Abstract
The earliest steps in nascent protein maturation greatly affect its overall efficiency. Constraints placed on maturing proteins at these early stages limit available conformations and help to direct the native maturation process. For type II membrane proteins, these cotranslational constraints include N- and C-terminal membrane tethering, chaperone binding, and disulfide bond formation. The cotranslational maturation process for the type II membrane glycoprotein influenza neuraminidase (NA) was investigated to provide a deeper understanding of these initial endoplasmic reticulum events. The type II orientation provides experimental advantages to monitor the first maturation steps. Calnexin was shown to cotranslationally interact with NA prior to calreticulin. These interactions were required for the efficient maturation of NA as it prematurely formed intramolecular disulfides and aggregated when calnexin and calreticulin interactions were abolished. Lectin chaperone binding slowed the NA maturation process, increasing its fidelity. Carbohydrates were required for NA maturation in a regio-specific manner. A subset of NA formed intermolecular disulfides and oligomerized cotranslationally. This fraction increased in the absence of calnexin and calreticulin binding. NA dimerization also occurred for an NA mutant lacking the critical large loop disulfide bond, indicating that dimerization did not require proper NA oxidation. The strict evaluation of proper maturation carried out by the quality control machinery was instilled at the tetramerization step. This study illustrates the type II membrane protein maturation process and shows how important cotranslational events contribute to the proper cellular maturation of glycoproteins.
Protein folding in living cells begins during synthesis or cotranslationally (1, 2). By folding cotranslationally, a protein can fold vectorially with the N-terminal region folding prior to completion of the synthesis of its C terminus. Coupling protein folding and synthesis tapers the possible ensemble of the available folding intermediates during progression to the native state, thereby minimizing nonproductive options, which helps to increase the overall fidelity of the folding reaction (3). Additionally, cotranslational folding ensures that the vulnerable nascent chain initially resides in a controlled environment optimized for proper folding (4, 5).
For proteins that traverse the secretory pathway, translation is linked to translocation producing a cotranslational and cotranslocational process that supports folding upon entrance into the endoplasmic reticulum (ER)4 lumen through the Sec61 translocon. The translocon and its associated proteins provide a privileged folding environment that favors proper maturation and limits interchain aggregation (6–9). Understanding cotranslational protein maturation and the organization of the ER translocon folding environment is of fundamental biological concern.
Nascent proteins can start to fold or form disulfide bonds as soon as the completed domain or Cys residues are translocated into the oxidizing environment of the ER lumen (1, 10). The ER houses various molecular chaperones, foldases, folding sensors, and covalent modifiers that assist with the proper folding and assembly of nascent proteins (11–14). It also provides a quality control system that permits properly folded and completely assembled proteins to exit the ER for trafficking to the Golgi (12, 15). Proteins that fail to reach their native state are generally retained in the ER and eventually degraded through the ER-associated protein degradation pathway (16).
The vast majority of proteins that traverse the secretory pathway are glycosylated. N-linked glycans are cotranslationally added when the consensus glycosylation site (Asn-X-Ser/Thr) resides ∼12–14 amino acids away from the translocon (17). The bulky, hydrophilic, and flexible N-linked glycans act as maturation and quality control tags that mediate interactions with molecular chaperones or quality control sorting receptors (13, 18–20). In the ER, the carbohydrate-binding molecular chaperones calnexin (CNX) and calreticulin (CRT) assist glycoproteins with maturation. They recognize monoglucosylated N-linked carbohydrates (Glc1ManxGlcNAc2) (21–23), which are rapidly generated through sequential trimming of triglucosylated species (Glc3Man9GlcNAc2) by the ER glucosidases I and II. Lectin chaperone binding persists until glucosidase II trims the final glucose. The unbound substrate is then free to fold and assemble to its native state (23, 24). Proteins with non-native or immature conformations are reglucosylated by the UDP-glucose:glycoprotein glucosyltransferase (GT1) to re-enter the CNX binding cycle (25–27). Therefore, glucosidase II and GT1 act antagonistically to support retention of immature, misfolded, or unassembled proteins in the CNX cycle and the ER (18, 28).
Viral proteins have been used as model substrates to advance our general knowledge of cellular protein maturation because they utilize the host cellular machinery for their maturation and are expressed at high levels in infected cells (29, 30). Little is known about the early maturation steps for type II membrane proteins. Initially, they are dually constrained by the N-terminal hydrophobic transmembrane segment that tethers the polypeptide to the membrane and the C-terminal attachment to the ribosome and translocon. The type II orientation provides technical advantages for live cell cotranslational protein maturation studies, which support the ability to monitor these critical early events in detail.
Influenza neuraminidase (NA) is a type II membrane glycoprotein. The structure of the enzymatically active head domain for the avian NA subtype 9 has been solved (31). N9 NA contains seven N-linked carbohydrates, nine intramolecular disulfide bonds, and two additional Cys residues that might be involved in intermolecular linkages. In this study, we investigated the molecular chaperone-assisted cotranslational folding and maturation processes of NA, uncovering critical early steps in the maturation process for a type II membrane protein.
EXPERIMENTAL PROCEDURES
Reagents—The T7 transcription kit was obtained from Ambion (Austin, TX). Reagents for cell-free translation (Flexi rabbit reticulocyte lysate, DTT, RNasin) were from Promega Corp. (Madison, WI). EasyTag [35S]Met/Cys was from PerkinElmer Life Sciences. Tissue culture and transfection reagents are as follows. Minimum essential alpha medium, fetal bovine serum, Lipofectamine 2000, Dulbecco's modified Eagle's medium, and Opti-MEM were from Invitrogen (Carlsbad, CA). Protein A-Sepharose and G-Sepharose were from Amersham Biosciences. NA and CRT antiserum were from Dr. G. M. Air (Oklahoma City, OK) and Affinity Bioreagents (Golden, CO), respectively. Anti-CNX antibodies were described previously (32). All other reagents were from Sigma-Aldrich.
Construction of His-tagged NA and Site-directed Mutagenesis—The cDNA encoding wild type N9 subtype NA (NA WT) of the influenza virus A/tern/Australia/G70C/75 in the plasmid pBluescript KS+ was from Dr. G. Laver (Canberra, Australia). Site-directed mutagenesis was performed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). For affinity purification of full-length and nascent chain NA peptides, a His8 tag was added to the N terminus to generate NA WT*. Site-directed mutagenesis using pBluescript-NA WT* as the template was used to generate the following mutants: C41S*, C43S*, C41/43S*, C93/419S*, and to generate the glycosylation site mutations S44A*, S54A*, T65A*, T68A*, T89A*, T149A*, S204A*, Δstem glycan*, Δhead glycan*, and glycan null*.
Translations—NA WT* and single glycan mutants* in pBluescript were linearized by digestion with KpnI, and the C terminus truncations were created by digestion with BsaI, BstZ17I, BglII, and EcoRI. The digestion products were in vitro transcribed and translated in the presence of [35S]Met/Cys and translocated for 1 h at 27 °C into rough ER microsomes or semipermeabilized Chinese hamster ovary (CHO) cells, as described previously (32, 33).
