Abstract
The mitochondrial tRNA genes are hot spots for mutations that lead to human disease. A single point mutation (T4409C) in the gene for human mitochondrial tRNAMet (hmtRNAMet) has been found to cause mitochondrial myopathy. This mutation results in the replacement of U8 in hmtRNAMet with a C8. The hmtRNAMet serves both in translational initiation and elongation in human mitochondria making this tRNA of particular interest in mitochondrial protein synthesis. Here we show that the single 8U→C mutation leads to a failure of the tRNA to respond conformationally to Mg2+. This mutation results in a drastic disruption of the structure of the hmtRNAMet, which significantly reduces its aminoacylation. The small fraction of hmtRNAMet that can be aminoacylated is not formylated by the mitochondrial Met-tRNA transformylase preventing its function in initiation, and it is unable to form a stable ternary complex with elongation factor EF-Tu preventing any participation in chain elongation. We have used structural probing and molecular reconstitution experiments to examine the structures formed by the normal and mutated tRNAs. In the presence of Mg2+, the normal tRNA displays the structural features expected of a tRNA. However, even in the presence of Mg2+, the mutated tRNA does not form the cloverleaf structure typical of tRNAs. Thus, we believe that this mutation has disrupted a critical Mg2+-binding site on the tRNA required for formation of the biologically active structure. This work establishes a foundation for understanding the physiological consequences of the numerous mitochondrial tRNA mutations that result in disease in humans.
Human mitochondria are subcellular organelles that produce more than 90% of the energy required by the cell. The mitochondrial genome encodes 13 proteins necessary for energy production, two rRNAs and all of the 22 tRNAs required for the synthesis of these proteins (1, 2). Mammalian mitochondrial tRNAs have several unusual features that distinguish them from canonical tRNAs. In many cases, they lack a number of the conserved or semi-conserved nucleotides that play important roles in creating the L-shaped tertiary structure of prokaryotic and eukaryotic cytoplasmic tRNAs (3). There is little detailed structural information on these tRNAs. No data are currently available that examine the structure of mammalian mitochondrial tRNAs with single nucleotide resolution. However, chemical and enzymatic probing has lead to the idea that these tRNAs have retained the basic cloverleaf structure of canonical tRNAs but that they lack several conserved tertiary interactions leading to a weaker three-dimensional structure (4-8). In particular, a number of the long range interactions between the D- and T-arms of the tRNAs appear to be missing.
All 22 tRNAs that function in mammalian mitochondria are encoded in the mitochondrial DNA. Considerable interest in mitochondrial tRNAs centers on the occurrence of diseases arising from mutations in their genes that lead to maternally inherited genetic disorders (9-12). The diseases associated with mitochondrial tRNA mutations may arise from failure in the processing of the tRNA (13), from reduced stability of the tRNA (14, 15), from a reduction in aminoacylation (12, 16, 17), from a reduced ability of the mutated aminoacyl-tRNA to interact with mitochondrial elongation factor Tu (EF-Tumt)3 (the corresponding prokaryotic factor is also designated EF1A) (16), and from the failure of the tRNA to be correctly modified leading to translational defects (18).
Normally, protein biosynthetic systems have two tRNAMet species. One is used solely for initiation, and the other functions in polypeptide chain elongation. Animal mitochondria are quite unusual in that they contain a single gene for tRNAMet, which functions in both polypeptide chain initiation and chain elongation. As a result of this dual role, mitochondrial Met-tRNAMet must be recognized by the mitochondrial Met-tRNA transformylase (MTFmt) and be brought as fMet-tRNAMet to the ribosome for translational initiation (19). In addition, Met-tRNAMet must interact with elongation factor EF-Tumt and bind to the A-site of the ribosome during translational elongation. Thus, this tRNAMet is of central importance in mitochondrial translation.
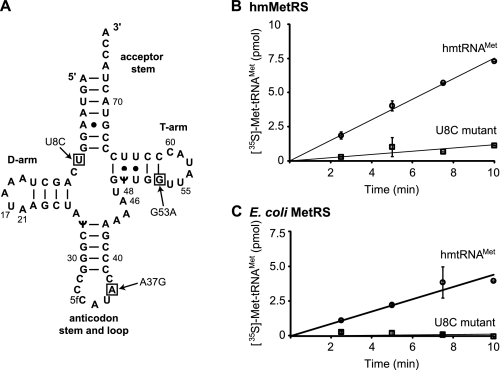
Human tRNAMet has a number of interesting features (Fig. 1A). The D-loop is somewhat small and lacks the G residues at positions 18 and 19 that facilitate interactions with the T-loop in the tertiary structure. The first position of the anticodon contains the rare modified base 5-formylcytidine. This modification may play a role in the unusual codon recognition requirements of this tRNA, which must recognize both AUG and AUA codons. The minor loop is short lacking the usual G47, and the T-stem has two adjacent pyrimidine:pyrimidine pairs (U-U and U-Ψ). Furthermore, the T-loop lacks the TΨC sequence and contains only six nucleotides instead of the normal seven. These unusual structural features suggest that human mitochondrial tRNAMet may have an intrinsically weak tertiary structure.
FIGURE 1.
The sequence of the normal and 8U→C hmtRNAMet and the effect of the mutation on the aminoacylation of the tRNA. A, primary sequence of hmtRNAMet indicating the position of the 8U→C mutation. The Sprinzl numbering system is used throughout (53). B, aminoacylation of the normal (circles) and 8U→C (U8C) transcripts (squares) of hmtRNAMet by hmMetRS. C, aminoacylation of normal (circles) and 8U→C (U8C) mutated (squares) hmtRNAMet by E. coli MetRS.
Three interesting point mutations (T4409C, A4435G, and G4450A) occur in the gene for human tRNAMet (hmtRNAMet). The T4409C mutation (Fig. 1A) results in a U8 to C change at the corner of the acceptor stem and D-stem of hmtRNAMet. This mutation leads to mitochondrial myopathy resulting in dystrophic muscles and exercise intolerance (20). The A4435G mutation leads to the change of A37 to G37 in the anticodon loop of the tRNA (21). This mutation acts as a modulator of Leber's Hereditary Optic Neuropathy increasing the severity of this condition when it arises because of other mutations in the mitochondrial DNA. The G4450A mutation leads to loss of the final base pair in the T-stem (Fig. 1A). This mutation presents as splenic lymphoma, is largely confined to lymphocyte cells, and results in severely abnormal mitochondria leading to serious defects in energy production (22). A systematic examination of the structural and biochemical consequences of these mutations is lacking. Here we examine the structure of human mitochondrial tRNAMet and probe the effects of the 8U→C mutation on the structure and function of this tRNA.
EXPERIMENTAL PROCEDURES
RNA Synthesis—Human mitochondrial tRNAMet transcripts for aminoacylation experiments were prepared by in vitro transcription using the hammerhead ribozyme construct described previously (23). The hmtRNAMet was purified by denaturing (10%) PAGE (29:1 acrylamide:bisacrylamide prepared with 7 m urea, 90 mm Tris borate, 2 mm EDTA), visualized by UV shadowing, excised from the gel, and recovered by passive elution in water followed by ethanol precipitation. hmtRNAMet transcripts for selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) experiments were prepared in the context of the structure cassette as described (24). D- and T-half-molecules were chemically synthesized (Dharmacon), purified, and analyzed as described previously (25).
Purification of E. coli Methionyl-tRNA Synthetase (MetRS) and Human Mitochondrial MetRS (hmMetRS)—A saturated overnight culture of JM109 cells carrying the pQE60-Escherichia coli MetRS plasmid construct (kindly provided by Uttam RajBhandary, Massachusetts Institute of Technology) was grown at 37 °C in 2× YT media (20 ml) supplemented with 50 μg/ml ampicillin and used to inoculate 2 liters of 2×YT media (50 μg/ml ampicillin). The cells were grown at 37 °C for 4 h (A600 = 0.6), induced with 50 μm isopropyl β-d-thiogalactopyranoside and then grown at 37 °C for 4 h post-induction. The cells were harvested by centrifugation at 4,000 rpm for 30 min. The cell pellet was resuspended in 500 ml of 10 mm Tris-HCl, pH 7.6, and then re-collected by low speed centrifugation. The cell pellet was fast frozen and stored at -80 °C until use.
