Abstract
Isoprenylated proteins bear an isoprenylcysteine methyl ester at the C terminus. Although isoprenylated proteins have been implicated in meristem development and negative regulation of abscisic acid (ABA) signaling, the functional role of the terminal methyl group has not been described. Here, we show that transgenic Arabidopsis thaliana plants overproducing isoprenylcysteine methyltransferase (ICMT) exhibit ABA insensitivity in stomatal closure and seed germination assays, establishing ICMT as a negative regulator of ABA signaling. By contrast, transgenic plants overproducing isoprenylcysteine methylesterase (ICME) exhibit ABA hypersensitivity in stomatal closure and seed germination assays. Thus, ICME is a positive regulator of ABA signaling. To test the hypothesis that ABA signaling is under feedback regulation at the level of isoprenylcysteine methylation, we examined the effect of ABA on ICMT and ICME gene expression. Interestingly, ABA induces ICME gene expression, establishing a positive feedback loop whereby ABA promotes ABA responsiveness of plant cells via induction of ICME expression, which presumably results in the demethylation and inactivation of isoprenylated negative regulators of ABA signaling. These results suggest strategies for metabolic engineering of crop species for drought tolerance by targeted alterations in isoprenylcysteine methylation.
INTRODUCTION
Protein farnesylation is the process by which proteins bearing a C-terminal CaaX motif (where C = Cys, a = aliphatic, and X = Met, Ala, Gln, Ser, or Cys) are posttranslationally modified by the covalent attachment of a 15-carbon farnesyl group (Clarke, 1992; Zhang and Casey, 1996; Crowell, 2000). This modification results in the formation of a stable thioether bond between the Cys of the CaaX motif and the farnesyl moiety, with farnesyl diphosphate serving as the farnesyl donor (Figure 1). This lipidation reaction is catalyzed by protein farnesyltransferase (PFT), which is a cytosolic enzyme consisting of α- and β-subunits (Clarke, 1992; Zhang and Casey, 1996; Crowell, 2000). In a similar process, proteins bearing a C-terminal CaaX motif, where X is Leu or Ile, are modified by the covalent attachment of a 20-carbon geranylgeranyl group to the Cys of the CaaX motif. This modification is catalyzed by protein geranylgeranyltransferase type I (PGGT I), which is also a cytosolic enzyme consisting of an α-subunit identical to that of PFT and a distinct β-subunit (Clarke, 1992; Zhang and Casey, 1996; Crowell, 2000). A third enzyme, protein geranylgeranyltransferase type II (PGGT II), also called RAB geranylgeranyltransferase (RAB GGT), catalyzes the geranylgeranylation of RAB proteins bound to the RAB ESCORT PROTEIN. All three enzymes have been found in protozoans, metazoans, fungi, and plants, including pea (Pisum sativum) (Yang et al., 1993; Qian et al., 1996), tomato (Solanum lycopersicum) (Loraine et al., 1996; Yalovsky et al., 1996), and Arabidopsis thaliana (Cutler et al., 1996; Pei et al., 1998; Caldelari et al., 2001; Running et al., 2004; Johnson et al., 2005).
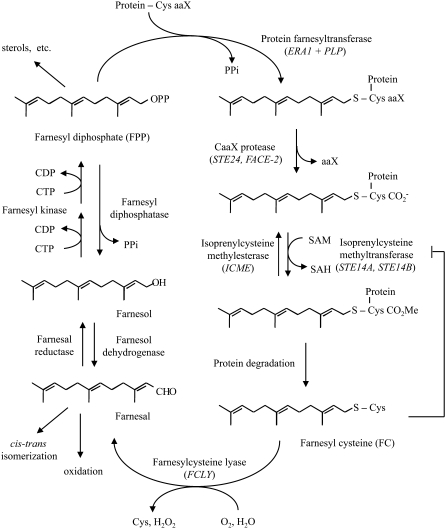
Figure 1.
Protein Farnesylation, Proteolysis, Methylation, Demethylation, Degradation, and Recycling of the Farnesyl Group.
The transfer of a farnesyl group from farnesyl diphosphate to a CaaX protein is followed by postisoprenylation processing (proteolysis and reversible methylation). Degradation of the farnesylated protein generates FC, which is oxidized by FC lyase to farnesal. Farnesal is then reduced to farnesol, which is phosphorylated to farnesyl diphosphate to complete the cycle. Gene names are indicated in italics. CDP, cytidine diphosphate; CTD, cytidine triphosphate; CHO, aldehyde; OPP, diphosphate; SAH, S-adenosyl-l-homocysteine; SAM, S-adenosyl-l-methionine.
In Arabidopsis, a single gene encodes the common α-subunit of PFT and PGGT I (PLURIPETALA [PLP]; Running et al., 2004), a second gene encodes the β-subunit of PFT (ENHANCED RESPONSE TO ABA1 [ERA1]; Cutler et al., 1996; Pei et al., 1998), and a third gene encodes the β-subunit of PGGT I (GERANYLGERANYLTRANSFERASE BETA [GGB]; Caldelari et al., 2001; Johnson et al., 2005). The ERA1 gene was so named because knockout mutations in this gene cause an enhanced response to abscisic acid (ABA) in both seed germination and stomatal closure assays. Consequently, era1 mutants exhibit increased seed dormancy and stomatal closure in response to ABA and are drought-tolerant (Cutler et al., 1996; Pei et al., 1998). These observations suggest that at least one farnesylated protein functions as a negative regulator of ABA signaling. However, to date, a farnesylated negative regulator of ABA signaling has not been identified. era1 plants also exhibit enlarged meristems and supernumerary floral organs, especially petals, and this phenotype is greatly exaggerated in plp mutants lacking the common α-subunit of PFT and PGGT I (Running et al., 1998, 2004; Bonetta et al., 2000; Yalovsky et al., 2000; Ziegelhoffer et al., 2000). The more severe developmental phenotype of plp mutants compared with that of era1 mutants suggests that PGGT I partially compensates for loss of PFT in era1 plants (Running et al., 2004). This cross-specificity was later confirmed by the observation that overexpression of the GGB gene partially suppressed the developmental phenotype of era1 plants (Johnson et al., 2005). Plants with defects in the GGB gene exhibit an enhanced response to ABA in guard cells, but not seeds, and an enhanced response to auxin with respect to lateral root formation, but not inhibition of primary root growth (Johnson et al., 2005). These observations suggest that at least one geranylgeranylated protein functions as a negative regulator of ABA signaling and at least one functions as a negative regulator of auxin signaling. Indeed, ROP2 and ROP6, which are geranylgeranylated small GTPases (Lemichez et al., 2001; Li et al., 2001), have been shown to function as negative regulators of ABA signaling. In addition, AUX2-11 (IAA4), which is a geranylgeranylated member of the AUX/IAA family, and ROP2 function as negative regulators of auxin signaling (Wyatt et al., 1993; Caldelari et al., 2001; Li et al., 2001), and Arabidopsis plants lacking either of two geranylgeranylated G-protein γ-subunits exhibit an enhanced response to auxin-induced lateral root formation (Trusov et al., 2007). Isoprenylated proteins have also been implicated in a plethora of other processes, such as calcium signal transduction (Rodríguez-Concepción et al., 1999; Xiao et al., 1999), response to heat and heavy metal stress (Zhu et al., 1993; Dykema et al., 1999; Suzuki et al., 2002), cytokinin biosynthesis (Galichet et al., 2008), and regulation of the cell division cycle (Qian et al., 1996; Hemmerlin and Bach, 1998; Hemmerlin et al., 2000). Given these multiple roles, it is surprising that, unlike other organisms, Arabidopsis plants lacking detectable PFT and PGGT I activities survive (Running et al., 2004).