Transfection of CHO Cells and Metabolic Labeling—CHO cells were infected with recombinant vaccinia virus expressing T7 RNA polymerase (34). Following infection and 4 h of transient transfection, CHO cells were washed twice with phosphate-buffered saline (PBS) (137 mm NaCl, 4.3 mm Na2HPO4, 2.7 mm KCl, 1.4 mm KH2PO4) and starved in Dulbecco's modified Eagle's medium lacking Cys and Met, with or without 1 mm N-butyldeoxynojirimycin (DNJ). 5 μg/ml of tunicamycin was added 3 h after infection as indicated. Cells were pulsed with 220 μCi of [35S]Met/Cys, and 5 mm DTT was used for reducing pulse experiments when indicated. After pulse/chase, cells were washed twice with ice-cold PBS/20 mm N-ethylmaleimide and incubated for 10 min on ice with PBS/20 mm N-ethylmaleimide/1 mm phenylmethylsulfonyl fluoride. Cells were then scraped, pelleted in a microcentrifuge at 4,000 rpm for 5 min in 4 °C, and lysed in 1% Nonidet-P40, 20 mm N-ethylmaleimide, 1 mm phenylmethylsulfonyl fluoride, 10 ng/μl leupeptin, and 10 ng/μl pepstatin, 50 mm imidazole in PBS for nickel-Sepharose purification. Immunoprecipitations were performed as described previously (7, 32). Co-immunoprecipitants were eluted by heating to 95 °C for 5 min in 100 μl of 1% SDS, 100 mm Tris, pH 7.4, cooled to room temperature. Samples were subsequently diluted with freshly made 2% Triton X-100, 50 mm Tris, pH 7.4, and 250 mm NaCl, incubated at room temperature for 10 min, and centrifuged for 15 min at 14,000 rpm at 4 °C. Affinity purification by nickel chelation was performed according to the manufacturer's instructions. Glycosidase digestion was performed as described previously (23).
Two-dimensional SDS-PAGE—Tube Gel Adaptor Kit SE 220 from Hoefer Pharmacia Biotech, Inc. (San Francisco, CA) was used for casting of 4% acrylamide stacking and 7.5%/11% acrylamide-resolving tube gels; discontinuous SDS-PAGE was performed under nonreducing conditions. Following electrophoresis, the tube gels were extruded and heated for 5 min at 95 °C in sample buffer with 100 mm DTT, layered onto 1.5-mm-thick minigels, and subjected to a second dimension of 7.5/11% SDS-PAGE, as indicated. Signal was visualized by phosphorimaging (FLA-500; Fuji). Data were analyzed and quantified using Multi-Gauge V. 2.02 (Fuji).
RESULTS
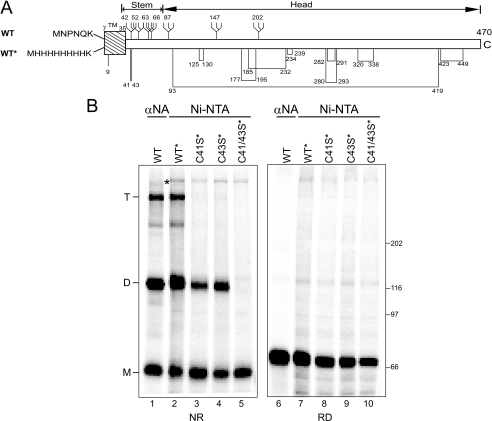
Intermolecular Disulfide Bonds Link NA Dimers and Tetramers—Cotranslational studies require a method to equally isolate the shortest translation intermediates and longer or full-length nascent chains. Polyclonal antibodies raised against purified proteins generally favor the isolation of longer chains because they often recognize more epitopes on the longer chains when compared with shorter nascent chains. The employment of antibodies that solely recognize an N-terminal epitope is complicated by the fact that most secretory cargoes contain cleavable N-terminal targeting sequences. An antibody generated against the mature N-terminal amino acids, in the case of influenza hemagglutinin, only recognized the substrate after signal sequence cleavage (7). The employment of a class II membrane protein with a noncleavable N-terminal signal sequence as a model maturation substrate circumvents these problems. The N-terminal six amino acids of NA subtype N9 reside in the cytoplasm, and therefore, are unlikely to contribute to the proper folding of the ectodomain of the protein within the ER lumen (Fig. 1A) (for review, see Ref. 35). To create a method for efficient isolation of the shortest translation and maturation intermediates, the four N-terminal cytosolic amino acids that follow the initiating Met were exchanged for a poly-His affinity tag.
FIGURE 1.
NA oligomerization. A, a schematic representation of NA subtype 9 WT construct and the His-tagged wild type (WT*) construct. The signal anchor sequence and glycans are indicated by a hatched box and branched structures, respectively. Intramolecular disulfide bonds and proposed lone Cys residues are displayed by lines with their Cys residues numbered. The stem and head domains are indicated based on the NA crystal structure (7nn9) (Colman and Ward (43). TM, tunicamycin; C, C terminus. B, NA WT, WT*, C41S*, C43S*, C41/43S* overexpressed in CHO cells were purified either by immunoprecipitation with NA antisera (lane 1) or with Ni-NTA agarose (lanes 2–5) and resolved by 6% nonreducing (NR) and reducing (RD) SDS-PAGE followed by autoradiography. NA monomers (M), dimers (D), and tetramers (T) are indicated.
To determine whether the N-terminal tag altered NA maturation, the oxidation and oligomerization of wild type NA (WT) or poly-His-NA (WT*) were monitored in CHO cells. These cells were transfected with NA WT or WT* and pulse-labeled with [35S]Met/Cys for 30 min before alkylation with N-ethylmaleimide to block free thiol reactive groups. WT NA was immunoprecipitated with NA antisera, whereas WT* was affinity-purified with Ni-NTA agarose. 35S-labeled NA was then analyzed by nonreducing and reducing SDS-PAGE and autoradiography.
Three bands were observed by nonreducing SDS-PAGE with WT NA (Fig. 1B, lane 1). The fastest migrating band of ∼60 kDa corresponded to NA monomer. This nonreduced band migrated slightly faster than the reduced protein (Fig. 1B, lane 6), suggestive of the oxidized sample containing intramolecular disulfides that created a more compact structure (36). Two additional bands migrated at ∼120 and ∼240 kDa. These bands were both reduced by the addition of the reducing agent dithiothreitol (DTT; Fig. 1B, lane 6), suggesting that they are homodimers (denoted as D) and homotetramers (designated as T). Equivalent results were obtained for WT*-NA (Fig. 1B, lanes 2 and 7), indicating that the alteration of the NA N terminus did not appear to affect its maturation.