The cell pellet (7 g) was resuspended in 100 ml of lysis buffer (50 mm Tris-HCl, pH 7.6, 50 mm KCl, 10 mm MgCl2, 200 μm phenylmethylsulfonyl fluoride (PMSF), 0.1% Triton X-100, and 7 mm β-mercaptoethanol (βME)) and sonicated on ice for 7 min with 10-s bursts followed by 50-s cooling periods. The cell lysate was centrifuged at 15,000 rpm for 30 min at 4 °C. E. coli MetRS was purified from the supernatant using 400 μl of a 50% nickel-nitrilotriacetic acid (Qiagen) slurry in wash buffer (100 mm Tris-HCl, pH 7.6, 1 m KCl, 10 mm MgCl2, 10 mm imidazole, 200 μm PMSF, and 7 mm βME). The resin was washed with 200 ml of wash buffer. The protein was eluted with 4 ml of elution buffer (100 mm Tris-HCl, pH 7.6, 50 mm KCl, 10 mm MgCl2, 150 mm imidazole, 200 μm PMSF, and 7 mm βME). The protein sample was dialyzed against 2 volumes of 500 ml of dialysis buffer (50 mm Tris-HCl, pH 7.6, 50 mm KCl, 2.5 mm MgCl2, 0.1 mm EDTA, 10% glycerol and 7 mm βME) for 1 h.
Cells carrying a plasmid encoding the His6-tagged human mitochondrial MetRS were grown as described (23). The cells were lysed as described above, and the hmMetRS was further purified as described (23).
Purification of Bovine Mitochondrial Methionyl-tRNA Transformylase (MTFmt)—E. coli BL21 cells, carrying the pET15-bovine MTFmt plasmid construct, were grown as described (19). Cells were harvested and lysed as described for E. coli MetRS above. The protein was purified as described for the E. coli MetRS except that the buffers contained 50 mm Tris-HCl, pH 7.6. The purified protein sample was dialyzed against 2 volumes of 500 ml of MTF dialysis buffer (20 mm Tris-HCl, pH 7.6, 100 mm KCl, 10% glycerol, and 3 mm βME) for 1 h, fast-frozen, and stored at -80 °C.
Assay for the Aminoacylation of Human Mitochondrial tRNAMet—The aminoacylation reactions for both the normal and 8U→C mutated tRNAMet transcripts were performed essentially as described (23). Reaction mixtures (100 μl) contained 50 mm Tris-HCl, pH 7.6, 2.5 mm MgCl2, 2.5 mm ATP, 0.2 mm spermine, 200 μg/ml bovine serum albumin, 0.2 units/μl SUPERase·In RNase inhibitor, 40 μm [35S]methionine (4,000 cpm/pmol), 50 nm human mitochondrial MetRS or 8 nm E. coli MetRS, and 1 μm U8 or 8U→C hmtRNAMet. The amount of aminoacylated tRNA formed was determined by trichloroacetic acid-precipitable counts at the indicated times.
Preparative Aminoacylation of [35S]Met-tRNAMet—Reaction mixtures (2 ml) were prepared as described above except that 20 μm [35S]methionine (20,000 cpm/pmol), 0.5 μm U8, or 8U→C hmtRNAMet, and saturating amounts of human mitochondrial MetRS were used. Reactions were incubated for 15 min at 37 °C, followed by phenol/chloroform extraction. The tRNAMet was collected by ethanol precipitation and then dissolved in 10 mm potassium succinate, pH 6.0, before use.
Formylation of Human Mitochondrial Met-tRNAMet—Formylation reactions (5 μl) contained 20 mm Tris-HCl, pH 7.6, 100 μm EDTA, 150 mm KCl, 7 mm MgCl2, 10 mm βME, 125 μm folinic acid (Sigma), 100 nm normal or 8U→C mutated [35S]Met-hmtRNAMet and 8 nm MTFmt. Reactions were performed at 37 °C for 0-8 min (0-min time point was taken in the absence of enzyme). At the indicated time, 83 mm NaOH (1 μl of 500 mm) was added, and incubation was continued at 37 °C for 30 min. The [35S]Met and [35S]fMet in 5 μl of each reaction were separated on Partisil LK5D TLC plates (Whatman) with a butanol:acetic acid:water (4:1:1) mixture. TLC plates were visualized by phosphorimaging (GE Healthcare) and the spots were analyzed using the ImageQuant program.
Binding of Human Mitochondrial Met-tRNAMet to Bovine Mitochondrial EF-Tu (EF-Tumt)—EF-Tumt was prepared as described (26), except that the cells were lysed as described above for E. coli MetRS, and the high speed centrifugation step was omitted. Where indicated the normal U8 or 8U→C mutated hmtRNAs were phosphorylated with cold ATP using polynucleotide kinase (New England Biolabs) prior to large scale aminoacylation.
To measure ternary complex formation, reaction mixtures (50 μl) were prepared as reported (27) except that 20 mm Hepes-KOH, pH 7, and the indicated amounts of EF-Tumt were used. The reactions were incubated for 15 min at 0 °C or 6 min at 37 °C as indicated. Free [35S]Met-hmtRNAMet was digested by a 30-s incubation with 10 μg of RNase A, and the reaction was terminated by the addition of cold 5% trichloroacetic acid. Following a 10-min incubation on ice, the [35S]Met-hmtRNAMet precipitate was collected on nitrocellulose filters and quantified by liquid scintillation counting. Determination of the Kd for ternary complex formation was carried out as described previously (28).
Degradation of hmtRNAMet in a Mitochondrial Extract—Bovine mitoplasts (0.2 g) were prepared as described (29). Mitoplasts were lysed in buffer (2 ml) containing 15 mm Tris-HCl, pH 7.6, 40 mm KCl, 6 mm MgCl2, 6 mm βME, 0.8 mm EDTA, and 1.6% Triton X-100 by hand homogenization. The extract was clarified by centrifugation at 10,000 rpm for 15 min at 4 °C. Normal U8 and 8U→C mutated hmtRNAMet were 32P-end-labeled using polynucleotide kinase (New England Biolabs) according to the manufacturer's instructions. Reaction mixtures (10 μl) contained 100 nm normal U8 or 8U→C mutated [32P]hmtRNAMet and the indicated amount of the extract in the lysis buffer above. Incubation was for 10 min at 37 °C, and cold trichloroacetic acid precipitation was used to quantitate the amount of [32P]hmtRNAMet remaining.
Selective 2′-Hydroxyl Acylation Analyzed by Primer Extension (SHAPE) Analysis of Normal U8 and 8U→C Mutated hmtRNAMet Transcripts—Normal U8 or 8U→C mutated hmtRNAMet (12 pmol, 0.33 μm) in 36 μl of nuclease-free water (Ambion) was incubated at 50 °C for 2 min and then cooled on ice for 2 min. The RNA was divided into 2 aliquots of 4 pmol (12 μl) and 8 pmol (24 μl). Folding buffer (6 μl; 333 mm Hepes-KOH, pH 8, 333 mm NaCl) was added to the 4 pmol of RNA, and folding buffer with 20 mm Mg2+ (12 μl) was added to the 8 pmol of RNA, and the two samples were incubated at 37 °C for 20 min. To 1 μl of 100 mm 1-methyl-7-nitroisotoic anhydride (1M7) in anhydrous DMSO or 1 μl of anhydrous DMSO (control), 9 μl (2 pmol) of folded RNA was added and allowed to react at 37 °C for 70 s (5 half-lives). The balance of the RNA folded in the presence of Mg2+ (18 μl) was divided into 2 aliquots of 2 pmol (9 μl) each and stored at 37 °C for sequencing. MgCl2 (1 μl; 64 mm) was added to the RNA treated with the folding buffer in the absence of Mg2+. Radiolabeled oligonucleotide (0.3 μm;3 μl; 5′-32P-GAACCGGACCGAAGCCCG, obtained from the Nucleic Acids Core Facility at University of North Carolina) was added to the 1M7-treated, DMSO-treated, or untreated RNA (2 pmol), and the samples were incubated at 65 °C for 5 min and then at 35 °C for 20 min for primer annealing. To each reaction, reverse transcription buffer (6 μl; 250 mm KCl, 167 mm Tris-HCl, pH 8.3, 17 mm dithiothreitol, and 0.42 mm each dNTP) was added. Then either ddCTP or ddTTP (1 μl; 5 mm; Amersham Biosciences) was added to the untreated RNA. After heating to 52 °C, reverse transcriptase (1 μl; 200 units; Superscript III, Invitrogen) was added, and the primer extension reactions were performed at 52 °C for 5 min. Reactions were quenched with 4 m NaOH (1 μl) and heated at 95 °C for 5 min. For gel analysis, a gel loading solution (29 μl; 138 mm unbuffered Tris-HCl, 73% (v/v) formamide, 2 mm Tris borate, 86 mm EDTA, pH 8, with xylene cyanol and bromphenol blue) was added, and the samples were heated at 95 °C for an additional 5 min. The cDNA products from the + and - 1M7 and sequencing reactions were separated by denaturing gel electrophoresis (10% polyacrylamide). Samples on gels (21 cm × 40 cm × 0.4 mm) were subjected to electrophoresis at 1400 V for ∼2.5 h. Gels were visualized by phosphorimaging (GE Healthcare). The + and - 1M7 band intensities were quantified using SAFA (30) and corrected for signal drop-off (31). SHAPE reactivities were normalized by subtracting intensities for the -1M7 control from the +1M7 reaction and dividing each by the average reactivity of the most reactive 7% of the nucleotides. To facilitate comparison of the normal U8 and 8U→C mutant tRNAs, the two data sets were normalized to the reactivity of the -CCA end nucleotides. The reactivity of each nucleotide was assigned a value between 0 and 1. Nucleotides fall into one of four categories (32, 33) as follows: unreactive (0.000-0.055), low reactivity (0.055-0.110), moderately reactive (0.110-0.220), or highly reactive (0.220-1.000).
