced signaling in cells (14, 21-23). However, very little is known about the role of individual PTPs in regulating ROS-induced signaling.
The mammalian leukocyte common antigen-related receptor (LAR) PTP family includes LAR, PTPδ, and PTPσ (24). LAR is expressed on the cell surface as a complex of two noncovalently associated subunits of 150 and 80 kDa, which are derived by the action of an endogenous protease on a preprotein (25, 26). The 150-kDa extracellular subunit comprises the N terminus of the protein, contains three tandem immunoglobulin-like domains and eight fibronectin-III like domains, and is modified by N-linked glycosylation. The 85-kDa C-terminal subunit contains a short ectodomain, a transmembrane domain, and two tandem phosphatase domains: the membrane-proximal D1 domain has phosphatase activity and the distal D2 domain is catalytically inactive but may regulate substrate specificity (27).
LAR has wide tissue distribution, including the lung, heart, brain, liver, and kidney, and it is expressed in various cell lineages, including epithelial cells, smooth muscle cells, and cardiomyocytes (25, 28). Cell culture and animal model studies have shown that this PTP acts as a negative regulator of insulin signaling (29-31). Recently, we reported that LAR regulates insulin-like growth factor-1 receptor signaling in VSMCs, and dysregulation of this PTP affects vascular pathophysiology (32).
In the present study, we examined the effect of LAR on H2O2-induced signal transduction pathways and apoptosis using aortic VSMCs derived from LAR knockout mice. Our results indicate that LAR regulates the viability of VSMCs under oxidative stress conditions. Absence of LAR enhances H2O2-induced VSMC apoptosis, by allowing hyperactivation of the Fyn/JAK2/STAT3 and Fyn/p38 MAPK pathways.
EXPERIMENTAL PROCEDURES
Materials—AG490, PP2, and H2O2 were purchased from Calbiochem. A MitoCapture™ mitochondrial apoptosis detection kit was from BioVision. LAR enzyme was obtained from New England Biolabs. Two distinct anti-LAR antibodies, anti-LAR monoclonal (Cat. No. 610350, BD Transduction Laboratories) and goat anti-LAR polyclonal (sc-1119, Santa Cruz Biotechnology, Santa Cruz, CA), were used in this study. The monoclonal antibody, raised against an epitope corresponding to amino acids 24-194 of human LAR, recognizes 150-kDa extracellular segment, whereas goat polyclonal antibody, raised against an epitope in the C-terminal cytoplasmic domain of rat LAR, recognizes the 85-kDa C-terminal subunit. The other antibodies used were as follows: anti-Src (Tyr-416), anti-Src (Tyr-527), anti-STAT3, anti-phospho-STAT3 (Tyr-705), anti-ERK1/ERK2, anti-phosphospecific ERK1/2 (Thr-202 and Tyr-204), anti-p38 MAPK, anti-phosphospecific p38 MAPK (Thr-180/Tyr-182) and anti-phosphospecific Lck (Tyr-505) (Cell Signaling Technology); anti-JAK2, anti-phosphospecific JAK2 (Tyr-1007 and Tyr-1008), and anti-phosphotyrosine (PY20) (Upstate Biotechnology, Inc.); and anti-Lck and anti-Fyn (Santa Cruz Biotechnology).
LAR-deficient Mice—LAR-deficient mice (kindly provided by Dr. Frank Longo, University of North Carolina, Chapel Hill) were generated by using the gene trap method (33, 34). LAR-/- littermates were derived via heterozygous crosses and were backcrossed at least eight times into the DBA background. Genotyping was done using reverse transcription-PCR as described by Yeo et al. (35).
Cell Culture—Aortic VSMCs were isolated from 4-month-old male LAR+/+ and LAR-/- mice as previously described by our group (36). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS), as described previously (37). Human aortic VSMCs (Cambrex Corp.) were cultured in smooth muscle cell basal medium containing 5% FBS, 100 ng/ml insulin, 200 ng/ml human fibroblast growth factor, 100 ng/ml human epidermal growth factor, 50 μg/ml gentamicin, and 50 ng/ml amphotericin. All experiments were conducted using VSMCs between passages 4 and 11 that were growth-arrested by incubation in DMEM containing 0.1% FBS for 72 h.
Immunoprecipitation and Western Analysis—Cells were lysed either in radioimmune precipitation buffer (20 mm Tris-HCl, pH 7.6, 150 mm NaCl, 0.05 mm sodium fluoride, 1 mm EDTA, 1% Igepal CA-630, 0.05% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) or Triton lysis buffer (20 mm Tris-HCl, pH 7.4, 137 mm NaCl, 2 mm EDTA, 10% glycerol, and 1% Triton X-100) containing protease inhibitors. Immunoprecipitation and Western analysis were performed as described previously (36).
Measurement of LAR Activity—LAR activity was measured using the fluorescence-based RediPlate 96 EnzChek Tyrosine Phosphatase Assay Kit from Molecular Probes. VSMCs were lysed in lysis buffer containing 20 mm Tris-Cl, pH 7.4, 132 mm NaCl, 2 mm EDTA, 10% glycerol, 1% Triton X-100, and a protease inhibitor mixture. Cell lysates containing 500 μg of protein were immunoprecipitated with goat anti-LAR polyclonal antibody overnight at 4 °C, and then incubated with Protein A-Sepharose beads for another 2 h. The immunoprecipitates were washed three times with lysis buffer, and resuspended in 80 μl of assay buffer (20 mm MOPS, pH 7.0, 50 mm NaCl, 1 mm dithiothreitol, and 0.05% Tween 20) containing 50 μm substrate, 6,8-difluoro-4-methylumbelliferyl phosphate. The reaction was performed at 37 °C for 30 min in the dark. 6,8-Difluoro-4-methylumbelliferyl fluorescence was measured at an excitation wavelength of 355 nm and an emission wavelength of 460 nm using a WALLAC 1420 Multilabel Counter. Phosphatase activity was calculated using a 6,8-difluoro-4-methylumbelliferyl phosphate standard curve.
Measurement of Recombinant LAR Activity in the Presence of H2O2—Recombinant LAR activity was assayed by measuring the dephosphorylation of universal phosphatase substrate, p-nitrophenyl phosphate. Formation of p-nitrophenolate product was monitored in real-time by measuring absorbance at 410 nm in a Beckman BDU-7500 spectrophotometer. All assays were performed at pH 7.0 in a reaction buffer containing 25 mm MOPS, 50 mm NaCl, and 0.05% Tween 20. LAR (6.25 nm) was reacted with 1 mm H2O2 in the absence of reducing agents. The reaction was allowed to proceed for 10 min at 25 °C in the presence of 0.5 mm p-nitrophenyl phosphate.
In Vitro Dephosphorylation Assay—Cell lysates obtained from growth-arrested LAR-/- VSMCs, treated with H2O2 for 10 min, were immunoprecipitated with either anti-Fyn or anti-STAT3 antibodies and immobilized on protein A-agarose beads. The beads were washed three times with lysis buffer, and then either left untreated or incubated with purified recombinant LAR (New England Biolabs), in the presence or absence of 0.1 mm sodium orthovanadate, at 30 °C. The reaction was stopped by adding Laemmli sample buffer and boiling for 5 min. Dephosphorylation of tyrosine-phosphorylated immunoprecipitates was observed by Western analysis with anti-PY20 antibody.
Construction of Recombinant Adenoviruses—Adenovirus-encoding LAR (AdLAR) was constructed by subcloning full-length human LAR cDNA (generously provided by Robert A. Mooney, University of Rochester, Rochester, NY) into adenoviral shuttle vector, pShuttle-CMV. After homologous recombination, by electroporation of pShuttle-CMV containing LAR cDNA into BJ5183 Escherichia coli (Stratagene) possessing the adenoviral backbone plasmid pADEasy-1, recombinants were selected. Recombinant (E1/E3-deficient) adenoviruses were generated by transfection of human embryonic kidney 293 cells with the recombinant plasmid using Lipofectamine (Invitrogen). Then the virus was serially amplified, purified on a CsCl density gradient by ultracentrifugation, and titered (38). A control adenovirus, consisting of the identical adenovirus backbone with β-galactosidase cDNA insert (Adβ-galactosidase), was provided by Dr. Huang (University of North Carolina, Chapel Hill).
Adenovirus Infection—Adenoviral infection of nearly confluent VSMCs was performed at a multiplicity of infection of 100 in DMEM containing 2% FBS. After 16-h incubation, the cells were made quiescent in DMEM containing 0.1% FBS for 72 h.
Assessment of Apoptosis—For detection of apoptosis, growth-arrested VSMCs were harvested after 16-h treatment with 1 mm H2O2. Histone-associated DNA fragmentation in cell lysates was determined using the Cell Death Detection ELISAPLUS kit (Roche Applied Science) in accordance with the manufacturer's instructions.
Cell death was also assessed by staining H2O2-treated (1 mm H2O2 for 3 h) VSMCs with MitoCapture reagent, a fluorescent lipophilic dye that indicates loss of mitochondrial membrane potential, which is a marker of apoptosis. MitoCapture reagent accumulates and aggregates in the mitochondria of healthy cells fluorescing red (590 nm), whereas in the apoptotic cells it remains in the cytoplasm fluorescing green (530 nm). The pixel intensity of photomicrographs obtained by fluorescent microscopy was quantified using ImageQuaNT software.
Determination of Cell Viability—Cell viability was measured by using a crystal violet staining assay. Cells cultured in 96-well plates at 8000 cells/well were treated with or without H2O2. After 16-h treatment, the media were decanted, and the cells were washed once with phosphate-buffered saline, followed by crystal violet staining (0.5% crystal violet in 1% ethanol and 1.5% formaldehyde) for 30 min. The cells were rinsed with H2O, and the bound dye was solubilized by incubation at 37 °C for 30 min with 100 μl of 1.0% deoxycholate. The absorbance was measured at 550 nm using a WALLAC 1420 Multilabel Counter.