The role of the stem domain Cys residues 41 and 43 in supporting intermolecular interactions was investigated by mutating them to Ser. When the Cys were mutated individually (Fig. 1B, lanes 3 and 4), NA formed monomers and dimers. However, when both Cys were altered (C41/43S*; Fig. 1B, lanes 5 and 10), no covalent oligomers were detected. The monomeric band, however, migrated with identical mobility to WT* upon nonreducing SDS-PAGE, indicating intramolecular disulfide pairing. In summary, NA of subtype N9 forms intramolecular and intermolecular disulfides. The stem domain residues Cys-41 and Cys-43 support the formation of covalent dimers and tetramers.
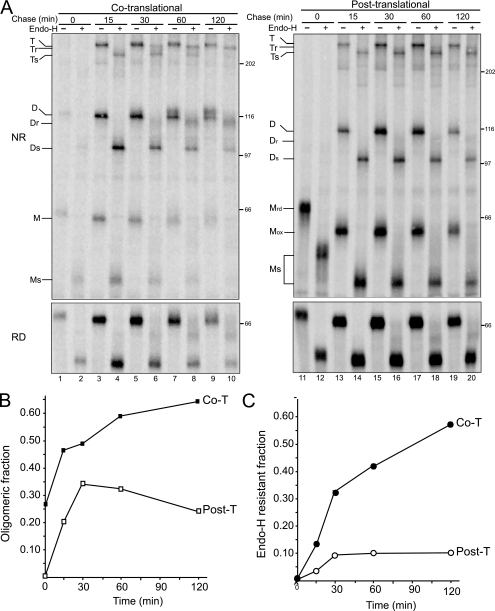
Cotranslational Oxidation Increases the Maturation Efficiency of NA—To address the necessity for maturation to commence cotranslationally, the oxidation of NA initiated cotranslationally was compared with post-translational initiation. Post-translational oxidation requires that the protein be synthesized under reducing conditions during the radioactive pulse and subsequently chased for various times under oxidizing conditions. The oxidizing environment of the ER can be rapidly re-established after DTT removal (36). The NA maturation efficiency under co- or post-translational disulfide bond-forming conditions was compared by quantifying the efficiency of oligomerization, including the relative amounts of dimer and tetramer, and trafficking to the Golgi using an endoglycosidase H (Endo H) sensitivity assay (Fig. 2).
FIGURE 2.
Cotranslational oxidation supports efficient maturation of NA. A, NA WT* was overexpressed in CHO cells and radiolabeled with [35S]Met/Cys for 5 min in the absence (left panel, cotranslational, lanes 1–10) or presence of 5 mm DTT (right panel, post-translational, lanes 11–20) and chased under oxidizing conditions for the indicated times. NA isolated with Ni-NTA agarose was then subjected to Endo H digestion where indicated. Radiolabeled NA was resolved on 7.5% nonreducing (NR) and reducing (RD) SDS-PAGE followed by autoradiography. The Endo H-sensitive and -resistant monomer (Ms/Mr), dimer (Ds/Dr), tetramer (Ts/Tr), and oxidized/reduced (Mox/Mrd) monomers are indicated. T, tetramers; M, monomers; D, dimers. B, the ratio of oligomers to total protein (dimer and tetramer) following chase was quantified. Closed and open boxes designate cotranslational and post-translational oligomers, respectively. C, the ratio of Endo H resistance (dimer and tetramer to the total protein) following chase time was quantified. Closed and open circles indicate cotranslational (Co-T) and post-translational (Post-T) Endo H-resistant NA, respectively.
NA was able to form oligomers under both cotranslational and post-translational oxidizing conditions. However, the efficiency of oligomerization was greatly diminished when oxidation was initiated post-translationally. Cotranslational oxidation conditions supported a peak oligomerization level of 65% after 120 min (Fig. 2A, lanes 1–10, and 2B). Maximal oligomerization with post-translational oxidation was approximately half that observed for cotranslational oxidation (Fig. 2A, lanes 11–20, and 2B). Also, a large fraction of the oligomers formed under post-translational oxidation conditions were ER-export deficient, as indicated by the persistent Endo H sensitivity observed after 120 min of chase (Fig. 2A, right panel, and 2C). In contrast, NA oxidized under cotranslational conditions was transported to the Golgi in a time-dependent manner, increasing to a maximum level of 55% after 120 min (Fig. 2A, lanes 1–10, and 2C). Interestingly, both the dimer and the tetramer were export-competent, indicating that intermolecular disulfide-linked tetramerization is not required for NA export. Together, these results show that cotranslational oxidation provides a more efficient mechanism for NA maturation.
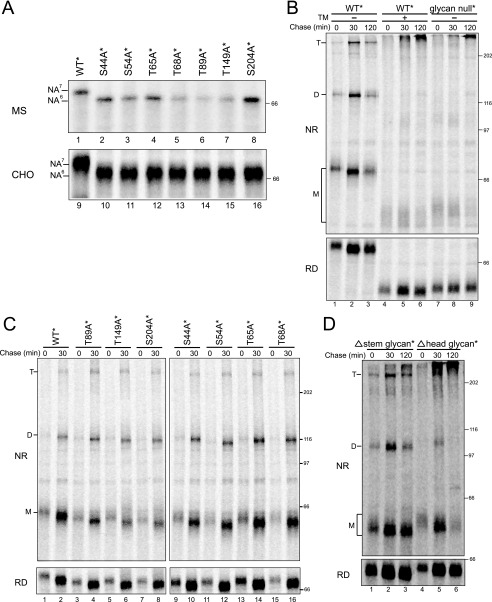
Head Domain Glycans Are Required for NA Maturation—N-linked glycans play key roles in the maturation of glycoproteins (13, 19, 20). NA has four consensus sites for glycosylation in its stem domain (Asn-42, -52, -63, and -66) and three in its head domain (Asn-87, -147, and -202) (Fig. 1A). To determine which N-linked glycosylation sites on NA were utilized, a panel of NA point mutants was constructed with Ser or Thr mutated to Ala within the individual glycosylation consensus sequence. The mutants were in vitro translated in the presence of ER-derived microsomes and glucosidase (N-butyl deoxynojirimycin, DNJ) and mannosidase (deoxymannojirimycin) inhibitors to remove any heterogeneity in protein mobility created by glycan trimming (23, 37).
All seven single site mutants migrated ∼3 kDa faster upon reducing SDS-PAGE, indicative of the absence of the corresponding glycan (Fig. 3A, lanes 1–8). The slower rate of in vitro translation can allow for prolonged exposure of the nascent protein to the ER translocon, increasing occupancy efficiency of suboptimal N-linked glycosylation sites (33). However, similar results were also obtained when the NA glycosylation mutants were expressed in live cells (Fig. 3A, lanes 9–16), demonstrating that all seven glycosylation sites of NA are efficiently occupied.
FIGURE 3.