Structural Studies of hmtRNAMet Half-molecules—Reconstitution of hmtRNAMet from U8 and 8U→C D-half-molecules with T-half-molecules required Mg2+ and was assessed by gel mobility shift assays using native 15% PAGE in Tris borate buffer (89 mm Tris base, 89 mm boric acid, pH 8.3) with and without 3 mm Mg2+ at 4 °C (25). The concentration of the D-half-molecule was held constant at 31.2 μm, whereas the concentration of the T-half-molecule was varied from 4.2 to 112 μm.
UV-Monitored Thermodynamic Experiments—The half-molecule RNA samples were dissolved in the above Tris borate buffer used for the PAGE experiments to obtain an RNA concentration of 1.2 μm. MgCl2 was added to a concentration of 3 mm. UV-monitored thermal denaturations and re-naturations were replicated 10 times and monitored by measuring UV absorbance (260 nm) using a Cary 3 spectrophotometer as published (34, 35). The data points were averaged over 20 s and recorded with a temperature change of 1 °C per min from 4 to 90 °C. The one most inconsistent of the 10 melting transitions (either a denaturation or renaturation) was discarded from each set, and the resulting data were averaged on a point-by-point basis.
For UV melts of the hmtRNAMet transcripts, the normal U8 and the 8U→C hmtRNAMet were dialyzed against water using 10-kDa cutoff dialysis cups (Stratagene). The U8 and 8U→C hmtRNAMet transcripts were diluted to 0.5 μm in a buffer containing 10 mm NaCl and 10 mm Hepes-KOH, pH 8.0. The thermal denaturation of the tRNAs was monitored by UV absorbance at 260 nm using a Cary 3 spectrophotometer. Data points were recorded once per min from 4 to 95 °C with a temperature change of 1 °C per min. Following thermal renaturation, 6 mm Mg2+ was added to the U8 and 8U→C transcripts, and the UV-monitored thermal denaturation experiments were repeated.
RESULTS
Aminoacylation of the Normal and 8U→C Mutated tRNAMet—Previous studies (23) have shown that the transcript of mitochondrial tRNAMet has aminoacylation properties similar to those observed with the native tRNA. Thus, it was possible to use the normal transcript and a transcript containing the 8U→C mutation for studies on the effect of the mutation on the properties of the tRNA. The 8U→C mutation leads to a myopathy presumably arising from a reduction in translational activity in mitochondria. To determine the biochemical consequence of the 8U→C mutation, the abilities of the U8 and 8U→C hmtRNAMet transcripts to be aminoacylated by the human mitochondrial methionyl-tRNA synthetase (hmMetRS) were tested. Aminoacylation is an early step required for the tRNA to be used in either the elongation or initiation phase of protein synthesis and is thus of central importance for protein synthesis in mitochondria. The normal U8 transcript was aminoacylated as expected (23); however, the 8U→C mutation caused a significant reduction in the rate of aminoacylation of the tRNA by hmMetRS (Fig. 1B). This observation provides one clear rationale for the failure of this tRNA to function in mitochondrial protein biosynthesis.
Not unexpectedly, the normal hmtRNAMet was aminoacylated by the E. coli MetRS (Fig. 1C). Interestingly, whereas the 8U→C hmtRNAMet was poorly aminoacylated by the hmMetRS, it was not aminoacylated at all by the E. coli MetRS (Fig. 1C) suggesting that the mutated tRNA had a significantly altered structure. The hmMetRS is believed to be both structurally and functionally homologous to its prokaryotic counter-part (23). However, this work demonstrates that the hmMetRS is less discriminatory than E. coli MetRS for the structure of the tRNA. Because a major determinant in the recognition of tRNAMet by the MetRS is thought to lie in the anticodon sequence that is unchanged (36), the weak aminoacylation most likely reflects significant structural alterations in the tRNA as a result of the mutation.
Formylation of Normal and 8U→C Mutated Met-tRNAMet—The defective aminoacylation of the 8U→C hmtRNAMet made it difficult to assess the effects of the mutation on additional steps in protein biosynthesis. However, small amounts of the aminoacylated 8U→C mutated hmtRNAMet could be isolated, permitting a limited investigation of additional steps in translation.
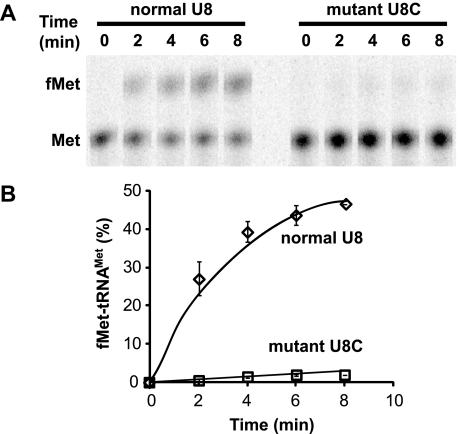
In the mammalian mitochondrial system, the Met-tRNAMet must be formylated by the mitochondrial transformylase (MTFmt) to be used in initiation (19, 37). The abilities of the U8 and 8U→C Met-tRNAMet to be formylated were tested by incubation of the [35S]Met-tRNA with the bovine MTFmt in the presence of folinic acid to provide the formyl group. The conversion of [35S]Met to [35S]fMet was monitored by TLC (Fig. 2). The normal hmtRNAMet formylates well, but interestingly, formylation of the 8U→C mutated tRNA is barely detectable (Fig. 2). The inability of the 8U→C mutated tRNA to be efficiently aminoacylated and formylated suggests there would be little fMet-tRNAMet available for initiation of protein synthesis.
FIGURE 2.
Formylation of the normal and 8U→C mutated Met-hmtRNAMet. A, conversion of the normal and 8U→C (U8C) mutated [35S]Met-hmtRNAMet to [35S]fMet-hmtRNAMet as a function of time. The aminoacylated tRNAs were incubated with bovine MTFmt for the indicated times. The amino acid was hydrolyzed from the tRNA using alkali and the [35S]Met separated from [35S]fMet by TLC. B, percentage of Met converted to fMet, over time, for the normal (diamonds) and 8U→C (U8C) mutated (squares) hmtRNAMet.
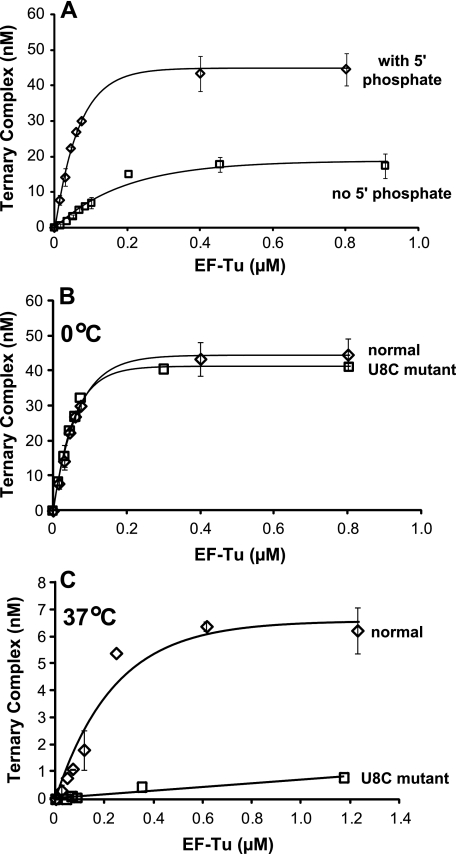
Binding of Normal and 8U→C Mutated Met-tRNAMet to EF-Tumt—The hmtRNAMet must be partitioned between the initiation and elongation stages of protein synthesis. To function in elongation, the formylation step must be by-passed and the Met-tRNAMet must form a ternary complex with EF-Tumt (also known as mitochondrial EF1A). The binding of amino-acyl-tRNA to EF-Tumt is normally studied at 0-4 °C to protect the aminoacyl bond from hydrolysis (38, 39). Because the strength of the interaction of Met-hmtRNAMet with EF-Tumt had never been determined, these experiments were begun with an investigation of the binding of EF-Tumt to the normal Met-tRNAMet. Because the transcript begins with a 5′-terminal A rather than the G residue preferred by the T7 RNA polymerase, the construct used for transcription contained a hammerhead ribozyme and T7 promoter gene sequence upstream of the tRNA sequence. Following transcription, the hammerhead ribozyme was cleaved leaving behind a tRNA beginning with a 5′-OH rather than the 5′-phosphate normally present in tRNAs. The presence of a 5′-phosphate is not required for aminoacylation or formylation of this tRNA (data not shown). The formation of the ternary complex with E. coli EF-Tu is not affected by the 5′-phosphate (40), but no studies were available on the importance of this group for EF-Tumt. The first issue addressed was whether the ability of EF-Tumt to bind the aminoacylated tRNA in the ternary complex was affected by the presence of the 5′-phosphate. For this experiment, the aminoacylated transcript carrying the 5′-OH was tested in ternary complex formation and compared with an aminoacylated transcript that carried a 5′-phosphate following phosphorylation by polynucleotide kinase. As indicated in Fig. 3A, the Met-tRNA transcript carrying the 5′-phosphate formed a ternary complex with EF-Tumt efficiently. However, the transcript with the 5′-OH was noticeably less active in interacting with EF-Tumt. This observation indicates that, unlike E. coli EF-Tu, the interaction of EF-Tumt with Met-tRNA benefits from contact with the phosphate at the 5′-end of the aminoacyl-tRNA. Examination of the residues in the binding pocket for the aminoacyl-tRNA in the bacterial and mitochondrial factors reveals that Glu-287 in E. coli EF-Tu is replaced by the oppositely charged residue Arg-335 in EF-Tumt (27). This change alters the charge balance in the region of EF-Tu in close proximity to the 5′-end of the tRNA in the ternary complex and may provide an additional stabilizing interaction enhancing the binding of EF-Tumt to the conformationally fragile mitochondrial tRNAs.