Proteins that are isoprenylated by either PFT or PGGT I are further modified. First, the aaX portion of the CaaX motif is proteolytically removed by specific CaaX proteases (STE24 and FACE-2 in Arabidopsis) (Boyartchuk et al., 1997; Bracha et al., 2002; Cadiñanos et al., 2003); second, the isoprenylcysteine at the newly formed C terminus is methylated (Figure 1) (Hancock et al., 1991; Sapperstein et al., 1994; Parish and Rando, 1996; Parish et al., 1996; Crowell et al., 1998; Bergo et al., 2000; Rodríguez-Concepción et al., 2000). Two distinct isoprenylcysteine methyltransferase (ICMT) enzymes, encoded by the STE14A and STE14B (ICMT) genes, catalyze the methylation of C-terminal isoprenylcysteines in Arabidopsis (Crowell et al., 1998; Rodríguez-Concepción et al., 2000; Crowell and Kennedy, 2001; Chary et al., 2002). The products of these genes, expressed as recombinant proteins in Saccharomyces cerevisiae, have been shown to methylate N-acetyl-S-trans,trans-farnesyl-l-cysteine (AFC) and N-acetyl-S-all trans-geranylgeranyl-l-cysteine (AGGC), which are biologically relevant isoprenylcysteine methyl acceptor substrates, but not N-acetyl-S-trans-geranyl-l-cysteine (AGC), a biologically irrelevant isoprenylcysteine substrate (Chary et al., 2002). Demethylation of isoprenylcysteine methyl esters is catalyzed by isoprenylcysteine methylesterase (ICME), which is encoded by the ICME gene (Deem et al., 2006). The ICME gene encodes a 427–amino acid polypeptide with a carboxyl esterase type B Ser active site and relatedness to sterol esterases, juvenile hormone esterases, and carboxyl ester lipases. Furthermore, the ICME gene was shown, by expression of recombinant protein in S. cerevisiae, to encode an isoprenylcysteine carboxyl methylesterase with high selectivity for biologically relevant isoprenylcysteine methyl ester substrates (i.e., AFC methyl ester and AGGC methyl ester, but not AGC methyl ester) (Deem et al., 2006). Two additional genes exist in the Arabidopsis genome with sequence relatedness to ICME (At3g02410 and At1g26120), but the protein products of these genes have not been tested for ICME activity.
As described above, two geranylgeranylated proteins (ROP2 and ROP6) and at least one farnesylated protein negatively regulate ABA signaling in Arabidopsis. However, it is not clear at present where, or how, these isoprenylated proteins function in ABA signaling. The observation that ggb mutations affect a subset of ABA responses suggests that geranylgeranylated negative regulators of ABA signaling more than likely regulate events downstream of branch points in the ABA signaling pathway or regulate some, but not all, ABA signaling pathways. The stomata of ggb plants were found to exhibit an enhanced response to ABA, consistent with the known role of ROP6 in negative regulation of ABA-induced stomatal closure (Lemichez et al., 2001; Johnson et al., 2005), but the response of ggb seeds to ABA was normal, despite a report that ROP2 is involved in negative regulation of ABA signaling in seeds (Li et al., 2001). While this may seem like a contradiction, it is possible that PFT activity in ggb plants is sufficient for the isoprenylation and function of certain isoprenylated proteins, such as ROP2, but not for others. Indeed, numerous reports exist of isoprenylated proteins that are substrates of both PFT and PGGT I and others that are strictly substrates of PFT or PGGT I (Trueblood et al., 1993; Armstrong et al., 1995). Given this heterogeneity in the specificity of PFT and PGGT I for certain CaaX proteins and the existence of at least two geranylgeranylated and one farnesylated protein involved in negative regulation of ABA signaling, deconvoluting the complex roles of isoprenylcysteine methylation and demethylation in negative regulation of ABA signaling poses a significant challenge. Nevertheless, to begin to address this problem, and to clearly establish whether ICMT and ICME expression influence ABA signaling, we analyzed Arabidopsis plants that overexpress either ICMT or ICME. These analyses demonstrated conclusively that ICMT is a negative regulator of ABA signaling and ICME is a positive regulator of ABA signaling. Furthermore, ABA was found to induce the expression of the ICME gene, thereby increasing the ABA responsiveness of Arabidopsis plants.
RESULTS
ICMT Is a Negative Regulator of ABA Signaling
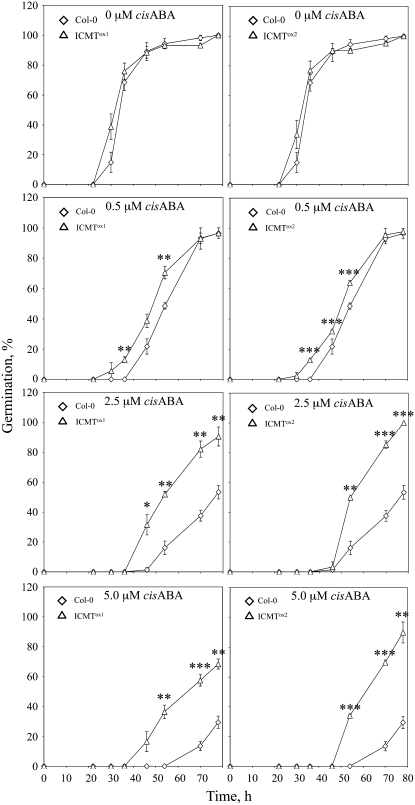
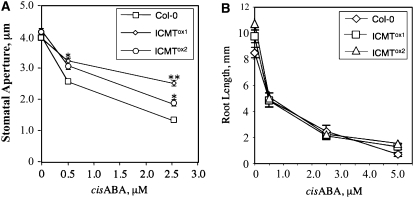
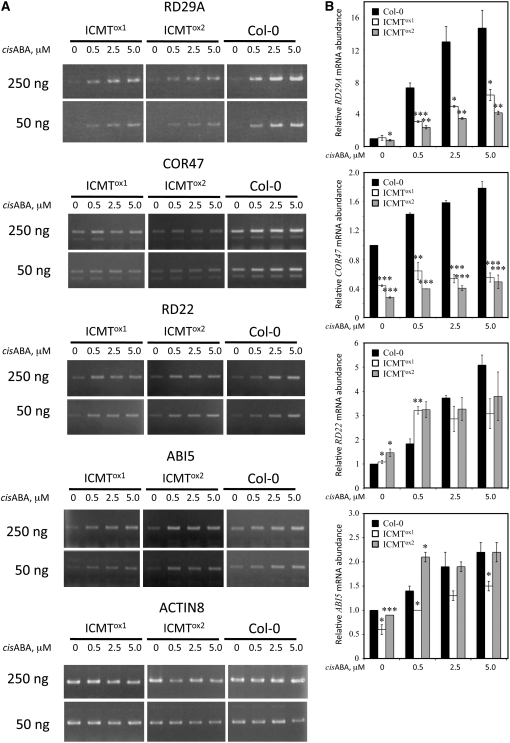
Competitive inhibitors of ICMT, including AFC, cause an enhanced response to ABA in seed germination and stomatal closure assays compared with solvent controls (Chary et al., 2002). These results are consistent with the hypothesis that ICMT is a negative regulator of ABA signaling. However, the effects of farnesylcysteine (FC) analog inhibitors of ICMT could be nonspecific. To definitively establish whether or not ICMT is a negative regulator of ABA signaling, we generated transgenic Arabidopsis plants that overexpress the STE14B (ICMT) gene using a cauliflower mosaic virus (CaMV) 35S promoter to drive transcription of the transgene. STE14B was chosen for this purpose because it encodes an ICMT with lower apparent Km values for biologically relevant isoprenylcysteine substrates and dramatically higher specific activities than the