Glycans are required for NA maturation. A, all the putative N-linked glycosylation sites are occupied on NA. NA WT* and a single glycosylation site mutant were either in vitro translated in a rabbit reticulocyte lysate system with canine pancreas-derived rough ER microsomes and displayed by SDS-PAGE directly (MS, top panel) or overexpressed in CHO cells and radiolabeled with [35S]Met/Cys for 30 min followed by purification with Ni-NTA agarose (CHO, bottom panel). The number of glycans added is indicated. B, NA WT* treated without and with tunicamycin or a NA mutant that lacks all the N-linked glycan sites (glycan null*) were overexpressed in CHO cells and radiolabeled with [35S]Met/Cys for 5 min followed by chasing for the indicated times. NA purified with Ni-NTA agarose is displayed on 7.5% nonreducing (NR) and reducing (RD) SDS-PAGE followed by autoradiography. The monomers (M), dimers (D), and tetramers (T) are indicated. TM, tunicamycin. C, NA WT* and single glycan knockouts (S44A*, S54A*, T65A*, T68A*, T89A*, T149A*, S204A*) were overexpressed and purified and displayed as in B. D, NA mutant that lack the stem domain glycans (Δstem glycan*) and head domain glycans (Δhead glycan*) were overexpressed and displayed as in B.
Some glycoproteins can undergo improper folding, misassembly, intracellular retention, and degradation if glycosylation is inhibited, whereas for others, glycosylation is dispensable or only a subset of the glycosylation sites are essential (20). To evaluate the necessity of NA glycans for proper maturation, a chemical inhibitor of glycosylation (tunicamycin) and additional NA mutants were utilized. NA was radiolabeled for 5 min and then chased for various times. WT* NA initially migrated predominantly as a monomeric protein (Fig. 3B, lane 1); mobility increased with time, indicative of glycan trimming because the mobility difference persisted upon reducing SDS-PAGE (Fig. 3B, lanes 1–3). The level of NA oligomerization also increased with time. In sharp contrast, when glycosylation was inhibited by tunicamycin treatment, most of the protein was found in high molecular weight intermolecularly cross-linked aggregates at the top of the gel under nonreducing conditions (Fig. 3B, lanes 4–6). The monomeric protein migrated ∼15 kDa faster by reducing SDS-PAGE, indicative of the absence of all seven glycans.
Tunicamycin abolishes the transfer of N-linked glycans to all glycoproteins. This activates an ER stress response due to accumulation of unfolded proteins, which can disrupt the activity of the ER (38). To ensure that the requirement for NA glycans was a direct effect, the maturation of mutant NA missing all seven glycosylation sites was analyzed (Fig. 3B, lanes 7–9; glycan null*). Equivalent results were obtained with glycan null* and WT* plus tunicamycin. Glycan null* NA was predominantly found in large intermolecularly linked aggregates. Therefore, NA glycosylation is essential for its proper maturation in the ER.
Next we asked, which glycans are essential for proper NA folding and maturation? To address this question, the maturation of NA single glycan mutants was analyzed (Fig. 3C). All mutants expressed in CHO cells were able to form dimers and tetramers with similar efficiencies to wild type NA, demonstrating that no individual glycan was required for NA maturation.
To determine whether glycan clusters were required for NA maturation, mutants lacking either stem domain glycans (Asn-42, -52, -63, -66; Δstem glycan*) or head domain glycans (Asn-87, -147, -202; Δhead glycan*) were expressed in CHO cells (Fig. 3D). Interestingly, NA was able to efficiently oligomerize without the four stem domain glycans, but the majority of NA lacking its three head domain glycans was found in intermolecularly linked aggregates at the top of the nonreducing gel. These results demonstrated that the head domain glycans were required for the proper maturation of NA but that the stem domain glycans were dispensable.
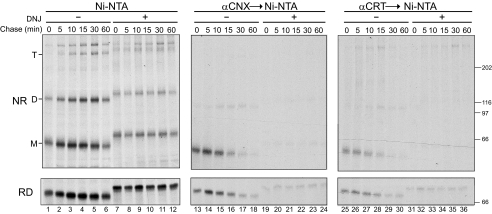
Temporal CNX and CRT Binding and NA Oligomerization—The lectin chaperones CNX and CRT can promote protein folding, oligomeric assembly, and quality control in the ER through their recognition of monoglucosylated N-linked glycans on maturing substrates (21–24, 39). To analyze binding of CNX and CRT to NA, CHO cells were radiolabeled with [35S]Met/Cys for 5 min and then chased for various times. NA was directly isolated with Ni-NTA agarose or co-immunoprecipitated with either CNX or CRT antisera prior to affinity purification.
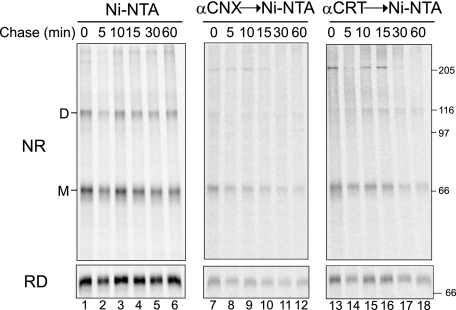
Both CNX and CRT transiently bound monomeric NA with a half-time of ∼7.5 min (Fig. 4, lanes 13–18 and 25–30). This binding required glucose trimming because the addition of the glucosidase inhibitor DNJ greatly diminished CNX and CRT interactions (Fig. 4, lanes 19–24 and 31–36). In the presence of DNJ, NA displayed a decreased mobility, indicating inhibition of glucose trimming. NA oligomerized in a time-dependent manner with increasing levels of both dimers and tetramers observed with time (Fig. 4, lanes 1–6). Interestingly, a significant amount of NA dimers (38%) was observed immediately after the 5-min radioactive pulse, demonstrating rapid NA oligomerization (Fig. 4, lane 1). Dimerization reached 50% after 30 min of chase. NA also dimerized in the absence of CNX and CRT binding (+DNJ); however, no time-dependent dimerization increase was observed. The initial dimer level (25%) that accumulated immediately after the radioactive pulse persisted during the 1-h chase. These results indicated that CNX and CRT transiently interact with NA in a glucosidase-dependent manner, promoting its efficient maturation and oligomerization.
FIGURE 4.
Transient interactions between NA and CNX/CRT. NA WT* was overexpressed in CHO cells and radiolabeled with [35S]Met/Cys for 5 min and chased for the indicated times in the absence or presence of glucose trimming inhibitor DNJ. NA isolated with Ni-NTA (lanes 1–12) and CNX/CRT interactions were detected by immunoprecipitation with anti-CNX or CRT antisera followed by Ni-NTA affinity purification (lanes 13–24, lanes 25–36). Samples were displayed on 6% nonreducing (NR) and reducing (RD) SDS-PAGE followed by autoradiography. The monomer (M), dimer (D), and tetramer (T) are indicated.
CNX and CRT Sequentially Interact with NA Cotranslationally—NA appears to mature extensively cotranslationally, which evidently contributes to its efficient proper maturation (Figs. 2 and 4). The lectin chaperones CNX and CRT can bind nascent chains cotranslationally and cotranslocationally to protect them from deleterious associations (1, 6, 7, 32, 37, 40). In vitro translated nascent chains lacking a C-terminal stop codon remain trapped on ribosomes and translocons when synthesized in the presence of ER membranes, providing a powerful method to simulate the cotranslational process (7, 32, 44).