Caspase-3/7 Assay—Caspase-3/7 activities were measured using an Apo-ONE™ Homogeneous Caspase-3/7 Assay kit (Promega). Briefly, cells seeded in 24-well plates at a density of 2.0 × 104 cells/well, were grown in DMEM containing 10% FBS, and then starved with serum-free medium for 24 h prior to treatment with 1 mm H2O2 for 2 h. The cells were lysed using a bifunctional cell lysis/activity buffer, which contained a profluorescent caspase-3/7 consensus substrate, rhodamine 110-bis-N-CBZ-l-aspartyl-l-glutamyl-l-valyl-l-aspartic acid amide (Z-DEVD-R110). After incubation at room temperature for 1 h, aliquots (150 μl) were transferred to a 96-well clear-bottom plate. Fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a WALLAC 1420 Multilabel Counter.
Retroviral Fyn shRNA Constructs and Infection of Mouse Aortic VSMCs—Two mouse pSM2 retroviral shRNAs for Fyn and one retroviral scrambled shRNA constructs were purchased from Open Biosystems. The sense sequences of Fyn shRNA were 5′-CCGATGTTATGTCAAAGGCCAA-3′ (RMM1766-96740266) and 5′-CCCACACTAACTTCCTGTATAA-3′ (RMM1766-96740343). The scrambled shRNA encoded a 19-bp sequence (5′-GCGCGCTTTGTAGGATTCG-3′) with no significant homology to any mouse gene, and was cloned into pSUPER.retro.puro vector. All retroviruses were prepared in [Phi]Nx-eco cells (Orbigen) by calcium phosphate transfection. Puromycin-resistant clones (selected with 2 μg/ml puromycin for 10 days) were expanded prior to their use in experiments. The effectiveness of Fyn shRNA was confirmed by Western blotting of cell lysates with anti-Fyn antibodies.
Fyn Kinase Assay—After appropriate treatments, cells were washed with cold phosphate-buffered saline, and lysed on ice for 15 min in lysis buffer containing 20 mm HEPES, pH 7.4, 2 mm EGTA, 1 mm dithiothreitol, 50 mm β-glycerophosphate, 1% Triton X-100, 10 units/ml aprotinin, 2 μm leupeptin, 1 mm Na3VO4, and 400 μm phenylmethylsulfonyl fluoride. Cell lysates containing 500 μg of protein were immunoprecipitated with anti-Fyn antibody for 2 h at 4 °C and then incubated with 40 μl of 50% (w/v) protein A-Sepharose beads for an additional 1 h. The beads were washed three times with lysis buffer and resuspended in 30 μl of kinase buffer (20 mm Tris, pH 7.5, 10 mm MnCl2, 10 mm MgCl2) containing 2.5 μg of denatured human non-neuronal enolase, 5 μm ATP, and 5 μCi of [γ-32P]ATP and incubated at 37 °C for 15 min. The reactions were stopped by adding 20 μl of 4× Laemmli sample buffer. The samples were heated at 95 °C for 5 min and analyzed by SDS-gel electrophoresis on 10% acrylamide gels. The dried gel was exposed to x-ray film and developed. Levels of phosphorylated enolase were quantified by densitometry.
Statistical Analysis—All numerical data are expressed as mean ± S.E. Data were analyzed with one-way and two-way analysis of variance (ANOVA) and in case of one-way ANOVA, post-hoc analysis was performed using the Newman-Keuls test. Statistical significance was accepted at p < 0.05.
RESULTS
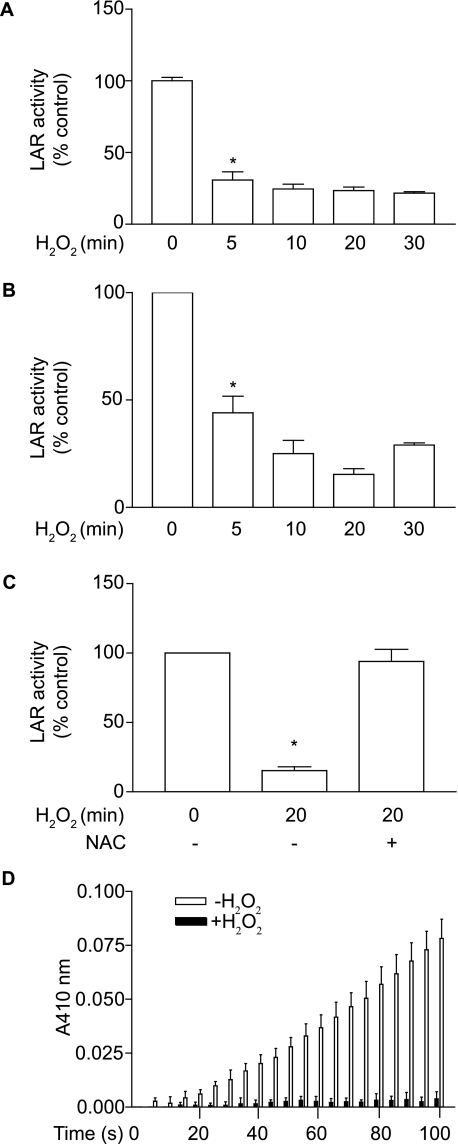
LAR Activity Is Inhibited under Oxidative Stress Condition—Because ROS are known to induce apoptosis by inhibiting PTP activity (39), we investigated whether H2O2 treatment inhibits the activity of LAR in VSMCs. We immunoprecipitated LAR in VSMCs lysates with an antibody raised against the C-terminal cytoplasmic domain of LAR and measured its activity using a Tyrosine Phosphatase Assay Kit (Molecular Probes). A decrease of ∼70% in LAR activity (p < 0.001) was observed 5 min after treatment with 1 mm H2O2 (Fig. 1A). LAR activity remained inhibited during the 30-min H2O2 treatment. We also measured the effect of H2O2 on LAR activity in LAR -/- VSMCs expressing AdLAR. LAR activity decreased significantly (p < 0.001) 5 min after H2O2 treatment, and a maximal inhibition of 80% was observed at 20 min (Fig. 1B). Pretreatment with N-acetyl cysteine, a thiol-reducing agent, abrogated H2O2-induced inhibition of LAR activity (Fig. 1C). Further, incubation of recombinant LAR with 1 mm H2O2, in the absence of reducing agents, resulted in complete inactivation of this PTP (Fig. 1D). These data support the notion that LAR is a redox-sensitive phosphatase, and the residual activity of this PTP in H2O2-treated VSMCs might be due to the inherent reducing capacity in VSMCs.
FIGURE 1.
H2O2 treatment inactivates LAR in mouse aortic VSMCs. A, growth-arrested VSMCs were treated with 1 mm H2O2 for the indicated time, and cell lysates were immunoprecipitated with anti-LAR antibody. Phosphatase activity was measured using 6,8-difluoro-4-methylumbelliferyl phosphate as a substrate. Data presented are mean ± S.E., (n = 3) of three separate experiments (*, p < 0.001 compared with control). B, LAR-/- VSMCs, infected with AdLAR, were growth-arrested and then treated with 1 mm H2O2 for the indicated times. LAR activity was measured as described above, and data presented are mean ± S.E., (n = 3) and representative of three separate experiments (*, p < 0.001 compared with control). C, LAR activity was assayed in lysates from growth-arrested VSMCs that were pretreated with 10 mm N-acetyl cysteine for 60 min and then treated with 1 mm H2O2 for 20 min. Data presented are mean ± S.E. (n = 3) of three separate experiments (*, p < 0.001 compared with control). D, the catalytic activity of recombinant LAR, in the absence of reducing agents, was assayed by monitoring the increase in absorbance resulting from the hydrolysis of substrate, p-nitrophenyl phosphate. A significant change in activity was observed for time and treatment (two-way ANOVA, p < 0.0001).
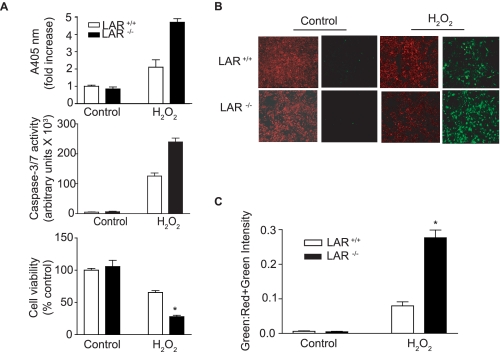
Deficiency of LAR Enhances H2O2-induced Apoptosis—ROS are known to induce apoptosis by inhibiting PTP activity (39). Further, H2O2-induced VSMC apoptosis is inhibited by Na3VO4, an inhibitor of PTP (40). To investigate the role of LAR in oxidative-stress induced apoptosis, we characterized the effect of H2O2 on VSMC apoptosis using cells derived from LAR+/+ (wild-type) and LAR-/- (knockout) mice. VSMCs were treated with 1 mm H2O2 for 16 h, and apoptosis was assessed by analyzing histone-associated DNA fragmentation from cellular extracts (Fig. 2A, upper panel). There was no difference in the basal level apoptosis between wild-type and LAR knockout VSMCs. H2O2 significantly increased DNA fragmentation in both wild-type and LAR knockout VSMCs compared with their respective untreated controls. However, apoptosis in response to H2O2 was significantly greater in LAR knockout VSMCs compared with the wild-type VSMCs (p < 0.001).
FIGURE 2.