STE14A-encoded enzyme (Chary et al., 2002). As shown in Figure 2, two independent ICMT-overproducing lines, called ICMTox1 and ICMTox2, contained significantly higher levels of STE14B mRNA and ICMT activity than control plants. As expected, the ICMT activity in these plants efficiently methylated AFC and AGGC, but not AGC. Interestingly, ICMTox1 and ICMTox2 plants exhibited a significant reduction in ABA inhibition of seed germination (Figure 3). Even in the absence of exogenous ABA, ICMTox1 and ICMTox2 seeds germinated more rapidly than control seeds, suggesting insensitivity to endogenous ABA. The ABA-insensitive phenotype of ICMTox1 and ICMTox2 seeds was apparent at all concentrations of ABA tested, up to and including 5.0 μM. The ICMTox1 and ICMTox2 lines also exhibited a significant decrease in ABA-induced stomatal closure compared with control plants (Figure 4A). No significant differences were observed in the absence of exogenous ABA, but statistically significant differences were observed in the presence of exogenous ABA. These results demonstrate that ICMT overexpression results in an ABA-insensitive phenotype in several ABA response assays (i.e., inhibition of seed germination and induction of stomatal closure). However, given that ggb mutants are affected in a subset of ABA responses (Johnson et al., 2005), we considered the possibility that ICMTox1 and ICMTox2 plants might also be affected in a subset of ABA responses. To test this hypothesis, ICMTox1 and ICMTox2 plants were tested for ABA inhibition of root growth and induction of ABA-induced genes. As shown in Figure 4B, ABA inhibition of root growth in ICMTox1 and ICMTox2 plants was identical to that in control plants. However, as shown in Figure 5, ICMTox1 and ICMTox2 seedlings exhibited reduced ABA induction of RD29A and COR47 gene expression (Yamaguchi-Shinozaki and Shinozaki, 1993; Baker et al., 1994). The ABA induction of ABI5, RD22, and COR15A, on the other hand, was not greatly altered in ICMTox plants (Figure 5; data not shown for COR15A). Thus, like ggb plants, ICMTox1 and ICMTox2 plants are affected in a subset of ABA responses, suggesting that isoprenylated negative regulators of ABA signaling function downstream of at least one branch point in the ABA signaling pathway or participate in some, but not all, ABA signaling pathways. Together, these observations establish ICMT as a negative regulator of ABA signaling and suggest that isoprenylated negative regulators of ABA signaling are incompletely methylated in planta (i.e., overproduction of ICMT would have no effect on ABA signaling if isoprenylated negative regulators of ABA signaling were completely methylated in untransformed plants).
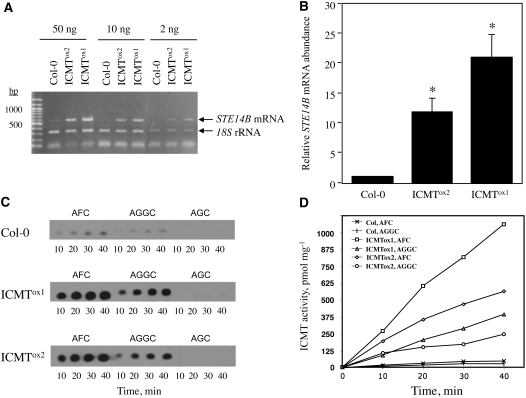
Figure 2.
ICMTox1 and ICMTox2 Plants Exhibit Increased Levels of STE14B mRNA and ICMT Activity.
(A) RT-PCR of STE14B mRNA from wild-type (Col-0), ICMTox1, and ICMTox2 lines is shown. The values at top indicate the amounts of input RNA added to the RT-PCR. RT-PCR of 18S rRNA served as an internal loading control.
(B) The relative mRNA abundances for the lines shown in (A) were determined by image analysis using Image J software (National Institutes of Health). The mRNA abundance of Col-0 is set to 1 and serves as the point of reference for ICMTox1 and ICMTox2. Error bars represent se of three replicates. Asterisks indicate significant differences from the control, as judged by Student's t test (* P < 0.05).
(C) Quantitative assay of ICMT activity in membranes of Col-0, ICMTox1, and ICMTox2 lines. AFC and AGGC are biologically relevant isoprenylcysteine methyl acceptor substrates. AGC is a biologically irrelevant negative control substrate. The TLC fluorogram shows product formation as a function of time.
(D) Product formation was quantified by liquid scintillation of the excised TLC spots shown in (C). The results shown are representative of two independent experiments.
Figure 3.
ICMT Overexpression Lines of Arabidopsis Exhibit Reduced ABA Inhibition of Seed Germination Compared with Control Col-0 Plants.
Seed germination was scored for Col-0, ICMTox1, and ICMTox2 lines as a function of time in the presence of 0, 0.5, 2.5, or 5.0 μM cis-ABA (each data point represents three independent trials; 40 < n < 83). The se is indicated for all data points. Asterisks represent significant differences from the Col-0 control of * P < 0.05, ** P < 0.01, and *** P < 0.001, as determined by Student's t test.
Figure 4.
ICMT Overexpression Lines of Arabidopsis Exhibit Reduced ABA Induction of Stomatal Closure Compared with Control Col-0 Plants.
(A) Stomatal apertures were measured for Col-0, ICMTox1, and ICMTox2 lines after incubation of excised leaves for 2 h in the presence of 0, 0.5, or 2.5 μM cis-ABA. Stomatal apertures were measured from photomicrographs of epidermal peels and reported as averages of 50 stomata per data point.
(B) Root growth was measured after transferring seedlings (21 < n < 51) to plates containing various concentrations of ABA. The seedlings were placed 90° from vertical, and growth from the bend to the tip was recorded after 5 d.
The se is shown for all data points. Asterisks represent significant differences from the control of * P < 0.05 and ** P < 0.001, as determined by Student's t test.
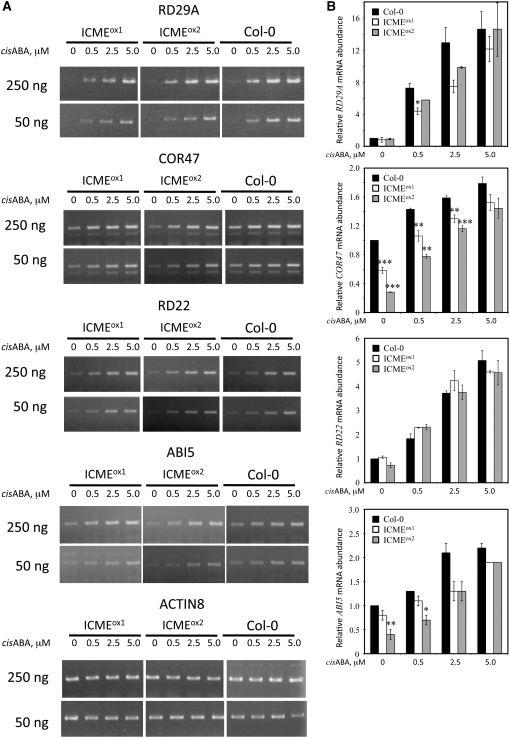
Figure 5.