This experimental system has been used to study the cotranslational maturation of type I membrane proteins (hemagglutinin and tyrosinase), a polytopic membrane protein (cystic fibrosis transmembrane conductance regulator, CFTR), and a soluble protein (factor V) (7, 32, 41). Studies using ribosome-arrested nascent chains show that CNX associates with shorter length proteins than CRT, suggesting that CNX is the first lectin chaperone an emerging nascent chain encounters in the ER lumen (7, 32).
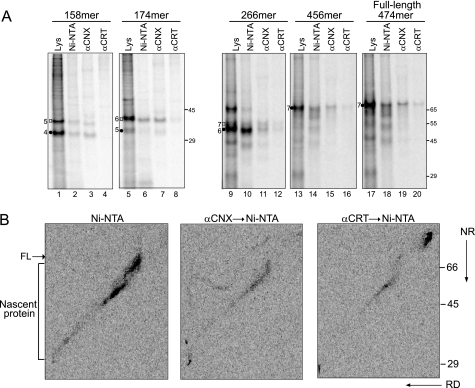
To determine the chaperone binding profile for a type II membrane protein, C-terminal NA truncations of increasing size were in vitro translated in semipermeabilized CHO cells and examined for association with CNX and CRT by co-immunoprecipitation (Fig. 5A). CNX binding was observed for the shortest NA construct (158-mer), possessing four N-linked glycans (Fig. 5A, lane 3). CRT binding was first detected with the 266-mer bearing six glycans (Fig. 5A, lane 12). Once interactions with the chaperones were observed, they persisted with all longer constructs including the full-length ribosome and translocon-released protein.
FIGURE 5.
CNX and CRT interact with NA cotranslationally in a sequential manner. A, NA C terminus truncations 158-mer, 174-mer, 266-mer, 456-mer, and the full-length protein radiolabeled with [35S]Met/Cys were translated in the presence of semipermeabilized CHO cells and then affinity-purified with Ni-NTA or immunoprecipitated with anti-CNX or CRT antisera. The translation time for each construct was optimized to gain the maximum fraction of ribosome-arrested proteins. A 15-min translation time was applied for the 158-mer and 174-mer; a 30-min translation time was applied for the 266-mer; and a 60-min translation time was applied for the 456-mer and the full-length construct. Samples were analyzed by 12% (left) and 8% (right) Tris-Tricine SDS-PAGE followed by autoradiography. Closed circles and open square indicate translocated and ribosome-released proteins, respectively. The number of glycans of the ER-translocated NA is indicated by the numbers next to the closed circles. B, NA WT* was overexpressed in CHO cells and radiolabeled with [35S]Met/Cys for 1 min. NA was purified with Ni-NTA (left panel), or CNX/CRT interactions were detected by immunoprecipitation with the corresponding antisera followed by Ni-NTA affinity purification (middle panel and right panel). Samples were resolved on 11% two-dimensional SDS-PAGE with the first dimension under nonreducing condition (NR) and the second dimension under reducing condition (RD) followed by autoradiography. The full-length (FL) and nascent proteins are indicated.
In live mammalian cells, NA is estimated to be synthesized in ∼2 min (5 amino acids/s) (33, 42). To test whether NA is bound by CNX and CRT cotranslationally in living cells, NA was radiolabeled using a brief 1-min radioactive pulse, and interactions with CNX and CRT were probed by sequential purification with CNX or CRT antisera followed by isolation with Ni-NTA agarose. Samples were resolved by two-dimensional nonreducing and reducing SDS-PAGE, which provides a more efficient technique for resolving nascent chains (diagonal electrophoresis) (1). Disulfide bond formation can also be monitored by diagonal electrophoresis. Intermolecular disulfides are localized above the main diagonal on the gel, proteins possessing large intramolecular disulfides can be found below the diagonal, and proteins lacking disulfides are found directly on the diagonal.
Nascent NA purified with Ni-NTA-agarose was located along the diagonal with increasing intensity as the chains approached full length (Fig. 5B, left panel, see FL). CNX and CRT bound to these elongating NA nascent chains, indicative of their cotranslational association with NA (Fig. 5B, middle and right panels). The diagonal smear corresponding to cotranslational interactions between CNX and NA extended longer than the diagonal smear for CRT, suggesting an earlier interaction with CNX. These results demonstrated that NA nascent chains cotranslationally interact with CNX and CRT both in live and in semipermeabilized cells, engaging the membrane protein CNX prior to the soluble CRT.
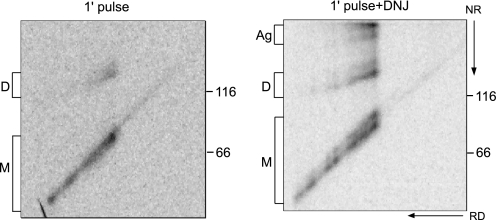
NA Oligomerization Commences Cotranslationally—Oligomeric proteins are thought to achieve their native structure after the monomers have properly folded (12). The rapid oligomerization of NA observed during the 5-min radioactive pulse period (Fig. 4, lane 1) suggested that extensive maturation may occur cotranslationally. To test this hypothesis, diagonal electrophoresis was employed to analyze NA oxidation after a 1-min radioactive pulse.
The majority of the radiolabeled protein was observed along the diagonal, culminating in full-length monomeric NA at the center of the gel (Fig. 6, left panel). This region corresponded to monomeric elongating chains lacking discernable intramolecular or intermolecular disulfide bonds. A weaker signal that migrated at the size of full-length dimeric NA was also observed at the top of the gel. A faint spur was visible leading up to this spot, demonstrating that a subset of NA formed dimers cotranslationally.
FIGURE 6.
A subset of NA dimerized cotranslationally. NA WT* was overexpressed in CHO cells by modified vaccinia virus T7 expression system and radiolabeled with [35S]Met/Cys for 1 min in the absence or presence of DNJ. NA was purified with Ni-NTA agarose and resolved on 7.5% two-dimensional SDS-PAGE followed by autoradiography. NA monomer (M), dimer (D), and aggregates (Ag) are indicated.
CNX and CRT binding slow the folding and oligomerization of maturing substrates (24); trapping full-length proteins on these chaperones by post-translational treatment with glucosidase inhibitors blocks their oxidation. The effect of inhibiting binding to CNX and CRT on NA oligomerization was analyzed by pretreatment of cells with DNJ. The dimer spur in DNJ-treated cells was more pronounced and extended farther than the spur observed in the absence of DNJ (Fig. 6, right panel), indicating that nascent chains with triglucosylated glycoforms dimerized earlier than those permitted to bind the lectin chaperones. Additionally, aggregates were observed at the top of the gel, suggesting that disrupting lectin chaperone interactions also increased aggregation. DNJ treatment also supported the formation of a doublet on the central diagonal, likely indicating premature formation of intramolecular disulfides. Together, these results demonstrate that CNX and CRT binding slow the intramolecular and intermolecular oxidation of NA, allowing for more efficient maturation overall.