FIGURE 3.
Effect of the 8U→C mutation and the 5′-phosphate on binding of Met-hmtRNAMet to EF-Tumt at 0 and 37 °C. A, binding of normal Met-tRNAMet with (diamonds) and without (squares) the 5′-phosphate to EF-Tumt: GTP. B, binding of EF-Tumt:GTP to normal (diamonds) and 8U→C (U8C) mutated (squares) Met-tRNAMet at 0 °C, forming the ternary complex. C, ternary complex formation for normal U8 (diamonds) and 8U→C (U8C) mutated (squares) Met-tRNAMet at 37 °C.
Analysis of the binding curve between EF-Tumt and the phosphorylated Met-hmtRNAMet indicates a binding constant of 27 ± 6 nm at 0 °C. This value is in good agreement with the Kd value observed for the binding of several other mitochondrial aminoacyl-tRNAs to EF-Tumt (41).
The interaction of the 8U→C Met-hmtRNAMet was then investigated at 0 °C using the phosphorylated transcript (Fig. 3B). Rather surprisingly, the mutated tRNA was as effective as the normal transcript in interacting with EF-Tumt under these conditions suggesting that the 8U→C Met-tRNA had adopted a tRNA-like conformation recognized by this factor. This observation appeared to be in conflict with the poor activity of the 8U→C hmtRNAMet in aminoacylation and formylation. However, the previous assays were carried out at 37 °C, whereas the ternary complex assay was carried out at 0 °C. Hence, ternary complex formation was tested at 37 °C with both the phosphorylated U8 and 8U→C Met-hmtRNAMet species (Fig. 3C). At this higher temperature, the 8U→C Met-hmtRNAMet was almost inactive in ternary complex formation, whereas the U8 Met-hmtRNAMet had significant activity in ternary complex formation. These data suggest a temperature-dependent destabilization of the structure of the 8U→C mutated tRNA. Structural probing provided insight into this question as described below.
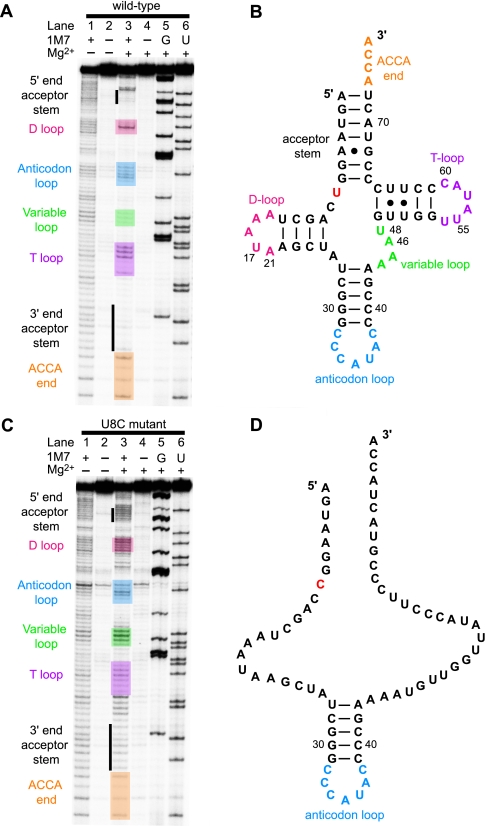
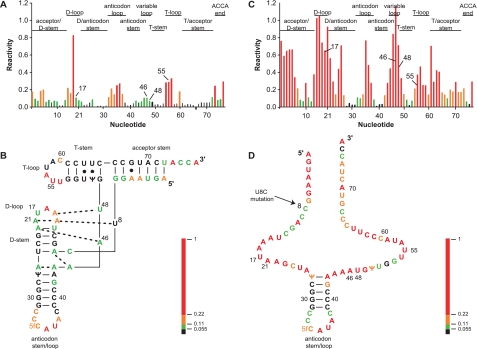
Chemical Probing of the Structure of the Normal U8 tRNAMet in the Presence and Absence of Mg2+—The structures of the normal U8 and 8U→C mutant hmtRNAMet transcripts were probed using SHAPE chemistry at 37 °C (24, 42). SHAPE chemistry relies on the differential reactivity of the nucleotide 2′-OH to the small molecule 1M7 (24, 42). Nucleotides in flexible, single-stranded regions are preferentially modified by the reagent, revealing different conformational states of residues within the RNA. The chemically treated tRNA transcripts were reverse-transcribed using a radiolabeled primer. Sites of modification were identified based on their ability to obstruct reverse transcription. The cDNA products were analyzed on a denaturing polyacrylamide gel. When residues in unstructured or flexible regions of the tRNA are modified, the reverse transcriptase stops, leading to a labeled band observed on the gel one residue shorter than the position of the modification. Comparison of the position of these bands to a sequencing ladder allows the identification of residues susceptible to modification (32). The reactivity of each nucleotide toward 1M7 was quantified, and the relative reactivity of each nucleotide was assigned a value between 0 and 1. The higher the value, the greater the propensity the nucleotide has to be in an unstructured region. This information allowed us to develop a model of the tRNA structure (43).
The reactivity of 1M7 is not Mg2+-sensitive; hence, SHAPE chemistry was used to examine the influence of Mg2+ on the structure formed by the normal and 8U→C mutant hmtRNAMet transcripts. In the presence of Mg2+, a number of distinct structural features were observed for the normal hmtRNAMet (Fig. 4, A and B). The 2′-OH of residues predicted to form the acceptor stem and the D-stem were not accessible to the reagent. This indicated that they were largely paired as expected. Only a single residue in the D-loop was highly reactive suggesting that most of the residues in this region of the tRNA were involved in tertiary interactions that reduced their reactivity. The anticodon stem was clearly protected, but as expected residues in the anticodon loop were available for modification. The variable loop again showed limited modification indicating tertiary interactions. These interactions were expected to take place primarily with residues in the D-loop as would be expected from tertiary contacts observed in canonical tRNAs. The T-stem apparently formed despite the presence of the U-U pairs. However, in contrast to other tRNAs (33), residues in the T-loop were quite reactive indicating that they were accessible in the tertiary structure of this tRNA.
FIGURE 4.
Structural consequence of the 8U→C mutation in hmtRNAMet and the effect of Mg2+. A, gel image showing the reactivity of the U8 hmtRNAMet transcript to 1M7 at 37 °C. Reactions were performed in the presence and absence of Mg2+, and control reactions were performed lacking 1M7. Dideoxy-NTP sequencing reactions were also performed, and the results show the positions of the G and U residues obtained using ddCTP and ddATP. The oligonucleotides in the gel bands are one nucleotide longer than those in the corresponding bands in the reaction lanes. The reactivities of the various tRNA loops are highlighted. The vertical line on the gel indicates the nucleotides within the acceptor stem. Bands above the 5′-end of the acceptor stem correspond to the structure cassette (24). B, structure of the hmtRNAMet transcript indicating structural regions of interest. The U8, which is changed in the 8U→C tRNA, is shown in red. C, gel image showing the 1M7 reactivity of the 8U→C hmtRNAMet in the presence (lane 4) and absence (lane 2) of Mg2+ at 37 °C. The gel is annotated as in A above. D, unstructured form of the 8U→C hmtRNAMet transcript in the presence of Mg2+ with C8 shown in red.