ICMT Overexpression Lines of Arabidopsis Exhibit Reduced ABA Induction of RD29A and COR47 Gene Expression Compared with Control Col-0 Plants.
Total RNA from Col-0, ICMTox1, and ICMTox2 seedlings was analyzed by RT-PCR for the presence of RD29A, COR47, RD22, ABI5, and Actin8 mRNA (as a loading control). The bands shown were photographed from the same gel but were not side by side. The se of two replicates is indicated for all data points. Asterisks represent significant differences from the corresponding Col-0 control of * P < 0.05, ** P < 0.01, and *** P < 0.001, as determined by Student's t test.
ICME Is a Positive Regulator of ABA Signaling
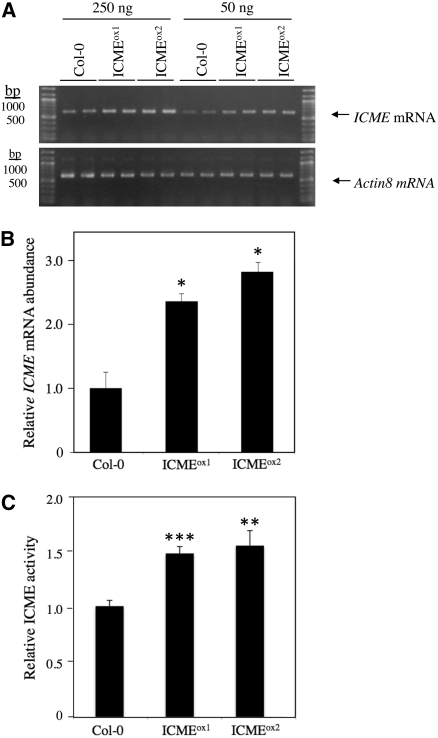
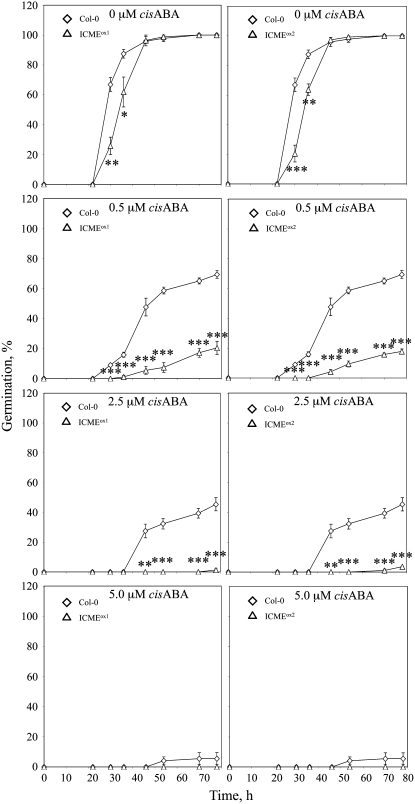
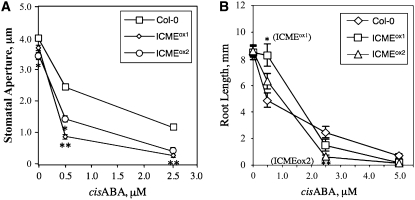
If ICMT is a negative regulator of ABA signaling, it follows that ICME is a positive regulator of ABA signaling. To test this hypothesis, we generated Arabidopsis plants that overexpress the ICME gene, again using a CaMV 35S promoter to drive transgene expression. As shown in Figure 6, two independent ICME-overproducing lines, called ICMEox1 and ICMEox2, contained significantly higher ICME mRNA levels and ICME activity than control plants. In all of the independent transgenic lines analyzed, none exhibited higher ICME expression than ICMEox1 and ICMEox2. This observation suggests that strong overexpression of the ICME gene is deleterious to the growth and development of T1 transformants. Interestingly, the increase in ICME activity in these lines (∼50%) was less than the increase in ICME mRNA abundance (∼150%), suggesting the possibility of translational or posttranslational control of ICME expression. As expected, ICMEox1 and ICMEox2 lines exhibited a significantly enhanced response to ABA inhibition of seed germination (Figure 7). Even in the absence of exogenous ABA, ICMEox1 and ICMEox2 seeds germinated more slowly than control seeds, suggesting an enhanced response to endogenous ABA. The ABA-hypersensitive phenotype of ICMEox1 and ICMEox2 seeds was apparent at all concentrations of ABA tested, up to and including 5.0 μM. The ICMEox1 and ICMEox2 lines also exhibited a significant increase in ABA-induced stomatal closure compared with control plants (Figure 8A). In the absence of exogenous ABA, the stomatal apertures of ICMEox1 and ICMEox2 plants were smaller than those of control plants, suggesting hypersensitivity to endogenous ABA. In the presence of exogenous ABA, a dramatic overresponse was observed in the ICMEox lines, resulting in stomata that were almost completely closed at 2.5 μM ABA. These results demonstrate that ICME overexpression results in an ABA-hypersensitive phenotype in multiple ABA response assays (i.e., inhibition of seed germination and induction of stomatal closure). However, as with the ICMTox1 and ICMTox2 lines, ICMEox1 and ICMEox2 did not show a clear change in ABA inhibition of root growth (ICMEox1 and ICMEox2 exhibited statistically significant differences in ABA inhibition of root growth compared with ecotype Columbia [Col-0] at 0.5 and 2.5 μM ABA, respectively, but not at other ABA concentrations; Figure 8B). As shown in Figure 9, the expression of ABA-induced genes was not higher in ICMEox1 and ICMEox2 seedlings than in Col-0 seedlings. Given that the ICMTox1 and ICMTox2 lines exhibited reduced ABA induction of RD29A and COR47 gene expression, it was somewhat surprising that expression of these genes was not higher in the presence of ABA in ICMEox1 and ICMEox2. This result either means that the modest overexpression of ICME in ICMEox1 and ICMEox2 is insufficient to affect this particular ABA response or other factors limit the ABA induction of RD29A and COR47 gene expression. Thus, like the ICMTox1 and ICMTox2 lines, ICMEox1 and ICMEox2 are affected in a subset of ABA responses. Taken together, these observations support the hypothesis that ICME is a positive regulator of ABA signaling, at least with respect to ABA inhibition of seed germination and ABA-induced stomatal closure.
Figure 6.
ICMEox1 and ICMEox2 Plants Exhibit Increased Levels of ICME mRNA and ICME Activity.
(A) RT-PCR of ICME mRNA from wild-type (Col-0), ICMEox1, and ICMEox2 lines is shown. The values at top indicate the amounts of input RNA added to the RT-PCR. RT-PCR of Actin8 mRNA served as a loading control.
(B) The relative mRNA abundances for the lines shown in (A) were determined by image analysis using Image J software. The mRNA abundance of Col-0 is set to 1 and serves as the point of reference for ICMEox1 and ICMEox2.
(C) Quantitative assay of ICME activity in membranes of Col-0, ICMEox1, and ICMEox2 lines. The activity of Col-0 is set to 1 and serves as the point of reference for ICMEox1 and ICMEox2.