LAR deficiency increases H2O2-induced apoptosis and caspase-3/7 activity, and decreases cell viability in VSMCs. A, growth-arrested VSMCs were treated with 1 mm H2O2 for 16 and 2 h, and lysates were used to measure histone-associated DNA fragmentation (upper panel) and caspase-3/7 activity (middle panel), respectively. Viability of VSMCs treated with 1 mm H2O2 for 16 h was assayed by staining with crystal violet (lower panel). The absorbance was measured at 550 nm, and cell survival data are expressed as percent of untreated cells (mean ± S.E., n = 3; *, p < 0.01 compared with LAR+/+ VSMCs treated with H2O2). B, H2O2-induced apoptosis as measured by mitochondrial membrane injury. VSMCs were either untreated or treated with 1 mm H2O2 for 3 h, and then stained with MitoCapture reagent and visualized using a fluorescence microscope. Predominant red staining is indicative of healthy cells, whereas predominant green staining indicates apoptotic cells. C, pixel intensity of MitoCapture-treated LAR+/+ and LAR-/- cells was determined, and the data are presented as the ratio of green to red plus green pixel intensity (mean ± S.E., n = 3; *, p < 0.001 compared with wild-type cells treated with H2O2).
To confirm the role of LAR in H2O2-induced apoptosis, we measured caspase-3/7 activity (Fig. 2A, middle panel), a fairly early marker of apoptosis (41, 42). Caspase-3/7 activity levels were very low in untreated wild-type and LAR knockout VSMCs but increased in both cell types in response to H2O2 treatment. The observed increase in caspase-3/7 activity was significantly greater in LAR knockout VSMCs than in wild-type VSMCs following treatment with H2O2 for 2 h (p < 0.001). Caspase-3/7 activity was much lower in both VSMCs after 16-h treatment with H2O2. However, LAR knockout VSMCs had significantly higher (p < 0.001) caspase-3/7 activity than wild-type cells 16 h after H2O2 treatment (data not shown). These results indicate that deficiency of LAR results in hyperactivation of cellular signaling pathways that cause apoptosis under oxidative stress conditions.
We further confirmed that absence of LAR enhances H2O2-induced VSMC apoptosis by measuring changes in mitochondrial membrane potential using MitoCapture reagent (Fig. 2B). VSMCs were treated with or without 1 mm H2O2 for 3 h prior to MitoCapture staining. In untreated wild-type and LAR knockout VSMCs, the fluorescence of MitoCapture was red indicating mitochondrial accumulation and healthy cells. Following H2O2 treatment, increase in green fluorescence was evident in both wild-type and LAR knockout cells, indicating apoptosis. Consistent with other markers of apoptosis, the ratio of green-to-red plus green fluorescence was significantly higher in LAR knockout VSMCs than in wild-type VSMCs (p < 0.001) (Fig. 2C).
Finally, we tested whether the increase in apoptosis after H2O2 treatment was associated with the decrease in VSMC viability using crystal violet staining (Fig. 2A, lower panel). Viability decreased significantly in wild-type cells after H2O2 treatment (p < 0.01). However, cell viability was significantly lower in LAR knockout VSMCs than in wild-type VSMCs, following H2O2 treatment (p < 0.01). Together, these data indicate that LAR regulates the apoptotic pathways in VSMCs and plays an important role in protecting these cells from oxidative stress-induced death.
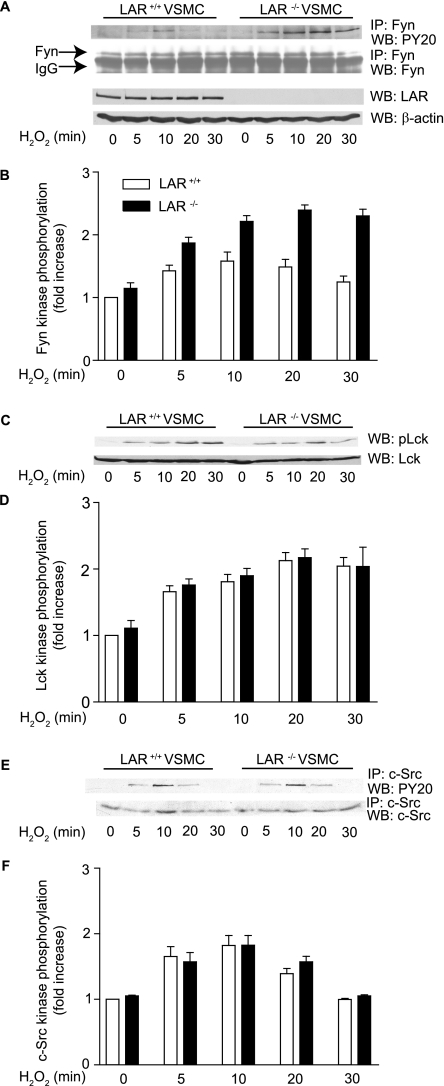
Deficiency of LAR Enhances H2O2-induced Tyrosine Phosphorylation of Fyn but Not Lck and c-Src—Berk and colleagues (43, 44) previously demonstrated the activation of Src family kinases in ROS-initiated signaling events. It was reported that Fyn, a Src family kinase, is required for H2O2-mediated activation of intracellular signaling events (45). Further, it was recently demonstrated that LAR binds both Fyn and Lck, another Src family kinase, and that LAR dephosphorylates Fyn both in vitro and in thymocytes (46). To determine whether LAR deficiency has differential effects on activation of Fyn and other Src family kinases, Lck and c-Src, we analyzed tyrosine phosphorylation of these kinases in cellular extracts of wild-type and LAR knockout VSMCs treated with 1 mm H2O2. Tyrosine phosphorylation of Fyn and c-Src was assessed by immunoprecipitation of cell lysates with their respective antibodies and then probing the immunoprecipitates with anti-phosphotyrosine (PY20) antibody, whereas Lck tyrosine phosphorylation was assessed by immunoblotting the cell lysates with anti-phosphospecific Lck antibody. A significant increase in Fyn tyrosine phosphorylation was observed with time and cell type (p < 0.0001, two-way ANOVA) in response to H2O2 treatment (Fig. 3, A and B). Sustained increase in Lck and c-Src phosphorylation was observed with time in both cell types when treated with H2O2 (p < 0.0001 for each kinase, two-way ANOVA) (Fig. 3, C-F). However, there was no significant difference in tyrosine phosphorylation of Lck and c-Src between wild-type and LAR knockout VSMCs (Fig. 3, D and E). It is unlikely that other PTPs are compensating/regulating the phosphorylation of these kinases in the absence of LAR, because LAR knockout mice did not exhibit any changes in the expression of other LAR family PTPs (47), and we did not observe any difference in the activity of a related PTP, PTP1B, between LAR+/+ and LAR-/- VSMCs (data not shown). Although we do not know the exact mechanism, our results indicate that LAR specifically regulates H2O2-induced Fyn, but not Lck and c-Src in VSMCs, through tyrosine phosphorylation.
FIGURE 3.
LAR deficiency increases tyrosine phosphorylation of Fyn. A, VSMCs were either left untreated or treated with 1 mm H2O2, and the cell lysates containing equal amounts of protein were immunoprecipitated with anti-Fyn antibody followed by Western analysis with either anti-phosphotyrosine (PY20) antibody or anti-Fyn antibody. Cell lysates were also analyzed by Western blotting with anti-LAR monoclonal antibody, and the membrane was reprobed with anti-β-actin antibody. B, densitometric analysis of normalized Fyn phosphorylation (mean ± S.E., n = 3; p < 0.0001 for cell type and time in two-way ANOVA). C, VSMCs were stimulated with 1 mm H2O2, and cell lysates were analyzed by Western blotting with either anti-phospho Lck or anti-Lckantibody. D, densitometric analysis of normalized Lck phosphorylation (mean ± S.E., n = 3; p < 0.0001 for time in two-way ANOVA). E, H2O2-treated VSMC lysates were immunoprecipitated with anti-c-Src antibody followed by Western analysis with either anti-PY20 antibody or anti-c-Src antibody. F, densitometric analysis of normalized c-Src phosphorylation (mean ± S.E., n = 3; p < 0.0001 for time in two-way ANOVA).
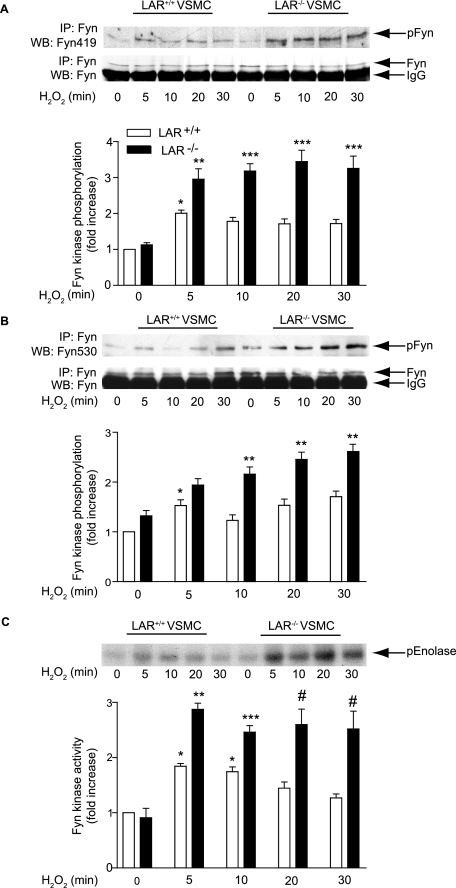
H2O2 Treatment Increases Autophosphorylation of Tyr-419 and Activity of Fyn Kinase in LAR-deficient VSMCs—Fyn contains two tyrosine phosphorylation sites, Tyr-419, an autophosphorylation site in the tyrosine kinase domain, and Tyr-530, a regulatory phosphorylation site within the C terminus (46). Autophosphorylation of Tyr-419 increases kinase activity, whereas phosphorylation of Tyr-530 inhibits the kinase via an intramolecular interaction with the SH2 domain (48, 49). To determine whether absence of LAR results in increased autophosphorylation of Fyn in cells treated with H2O2, we immunoprecipitated Fyn and probed Tyr-419 phosphorylation using phosphospecific anti-Src (pY416) antibody, which cross-reacts with autophosphorylated Fyn kinase. Fyn autophosphorylation increased significantly, but transiently 5 min after H2O2 treatment (p < 0.05 versus respective control) in wild-type VSMCs (Fig. 4A). However, the absence of LAR caused significant and sustained increase in autophosphorylation of Fyn in VSMCs treated with H2O2 (p < 0.001 versus untreated LAR knockout VSMCs and wild-type VSMCs following H2O2 treatment).