The folding of the hmtRNAMet structure required the presence of Mg2+ (Fig. 4A, lanes 1 and 3 compared). Mg2+ not only stabilizes the negatively charged backbone but is known to bind to specific sites of canonical cytoplasmic tRNAs to aid in folding their tertiary structures. In the absence of Mg2+, the hmtRNAMet structure was open and highly reactive to 1M7, indicating that both secondary and tertiary interactions had been lost (Fig. 4A, lane 1).
A more thorough analysis of the SHAPE data through quantitation of the individual nucleotide reactivities provided additional insight into the three-dimensional structure formed by the unusual hmtRNAMet. The SHAPE reactivity pattern was suggestive of a folded cloverleaf-shaped tRNA and was similar to the reaction pattern observed with other tRNAs (33) (Fig. 5A). Superimposition of the nucleotide reactivities on the L-shaped tRNA structure (43) (Fig. 5B) provided additional information about possible tertiary interactions within the tRNA. For clarity, the nucleotides have been colored according to their level of reactivity. As expected, the most reactive portion of the tRNA was the anticodon loop. The reactivity data support a structure in which many of the conserved canonical tertiary interactions are preserved. For example, nucleotides U8, A14, and A21 which, in canonical tRNAs form a triple base pair, were all modestly reactive or nonreactive in hmtRNAMet. Other possible preserved tertiary interactions are shown in Fig. 5B and include the expected interaction between nucleotides in the D-loop and the variable loop such as between A15 and U48.
FIGURE 5.
Tertiary interactions in the hmtRNAMet and the effect of mutating U8 to C on these interactions. A, histogram showing the reactivity of nucleotides in the U8 hmtRNAMet transcript. Nucleotides with high, medium, low, and no reactivity are shown in red, orange, green, and black, respectively (see “Experimental Procedures”). Structural regions in the tRNA are shown above the histogram. The numbering shown is from the Sprinzl system (53). B, L-shaped structure of hmtRNAMet. Possible tertiary interactions occurring in the tRNA are shown and are based on conserved interactions that occur in the canonical cytoplasmic tRNAs. The nucleotides are colored based on reactivity as in A above. C, reactivity of nucleotides in 8U→C hmtRNAMet. The histogram is labeled and colored as in A above. D, unstructured form of the 8U→C mutated hmtRNAMet. The colors of the nucleotides are based on their reactivity to 1M7 as in the histogram in C. In contrast to the reactivity of the nucleotides of the normal U8 hmtRNAMet to 1M7, the reactivity data for the 8U→C mutant hmtRNAMet clearly did not fit the L-shaped structure.
Despite the likely presence of many conserved tertiary interactions in hmtRNAMet, other interactions are probably not occurring because of the shortened sequence of this tRNA. For example, interactions between the D- and T-loops may be different from canonical tRNAs because the D-loop is short and lacks the common GG sequence, whereas the T-loop is only six nucleotides instead of the universal seven nucleotides found in classical tRNAs. These deviations from classical tRNAs indicate that hmtRNAMet cannot have the G18-U55 and G19-C56 interactions that normally stabilize the corner of the L-shaped structure (3). Also, A58 in the T-loop was not reactive, whereas its expected partner U54 showed significant reactivity. This suggests that the canonical tertiary interaction of U54 with A58 may not occur. The tertiary interactions occurring in the T-loop of the hmtRNAMet may be significantly different from that of the canonical cytoplasmic tRNAs.
Chemical Probing of the Structure of the 8U→C Mutated tRNA in the Presence and Absence of Mg2+—SHAPE analysis of the 8U→C mutated hmtRNAMet carried out at 37 °C in the presence of Mg2+ indicated that all of the nucleotides in the tRNA were susceptible to modification except the three G-C pairs in the anticodon stem. The single nucleotide mutation (8U→C) at the corner of the acceptor stem and D-stem of the hmtRNAMet thus resulted in a drastic loss of structure that is seen even in the presence of Mg2+ (Fig. 4C, lane 3). The regions of the D-loop, the variable loop, acceptor stem, and T-stem all exhibited an increased reactivity to the reagent 1M7. For example, all of the residues in the D-loop and most of the residues in the variable loop were now reactive. Furthermore, the residues in the acceptor stem, which were base-paired in the normal U8 tRNA, were reactive. These changes indicated that the tertiary structure and a large portion of the secondary structure had been lost because of the mutation. As expected, the 8U→C mutant hmtRNAMet had essentially no structure in the absence of Mg2+, as was observed for the normal hmtRNAMet (Fig. 4C, lane 1). Even the anticodon stem had lost its base-paired structure. Clearly, the folding of the mutated tRNA was not responding to the presence of Mg2+ and thus may have lost one or more Mg2+-binding sites critical to the structure. It is likely that the loss in structure observed upon mutation of 8U→C results from a loss of stabilizing tertiary interactions that were present in the normal hmtRNAMet and that are dependent on the presence of Mg2+. It should be noted that, alternatively, the tRNA could be folding into a dynamic mixture of transient conformations that do not reflect the structure of the normal U8 hmtRNAMet.
Analysis of the 8U→C mutated tRNA clearly showed a global increase in reactivity (Fig. 5, C and D). This increase was seen in all of the stems except the anticodon stem suggesting a loss of these secondary structural elements. Both the D- and T-loops also significantly increased in reactivity, suggesting a loss in stabilizing tertiary interactions. The variable loop, which makes extensive tertiary interactions in the U8 hmtRNAMet, became highly reactive in the 8U→C tRNA. Although the mutated 8U→C residue itself showed only a slight increase in reactivity, its tertiary interaction partners, A14 and A21, became highly reactive suggesting that they were unable to form the necessary stabilizing tertiary interactions in this region of the tRNA. When these reactivities were superimposed on the L-shaped hmtRNAMet structure, it was clear that changing the single U8 nucleotide to a C lead to a loss of both secondary and tertiary interactions resulting in a tRNA that was largely unstructured (Fig. 5D).
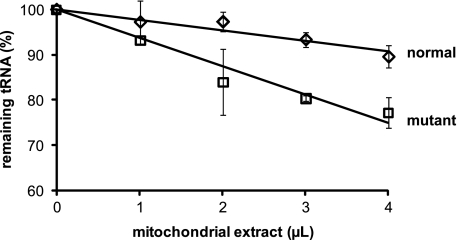
Stability of Normal and 8U→C Mutated hmtRNAMet in a Mitochondrial Extract—A number of mutated mitochondrial tRNAs are more rapidly degraded in vivo than are the normal tRNAs (43, 44). To determine how the 8U→C mutation might affect the stability of the tRNA within mitochondria, the degradation of the normal and 8U→C mutated hmtRNAMet was monitored in an extract prepared from the mitochondrial matrix. For this analysis, the U8 and 8U→C transcripts were labeled at the 5′-end with 32P-phosphate and incubated with varying concentrations of the mitochondrial extract. The amount of the tRNA remaining intact was determined by precipitation with trichloroacetic acid. The 8U→C mutated tRNA was more readily degraded by the mitochondrial nucleases than the normal U8 tRNA (Fig. 6). When visualized on a sequencing gel, it was apparent that the normal tRNA was degraded in discrete locations corresponding to loops, whereas the mutated tRNA was degraded throughout the body of the tRNA (data not shown). The differential degradation of the two tRNAs was in agreement with the SHAPE data showing an overall lack of structure in the mutated tRNA. Furthermore, these data suggested that, in addition to being incorrectly folded, the mutated tRNA will also be more rapidly degraded within the milieu of the mitochondrial matrix.
FIGURE 6.
Stability of the normal and 8U→C mutated hmtRNAMet in a mitochondrial extract. The percentage of trichloroacetic acid-precipitable counts for the normal U8 transcript (diamonds) and the 8U→C mutated (squares) hmtRNAMet remaining after incubation of 32P-labeled transcripts with increasing amounts of a mitochondrial matrix extract.
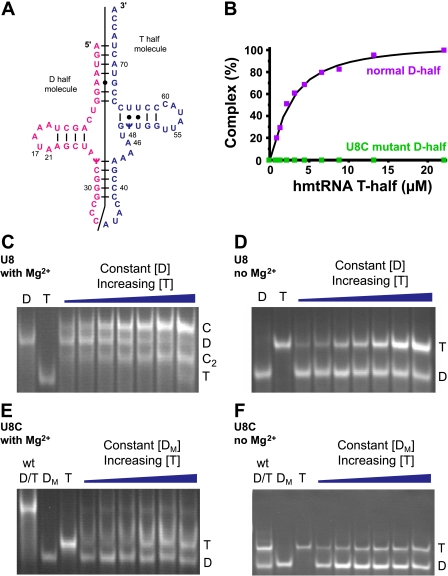
Association of T-half-molecules with U8 and 8U→C D-half-molecules—To further probe the effect of the 8U→C mutation on the structure of the tRNA in the presence and absence of Mg2+, D- and T-domain hmtRNAMet half-molecules were chemically synthesized with the two pseudouri-dines, Ψ, and either U8 or 8U→C in the D-half-molecule (Fig. 7A). Previously, we demonstrated that incubation of the normal D- and T-half-molecules of bovine mtRNAMet in the presence of Mg2+ resulted in reconstitution of the mtRNAMet (25). The reconstituted tRNA could be observed as a slow moving band under native conditions. The effect of the 8U→C mutation on the reconstitution of the human mtRNAMet was assessed using PAGE migration in the presence and absence of Mg2+.