Error bars represent se of five replicates. Asterisks indicate significant differences from the control of * P < 0.05, ** P < 0.01, and *** P < 0.001, as judged by Student's t test.
Figure 7.
ICME Overexpression Lines of Arabidopsis Exhibit Enhanced ABA Inhibition of Seed Germination Compared with Control Col-0 Plants.
Seed germination was scored for Col-0, ICMEox1, and ICMEox2 lines as a function of time in the presence of 0, 0.5, 2.5, or 5.0 μM cis-ABA (each data point represents four independent trials; 91 < n < 138). The se is indicated for all data points. Asterisks represent significant differences from the Col-0 control of * P < 0.05, ** P < 0.01, and *** P < 0.001, as determined by Student's t test.
Figure 8.
ICME Overexpression Lines of Arabidopsis Exhibit Enhanced ABA Induction of Stomatal Closure Compared with Control Col-0 Plants.
(A) Stomatal apertures were measured for Col-0, ICMEox1, and ICMEox2 lines after incubation of excised leaves for 2 h in the presence of 0, 0.5, or 2.5 μM cis-ABA. Stomatal apertures were measured from photomicrographs of epidermal peels and reported as averages of 50 stomata per data point.
(B) Root growth was measured after transferring seedlings (22 < n < 32) to plates containing various concentrations of ABA. The seedlings were placed 90° from vertical, and growth from the bend to the tip was recorded after 5 d.
The se is shown for all data points. Asterisks represent significant differences from the control of * P < 0.01 and ** P < 0.001, as determined by Student's t test.
Figure 9.
ICME Overexpression Lines of Arabidopsis Do Not Exhibit Dramatically Altered ABA Induction of Gene Expression Compared with Control Col-0 Plants.
Total RNA from Col-0, ICMEox1, and ICMEox2 seedlings was analyzed by RT-PCR for the presence of RD29A, COR47, RD22, ABI5, and Actin8 mRNA (as a loading control). The bands shown were photographed from the same gel but were not side by side. The se of two replicates is indicated for all data points. Asterisks represent significant differences from the corresponding Col-0 control of * P < 0.05, ** P < 0.01, and *** P < 0.001, as determined by Student's t test.
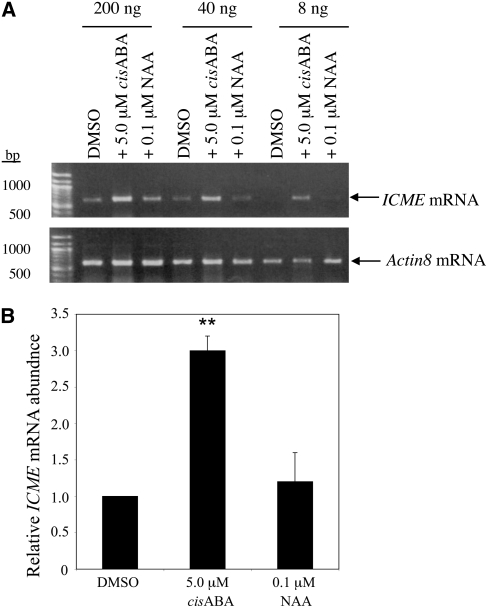
ABA Induces ICME Gene Expression
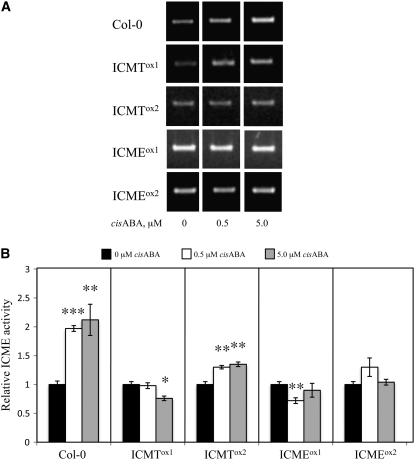
The results described above firmly establish that ICMT and ICME regulate ABA responsiveness in Arabidopsis. To determine whether ABA affects the expression of the ICMT and/or ICME genes, seedlings were grown for 4 d on half-strength Murashige and Skoog (MS) plates containing 1% sucrose and 0.8% agar and then transferred to plates containing 0.5 or 5.0 μM cis-ABA, 0.1 μM naphthalene acetic acid (NAA), or an equivalent volume of DMSO for 24 h. These concentrations were chosen because they induce responses in standard physiological assays (e.g., inhibition of seed germination and induction of stomatal closure for ABA and initiation of lateral root formation for NAA; Johnson et al., 2005). Interestingly, 5.0 μM ABA caused an approximately threefold accumulation of ICME mRNA (Figure 10) and a twofold increase in ICME activity in wild-type Col-0 seedlings (Figure 11). As observed in Figure 6, the increase in ICME mRNA abundance was greater than the increase in ICME activity, suggesting translational or posttranslational control of ICME gene expression. These results are consistent with the hypothesis that ABA promotes ABA responsiveness of plant cells via induction of ICME gene expression and demethylation (i.e., inactivation) of isoprenylated negative regulators of ABA signaling. Having established that ABA induces ICME gene expression in wild-type seedlings, we investigated whether ABA induction of ICME expression was affected in ICMTox and ICMEox seedlings. As shown in Figure 11, ABA induction of ICME expression was decreased in ICMTox1 and ICMTox2 seedlings, confirming the ABA-insensitive phenotype of these plants. Not surprisingly, ABA induction of ICME expression was also decreased in ICMEox seedlings, presumably because of the elevated ICME expression in these plants. By contrast, no regulation of STE14A or STE14B expression by ABA or NAA was observed (data not shown). These results are supported by publicly available microarray data, which reveal a fourfold to fivefold increase in ICME mRNA abundance following ABA treatment (microarray data visualized using the AtGenExpress Visualization Tool at http://jsp.weigelworld.org/expviz/expviz.jsp). Microarray data also suggest roles for ICME in leaf senescence, seed maturation, abiotic stress responses (i.e., osmotic, salt, UV-B light, and heat stress), and defense responses to bacterial and fungal pathogens (see Supplemental Data Set 1 online). Consistent with our findings, microarray data do not support the hypothesis that expression of STE14A or STE14B is ABA- or auxin-responsive.
Figure 10.
ABA Induces ICME Gene Expression.
(A) RT-PCR of ICME mRNA from Arabidopsis seedlings (Col-0) grown for 4 d and then exposed to 5.0 μM cis-ABA, 0.1 μM NAA, or an equivalent volume of DMSO for 24 h. The values at top indicate the amounts of input RNA added to the RT-PCR. RT-PCR of Actin8 mRNA served as a loading control.
(B) The relative mRNA abundances for the treatments shown in (A) were determined by image analysis using Image J software. Error bars represent se. Asterisks indicate a significant difference from the control, as judged by Student's t test (** P < 0.01). The results shown are representative of three independent experiments.
Figure 11.
Analysis of ABA Induction of ICME mRNA Accumulation and ICME Activity in Col-0, ICMTox, and ICMEox Seedlings Treated with 0.5 or 5.0 μM cis-ABA or an Equivalent Volume of DMSO.
(A) RT-PCR of ICME mRNA from Col-0, ICMTox, and ICMEox lines treated with 0, 0.5, or 5.0 μM cis-ABA is shown.