FIGURE 4.
H2O2-induced Fyn autophosphorylation and kinase activity are enhanced in the absence of LAR. A, growth-arrested LAR+/+ and LAR-/- VSMCs were either left untreated or treated with 1 mm H2O2 for the indicated times, and the cell lysates were immunoprecipitated with anti-Fyn antibody followed by Western analysis with anti-phosphospecific Fyn (pY419) antibody (top panel). The membrane was reprobed with anti-Fyn antibody (middle panel). Densitometric analysis (lower panel) of Fyn autophosphorylation (mean ± S.E., n = 3; *, p < 0.05 versus untreated LAR+/+ VSMCs, **, p < 0.01 versus respective control, ***, p < 0.001 versus respective control). B, lysates from VSMCs, treated as described above, were immunoprecipitated with anti-Fyn antibody followed by Western analysis with anti-phosphospecific Fyn (pY530) antibody (top panel). The membrane was reprobed with anti-Fyn antibody (middle panel). Densitometric analysis (lower panel) of Fyn (pY530) phosphorylation (mean ± S.E., n = 3; *, p < 0.05 versus untreated LAR+/+ VSMCs, **, p < 0.001 versus respective control). C, growth-arrested VSMCs were treated with 1 mm H2O2 for the indicated times. Cell lysates containing equal amount of protein were immunoprecipitated with anti-Fyn antibody, and Fyn kinase activity was measured by an immunocomplex kinase assay using denatured enolase as the substrate (top panel). Densitometric analysis (lower panel) of Fyn activity (mean ± S.E., n = 3; *, p < 0.05 versus untreated LAR+/+ VSMCs; ** p < 0.05 versus respective control; ***, p < 0.05 versus respective control; #, p < 0.001 versus respective control).
Next, we examined the effect of H2O2 on phosphorylation of Fyn Tyr-530 by immunoprecipitating the VSMC lysates with anti-Fyn antibody, followed by Western analysis with phosphospecific anti-Src (pY527) antibody, which cross-reacts with phosphorylated Fyn Tyr-530. Consistent with the report of Sanguinetti et al. (49), H2O2 treatment of wild-type VSMCs increased phosphorylation of Fyn on its inhibitory Tyr residue in the C terminus (p < 0.05 at 5 min versus untreated cells) (Fig. 4B). Phosphorylation of Fyn Tyr-530 was significantly higher in LAR-/- VSMCs than in wild-type VSMCs 10, 20, and 30 min after H2O2 treatment (p < 0.001 for each time point).
Because LAR deficiency resulted in increase in both the autophosphorylation as well as phosphorylation of inhibitory Fyn Tyr-530 residue, we measured Fyn activity in untreated and H2O2-treated VSMCs using an immunocomplex kinase assay with enolase as the substrate. Fyn kinase activity, after H2O2 treatment, increased significantly in wild-type VSMCs for 10 min (p < 0.05 versus untreated control) (Fig. 4C). However, the activity of Fyn kinase decreased thereafter and returned to near basal levels 30 min after H2O2 treatment. Fyn kinase activity in LAR knockout VSMCs was significantly higher under basal conditions (p < 0.05 versus untreated wild type) and increased further with H2O2 treatment (p < 0.05 30 min after treatment versus untreated LAR knockout VSMCs). Deficiency of LAR resulted in significant and sustained activation of Fyn kinase after H2O2 treatment (p < 0.001 versus wild-type VSMCs 30 min after H2O2 treatment). Together, these data indicate that increase in autophosphorylation overrides the increase in phosphorylation of inhibitory Tyr-530 residue in modulating Fyn activity in the absence of LAR.
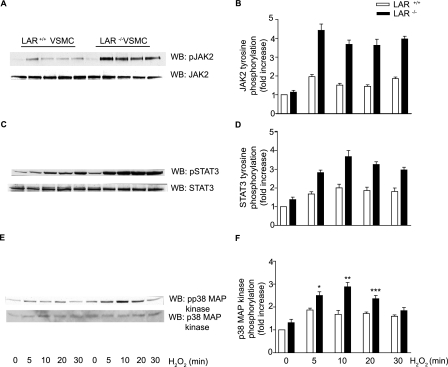
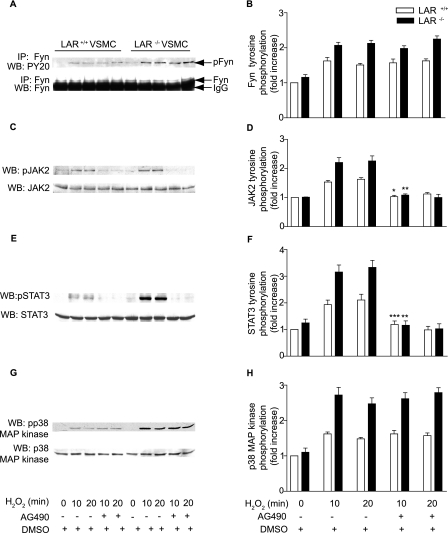
Enhanced Activation of JAK2-STAT3 Pathway and p38 MAPK in H2O2-treated LAR-deficient VSMCs—Fyn is required for H2O2-mediated activation of Janus kinase 2 (JAK2) (43), which is essential for H2O2-induced apoptosis (50). Therefore, we investigated whether sustained activation of Fyn by H2O2 in LAR knockout cells would result in higher activation of JAK2 as well. Western analysis with phosphospecific antibodies demonstrated a significant increase in JAK2 phosphorylation with time and cell type in VSMCs treated with H2O2 (p < 0.0001, two-way ANOVA) (Fig. 5, A and B). To determine whether enhanced activation of JAK2 in LAR knockout VSMCs treated with H2O2 leads to tyrosine phosphorylation of STAT3, a JAK2 substrate, we measured STAT3 tyrosine phosphorylation in wild-type and LAR knockout VSMCs treated with H2O2 (Fig. 5C). H2O2 treatment caused a significant increase in STAT3 tyrosine phosphorylation with time and cell type (p < 0.0001, two-way ANOVA) in VSMCs (Fig. 5D).
FIGURE 5.
JAK2, STAT3, and p38 MAPK phosphorylation are enhanced in the absence of LAR. Growth-arrested LAR+/+ and LAR-/- VSMCs were either left untreated or treated with 1 mm H2O2, and phosphorylation was determined by immunoblotting with either anti-phospho JAK2 (A), anti-phospho STAT3 (C), or anti-phospho p38 MAPK (E) antibodies. Densitometric analysis of normalized phospho JAK2 (B), phospho STAT3 (D), and phospho p38 MAPK (F) (mean ± S.E., n = 3; *, p < 0.01, **, p < 0.001, and ***, p < 0.05 versus respective controls).
Fyn tyrosine kinase is upstream of p38 MAPK in the signal transduction pathways of several cells, including vascular cells (51, 52). Because the activation of p38 MAPK has been implicated in apoptosis of VSMCs (53, 54), we examined the activation of this kinase in wild-type and LAR knockout VSMCs treated with H2O2 (Fig. 5E). Phosphorylation of p38 MAPK was significantly higher in LAR knockout VSMCs than in wild-type cells 5 (p < 0.01), 10 (p < 0.001), and 20 min (p < 0.05) after H2O2 treatment (Fig. 5F).
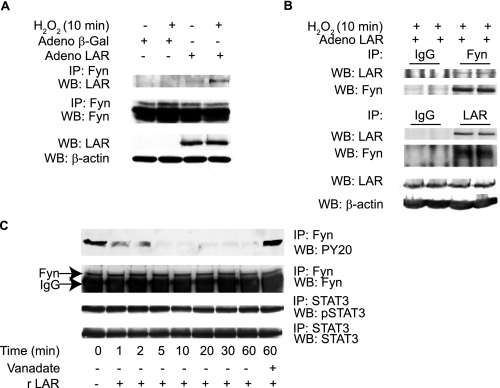
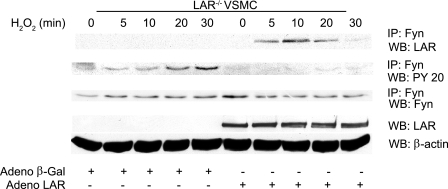
LAR Binds to and Dephosphorylates Activated Fyn—Because Fyn kinase tyrosine phosphorylation was increased in LAR knockout VSMCs and LAR was shown to bind Fyn in other cell types (46), we investigated whether the regulatory function of LAR on Fyn is mediated by a direct physical interaction between the two proteins in VSMCs treated with H2O2. LAR knockout VSMCs, infected with either human AdLAR or Adβ-galactosidase, were treated with or without H2O2 for 10 min. Immunoprecipitation of these cell lysates with anti-Fyn antibody followed by Western analysis with anti-LAR C-terminal antibody revealed enhanced association of LAR with activated Fyn kinase in VSMCs following treatment with H2O2 (Fig. 6A). The association of LAR with activated Fyn was corroborated using lysates from LAR knockout VSMCs infected with human AdLAR and treated with H2O2. Immunoprecipitation of cell lysates with anti-Fyn antibody followed by Western analysis with anti-LAR C-terminal antibody and reciprocal immunoprecipitation with anti-LAR C-terminal antibody and Western analysis with Fyn antibody confirmed enhanced association of LAR with activated Fyn (Fig. 6B).
FIGURE 6.