FIGURE 7.
Effect of Mg2+ and the 8U→C (U8C) mutation on reconstitution of the hmtRNAMet from the D-half- and T-half-molecules. A, D-half-molecule (A1-C32, pink) and T-half-molecule (A33-A76, blue) were chemically synthesized to include Ψ27 and Ψ50. B, summary of the efficiency of reconstituting hmtRNAMet from the normal (purple) and mutated (green) D-half- and T-half-molecules in the presence of Mg2+. C, titration of the U8 D-half-molecule (D) with increasing concentrations of the T-half-molecule (T) at 3 mm Mg2+ showing the formation of two complexes (C1 and C2). D, titration of the U8 D-half-molecule with increasing concentrations of the T-half-molecule in the absence of Mg2+ showing that no complex is formed. E, titration of the 8U→C D-half-molecule with increasing concentrations of the T-half-molecule at 3 mm Mg2+ showing that little complex is formed. F, titration of the 8U→C D-half-molecule with increasing concentrations of the T-half-molecule in the absence of Mg2+ showing that no complex is formed. The 1st lane labeled wt D/T is a positive control showing the formation of the complex between the normal U8 D-half-molecule (37.6 μm) and the normal T-half-molecule (37.1 μm). The lane labeled Dm indicates the migration of the 8U→C mutated D-half-molecule alone, whereas the T lane indicates the migration of the normal T-half-molecule.
In PAGE, when the slower migrating mtRNAMet D-half-molecule containing the normal U8 was titrated with the faster migrating T-half-molecule at 3 mm Mg2+, a gel shift was observed that corresponded to the formation of a complex between the two half-molecules (Fig. 7C) (25). In fact, two new bands attributed to the reconstituted tRNA appeared to increase with increasing amounts of the T-half-molecule. This increase was accompanied by the expected decrease in the D-half-molecule (Fig. 7C). The two gel bands attributed to the reconstituted mtRNAMet contained both half-molecules, as determined by denaturing PAGE (data not shown). The slower moving mtRNAMet (∼70% of the reconstituted tRNA) migrated in a fashion similar to that of E. coli cytoplasmic tRNA (Fig. 7C), and to the migration found for the complex of bovine mtRNAMet half-molecules (25). The faster moving band representing some 30% of the reconstituted human mtRNAMet migrated faster than the D-half- and slower than the T-half-molecule. It is believed to be folded into a second, but non-native, conformation. In the absence of Mg2+, the migration of the D-half-molecule was more rapid than in the presence of Mg2+ suggesting a Mg2+-dependent conformational change in the half-molecule. In the absence of Mg2+, the two half-molecules could not form a complex (Fig. 7, C and D).
The mutated 8U→C D-half- and normal T-half-molecules were incapable of forming a complex either in the presence or absence of Mg2+ (Fig. 7, E, F, and B). The migration of the mutant 8U→C D-half-molecule was much faster than that of the normal D-half-molecule in the presence of Mg2+, suggesting a conformational difference in the half-molecule resulting from the 8U→C change. In addition, the rapid migration of the 8U→C D-half-molecule was not affected by Mg2+. These results suggested that the 8U→C D-half-molecule had lost the ability to bind one or more structurally important Mg2+ ions. The hmtRNAMet transcripts were subjected to gel electrophoresis under native conditions and in the presence and absence of Mg2+ (data not shown). In the presence of Mg2+, the 8U→C mtRNAMet transcript migrated more slowly than the normal transcript. In the absence of Mg2+, only a slight difference in migration was observed. These data again indicated that the 8U→C and normal mtRNAMet responded differently to Mg2+.
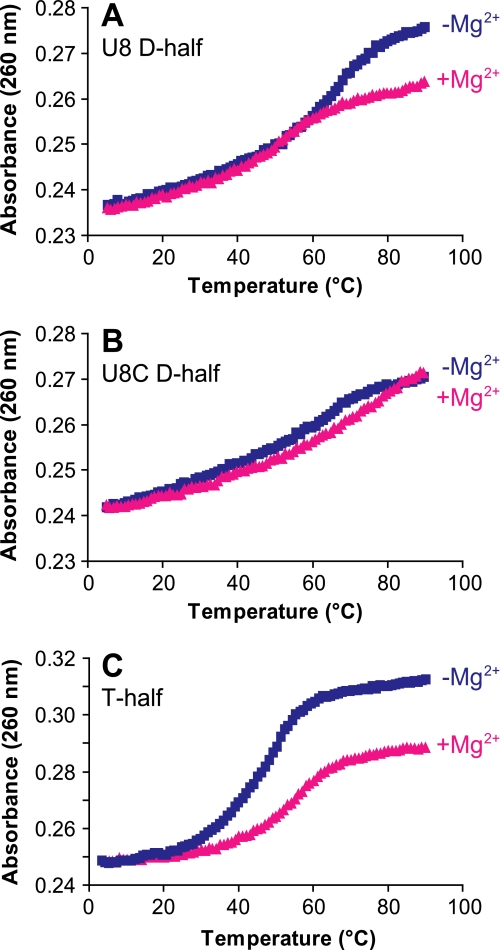
Thermal Denaturation of the Mutated tRNA—The thermal stabilities of the U8 and 8U→C D-half-molecules and the normal T-half-molecule were also determined in the presence and absence of Mg2+. The addition of counterions, particularly Mg2+, to RNA stabilizes its structure. The PAGE results suggested that the 8U→C D-half-molecule had lost the ability to bind one or more critical Mg2+. The U8 half-molecule gave different melting profiles in the presence and absence of Mg2+ (Fig. 8A). In contrast, the 8U→C D-half-molecule had the same thermal melting profile in both the presence and absence of Mg2+ (Fig. 8B) indicating that it was unable to respond to the addition of this counterion. This observation again argues that the mutation led to the loss of a Mg2+-binding site.
FIGURE 8.
Effect of Mg2+ on the thermal stabilities of the normal and 8U→C mutated hmtRNAMet D-half-molecules and the normal T-half-molecule. Normal U8 (A), 8U→C (U8C) mutated D-half-molecules (B), and normal T-half-molecule (C) were subjected repeatedly to thermal denaturation and renaturation monitored at 260 nm. The average of nine melt profiles conducted on different days are plotted for each RNA in the absence (blue) and presence (pink) of 3 mm Mg2+.
The normal T-half-molecule exhibited a major thermal transition that was stabilized by the presence of Mg2+ (Fig. 8C). This was surprising considering that the T-stem contained two adjacent U-U mismatches (Fig. 1A). However, tandem U-U mismatches form one of the most stable internal loops in RNA and, despite the stem distortion that results, their presence actually stabilizes duplex RNA (45, 46).
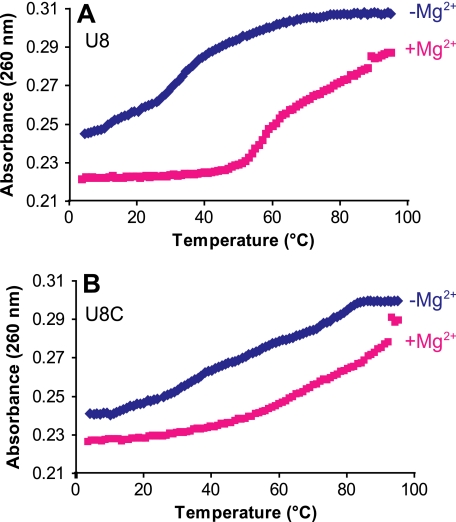
Thermal denaturation of the intact U8 hmtRNAMet transcript demonstrated the importance of Mg2+ for the correct folding of hmtRNAMet. In the absence of Mg2+, the U8 tRNA began melting at low temperatures but demonstrated a major transition at around 30 °C (Fig. 9A, blue). In the presence of Mg2+, the U8 tRNA was stably folded until about 50 °C where a major transition occurred (Fig. 9A, pink). The shift in melting temperature in the presence of Mg2+ demonstrated that the structure was stabilized by the presence of Mg2+. Conversely, melting the 8U→C hmtRNAMet transcript did not show a strong dependence on Mg2+. In both the presence and absence of Mg2+, the 8U→C transcript failed to show a major thermal transition, although significant hyperchromicity was observed (Fig. 9B). Clearly the mutant 8U→C tRNA did not show the same structural response to Mg2+ as did the normal U8 tRNA. In addition, the lack of a major thermal transition for the 8U→C mutated tRNA agrees with the results of the SHAPE experiments, which indicated that the 8U→C mutated tRNA lacks significant structure.