(B) Relative ICME activity in membranes of Col-0, ICMTox, and ICMEox lines. The ICME activity of the solvent control for each line is set to 1 and serves as the point of reference for the corresponding 0.5 and 5.0 μM ABA samples. Error bars represent se. Asterisks indicate significant differences from the 0 μM ABA control of * P < 0.05, ** P < 0.01, and *** P < 0.001, as judged by Student's t test. The results shown are representative of three independent experiments.
DISCUSSION
In this article, ICMT overexpression is shown to cause an ABA-insensitive phenotype in seed germination and stomatal closure assays and reduced ABA induction of RD29A, COR47, and ICME gene expression (Figures 3, 4, 5, and 11). However, ICMT overexpression had no effect on ABA inhibition of root elongation. These observations are consistent with the hypothesis that ICMT is a negative regulator of ABA signaling and, as suggested by the phenotype of ggb mutants, either functions downstream of at least one branch point in the ABA signaling pathway or functions in a subset of ABA signaling pathways (Bonetta and McCourt, 1998). These data also suggest that isoprenylated negative regulators of ABA signaling are not completely methylated in wild-type Arabidopsis plants, because overexpression of ICMT would not be expected to have an effect if they were. Consistent with this suggestion, immunoblots using a pan-ROP antibody demonstrated that ICMT overexpression increased the membrane association of ROP proteins, although the antiserum used did not allow for the identification of which ROP proteins were affected (see Supplemental Figure 1 online). Of the 11 known Arabidopsis ROP proteins, 8 are known or predicted to be isoprenylated, whereas 3 (i.e., the type II ROPs) are membrane-associated via an isoprenylation-independent mechanism (Lavy et al., 2002; Lavy and Yalovsky, 2006). ROP10, which has been implicated in negative regulation of ABA signaling (Zheng et al., 2002; Xin et al., 2005), is a type II ROP. However, ROP6, which has a CSIL motif at its C terminus with an upstream polybasic domain (Lemichez et al., 2001), and ROP2, which possesses a C-terminal CAFL motif and can be geranylgeranylated in vitro (see Supplemental Figure 2 online), are also negative regulators of ABA signaling (Li et al., 2001). Thus, more than one isoprenylated negative regulator of ABA signaling has been identified. These ROPs, however, are geranylgeranylated and thus do not explain the enhanced response to ABA of era1 mutants, which lack the β-subunit of PFT. Farnesylated negative regulators of ABA signaling have not, to date, been identified.
The data in Figures 7 and 8 demonstrate that overexpression of the ICME gene causes an enhanced response to ABA in seed germination and stomatal closure assays. It is interesting that ICME overexpression caused a slight, but significant, decrease in stomatal apertures even in the absence of exogenous ABA (Figure 8), suggesting an enhanced response to endogenous ABA. That ABA-induced gene expression in ICMEox1 and ICMEox2 did not exceed that in Col-0 attests to the modest overexpression of ICME in these lines or suggests the presence of other factors that limit ABA-induced gene expression. However, a close examination of the data in Figure 9 reveals significant differences in ABA-induced gene expression between Col-0 and ICMEox lines for COR47 and ABI5. Both genes were expressed at significantly lower levels in the absence of exogenous ABA and were induced to a greater extent in the presence of exogenous ABA in ICMEox1 and ICMEox2 seedlings. Consequently, at 5.0 μM ABA, no significant differences in COR47 or ABI5 gene expression were observed between Col-0 and ICMEox lines. These data can be interpreted, albeit with caution, to mean that increased ABA induction of COR47 and ABI5 gene expression was observed in ICMEox1 and ICMEox2. Overall, these data demonstrate that ICME is a positive regulator of ABA signaling and, like ICMT, affects some, but not all, ABA responses. In total, the data in Figures 3, 4, 5, 7, and 8 support the hypothesis that isoprenylcysteine methylation is required for the function of isoprenylated negative regulators of ABA signaling and isoprenylcysteine demethylation attenuates the function of these proteins.
Until recently, no T-DNA insertions in either STE14A or STE14B were available. However, a T-DNA insertion three nucleotides upstream of the TGA codon of STE14A was recently reported (Bracha-Drori et al., 2008). This mutant exhibited no detectable STE14A mRNA and no observable phenotype, suggesting that its function is either dispensable or redundant with that of STE14B. Transgenic plants containing an STE14B RNA interference (RNAi) construct did not exhibit a significant decrease in STE14B mRNA abundance, but a subtle phenotype was observed (Bracha-Drori et al., 2008), suggesting that either STE14B silencing occurred at the level of translation or another gene was affected by the RNAi construct (no data were presented to demonstrate reduced ICMT protein or activity). These plants exhibited altered phylotaxis, multiple axillary buds, and fasciated stems, but they did not exhibit an ABA-hypersensitive phenotype (Bracha-Drori et al., 2008). Given the ABA hypersensitivity of era1, plp, ggb, and ICMEox plants, this observation is surprising, but it is impossible to interpret without evidence of reduced ICMT activity in STE14B RNAi plants.
The SALK collection includes a mutant with a T-DNA insertion in the ICME gene, but the insertion does not cause a knockout phenotype and, therefore, was not analyzed in detail or included in the body of this report. Nevertheless, the SALK_010304 line, which has a T-DNA insertion in intron 1 of the ICME gene, exhibited decreased ICME mRNA abundance and an ABA-insensitive phenotype in seed germination assays (see Supplemental Figure 3 online). These results support the conclusion that ICME is a positive regulator of ABA signaling.
With known and unknown isoprenylated proteins affecting ABA signaling, it is not possible at this time to link isoprenylcysteine methylation and demethylation of a specific protein or set of proteins to the changes in ABA responsiveness observed in ICMTox and ICMEox plants. It is unlikely that the effects of ICMT or ICME overexpression are due to changes in the methylation state of free isoprenylcysteines (i.e., in lieu of isoprenylated proteins), because free isoprenylcysteines are present in wild-type Arabidopsis plants at virtually undetectable levels (data not shown). However, plants with T-DNA insertions in the FCLY gene, which encodes a specific FC lyase (Figure 1), accumulate FC and exhibit an enhanced response to ABA in seed germination and stomatal closure assays (Crowell et al., 2007). The ABA hypersensitivity of fcly plants, which establishes the FCLY gene as another negative regulator of ABA signaling, is presumably the result of FC inhibition of ICMT (Figure 1) (Chary et al., 2002). Even if the effects described here are the result of changes in the methylation state of free isoprenylcysteines, the conclusions that ICMT is a negative regulator, and ICME a positive regulator, of ABA signaling are nonetheless valid.
Surprisingly, like ggb plants, ICMTox and ICMEox plants exhibited no obvious developmental phenotype. Because era1-2 and plp plants exhibit enlarged meristems and supernumerary floral organs, this phenotype might be expected in ICMEox plants. That no such phenotype was observed suggests that the isoprenylated protein(s) involved in meristem development is (1) fully methylated, even in the presence of ICME overproduction (i.e., because of high ICMT activity in meristems), or (2) unaffected by isoprenylcysteine methylation/demethylation. Alternatively, it is also possible that the modest overproduction of ICME in ICMEox plants is insufficient to affect the function of this protein(s). It is noteworthy that clv3-7 mutants, which have enlarged meristems and increased floral organ numbers, exhibit decreased STE14A mRNA accumulation (see Supplemental Data Set 1 online), suggesting a potential role for the STE14A-encoded ICMT in meristem development.