NA Oligomerization Does Not Require Completed Oxidation of Monomers—The cotranslational dimerization of NA was unexpected because NA contains a large intramolecular disulfide loop (Cys-93–Cys-419) that links the N- and C-terminal regions of the 470-amino-acid protein (43). During synthesis, ∼60 amino acids reside in the ribosome and translocon tunnels; therefore, Cys-419 must emerge into the ER post-translationally after the C terminus has been released from the ribosome (44). If NA oligomerization begins cotranslationally, this step must have occurred prior to stabilization of the monomeric structure by large loop disulfide formation. To explore the necessity of monomer stabilization prior to oligomerization, the chaperone binding properties and oligomerization of an NA mutant lacking the large loop Cys residues (Cys-93–Cys-419), termed C93/419S* were analyzed.
Surprisingly, NA C93/419S* formed dimers immediately after a 5-min radioactive pulse (Fig. 7, lane 1), as observed previously with NA WT*, indicating that dimers could form before the completion of monomer oxidation. However, dimerization did not increase with time, and tetramers were not observed. CNX and CRT associated with NA C93/419S* for prolonged periods (binding half-times of ∼14 min for CNX and ∼17 min for CRT) (Fig. 7, lanes 7–18). CRT but not CNX persistently bound to NA C93/419S* dimers. The differential binding of CNX and CRT to the NA C93/419S* indicated that these lectin chaperones may serve different roles in NA maturation.
FIGURE 7.
A subset of NA mutant lacking the large loop (C93/419S*) can dimerize cotranslationally, and the dimer persistently interacts with CRT. NA C93/419S* was overexpressed in CHO cells and radiolabeled with [35S]Met/Cys for 5 min followed by chases for the indicated times. NA was purified with Ni-NTA directly (lanes 1–6) or after initial immunoprecipitation with anti-CNX or -CRT antisera (lanes 7–18). NA was resolved by 7.5% nonreducing (NR) and reducing (RD) SDS-PAGE followed by autoradiography. Monomers (M) and dimers (D) are indicated.
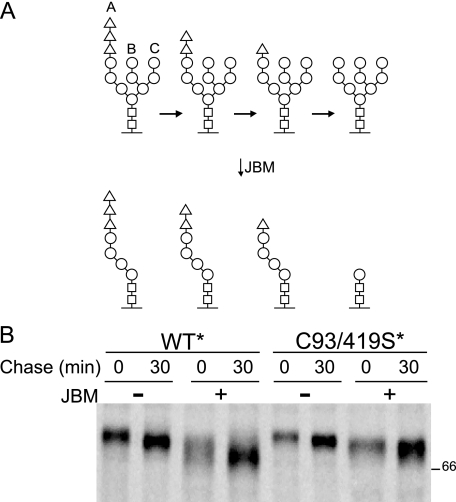
CNX and CRT retain misfolded proteins in the ER through their persistent binding of aberrant proteins or unassembled subunits (45). Because the CNX/CRT binding cycle is controlled by reglucosylation of high mannose side chains by GT1, the glycan status of NA WT* and NA C93/419S* was analyzed using a jack bean α-mannosidase (JBM) assay. This exomannosidase only cleaves mannoses on sugar branches that do not contain terminal glucose residues (Fig. 8A) (22, 23). JBM digestion therefore produces an accentuated mobility shift in non-glucosylated proteins (Fig. 8A, right-most structure) when compared with glucosylated proteins (Fig. 8A, the three structures on the left), providing a method to monitor the glucosylation state of a substrate.
FIGURE 8.
NA large loop mutant C93/419S* accumulates monoglucosylated glycans. A, schematic demonstrating the carbohydrates that are sensitive to endomannosidase digestion. The unglucosylated glycan opens up this branch for the removal of an additional three mannoses by α-mannosidase digestion, producing an accentuated shift in electrophoretic mobility of the treated proteins. B, NA WT* and C93/419S* were overexpressed in CHO cells and radiolabeled with [35S]Met/Cys for 5 min followed by chases of 0 or 30 min. NA purified with Ni-NTA was subjected to JBM digestion.
NA WT* and NA C93/419S* were subjected to 5-min labeling and 30-min chase followed by Ni-NTA agarose purification and JBM digestion. Both NA WT* and C93/419S* showed time-dependent mobility shifts after 30 min of chase without JBM digestion, corresponding to glycan trimming. After digestion, a larger shift was observed for the NA WT* than for the mutant NA, indicating that NA C93/419S* harbored more monoglucosylated glycans. In summary, NA C93/419S* could form dimers but not tetramers, and persisting in a monoglucosylated state supported prolonged binding by CNX and CRT.
DISCUSSION
We have investigated the poorly understood cotranslational folding and maturation processes for type II membrane proteins using influenza A NA as a model substrate. Carbohydrates were required for proper NA maturation in a regio-specific manner. The three head domain glycans were found to be essential for reaching the native state, whereas the four-stem domain glycans were dispensable. The efficient maturation of NA required assistance from lectin chaperones CNX and CRT that were cotranslationally and cotranslocationally recruited by N-linked glycans on NA. The lectin chaperones increase the fidelity of NA maturation by slowing overall maturation. Unexpectedly, the formation of disulfide-linked homodimers began cotranslationally demonstrating that oligomerization through the N-terminal stem domain does not require completion of the folding process. N9 NA post-translationally formed covalent homotetramers, and unlike the dimerization process, tetramerization appeared to involve a strict quality control test. Together, this study provides a detailed understanding of the type II membrane protein maturation process.
We have previously hypothesized that glycans localized in four orientations relative to Cys can assist the folding reaction (7). These include: 1) near an N-terminal Cys in a large loop disulfide that requires protection until its C-terminal partner Cys emerges; 2) proximal to an orphan Cys in a protein with multiple disulfide bonds (for protection from forming aberrant disulfides); 3) local to a Cys of an intermolecular disulfide; and 4) recruitment of ERp57 to a domain to catalyze disulfide bond formation. NA was chosen as a model protein, in part because it appeared to possess glycans located in all four of these proposed regions. Asn-87 provides a glycosylation site located nearby the N-terminal Cys (Cys-93) of the large loop disulfide bond (Cys-93–419). The two Cys in the stem region (Cys-41 and Cys-43) are the only Cys not involved in intramolecular disulfides. Previous studies suggested that one of these stem Cys residues supported covalent homodimerization, whereas the other was expected to remain unpaired (46). However, we determined that both stem domain Cys were involved in intermolecular disulfide bonds. Intriguingly, a glycan on NA is located immediately between these proximal Cys residues at Asn-42, with an additional three-glycan cluster found C-terminal to this region. Finally, most of the glycans are localized near disulfides and could therefore recruit ERp57 for oxidation.
Although no single glycan was essential for NA maturation, glycans were required for proper NA maturation. The highly conserved head domain glycans were more critical to the overall folding and oligomerization of NA than the stem domain glycans. Glycans and their subsequent trimming support the sequential binding of CNX followed by CRT (7, 32). The lectin chaperones first interact with NA cotranslationally and continue to associate with the full-length chain post-translationally. Inhibition of CNX and CRT binding disrupted proper cotranslational oxidation and oligomerization as intramolecular disulfides were found to occur prematurely, leading to the formation of intermolecular-linked aggregates of NA.