LAR associates with and dephosphorylates activated Fyn. A, lysates from growth-arrested LAR-/- VSMCs, either untreated or treated with 1 mm H2O2 in the presence of Adβ-galactosidase or AdLAR, were immunoprecipitated with anti-Fyn antibody and Western analysis was performed with anti-LAR antibody. The membrane was reprobed with anti-Fyn antibody. LAR overexpression was confirmed by Western analysis of cell lysates with either anti-LAR or anti-β-actin antibody (loading control). B, growth-arrested LAR-/- VSMCs infected with AdLAR were treated with H2O2, and cell lysates were immunoprecipitated with either anti-Fyn or anti-LAR antibodies or isotype-matched control antibodies. Western analysis was done with either anti-LAR (the membrane was reprobed with anti-Fyn) or anti-Fyn (membrane reprobed with anti-LAR) antibodies. LAR overexpression was confirmed as described above. C, Fyn or STAT3 were immunoprecipitated from lysates of VSMCs treated with H2O2, immobilized on protein A-agarose beads, and incubated with recombinant LAR in the phosphatase reaction buffer. Phosphorylation was examined with either anti-PY20 or anti-phospho STAT3 (Tyr-705) antibodies. Equal loading of Fyn and STAT3 in cell lysates was confirmed by immunoprecipitation/Western analysis of aliquots with respective antibodies.
Next we examined whether LAR dephosphorylates Fyn in vitro. LAR knockout VSMCs were treated with H2O2 for 10 min, and activated Fyn in the lysates was immunoprecipitated with anti-Fyn antibody and incubated with purified recombinant LAR (New England Biolabs) or vehicle. As shown in Fig. 6C (upper panel), Western analysis of immunoprecipitates with anti-PY20 antibody indicated that recombinant LAR dephosphorylated Fyn in a time-dependent manner. Fyn tyrosine dephosphorylation by LAR was inhibited in the presence of sodium vanadate, a potent inhibitor of PTP. In contrast, LAR did not dephosphorylate activated STAT3 immunoprecipitated from LAR knockout VSMCs treated with H2O2 (Fig. 6C, lower two panels). Taken together, these results suggest that activated Fyn is a possible substrate of LAR in vivo under oxidative stress conditions. Furthermore, LAR mainly regulates the activity of Fyn kinase by modulating autophosphorylation of Tyr-419 in the kinase domain.
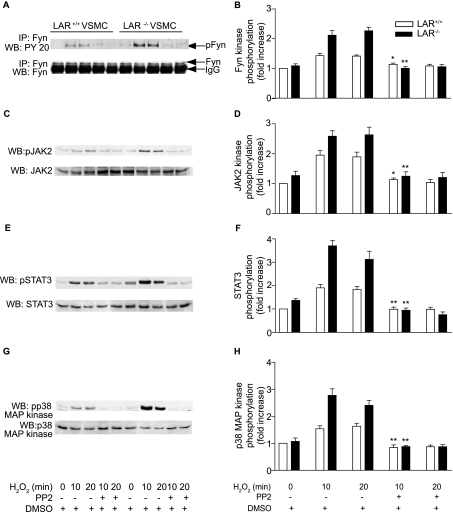
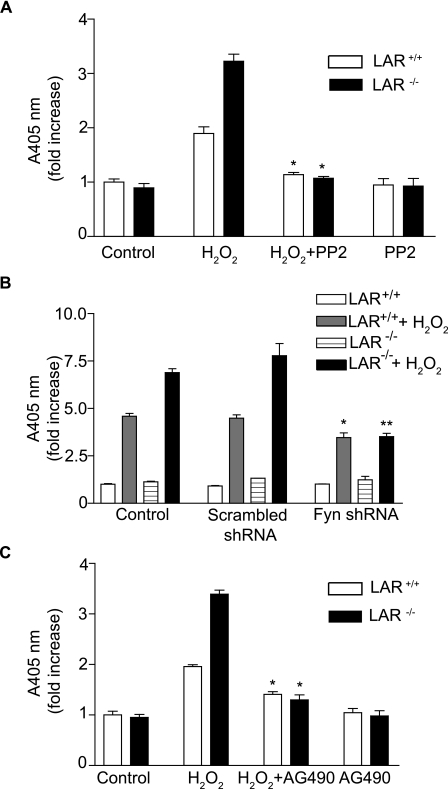
Src Family-selective Tyrosine Kinase Antagonist PP2 Inhibits H2O2-induced JAK2, STAT3, and p38 MAPK Phosphorylation—Because Fyn has been suggested to be upstream of JAK2 and p38 MAPK (43, 51, 52), we investigated whether Fyn activation is required for H2O2-induced stimulation of JAK2, STAT3, and p38 MAPKs in VSMCs (Fig. 7). Pretreatment of VSMCs with 1 μm PP2 abrogated H2O2-induced tyrosine phosphorylation of Fyn in wild-type VSMCs (p < 0.05 versus H2O2 treatment for 10 min, Fig. 7, A and B). H2O2-induced Fyn tyrosine phosphorylation in LAR-/- VSMCs was also significantly (p < 0.001 versus H2O2 treatment for 10 min) inhibited by PP2 pretreatment. Similarly, complete inhibition of H2O2-induced tyrosine phosphorylation of JAK2 was observed with PP2 pretreatment in both wild-type (p < 0.05 versus H2O2 treatment for 10 min) and LAR knockout VSMCs (p < 0.001 versus H2O2 treatment for 10 min, Fig. 7, C and D). Consistent with the inhibition of JAK2 activation, PP2 pretreatment significantly decreased H2O2-induced STAT3 tyrosine phosphorylation in both wild-type and LAR-/- VSMCs (p < 0.001 versus H2O2 treatment for 10 min for both the cell types) (Fig. 7, E and F). PP2 pretreatment also significantly inhibited H2O2-induced phosphorylation of p38 MAPK in both wild-type and LAR-/- VSMCs (p < 0.001 versus H2O2 treatment for 10 min for both the cell types) (Fig. 7, G and H). These data indicate that Fyn activation regulates H2O2-induced signaling events, and Fyn is an upstream kinase in the JAK-STAT pathway and p38 MAPK activation.
FIGURE 7.
H2O2-induced Fyn and JAK-STAT pathway and p38 MAPK activation is abrogated by a Fyn inhibitor. Growth-arrested VSMCs were pretreated with DMSO or PP2 (1 μm) for 2 h before treatment with 1 mm H2O2 for the indicated times. Lysates were immunoprecipitated with anti-Fyn antibody and subjected to Western analysis with anti-PY20 antibody (A). Lysates were subjected to Western analysis with either anti-phospho Jak2 (C), anti-phospho STAT3 (E), or anti-phospho p38 MAPK (G) antibodies. Densitometric analysis of normalized phospho Fyn (B), phospho JAK2 (D), and phospho STAT3 (F) and phospho p38 MAPK (H) (mean ± S.E., n = 3; *, p < 0.05, and **, p < 0.001 versus respective cells treated with H2O2 for 10 min).
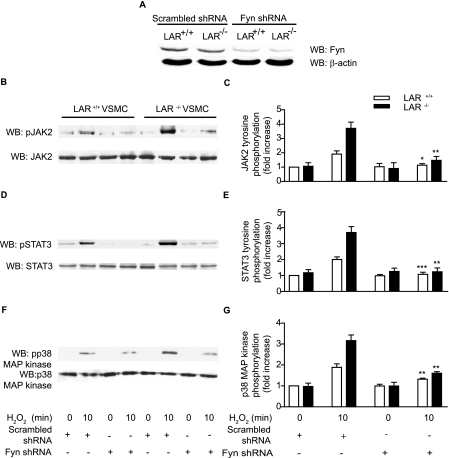
Silencing of Fyn Expression by shRNA Abrogates H2O2-induced JAK2, STAT3, and p38 MAPK Phosphorylation in VSMCs—To confirm the requirement for Fyn in H2O2-induced JAK2, STAT3, and p38 MAPK activation, we used retroviral expression of Fyn shRNAmir to silence the expression of the endogenous Fyn. Infection of wild-type and LAR-/- VSMCs with retroviral Fyn shRNA construct decreased Fyn expression by ∼70% compared with respective scrambled shRNA-expressing VSMCs (Fig. 8A). Expression of Fyn shRNA abrogated H2O2-induced increase in JAK2 phosphorylation in wild-type (p < 0.01 versus respective scrambled shRNA-expressing cells treated with H2O2 for 10 min) and LAR-/- VSMCs (p < 0.001 versus respective scrambled shRNA-expressing cells treated with H2O2 for 10 min) (Fig. 8, B and C). Consistent with JAK2 inhibition, Fyn shRNA expression inhibited H2O2-induced increase in STAT3 phosphorylation in wild-type (p < 0.05 versus respective scrambled shRNA-expressing cells treated with H2 O2 for 10 min) and LAR-/- VSMCs (p < 0.001 versus respective scrambled shRNA-expressing cells treated with H2O2 for 10 min) (Fig. 8, D and E). Expression of Fyn shRNA also significantly decreased H2O2-induced increase in p38 MAPK phosphorylation in wild-type (p < 0.001 versus respective scrambled shRNA-expressing cells treated with H2O2 for 10 min) and LAR-/- VSMCs (p < 0.001 versus respective scrambled shRNA-expressing cells treated with H2O2 for 10 min) (Fig. 8, F and G). However, expression of Fyn shRNA had no effect on the steady-state levels of JAK2, STAT3, and p38 MAPK expression. Together, these data indicate that LAR regulates H2O2-induced cell signaling in VSMCs by modulating Fyn activation.
FIGURE 8.
H2O2-stimulated tyrosine phosphorylation of JAK2 and STAT3 and p38 MAPK are inhibited in Fyn shRNA-expressing LAR+/+ and LAR-/- VSMC clones. Scrambled shRNA- or Fyn shRNA-expressing LAR+/+ and LAR-/- VSMCs lysates were analyzed by Western analysis with anti-Fyn antibody, and the membrane was reprobed with anti-β-actin antibody (A). Scrambled shRNA- or Fyn shRNA-expressing LAR+/+ and LAR-/- VSMCs clones were growth-arrested and then treated with 1 mm H2O2 for the time indicated, and lysates were analyzed by Western blotting with either anti-phospho JAK2 (B), anti-phospho STAT3 (D), or anti-phospho p38 MAPK (F) antibodies. Densitometric analysis of normalized phospho JAK2 (C), phospho STAT3 (E), and phospho p38 MAPK (G) (mean ± S.E., n = 3; *, p < 0.01, **, p < 0.001 and ***, p < 0.05 versus respective cells treated H2O2 for 10 min).