FIGURE 9.
Thermal denaturation of the U8 and 8U→C transcripts in the presence and absence of Mg2+. A, thermal denaturation of the U8 transcript was monitored at 260 nm in the absence (blue) and presence (pink) of 6 mm Mg2+. B, 8U→C (U8C) transcript in the absence (blue) and presence (pink) of 6 mm Mg2+ was subjected to thermal denaturation monitored at 260 nm.
DISCUSSION
Considerable information is now available on the sequences of mammalian mitochondrial tRNAs. With some exceptions, these tRNAs can be drawn as cloverleaf structures and are thought to be able to fold into a three-dimensional structure that resembles the classical L-shape of canonical tRNAs (43). In mammals, the mitochondrial tRNAs are A/U-rich reflecting the base composition of the genomes. In general, these tRNAs are shorter (71-72 nucleotides in length) than bacterial or eukaryotic cytoplasmic tRNAs (about 76 nucleotides for class I tRNAs). The D-loop tends to be smaller and most of them lack the GG sequence that is involved in tertiary interactions in normal tRNAs (8). The T-loop varies from five to nine residues deviating from the highly conserved seven residues found in cytoplasmic tRNAs. The cryo-EM study on the structure of the bovine mitochondrial ribosome (47) shows a tRNA tightly bound at the P-site. The structure of this tRNA can be fit into the crystallographic structure of a cytoplasmic tRNA except in the region of the elbow perhaps reflecting the smaller sizes of the T- and D-loops of many mitochondrial tRNAs. The D- and T-stems also vary significantly in size and often contain mismatches. Searches for tertiary interaction networks corresponding to those found in other tRNAs have not been successful, and no compensating set of interactions can be deduced from sequence alignments (43). As discussed in more detail under “Results,” our data indicate that hmtRNAMet forms a cloverleaf structure but that D-loop and T-loop interactions cannot follow the pattern observed in canonical tRNAs.
More than 130 pathogenic mutations (see MITOMAP available online) have been observed in mitochondrial tRNA genes (48). Numerous studies have been carried out in efforts to delineate the underlying causes for the defects observed in genetic diseases arising from mutations in mitochondrial tRNA genes (43). In many cases, numerous deleterious biochemical effects are observed with the mutated tRNAs making it difficult to assess the precise cause of the pathogenicity. There are several examples in which structural perturbations are believed to arise as a result of the mutations (14, 49). One of the more complete studies recently published (50) examined mutations in tRNATyr. These studies showed that mutation of G15 and A22 in the D-loop as well as U54 in the T-loop resulted in structural alterations in the mutated tRNAs. These mutations are thought to disrupt tertiary interactions and lead to the loss of aminoacylation of these tRNAs.
The data presented here indicate that the single point mutation 8U→C in hmtRNAMet causes the loss of stable structure at physiological temperatures. The inability of the 8U→C mutated tRNA to fold properly severely impedes aminoacylation, formylation, and interaction with EF-Tumt. Consequently, the mutated tRNA cannot function effectively in either translational initiation or elongation. With a significant proportion of the heterogeneous population of mitochondria having the 8U→C mutation, the result is the pathogenic manifestation of disease.
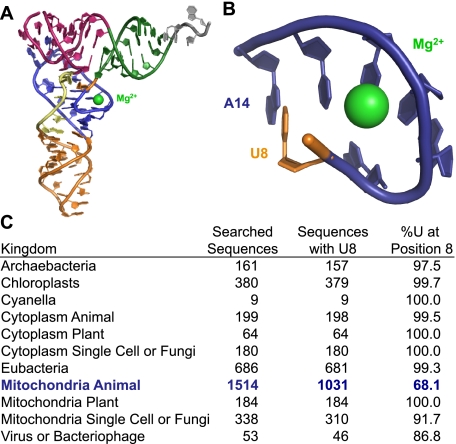
Residue U8 plays an important role in forming and stabilizing the tertiary structure of tRNA. Early work (51) indicated that Mg2+ also plays a critical role in stabilizing the structure of the D-arm and its tertiary interactions. The crystal structure of yeast tRNAPhe clearly shows that Mg2+ is tightly and site-specifically coordinated in binding pockets formed by tertiary interactions within the tRNA (52). It is believed that formation of these binding pockets precedes Mg2+ binding, and that the subsequent binding of Mg2+ then stabilizes the three-dimensional architecture of the tRNA (52). A single tightly coordinated Mg2+ is bound at the elbow of tRNAPhe. The binding site for this ion is formed by nucleotides in the D-arm and at the corner of the D-stem and acceptor stem (Fig. 10A). The phosphate of U8 contacts this Mg2+ through a bridging water molecule. U8 is also involved in a triple non-Watson-Crick pairing interaction with nucleotides A14 and A21 forming the binding pocket (Fig. 10B). Interestingly, in canonical tRNAs, U8 is highly conserved and is considered a universal nucleotide (Fig. 10C) underlying the critical role of this nucleotide in forming the site-specific Mg2+ binding pocket that leads to a correctly folded, functionally L-shaped tRNA. Our work suggests that mutation of this single nucleotide to cytosine (8U→C) in hmtRNAMet prevents formation of this Mg2+ binding pocket, resulting in a tRNA that fails to fold in the presence of Mg2+. The loss of the structure of the tRNA could occur because of a disruption in the base pairing between nucleotides U8 and either A14 or A21, which in turn would destabilize the sharp turn and the Mg2+ binding pocket. Alternatively, loss of structure could result from an inability to properly coordinate the Mg2+ ion via a water molecule in the pocket. Without the stabilizing effect of Mg2+ bound at this site, a drastic loss of structure results leading to a tRNA that does not effectively participate in protein biosynthesis. Although it is not possible to directly measure the specific binding of an individual Mg2+ ion, the data presented here are in agreement with this basic hypothesis.
FIGURE 10.
The location of the Mg2+ binding pocket in tRNAPhe involving the highly conserved U8. A, tertiary structure of yeast tRNAPhe showing the Mg2+ (green) bound near U8 (orange). The tRNA is colored by secondary structure domains. The acceptor arm is green; the T-arm is pink; the D-arm is blue; the anticodon arm is orange; the variable loop is yellow; and the ACCA end is gray. B, close-up view of the Mg2+ binding pocket colored as in A. C, conservation of U8 among the tRNAs from various organisms and organelles. The conservation of U8 in mammalian mitochondrial tRNA is shown in blue.
Of the 22 tRNAs present in human mitochondria, 16 of them have U at position 8 and 14 of them have the U8-A14-A21 combination seen in hmtRNAMet and canonical tRNAs. It is of interest to note that the most common mutation in human mitochondrial tRNAs (the A3243G mutation in the tRNALeu gene associated with MELAS) is the mutation of A14 to G. The extremely deleterious effect of this mutation is also likely to arise from an inability to form the tertiary interaction involving the U8-A14-A21 base triple and Mg2+ coordination. The absence of tertiary interactions between the D- and T-loop of the hmtRNAMet suggests a substantial role for U8 and its associated Mg2+-binding site in stabilizing the three-dimensional structure of the mitochondrial tRNA.
Acknowledgments
We thank members of the Spremulli and Agris laboratories for discussion and support. We thank K. Weeks, K. Wilkinson, and S. Mortimer for assistance with SHAPE experiments and for the 1M7.
This work was supported, in whole or in part, by National Institutes of Health Grants GM32734 (to L. L. S.) and GM23037. This work was also supported by a United Mitochondrial Disease Foundation grant (to L. L. S. and P. F. A.) and National Science Foundation Grant MCB 548602 (to P. F. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: EF-Tumt, mitochondrial elongation factor Tu; hmtRNAMet, human mitochondrial tRNAMet; MetRS, methionyl-tRNA synthetase; hmMetRS, human mitochondrial methionyl-tRNA synthetase; MTF, methionyl-tRNA transformylase; SHAPE, selective 2′-hydroxyl acylation analyzed by primer extension; PMSF, phenylmethylsulfonyl fluoride; βME, β-mercaptoethanol; 1M7, 1-methyl-7-nitroisatoic anhydride.