NA homo-oligomerization is mediated through N-terminal stem domain interactions. As chaperone binding can sterically hinder oligomerization, the rapid dimerization of NA suggests that the stem domain glycans were short term substrates of CNX and CRT. Therefore, it would follow that the stem domain glycans are not efficiently reglucosylated by GT1. This supports rapid chaperone release and the early formation of intermolecular disulfide linkages between N-terminal stem domain Cys residues. Studies using purified proteins have shown that GT1 preferentially reglucosylates non-native glycoproteins with hydrophobic amino acids exposed on their surface, a hallmark of folding intermediates or aberrantly folded proteins (25, 47, 48). The stem domain is predominantly hydrophilic, containing four carbohydrate consensus sites; therefore, these glycans are expected to be a poor substrate for reglucosylation (supplemental Fig. 1) (49). In contrast, all three head domain glycans are located proximal to hydrophobic patches, properties expected of glycans recognized by GT1, which would support prolonged CNX and/or CRT binding.
These results support an expanded model for glycan positioning controlling the molecular choreography of nascent chains. In the case of NA, the clustering of four glycans combined with the overall hydrophilicity of the polypeptide chain in the membrane proximal stem region dictates transient cotranslational tethering by lectin chaperones and controlled covalent oligomerization. Post-translational chaperone association mediated by GT1 reglucosylation is disfavored due to the lack of exposed hydrophobicity in this area. This glycan positioning thereby supports transient chaperone binding and dimerization prior to the achievement of native conformations. Therefore, we amend the hypothesis regarding the positioning of glycans near critical cysteines to include a subcategory whereby the grouping of glycans in a hydrophilic area precludes post-translational lectin chaperone binding, emphasizing the critical nature of cotranslational associations in these locales.
Slight levels of NA dimers appeared cotranslationally, with the extent of cotranslational dimerization drastically increasing when lectin chaperone binding was inhibited. NA dimerization involves the N-terminal region of NA and does not require proper folding of the complete protein. In some cases, dimerization occurred before the C terminus was synthesized and did not require formation of the large disulfide loop that closes off the head domain. The early formation of covalent dimers did not appear to require a strict quality control evaluation; rather, this test appeared to be deployed at the tetramer formation stage.
Nascent protein oligomerization has been observed for several proteins using cell-free systems. The T1 domain of voltage-gated potassium (Kv) channel, reovirus cell attachment protein s1, and the Rel homology domain of NF-κB1 p50 transcription factor can form oligomers, whereas the proteins are still attached to the ribosome (50–52). These conclusions were achieved using in vitro translated ribosome-arrested chains, which compromises the kinetics of the protein folding and maturation reactions. In this study, the application of a viral expression system and short radiolabeling period enabled us to detect cotranslational dimerization under cellular conditions.
Oligomerization is a concentration-dependent reaction; therefore, the high concentration of proteins reached during infection could favor early intermolecular interactions. Cotranslational assembly has been observed previously for viral polyproteins from hepatitis C and the alphaviruses Sindbis and Semliki Forest (30). Polypeptides from these viruses are cleaved to multiple proteins in the ER lumen. They form heterodimers shortly after entering the ER lumen. These hetero-oligomers are thought to be formed by proteins translocating through a single translocon, resulting in their colocalization with high apparent concentration supporting assembly. This mechanism of assembly is different from that observed for NA homodimerization because NA assembly involves nascent chains that might be the product of the same polysome but likely different ribosome-translocon complexes. This demonstrates that the full-length pool of NA is close enough spatially to the nascent chains to initiate assembly cotranslationally, especially if interactions with the lectin chaperones are inhibited.
The Levinthal paradox demonstrates that protein folding is not a random walk because there is not sufficient time to sample all possible conformations available for a given protein to reach its native state (53). Commencing the maturation program for a protein cotranslationally places temporal and spatial constraints on the maturing protein, providing important physical restrictions that can support a more direct route to the native state. N-linked glycans play critical roles in this process.
Viral proteins have evolved mechanisms to mature with optimal efficiencies when expressed at high levels during viral infection. Aberrant protein maturation could inhibit the infection process because it supports antigen production through proteasomal degradation and subsequent activation of the immune response. Viral immunoevasion strategies appear to include the positioning of glycans in locations that help to direct the folding and assembly processes and mask immunogenic epitopes (37, 54).
Membrane glycoproteins of enveloped viruses are essential for viral pathogenesis. NA, a critical envelope glycoprotein of the influenza virus, acts as an exoglycosidase that cleaves the viral receptor sialic acid from surface glycoproteins, thereby facilitating virion release. Common flu therapies target NA (55). Understanding the maturation of viral proteins provides insight into the general pathways for protein maturation and could lead to additional strategies for infection prevention and control.
Supplementary Material
Acknowledgments
We are grateful to Drs. G. Laver (Canberra, Australia) and G. Air (Oklahoma City, OK) for the generous gifts of plasmids and antibodies, respectively. We also acknowledge members of the Hebert laboratory including J. Cormier and R. Daniels for thoughtful discussions.
This work was supported, in whole or in part, by National Institutes of Health Chemistry-Biology Interface Training Grant T32GM00815 (to B. R. P.). This work was also supported by U. S. Public Health Service Grant CA79864 (to D. N. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental text and a supplemental figure.