JAK2 Activates STAT3, but Not p38 MAPK, in VSMCs Treated with H2O2—To determine whether JAK2 is upstream of STAT3 as well as p38 MAPK activation, we investigated the effect of AG490, a JAK2-specific inhibitor, on the activation of STAT3 and p38 MAPK in wild-type and LAR knockout VSMCs treated with H2O2. Pretreatment with 25 μm AG490 for 16 h prior to H2O2 treatment had no effect on phosphorylation of Fyn, the JAK2 upstream kinase, in wild-type and LAR-/- cells (Fig. 9, A and B). Pretreatment with AG490 significantly inhibited H2O2-induced tyrosine phosphorylation of JAK2 in both wild-type (p < 0.05 versus respective cells treated with H2O2 for 10 min) and LAR knockout (p < 0.001 versus respective cells treated with H2O2 for 10 min) VSMCs (Fig. 9, C and D). Consistent with the inhibition of JAK2 stimulation, STAT3 tyrosine phosphorylation was significantly inhibited in both wild-type (p < 0.05 versus respective cells treated with H2O2 for 10 min) and LAR knockout (p < 0.001 versus respective H2O2-treated cells) VSMCs (Fig. 9, E and F). In contrast, p38 MAPK phosphorylation induced by H2O2 was unaffected by AG490 in both the cell types (Fig. 9, G and H). These results indicate that LAR regulates two alternative oxidative stress-induced pathways in VSMCs: a Fyn/JAK2/STAT3 pathway and a Fyn/p38 MAPK pathway.
FIGURE 9.
H2O2-stimulated tyrosine phosphorylation of JAK2 and STAT3 but not Fyn and p38 MAPK are inhibited by AG490 in both LAR+/+ and LAR-/- VSMCs. Growth-arrested VSMCs were pretreated with 25 μm AG490 for 16 h and then treated with H2O2 for 10 min. Cell lysates were immunoprecipitated with anti-Fyn antibody followed by Western analysis with anti-phosphotyrosine (PY20) or anti-Fyn antibody (A). Cell lysates were also analyzed by Western blotting with either anti-phospho JAK2 (C), anti-phospho STAT3 (E), or anti-phospho p38 MAPK (G) antibodies. Densitometric analysis of normalized phospho Fyn (B), JAK2 (D), phospho STAT3 (F) and phospho p38 MAPK (H) (mean ± S.E., n = 3, *, p < 0.05, **, p < 0.001 and ***, p < 0.05 versus respective cells treated with H2O2).
Fyn and JAK2 Are Required for H2O2-induced Apoptosis of VSMCs—To elucidate the role of Fyn activation in H2O2-induced VSMC apoptosis, we pretreated wild-type and LAR knockout VSMCs with Fyn inhibitor PP2 (1 μm) for 2 h, prior to treatment with or without H2O2 for 16 h (Fig. 10A). PP2 pretreatment significantly decreased H2O2-induced apoptosis, as measured by histone-associated DNA fragmentation, in both wild-type and LAR knockout VSMCs (p < 0.001 versus respective cells treated with H2O2).
FIGURE 10.
H2O2-induced apoptosis in wild-type and LAR-/- VSMCs is inhibited by PP2, expression of Fyn shRNA, and AG 490. A, growth-arrested VSMCs were pretreated with 1 μm PP2 for 2 h and then treated with 1 mm H2O2 for 16 h. Cell lysates were assayed for histone-associated DNA fragmentation (mean ± S.E., n = 3; *, p < 0.001 respective H2O2-treated cells). B, mock-infected control VSMCs, scrambled shRNA- or Fyn shRNA-expressing LAR+/+ and LAR-/- VSMCs clones were growth-arrested and then treated with 1 mm H2O2 for 16 h. Cell lysates were assayed for histone-associated DNA fragmentation (mean ± S.E., n = 3; *, p < 0.01 versus mock-infected H2O2-treated LAR+/+ VSMCs; **, p < 0.001 versus mock-infected H2O2-treated LAR-/- VSMCs). C, growth-arrested VSMCs were pretreated with 25 μm AG490 for 16 h and then treated with 1 mm H2O2 for 16 h. Cell lysates were assayed for histone-associated DNA fragmentation (mean ± S.E., n = 3; *, p < 0.001 respective H2O2-treated cells).
Consistent with the effect of PP2 on H2O2-induced apoptosis in VSMCs, histone-associated DNA fragmentation upon H2O2 treatment was partially, but significantly decreased in wild-type VSMCs clones expressing Fyn shRNA compared with untreated wild-type cells or scrambled shRNA-expressing wild-type VSMCs clones (p < 0.01) (Fig. 10B). Similarly, a significant decrease in histone-associated DNA fragmentation upon H2O2 treatment was observed in LAR knockout VSMCs clones expressing Fyn shRNA compared with H2O2-treated LAR knockout VSMCs or scrambled shRNA expressing LAR knockout VSMCs clones (p < 0.001). Importantly, suppression of Fyn expression in LAR knockout VSMCs decreased H2O2-induced histone-associated DNA fragmentation to the same level observed in wild-type VSMCs clones expressing Fyn shRNA. Together, these results indicate that LAR regulates H2O2-induced apoptosis by modulating Fyn kinase activity.
Similar to the effect of PP2 on H2O2-induced apoptosis, pretreatment of VSMCs with 25 μm AG490 for 16 h significantly decreased H2O2-induced histone-associated DNA fragmentation in wild-type and LAR knockout VSMCs (p < 0.001 versus respective cells treated with H2O2) (Fig. 10C). Overall, these results suggest that LAR regulates VSMC apoptosis under oxidative stress conditions by inhibiting hyperactivation of Fyn/JAK2/STAT3 and Fyn/p38 MAPK pathways.
LAR Overexpression Attenuates H2O2-induced Fyn Activation in LAR-deficient VSMCs—To confirm the regulatory role of LAR in Fyn activation induced by H2O2, LAR knockout VSMCs were infected with either Adβ-galactosidase or AdLAR and then treated with H2O2. As shown in Fig. 11, overexpression of LAR in LAR knockout VSMCs led to enhanced association of LAR with activated Fyn (top panel) and decreased tyrosine phosphorylation of Fyn kinase (second panel). Increased association of LAR with Fyn and decreased phosphorylation of Fyn were also observed in human aortic SMC overexpressing LAR and treated with H2O2 (data not shown). Together, these results support the notion that LAR regulates Fyn activity in VSMCs under oxidative stress conditions.
FIGURE 11.
H2O2-induced tyrosine phosphorylation of Fyn is attenuated in LAR-/- VSMCs overexpressing LAR. Growth-arrested LAR-/- VSMCs, infected with either Adβ-galactosidase or AdLAR, were treated with 1 mm H2O2 for the indicated times. Cell lysates were immunoprecipitated with anti-Fyn antibody followed by Western analysis with either anti-LAR antibody, anti-PY20 antibody, or anti-Fyn antibody. Whole cell lysates were analyzed by Western blotting with anti-LAR monoclonal antibody, and the membrane was reprobed with anti-β-actin antibody.
DISCUSSION
ROS regulate various redox-sensitive cellular functions, including response to stress or growth signals (55). H2O2 is produced during activation of cognate receptors by various growth factors, cytokines, and hormones. The transient increase in intracellular H2O2 produced under these conditions is equivalent to exogenously added H2O2 concentrations of 0.1-1.0 mm (22, 56). When H2O2 is applied to cell cultures, a rapid equilibrium is established, from the metabolic actions of cellular peroxidases and catalases, in which the intracellular concentration of H2O2 is ∼7- to 10-fold less than the extracellular concentration (56). PTPs are the important redox sensors inside the cell, because localized reversible oxidation and consequent inactivation of these proteins ensures efficient intracellular signal propagation (14, 16, 57). ROS-induced inactivation of PTPs in VSMCs was reported (58, 59), however, the specific role of individual PTP in regulating apoptotic pathways has not yet been investigated. In the present report, we characterize the role of LAR in H2O2-induced apoptosis using VSMCs derived from mice that are deficient in this PTP. Our data indicate that LAR plays an important role in VSMC survival by negatively regulating H2O2-induced Fyn activation and downstream effector pathways of apoptosis.
Our results demonstrate that LAR is inactivated rapidly in VSMCs treated with H2O2. This is consistent with the report of Groen et al. (20), which demonstrated that both catalytically active membrane-proximal domain D1 and membrane-distal regulatory domain D2 of LAR are readily oxidized at low H2O2 concentrations, with maximal oxidation at 125-250 μm concentration. However, VSMCs contain several other redox-sensitive PTPs such as SHP-1, PTP1B, and low molecular weight PTP (13), which might become inactive at the H2O2 concentration we used in this study. The use of LAR-deficient VSMCs allowed us to circumvent the confounding effects of other inactivated PTPs following H2O2 treatment and investigate the specific role of LAR in regulating stress-induced apoptotic pathways. Our results show that activation of Fyn in response to H2O2 treatment is transient in wild-type VSMCs, whereas absence of LAR results in sustained activation of this proapoptotic kinase in H2O2-treated cells (Fig. 4).
The critical role of Fyn activation in redox-sensitive mechanisms is evident from the impaired ROS-induced cellular signaling pathways in various Fyn knockout cells (43, 45). Further, caspase activation and DNA fragmentation induced by various proapoptotic stimuli are significantly reduced in embryonic fibroblasts and thymocytes from Fyn knockout mice (60), which strengthens the idea that Fyn activation is a critical event in stress-induced apoptosis. These observations are in agreement with our data that silencing of Fyn expression by shRNA significantly attenuated H2O2-induced downstream signaling pathways (Fig. 8) and apoptosis in both wild-type and LAR knockout VSMCs (Fig. 10).