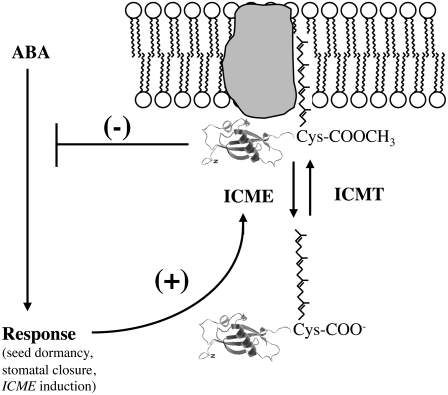
The data shown in Figures 10 and 11 demonstrate that ABA causes the accumulation of ICME mRNA, whereas NAA does not significantly affect ICME mRNA abundance. This result is supported by publicly available microarray data, which reveal a fourfold to fivefold increase in ICME mRNA abundance following ABA treatment (microarray data visualized using the AtGenExpress Visualization Tool at http://jsp.weigelworld.org/expviz/expviz.jsp). By contrast, no change in STE14A or STE14B mRNA abundance in the presence of exogenous ABA or NAA was observed (data not shown). Whether ABA regulation of ICME expression is due to increased ICME transcription, decreased ICME mRNA degradation, or both is not known, but the observation that ICME is upregulated in response to ABA supports the hypothesis that ABA promotes ABA responsiveness of plant cells via increased ICME expression and subsequent demethylation (i.e., loss of function) of isoprenylated negative regulators of ABA signaling. This positive feedback loop, shown in Figure 12, ensures that ABA signaling is not transient, a property that makes biological sense given the role of ABA in long-term responses, such as seed dormancy and stomatal closure under prolonged drought conditions. This finding is also consistent with previous reports of ABA-induced positive feedback regulation of ABA biosynthesis (Xiong et al., 2002; Barrero et al., 2006). Other microarray data indicate that ICME expression is initially downregulated by osmotic stress, salt stress, UV-B light stress, and heat stress but is strongly upregulated after several hours by these abiotic stresses (see Supplemental Data Set 1 online). This is particularly interesting in view of the role of ABA in abiotic stress responses. Future experiments will address the mechanism of regulation of ICME gene expression by ABA and environmental stimuli. Ultimately, the identification of all factors that regulate ICME and/or ICMT expression will be necessary for a complete understanding of how the balance between isoprenylcysteine methylation and demethylation affects ABA signaling and other physiological processes in plants.
Figure 12.
Model for Positive Feedback Regulation of ABA Signaling via Induction of ICME Expression and Demethylation (i.e., Inactivation) of Isoprenylated Negative Regulators of ABA Signaling.
ABA promotes seed dormancy and stomatal closure but also increases the expression of the ICME gene, a positive regulator of ABA signaling. To date, the only isoprenylated negative regulators of ABA signaling to be identified are ROP2 and ROP6 (Lemichez et al., 2001; Li et al., 2001). Consequently, the isoprenylated protein shown is modified by a geranylgeranyl moiety and represents either ROP2 or ROP6. However, at least one unidentified farnesylated negative regulator of ABA signaling also exists in Arabidopsis. A hypothetical integral membrane isoprenylprotein receptor is shown.
Drought is a major cause of variability in crop yields from year to year. The data shown here and elsewhere (Crowell et al., 2007) demonstrate that ICMT and FCLY are negative regulators, and ICME is a positive regulator, of ABA signaling. Thus, underexpression of ICMT or FCLY, or overexpression of ICME, would be expected to cause an enhanced response to ABA in transgenic plants. Using these strategies, singly or in combination, and controlling transgene expression with a drought-inducible, guard cell–specific promoter might result in plants that are resistant to drought, and possibly salt stress, without deleterious effects on germination or growth. This work has the potential to stabilize crop production, even in years of severe drought.
METHODS
Plant Growth Conditions
Arabidopsis thaliana seeds were surface-sterilized according to the following procedure: 95% ethanol for 5 min, 20 to 50% bleach for 5 to 10 min, and five washes in sterile deionized water. Seeds were then suspended in sterile 0.1% agar, imbibed on half-strength MS plates containing 1% sucrose and 0.8% agar, treated for 3 d at 4°C, and germinated in a vertical orientation at 22°C under long-day conditions (18 h of white light at 100 μmol·m−2·s−1 followed by 6 h of dark). Seedlings were harvested after 4 d of growth for extraction of RNA or microsomal membranes or transferred to ProMix and grown under the same conditions for propagation or analysis of stomatal function. Plants were irrigated and fertilized from below with a standard mixture of macronutrients and micronutrients.
Plant Transformation
To generate the ICMTox1 and ICMTox2 lines, the Col-0 ecotype of Arabidopsis was transformed with a pCL108 construct harboring the CaMV 35S promoter and the STE14B coding sequence. pCL108 was derived from plasmid YY217 (Yamamoto et al., 2001) by replacing the sm-RSGFP fragment of YY217 with an XbaI–KpnI fragment containing the STE14B coding sequence. To generate the ICMEox1 and ICMEox2 lines, the ICME coding sequence was amplified by RT-PCR, confirmed by DNA sequence analysis, and inserted into the EcoRI and HindIII sites of the vector pH2GW7 (Karimi et al., 2002), downstream of the CaMV 35S promoter. Agrobacterium tumefaciens strain GV3101 (pMP90) (Koncz and Schell, 1986) and the floral dip method (Clough and Bent, 1998) were used for plant transformation. T1 transformants were selected on half-strength MS plates containing 1% sucrose, 0.8% agar, and 200 μg/mL gentamycin (ICMTox1 and ICMTox2) or 50 μg/mL hygromycin (ICMEox1 and ICMEox2). Transgenic plant lines were propagated to the T3 generation and scored for homozygosity by PCR and nonsegregation of the antibiotic resistance marker.
RT-PCR Analysis of ICMTox and ICMEox Lines and ABA Induction of ICME
Total RNA was isolated from seedlings of ICMTox1, ICMTox2, ICMEox1, ICMEox2, and wild-type Col-0 plants using TRIzol reagent according to the manufacturer's instructions (Invitrogen/Life Technologies). RT-PCR was then performed to analyze ICMT or ICME transcript levels using different amounts of input RNA (250 to 2 ng), 5 pmol of forward primer, 5 pmol of reverse primer, 18S rRNA competimer/primer pairs (Ambion) or Actin8 primers as a loading control, and the Platinum Quantitative RT-PCR Thermoscript One-Step system (Invitrogen/Life Technologies) in a total reaction volume of 25 μL. The STE14B forward and reverse primers were ICMT-5′ (5′-GTGACACCGGGTTCAGACAGTTAA-3′) and ICMT-3′ (5′-CAGTTTACAAAAGGAACACCAGAA-3′), and the ICME forward and reverse primers were ICME-5′ (5′-GGTAGGCTATCGATGGATGACAA-3′) and ICME-3′ (5′-AGGACTCTTCTCCTTCCATTATG-3′). RT-PCR conditions included a 20-min reverse transcription step at 50°C, followed by a 5-min presoak at 95°C and 25 to 35 cycles of the following PCR program: 95°C for 30 s; 53°C for 30 s; and 72°C for 90 s. A postsoak was performed at 72°C for 7 min to ensure complete product synthesis. RT-PCR products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining.