References
- 1.Attardi, G. (1985) Int. Rev. Cytol. 93 93-145 [DOI] [PubMed] [Google Scholar]
- 2.Anderson, S., de Brujin, M., Coulson, A., Eperon, I., Sanger, F., and Young, I. (1982) J. Mol. Biol. 156 683-717 [DOI] [PubMed] [Google Scholar]
- 3.Dirheimer, G., Keith, G., Dumas, P., and Westhof, E. (1995) in tRNA: Structure, Biosynthesis and Function (RajBhandary, U., and Soll, D., eds) pp. 93-126, American Society for Microbiology, Washington, DC
- 4.Watanabe, Y.-I., Kawai, G., Yokogawa, T., Hayashi, N., Kumazawa, Y., Ueda, T., Nishikawa, K., Hirao, I., Miura, K.-I., and Watanabe, K. (1994) Nucleic Acids Res. 22 5378-5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokogawa, T., Watanabe, Y.-I., Kumazawa, Y., Ueda, T., Hirao, I., Miura, K.-I., and Watanabe, K. (1991) Nucleic Acids Res. 19 6101-6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakita, K., Watanabe, W., Yokogawa, T., Kumazawa, Y., Nakamura, S., Ueda, T., Watanabe, K., and Nishikawa, K. (1994) Nucleic Acids Res. 22 347-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helm, M., Giege, R., and Florentz, C. (1999) Biochemistry 38 13338-13346 [DOI] [PubMed] [Google Scholar]
- 8.Helm, M., Brule, H., Friede, D., Giege, R., Putz, D., and Florentz, C. (2000) RNA (N. Y.) 6 1356-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wittenhagen, L. M., and Kelley, S. O. (2003) Trends Biochem. Sci. 28 605-611 [DOI] [PubMed] [Google Scholar]
- 10.King, M., Koga, Y., Davidson, M., and Schon, E. (1992) Mol. Cell. Biol. 12 480-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sternberg, D., Chatzoglou, E., Laforet, P., Fayet, G., Jardel, C., Blondy, P., Fardeau, M., Amselem, S., Eymard, B., and Lombes, A. (2001) Brain 124 984-994 [DOI] [PubMed] [Google Scholar]
- 12.Enriquez, J., Chomyn, A., and Attardi, G. (1995) Nat. Genet. 10 47-55 [DOI] [PubMed] [Google Scholar]
- 13.Levinger, L., Jacobs, O., and James, M. (2001) Nucleic Acids Res. 29 4334-4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao, H., and Moraes, C. T. (1997) Mol. Cell. Biol. 17 6831-6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley, S. O., Steinberg, S. V., and Schimmel, P. (2000) Nat. Struct. Biol. 7 862-865 [DOI] [PubMed] [Google Scholar]
- 16.Ling, J., Roy, H., Qin, D., Rubio, M. A., Alfonzo, J. D., Fredrick, K., and Ibba, M. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 15299-15304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cenatiempo, Y., Deville, F., Dondon, J., Grunberg-Manago, M., Sacerdot, C., Hershey, J. W., Hansen, H. F., Petersen, H. U., Clark, B. F., Kjeldgaard, M., la Cour, T. F. M., Mortensen, K. K., and Nyborg, J. (1987) Biochemistry 26 5070-5076 [DOI] [PubMed] [Google Scholar]
- 18.Kirino, Y., Yasukawa, T., Ohta, S., Akira, S., Ishihara, K., Watanabe, K., and Suzuki, T. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 15070-15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer, A. C., and Spremulli, L. L. (2004) Nucleic Acids Res. 32 5464-5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vissing, J., Salamon, M. B., Arlien-Soborg, P., Norby, S., Manta, P., DiMauro, S., and Schmalbruch, H. (1998) Neurology 50 1875-1878 [DOI] [PubMed] [Google Scholar]
- 21.Qu, J., Li, R., Zhou, X., Tong, Y., Lu, F., Qian, Y., Hu, Y., Mo, J. Q., West, C. E., and Guan, M. X. (2006) Investig. Ophthalmol. Vis. Sci. 47 475-483 [DOI] [PubMed] [Google Scholar]
- 22.Lombes, A., Bories, D., Girodon, E., Franchon, P., Ngo, M., Breton-Gorious, J., Tulliez, M., and Goossens, M. (1998) Hum. Mutat. 1 S175-S183 [DOI] [PubMed] [Google Scholar]
- 23.Spencer, A. C., Heck, A. H., Takeuchi, N., Watanabe, K., and Spremulli, L. L. (2004) Biochemistry 43 9743-9754 [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson, K. A., Merino, E. J., and Weeks, K. M. (2006) Nat. Protoc. 1 1610-1616 [DOI] [PubMed] [Google Scholar]
- 25.Jones, C., Spencer, A. C., Hsu, J., Spremulli, L. L., Martinis, S. A., DeRider, M., and Agris, P. F. (2006) J. Mol. Biol. 362 771-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bullard, J. M., Cai, Y.-C., Zhang, Y., and Spremulli, L. L. (1999) Biochim. Biophys. Acta 1446 102-114 [DOI] [PubMed] [Google Scholar]
- 27.Hunter, S. E., and Spremulli, L. L. (2004) RNA Biol. 2 95-102 [DOI] [PubMed] [Google Scholar]
- 28.Cai, Y.-C., Bullard, J. M., Thompson, N. L., and Spremulli, L. L. (2000) J. Biol. Chem. 275 20308-20314 [DOI] [PubMed] [Google Scholar]
- 29.Schwartzbach, C., Farwell, M., Liao, H.-X., and Spremulli, L. L. (1996) Methods Enzymol. 264 248-261 [DOI] [PubMed] [Google Scholar]
- 30.Das, R., Laederach, A., Pearlman, S. M., Herschlag, D., and Altman, R. B. (2005) RNA (N. Y.) 11 344-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badorrek, C. S., and Weeks, K. M. (2006) Biochemistry 45 12664-12672 [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson, K. A., Merino, E. J., and Weeks, K. M. (2005) J. Am. Chem. Soc. 127 4659-4667 [DOI] [PubMed] [Google Scholar]
- 33.Merino, E. J., Wilkinson, K. A., Coughlan, J. L., and Weeks, K. M. (2005) J. Am. Chem. Soc. 127 4223-4231 [DOI] [PubMed] [Google Scholar]
- 34.Ashraf, S. S., Guenther, R. H., Ansari, G., Malkiewicz, A., Sochacka, E., and Agris, P. F. (2000) Cell Biochem. Biophys. 33 241-252 [DOI] [PubMed] [Google Scholar]
- 35.Yarian, C. S., Basti, M. M., Cain, R. J., Ansari, G., Guenther, R. H., Sochacka, E., Czerwinska, G., Malkiewicz, A., and Agris, P. F. (1999) Nucleic Acids Res. 27 3543-3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulman, L. H., and Pelka, H. (1988) Science 242 765-768 [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi, N., Kawakami, M., Omori, A., Ueda, T., Spremulli, L. L., and Watanabe, K. (1998) J. Biol. Chem. 273 15085-15090 [DOI] [PubMed] [Google Scholar]
- 38.Louie, A., Ribeiro, S., Reid, B., and Jurnak, F. (1984) J. Biol. Chem. 259 5010-5016 [PubMed] [Google Scholar]
- 39.LaRiviere, F. J., Wolfson, A. D., and Uhlenbeck, O. C. (2001) Science 294 165-168 [DOI] [PubMed] [Google Scholar]
- 40.Sprinzl, M., and Graeser, E. (1980) Nucleic Acids Res. 8 4737-4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunter, S. E., and Spremulli, L. L. (2004) Mitochondrion (Kidlington) 4 21-29 [DOI] [PubMed] [Google Scholar]
- 42.Mortimer, S. A., and Weeks, K. M. (2007) J. Am. Chem. Soc. 129 4144-4145 [DOI] [PubMed] [Google Scholar]
- 43.Florentz, C., Sohm, B., Tryoen-Toth, P., Putz, J., and Sissler, M. (2003) Cell. Mol. Life Sci. 60 1356-1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacman, S. R., Atencio, D. P., and Moraes, C. T. (2003) Biochem. J. 374 131-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlov, M. Y., Antoun, A., Lovmar, M., and Ehrenberg, M. (2008) EMBO J. 27 1706-1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baeyens, K. J., De Bondt, H. L., and Holbrook, S. R. (1995) Nat. Struct. Biol. 2 56-62 [DOI] [PubMed] [Google Scholar]
- 47.Sharma, M. R., Koc, E. C., Datta, P. P., Booth, T. M., Spremulli, L. L., and Agrawal, R. K. (2003) Cell 115 97-108 [DOI] [PubMed] [Google Scholar]
- 48.Zifa, E., Giannouli, S., Theotokis, P., Stamatis, C., Mamuris, Z., and Stathopoulos, C. (2007) RNA Biol. 4 38-66 [DOI] [PubMed] [Google Scholar]
- 49.Wittenhagen, L. M., Roy, M. D., and Kelley, S. O. (2003) Nucleic Acids Res. 31 596-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonnefond, L., Florentz, C., Giege, R., and Rudinger-Thirion, J. (2008) RNA 14 641-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stein, A., and Crothers, D. M. (1976) Biochemistry 15 160-168 [DOI] [PubMed] [Google Scholar]
- 52.Jovine, L., Djordjevic, S., and Rhodes, D. (2000) J. Mol. Biol. 301 401-414 [DOI] [PubMed] [Google Scholar]
- 53.Sprinzl, M., Horn, C., Brown, M., Ioudovitch, A., and Steinberg, S. (1998) Nucleic Acids Res. 26 148-153 [DOI] [PMC free article] [PubMed] [Google Scholar]