Regulation of Src-family kinase activity occurs through phosphorylation on two tyrosine residues (48). Autophosphorylation of tyrosine in the activation loop (Tyr-419 in Fyn) increases kinase activity, whereas phosphorylation of tyrosine residue in the C terminus (Tyr-530 in Fyn) inhibits activity through an intramolecular SH2 (Src homology 2)-phosphotyrosine interaction. It has been suggested that dephosphorylation of tyrosine in the C-terminal domain precedes the autophosphorylation of tyrosine in the activation loop for full kinase activity (61). However, Sanguinetti et al. (49) demonstrated that, concomitant with autophosphorylation, oxidative stress also increased phosphorylation of the inhibitory site of Fyn, which indicates that activation of Fyn is rapidly followed by inactivation. Activation of Fyn could also be regulated by LAR as this activated PTP is associated with Fyn and dephosphorylates the phosphotyrosine residues in both the kinase domain and C terminus (46). Consistent with these observations, our results demonstrate enhanced association of LAR with activated Fyn in H2O2-treated VSMCs (Fig. 5, A and B) and increased autophosphorylation and sustained activation of Fyn in LAR knockout VSMCs treated with H2O2. This occurred despite increased phosphorylation of Tyr-530 in Fyn. Furthermore, absence of LAR impaired mitochondrial membrane potential in VSMCs treated with H2O2 and enhanced caspase-3/7 activity and apoptosis (Fig. 2).
Our data indicate increased activation of JAK2 and STAT3 in wild-type VSMCs and significantly enhanced activation of these proteins in LAR knockout VSMCs compared with wild-type cells in response to H2O2 treatment. In addition, inhibition of Fyn activity with PP2 pretreatment abrogated H2O2-induced JAK2 and STAT3 stimulation in both wild-type and LAR knockout mice. These data are in consonance with the observations that H2O2-stimulated JAK2 activity was completely inhibited in Fyn knockout cells (43) and that Fyn directly interacts with activated JAK2 (62).
The JAK2-specific inhibitor, AG490, not only abrogated H2O2-induced stimulation of STAT3 (Fig. 8, C and D), but also significantly attenuated H2O2-induced apoptosis in both wild-type and LAR knockout cells (Fig. 9B). These results are in agreement with the observation of Sandberg and Sayeski (50) that activation of JAK2 is essential for H2O2-induced apoptosis of VSMCs. They have shown that JAK2 activation leads to induction of proapoptotic Bax expression, impaired mitochondrial membrane integrity, and activation of caspases. Further, AG490 treatment markedly reduced ischemia-induced infarct size and cardiomyocyte apoptosis (63). Although STAT3 activation is usually associated with cell proliferation, strong and prolonged activation of STAT3 induces apoptosis (64, 65), which further supports our conclusions that hyperactivation of the Fyn/JAK2/STAT3 pathway in the absence of LAR induces VSMC apoptosis.
Our data indicate that H2O2-induced p38 MAPK activity, which was increased in wild-type VSMCs, was further enhanced in the absence of LAR. PP2 pretreatment abrogated H2O2-induced p38 MAPK activity, whereas AG490 had no such inhibitory effect. These data confirm that Fyn is upstream of p38 MAPK activation (51, 52) and that Fyn/JAK2/STAT3 and Fyn/p38 MAPK are two distinct signaling pathways involved in oxidative stress-induced VSMC apoptosis. It is well documented that p38 MAPK plays a critical role in apoptosis induced by several agonists (54, 66, 67). Phosphorylation of Bcl-2 by activated p38 MAPK in mitochondrial compartment leads to cytochrome c release and caspase activation, and these processes are absent in p38 MAPK-deficient cells (68).
In conclusion, our results demonstrate that LAR is a redox-sensor and negative regulator of H2O2-induced apoptosis in VSMCs. Moreover, our report elucidates Fyn/JAK2/STAT3 and Fyn/p38 MAPK pathways as intracellular mediators of H2O2-induced VSMC apoptosis. Our data also suggest tissue/cell-specific effect of LAR as its activation/expression was correlated with increased apoptosis in other tissues (69-71). PTP inhibitor development is an active area of research for the treatment of obesity and diabetes. However, our data cautions on the use of such a strategy, because apoptosis, induced by exacerbated oxidative stress prevalent under such pathophysiological conditions, may profoundly affect many vascular diseases.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-5735. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: VSMC, vascular smooth muscle cell; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4-d] pyrimidine; ROS, reactive oxygen species; PTP, protein tyrosine phosphatase; JAK2, Janus kinase 2; STAT3, signal transducer and activator of transcription 3; MAPK, mitogen-activated protein kinase; FBS, fetal bovine serum; DMEM, Dulbecco's modified Eagle's medium; shRNA, short hairpin RNA; LAR, leukocyte antigen-related; ERK, extracellular signal-regulated kinase; MOPS, 4-morpholinepropanesulfonic acid; AdLAR, adenovirus-encoding LAR; CMV, cytomegalovirus; ANOVA, analysis of variance; SH2, Src homology 2.
References
- 1.Meier, P., Finch, A., and Evan, G. (2000) Nature 407 796-801 [DOI] [PubMed] [Google Scholar]
- 2.Han, D., Haudenschild, C., Hong, M., Tinkle, B., Leon, M., and Liau, G. (1995) Am. J. Pathol. 147 267-277 [PMC free article] [PubMed] [Google Scholar]
- 3.Geng, Y., and Libby, P. (1995) Am. J. Pathol. 147 251-266 [PMC free article] [PubMed] [Google Scholar]
- 4.Pollman, M. J., Hall, J. L., and Gibbons, G. H. (1999) Circ. Res. 84 113-121 [DOI] [PubMed] [Google Scholar]
- 5.Bauriedel, G., Hutter, R., Welsch, U., Bach, R., Sievert, H., and Luderitz, B. (1999) Cardiovasc. Res. 41 480-488 [DOI] [PubMed] [Google Scholar]
- 6.von der Thusen, J. H., van Vlijmen, B. J., Hoeben, R. C., Kockx, M. M., Havekes, L. M., van Berkel, T. J., and Biessen, E. A. (2002) Circulation 105 2064-2070 [DOI] [PubMed] [Google Scholar]
- 7.Clarke, M. C., Figg, N., Maguire, J. J., Davenport, A. P., Goddard, M., Littlewood, T. D., and Bennett, M. R. (2006) Nat Med. 12 1075-1080 [DOI] [PubMed] [Google Scholar]
- 8.Smith, C., Mitchinson, M. J., Aruoma, O. I., and Halliwell, B. (1992) Biochem. J. 286 901-905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, P. F., Dietz, R., and von Harsdorf, R. (1997) FEBS Lett. 404 249-252 [DOI] [PubMed] [Google Scholar]
- 10.Sorescu, D., Weiss, D., Lassegue, B., Clempus, R. E., Szocs, K., Sorescu, G. P., Valppu, L., Quinn, M. T., Lambeth, J. D., Vega, J. D., Taylor, W. R., and Griendling, K. K. (2002) Circulation 105 1429-1435 [DOI] [PubMed] [Google Scholar]
- 11.Guo, Z., Mitchell-Raymundo, F., Yang, H., Ikeno, Y., Nelson, J., Diaz, V., Richardson, A., and Reddick, R. (2002) Mech Ageing Dev. 123 1121-1131 [DOI] [PubMed] [Google Scholar]
- 12.Irani, K. (2000) Circ. Res. 87 179-183 [DOI] [PubMed] [Google Scholar]
- 13.Clempus, R. E., and Griendling, K. K. (2006) Cardiovasc Res. 71 216-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, S. R., Kwon, K. S., Kim, S. R., and Rhee, S. G. (1998) J. Biol. Chem. 273 15366-15372 [DOI] [PubMed] [Google Scholar]
- 15.Meng, T. C., Fukada, T., and Tonks, N. K. (2002) Mol Cell. 9 387-399 [DOI] [PubMed] [Google Scholar]
- 16.Meng, T. C., Buckley, D. A., Galic, S., Tiganis, T., and Tonks, N. K. (2004) J. Biol. Chem. 279 37716-37725 [DOI] [PubMed] [Google Scholar]
- 17.Tang, H., Hao, Q., Rutherford, S. A., Low, B., and Zhao, Z. J. (2005) J. Biol. Chem. 280 23918-23925 [DOI] [PubMed] [Google Scholar]
- 18.Denu, J. M., and Dixon, J. E. (1998) Curr. Opin. Chem. Biol. 2 633-641 [DOI] [PubMed] [Google Scholar]
- 19.Denu, J. M., and Tanner, K. G. (1998) Biochemistry 37 5633-5642 [DOI] [PubMed] [Google Scholar]
- 20.Groen, A., Lemeer, S., van der Wijk, T., Overvoorde, J., Heck, A. J., Ostman, A., Barford, D., Slijper, M., and den Hertog, J. (2005) J. Biol. Chem. 280 10298-10304 [DOI] [PubMed] [Google Scholar]