Footnotes
The abbreviations used are: ER, endoplasmic reticulum; NA, neuraminidase, CNX, calnexin; CRT, calreticulin; DTT, dithiothreitol; WT, wild type; DNJ, N-butyldeoxynojirimycin, CHO, Chinese hamster ovary; Ni-NTA, nickel-nitrilotriacetic acid; Endo H, endoglycosidase H; JBM, jack bean α-mannosidase; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
References
- 1.Chen, W., Helenius, J., Braakman, I., and Helenius, A. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 6229–6233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frydman, J., Erdjument-Bromage, H., Tempst, P., and Hartl, F. U. (1999) Nat. Struct. Biol. 6 697–705 [DOI] [PubMed] [Google Scholar]
- 3.Fedorov, A. N., and Baldwin, T. O. (1997) J. Biol. Chem. 272 32715–32718 [DOI] [PubMed] [Google Scholar]
- 4.Clark, P. L. (2004) Trends Biochem. Sci. 29 527–534 [DOI] [PubMed] [Google Scholar]
- 5.Schnell, D. J., and Hebert, D. N. (2003) Cell 112 491–505 [DOI] [PubMed] [Google Scholar]
- 6.Chen, W., and Helenius, A. (2000) Mol. Biol. Cell 11 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels, R., Kurowski, B., Johnson, A. E., and Hebert, D. N. (2003) Mol. Cell 11 79–90 [DOI] [PubMed] [Google Scholar]
- 8.Molinari, M., and Helenius, A. (1999) Nature 402 90–93 [DOI] [PubMed] [Google Scholar]
- 9.Woolhead, C. A., McCormick, P. J., and Johnson, A. E. (2004) Cell 116 725–736 [DOI] [PubMed] [Google Scholar]
- 10.Kowarik, M., Kung, S., Martoglio, B., and Helenius, A. (2002) Mol. Cell 10 769–778 [DOI] [PubMed] [Google Scholar]
- 11.Cabral, C. M., Liu, Y., and Sifers, R. N. (2001) Trends Biochem. Sci. 26 619–624 [DOI] [PubMed] [Google Scholar]
- 12.Ellgaard, L., and Helenius, A. (2003) Nat Rev. Mol. Cell Biol. 4 181–191 [DOI] [PubMed] [Google Scholar]
- 13.Hebert, D. N., Garman, S. C., and Molinari, M. (2005) Trends Cell Biol. 15 364–370 [DOI] [PubMed] [Google Scholar]
- 14.Schrag, J. D., Propopio, D. O., Cygler, M., Thomas, D. Y., and Bergeron, J. J. (2003) Trends Biochem. Sci. 28 49–57 [DOI] [PubMed] [Google Scholar]
- 15.Anelli, T., and Sitia, R. (2008) EMBO J. 27 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodsky, J. L. (2007) Biochem. J. 404 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson, I., and von Heijne, G. (1993) J. Biol. Chem. 268 5798–5801 [PubMed] [Google Scholar]
- 18.Caramelo, J. J., and Parodi, A. J. (2008) J. Biol. Chem. 10221–10225 [DOI] [PMC free article] [PubMed]
- 19.Hebert, D. N., and Molinari, M. (2007) Physiol. Rev. 87 1377–1408 [DOI] [PubMed] [Google Scholar]
- 20.Helenius, A., and Aebi, M. (2004) Annu. Rev. Biochem. 73 1019–1049 [DOI] [PubMed] [Google Scholar]
- 21.Ou, W. J., Cameron, P. H., Thomas, D. Y., and Bergeron, J. J. M. (1993) Nature 364 771–776 [DOI] [PubMed] [Google Scholar]
- 22.Hammond, C., Braakman, I., and Helenius, A. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebert, D. N., Foellmer, B., and Helenius, A. (1995) Cell 81 425–433 [DOI] [PubMed] [Google Scholar]
- 24.Hebert, D. N., Foellmer, B., and Helenius, A. (1996) EMBO J. 15 2961–2968 [PMC free article] [PubMed] [Google Scholar]
- 25.Sousa, M., and Parodi, A. J. (1995) EMBO J. 14 4196–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caramelo, J. J., and Parodi, A. J. (2007) Semin. Cell Dev. Biol. 18 732–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearse, B. R., Gabriel, L., Wang, N., and Hebert, D. N. (2008) J. Cell Biol. 181 309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helenius, A. (1994) Mol. Biol. Cell 5 253–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copeland, C. S., Zimmer, K.-P., Wagner, K. R., Healey, G. A., Mellman, I., and Helenius, A. (1988) Cell 53 197–209 [DOI] [PubMed] [Google Scholar]
- 30.Braakman, I., and van Anken, E. (2000) Traffic 1 533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tulip, W. R., Varghese, J. N., Baker, A. T., van Donkelaar, A., Laver, W. G., Webster, R. G., and Colman, P. M. (1991) J. Mol. Biol. 221 487–497 [DOI] [PubMed] [Google Scholar]
- 32.Wang, N., Daniels, R., and Hebert, D. N. (2005) Mol. Biol. Cell 16 3740–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Újvári, A., Aron, R., Eisenhaure, T., Cheng, E., Parag, H. A., Smicun, Y., Halaban, R., and Hebert, D. N. (2001) J. Biol. Chem. 276 5924–5931 [DOI] [PubMed] [Google Scholar]
- 34.Fuerst, T. R., Niles, E. G., Studier, F. W., and Moss, B. (1986) Proc. Natl. Acad. Sci. U. S. A. 83 8122–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colman, P. M. (1989) in The Influenza Viruses (Krug, R. M., ed), pp. 175–218, Plenum Press, New York
- 36.Braakman, I., Helenius, J., and Helenius, A. (1992) EMBO J. 11 1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hebert, D. N., Zhang, J.-X., Chen, W., Foellmer, B., and Helenius, A. (1997) J. Cell Biol. 139 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroder, M., and Kaufman, R. J. (2005) Annu. Rev. Biochem. 74 739–789 [DOI] [PubMed] [Google Scholar]
- 39.Vassilakos, A., Cohen-Doyle, M. F., Peterson, P. A., Jackson, M. R., and Williams, D. B. (1996) EMBO J. 15 1495–1506 [PMC free article] [PubMed] [Google Scholar]
- 40.Molinari, M., and Helenius, A. (2000) Science 288 331–333 [DOI] [PubMed] [Google Scholar]
- 41.Kleizen, B., van Vlijmen, T., de Jonge, H. R., and Braakman, I. (2005) Mol. Cell 20 277–287 [DOI] [PubMed] [Google Scholar]
- 42.Braakman, I., Hoover-Litty, H., Wagner, K. R., and Helenius, A. (1991) J. Cell Biol. 114 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colman, P. M., and Ward, C. W. (1985) Curr. Top. Microbiol. Immunol. 114 178–255 [DOI] [PubMed] [Google Scholar]
- 44.Gilmore, R., Collins, P., Johnson, J., Kellaris, K., and Rapiejko, P. (1991) Methods Cell Biol. 34 223–239 [DOI] [PubMed] [Google Scholar]
- 45.Rajagopalan, S., and Brenner, M. B. (1994) J. Exp. Med. 180 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kundu, A., Jabbar, M. A., and Nayak, D. P. (1991) Mol. Cell. Biol. 11 2675–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caramelo, J. J., Castro, O. A., Alonso, L. G., de Prat-Gay, G., and Parodi, A. J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sousa, M. C., Ferrero-Garcia, M. A., and Parodi, A. J. (1992) Biochemistry 31 97–105 [DOI] [PubMed] [Google Scholar]
- 49.Taylor, S. C., Thibault, P., Tessier, D. C., Bergeron, J. J., and Thomas, D. Y. (2003) EMBO Rep. 4 405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilmore, R., Coffey, M. C., Leone, G., McLure, K., and Lee, P. W. (1996) EMBO J. 15 2651–2658 [PMC free article] [PubMed] [Google Scholar]
- 51.Lin, L., DeMartino, G. N., and Greene, W. C. (2000) EMBO J. 19 4712–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu, J., and Deutsch, C. (2001) Biochemistry 40 13288–13301 [DOI] [PubMed] [Google Scholar]
- 53.Levinthal, C. (1968) J. Chim. Phys. 65 44–45 [Google Scholar]
- 54.Munk, K., Pritzer, E., Kretzschmar, E., Gutte, B., Garten, W., and Klenk, H. D. (1992) Glycobiology 2 233–240 [DOI] [PubMed] [Google Scholar]
- 55.De Clercq, E. (2006) Nat. Rev. Drug Discov. 5 1015–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.