- 21.Bae, Y. S., Kang, S. W., Seo, M. S., Baines, I. C., Tekle, E., Chock, P. B., and Rhee, S. G. (1997) J. Biol. Chem. 272 217-221 [PubMed] [Google Scholar]
- 22.Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K., and Finkel, T. (1995) Science 270 296-299 [DOI] [PubMed] [Google Scholar]
- 23.Hao, Q., Rutherford, S. A., Low, B., and Tang, H. (2006) Free Radic. Biol. Med. 41 302-310 [DOI] [PubMed] [Google Scholar]
- 24.Pulido, R., Serra-Pages, C., Tang, M., and Streuli, M. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 11686-11690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streuli, M., Krueger, N. X., Ariniello, P. D., Tang, M., Munro, J. M., Blattler, W. A., Adler, D. A., Disteche, C. M., and Saito, H. (1992) EMBO J. 11 897-907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu, Q., Lenardo, T., and Weinberg, R. A. (1992) Oncogene 7 1051-1057 [PubMed] [Google Scholar]
- 27.Tsujikawa, K., Kawakami, N., Uchino, Y., Ichijo, T., Furukawa, T., Saito, H., and Yamamoto, H. (2001) Mol. Endocrinol. 15 271-280 [DOI] [PubMed] [Google Scholar]
- 28.Longo, F. M., Martignetti, J. A., Le Beau, J. M., Zhang, J. S., Barnes, J. P., and Brosius, J. (1993) J. Biol. Chem. 268 26503-26511 [PubMed] [Google Scholar]
- 29.Kulas, D. T., Zhang, W. R., Goldstein, B. J., Furlanetto, R. W., and Mooney, R. A. (1995) J. Biol. Chem. 270 2435-2438 [DOI] [PubMed] [Google Scholar]
- 30.Ahmad, F., and Goldstein, B. J. (1997) J. Biol. Chem. 272 448-457 [PubMed] [Google Scholar]
- 31.Ren, J. M., Li, P. M., Zhang, W. R., Sweet, L. J., Cline, G., Shulman, G. I., Livingston, J. N., and Goldstein, B. J. (1998) Diabetes 47 493-497 [DOI] [PubMed] [Google Scholar]
- 32.Niu, X. L., Li, J., Hakim, Z. S., Rojas, M., Runge, M. S., and Madamanchi, N. R. (2007) J. Biol. Chem. 282 19808-19819 [DOI] [PubMed] [Google Scholar]
- 33.Skarnes, W. C., Moss, J. E., Hurtley, S. M., and Beddington, R. S. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 6592-6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang, T., Bernabeu, R., Xie, Y., Zhang, J. S., Massa, S. M., Rempel, H. C., and Longo, F. M. (2003) J. Neurosci. 23 3353-3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeo, T. T., Yang, T., Massa, S. M., Zhang, J. S., Honkaniemi, J., Butcher, L. L., and Longo, F. M. (1997) J. Neurosci. Res. 47 348-360 [DOI] [PubMed] [Google Scholar]
- 36.Moon, S. K., Thompson, L. J., Madamanchi, N., Ballinger, S., Papaconstantinou, J., Horaist, C., Runge, M. S., and Patterson, C. (2001) Am. J. Physiol. 280 H2779-H2788 [DOI] [PubMed] [Google Scholar]
- 37.Madamanchi, N. R., Li, S., Patterson, C., and Runge, M. S. (2001) J. Biol. Chem. 276 18915-18924 [DOI] [PubMed] [Google Scholar]
- 38.Channon, K. M., Blazing, M. A., Shetty, G. A., Potts, K. E., and George, S. E. (1996) Cardiovasc. Res. 32 962-972 [PubMed] [Google Scholar]
- 39.Kamata, H., Honda, S., Maeda, S., Chang, L., Hirata, H., and Karin, M. (2005) Cell 120 649-661 [DOI] [PubMed] [Google Scholar]
- 40.Lee, C. K., Park, H. J., So, H. H., Kim, H. J., Lee, K. S., Choi, W. S., Lee, H. M., Won, K. J., Yoon, T. J., Park, T. K., and Kim, B. (2006) Proteomics 6 6455-6475 [DOI] [PubMed] [Google Scholar]
- 41.Salvesen, G. S., and Dixit, V. M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 10964-10967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sgorbissa, A., Benetti, R., Marzinotto, S., Schneider, C., and Brancolini, C. (1999) J. Cell Sci. 112 4475-4482 [DOI] [PubMed] [Google Scholar]
- 43.Abe, J., and Berk, B. C. (1999) J. Biol. Chem. 274 21003-21010 [DOI] [PubMed] [Google Scholar]
- 44.Yoshizumi, M., Abe, J., Haendeler, J., Huang, Q., and Berk, B. C. (2000) J. Biol. Chem. 275 11706-11712 [DOI] [PubMed] [Google Scholar]
- 45.Abe, J., Okuda, M., Huang, Q., Yoshizumi, M., and Berk, B. C. (2000) J. Biol. Chem. 275 1739-1748 [DOI] [PubMed] [Google Scholar]
- 46.Tsujikawa, K., Ichijo, T., Moriyama, K., Tadotsu, N., Sakamoto, K., Sakane, N., Fukada, S., Furukawa, T., Saito, H., and Yamamoto, H. (2002) Mol. Cancer Res. 1 155-163 [PubMed] [Google Scholar]
- 47.Schaapveld, R. Q., Schepens, J. T., Robinson, G. W., Attema, J., Oerlemans, F. T., Fransen, J. A., Streuli, M., Wieringa, B., Hennighausen, L., and Hendriks, W. J. (1997) Dev. Biol. 188 134-146 [DOI] [PubMed] [Google Scholar]
- 48.Mayer, B. J. (1997) Curr. Biol. 7 R295-R298 [DOI] [PubMed] [Google Scholar]
- 49.Sanguinetti, A. R., Cao, H., and Corley Mastick, C. (2003) Biochem. J. 376 159-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandberg, E. M., and Sayeski, P. P. (2004) J. Biol. Chem. 279 34547-34552 [DOI] [PubMed] [Google Scholar]
- 51.Samayawardhena, L. A., Hu, J., Stein, P. L., and Craig, A. W. (2006) Cell Signal. 18 1447-1454 [DOI] [PubMed] [Google Scholar]
- 52.Lamalice, L., Houle, F., and Huot, J. (2006) J. Biol. Chem. 281 34009-34020 [DOI] [PubMed] [Google Scholar]
- 53.Diep, Q. N., Touyz, R. M., and Schiffrin, E. L. (2000) Hypertension 36 851-855 [DOI] [PubMed] [Google Scholar]
- 54.Mori-Abe, A., Tsutsumi, S., Takahashi, K., Toya, M., Yoshida, M., Du, B., Kawagoe, J., Nakahara, K., Takahashi, T., Ohmichi, M., and Kurachi, H. (2003) J. Endocrinol. 178 417-426 [DOI] [PubMed] [Google Scholar]
- 55.Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M., and Telser, J. (2007) Int. J. Biochem. Cell Biol. 39 44-84 [DOI] [PubMed] [Google Scholar]
- 56.Stone, J. R., and Yang, S. (2006) Antioxid Redox Signal. 8 243-270 [DOI] [PubMed] [Google Scholar]
- 57.Chiarugi, P., Fiaschi, T., Taddei, M. L., Talini, D., Giannoni, E., Raugei, G., and Ramponi, G. (2001) J. Biol. Chem. 276 33478-33487 [DOI] [PubMed] [Google Scholar]
- 58.Amiri, F., Venema, V. J., Wang, X., Ju, H., Venema, R. C., and Marrero, M. B. (1999) J. Biol. Chem. 274 32382-32386 [DOI] [PubMed] [Google Scholar]
- 59.Kappert, K., Sparwel, J., Sandin, A., Seiler, A., Siebolts, U., Leppänen, O., Rosenkranz, S., and Ostman, A. (2006) Arterioscler. Thromb. Vasc. Biol. 26 2644-2651 [DOI] [PubMed] [Google Scholar]
- 60.Ricci, J. E., Lang, V., Luciano, F., Belhacene, N., Giordanengo, V., Michel, F., Bismuth, G., and Auberger, P. (2001) FASEB J. 15 1777-1779 [DOI] [PubMed] [Google Scholar]
- 61.Xu, W., Harrison, S. C., and Eck, M. J. (1997) Nature 385 595-602 [DOI] [PubMed] [Google Scholar]
- 62.Uddin, S., Sher, D. A., Alsayed, Y., Pons, S., Colamonici, O. R., Fish, E. N., White, M. F., and Platanias, L. C. (1997) Biochem. Biophys. Res. Commun. 235 83-88 [DOI] [PubMed] [Google Scholar]
- 63.Mascareno, E., El-Shafei, M., Maulik, N., Sato, M., Guo, Y., Das, D. K., and Siddiqui, M. A. (2001) Circulation 104 325-329 [DOI] [PubMed] [Google Scholar]
- 64.Ahmed-Choudhury, J., Williams, K. T., Young, L. S., Adams, D. H., and Afford, S. C. (2006) Cell Signal. 18 456-488 [DOI] [PubMed] [Google Scholar]
- 65.Lu, Y., Fukuyama, S., Yoshida, R., Kobayashi, T., Saeki, K., Shiraishi, H., Yoshimura, A., and Takaesu, G. (2006) J. Biol. Chem. 281 36683-36690 [DOI] [PubMed] [Google Scholar]
- 66.Rosini, P., De Chiara, G., Bonini, P., Lucibello, M., Marcocci, M. E., Garaci, E., Cozzolino, F., and Torcia, M. (2004) J. Biol. Chem. 279 14016-14023 [DOI] [PubMed] [Google Scholar]
- 67.Grethe, S., Ares, M. P., Andersson, T., and Pörn-Ares, M. I. (2004) Exp. Cell Res. 298 632-642 [DOI] [PubMed] [Google Scholar]
- 68.De Chiara, G., Marcocci, M. E., Torcia, M., Lucibello, M., Rosini, P., Bonini, P., Higashimoto, Y., Damonte, G., Armirotti, A., Amodei, S., Palamara, A. T., Russo, T., Garaci, E., and Cozzolino, F. (2006) J. Biol. Chem. 281 21353-21361 [DOI] [PubMed] [Google Scholar]
- 69.Weng, L. P., Yuan, J., and Yu, Q. (1998) Curr. Biol. 8 247-256 [DOI] [PubMed] [Google Scholar]
- 70.Weng, L. P., Wang, X., and Yu, Q. (1999) Genes Cells 4 185-196 [DOI] [PubMed] [Google Scholar]
- 71.Tisi, M. A., Xie, Y., Yeo, T. T., and Longo, F. M. (2000) J. Neurobiol. 42 477-486 [PubMed] [Google Scholar]