ICMT Assays
For ICMT assays, Arabidopsis extracts were prepared by grinding 4-d-old seedlings with a mortar and pestle at 4°C in a homogenization buffer containing 50 mM HEPES (pH 7.4), 500 mM mannitol, 5 mM EDTA, 5 mM DTT, and Complete protease inhibitors (Roche Diagnostics). Extracts were filtered through four layers of cheesecloth and centrifuged at 8000g for 10 min at 4°C. Membranes were then sedimented from Arabidopsis extracts at 100,000g for 60 min at 4°C and resuspended in a resuspension buffer containing 2.5 mM HEPES (pH 7.0), 250 mM mannitol, and 1 mM DTT. Membrane aliquots were stored at −80°C in the presence of 15% glycerol. ICMT assays were performed essentially as described (Hrycyna and Clarke, 1990; Crowell et al., 1998) in the presence of 60 μg of membrane protein, S-adenosyl-l-[3H-methyl]methionine (GE Healthcare Life Sciences) as a methyl donor (24 μM, 2.5 Ci/mmol, 60 μCi/mL), 200 μM AFC or AGGC as a methyl acceptor (200 μM AGC was used as a negative control), and 100 mM HEPES (pH 7.0), in a total volume of 50 μL. Stock solutions of AFC, AGGC, and AGC (BioMol) were prepared in DMSO at a concentration of 10 mM. Reactions were incubated at 30°C for 1 h, terminated by the addition of 50 μL of 90% (v/v) methylene chloride, 9.75% (v/v) methanol, and 0.25% (v/v) acetic acid, mixed vigorously, and centrifuged for 1 min in a microcentrifuge. A 10-μL portion of the organic phase was analyzed by thin layer chromatography (TLC) using a Polygram Sil G plastic-backed silica gel plate (Aldrich) and 90% methylene chloride, 9.75% methanol, and 0.25% acetic acid as a mobile phase. Plates were sprayed with En3hance fluorographic reagent (NEN/DuPont), and fluorography was performed for 1 to 3 d at −80°C with X-Omat AR film (Eastman Kodak). ICMT activity was quantified by liquid scintillation of excised TLC spots.
ICME Assays
To prepare isoprenylcysteine methyl ester substrates, recombinant Arabidopsis ICMT was expressed in Saccharomyces cerevisiae strain SM1188 (MATa ste14∷TRP1, leu2, ura3, trp1, his4, can1) and used to generate the methyl ester of AFC. STE14B expression was induced by shifting log-phase cells grown at 30°C in CSM-ura medium containing 2% glucose into CSM-ura medium containing 2% galactose. After 14 h at 30°C in galactose medium, the cells were sedimented and lysed in lysis buffer containing 100 mM Tris-HCl (pH 7.5), 1 mM DTT, 20% (v/v) glycerol, and Complete protease inhibitors by vortexing in the presence of acid-washed glass beads. Yeast extracts were centrifuged at 8000g for 10 min at 4°C, and membranes were prepared from the supernatants by ultracentrifugation at 100,000g for 60 min at 4°C. ICMT reactions were performed as described above except that En3hance fluorographic reagent was not used. Plates were exposed directly to Kodak XAR-5 film (Eastman Kodak) for 3 to 5 d at −80°C, after which TLC spots corresponding to AFC methyl ester were excised, eluted into ethanol, and stored at −80°C at a radiochemical concentration of 10,000 cpm/μL. ICME assays were performed essentially as described (Deem et al., 2006) in the presence of 80 mM HEPES (pH 7.5), 5 mM MgCl2, 100 mM NaCl, up to 100 μg of membrane protein from 4-d-old Arabidopsis seedlings prepared as described above, and 30,000 cpm of AFC methyl ester substrate (2.5 Ci/mmol, 0.27 μCi/mL) in a reaction volume of 50 μL. Reactions were incubated at 30°C for various times in sealed vials, and volatile [3H]methanol was captured into scintillation cocktail.
Seed Germination Assays
Arabidopsis seeds were harvested from control and experimental plants, which were grown together under identical conditions. Seeds were surface-sterilized as described above, suspended in sterile 0.1% agar, and placed on half-strength MS plates containing 1% sucrose and 0.8% agar in the dark at 22°C as described (Cutler et al., 1996). Seeds from control and experimental plants were sown in rows on the same plates under identical conditions, and germination (radical emergence) was scored at various times in the presence of various concentrations of exogenous ABA with a dissecting microscope.
Stomatal Closure Assays
Mature rosette leaves of Arabidopsis were excised and incubated for 2 h in the presence of various concentrations of ABA or an equivalent volume of DMSO (as a solvent control) in 10 mL of water. Abaxial epidermal peels were then prepared by peeling away the leaf surface with Scotch tape. Epidermal peels were stained with toluidine blue, mounted on a microscope slide, and visualized with a Nikon Eclipse E600 microscope interfaced to a SPOT digital camera. Data are recorded as average widths of individual apertures (n = 50 per sample). In random order, D.N.C. excised and incubated leaves in the presence of various concentrations of ABA. D.H.H. prepared epidermal peels and photographed and measured stomatal apertures without knowledge of sample identities.
Root Growth Assays
Arabidopsis seeds were surface-sterilized as described above, suspended in sterile 0.1% agar, imbibed on half-strength MS plates containing 1% sucrose and 0.8% agar, treated for 3 d at 4°C, and germinated in a vertical orientation at 22°C. After 2 d, seedlings were transferred to plates containing various concentrations of ABA, turned 90°, and visualized after an additional 5 d of growth. Root length from the bend to the tip was measured in order to rule out differences in germination time.
Analysis of ABA-Induced Gene Expression
Arabidopsis seedlings were germinated as described above in a vertical orientation at 22°C under long-day conditions (18 h of white light at 100 μmol·m−2·s−1 followed by 6 h of dark), grown for 6 d, and then transferred to plates containing ABA (0.5, 2.5, or 5.0 μM) or an equivalent volume of DMSO for 24 h. RNA was extracted using TRIzol reagent according to the manufacturer's instructions, and RT-PCR was performed to determine the relative accumulation of mRNA corresponding to the ABA-induced genes RD29A, COR47, RD22, ABI5, and COR15A. RT-PCR was performed as described above with the following primers: RD29A-5′ (5′-TACCGGAGATTGCTGAGTCTTT-3′), RD29A-3′ (5′-CTCTGTTTTGGCTCCTCTGTTT-3′), COR47-5′ (5′-AGGTTACGGATCGTGGATTG-3′), COR47-3′ (5′-CTTCCTCTTCAGTGGTCTTGG-3′), RD22-5′ (5′-CCAACGTCCAAGTAGGTAAAGG-3′), RD22-3′ (5′-GCGAATGGGTACTTCTGTTTGT-3′), ABI5-5′ (5′-ATTGACCCTTGACGAGTTCC-3′), ABI5-3′ (5′-CAACCATTCCCATTTGCTGT-3′), COR15A-5′ (5′-ATGGCTTCTTCTTTCCACAGCG-3′), COR15A-3′ (5′-CTTTGTGGCATCCTTAGCCTCT-3′), ACT8-5′ (5′-ATTGCCTCGTCGTCTTCAGCTTC-3′), ACT8-3′ (5′-GTACGACCACTGGCGTAAAGTGA-3′).
Statistical Methods
Throughout this report, data are represented as means ± se. Unlike the sd, the se takes into account sample size and is, thus, a more meaningful measure of error. In all experiments, ICMTox and ICMEox plants are compared with the Col-0 control at the same time and/or ABA concentration. Because these are pair-wise comparisons, a Student's t test was performed to assess the statistical significance of all differences, and only differences with P < 0.05 (95% confidence) were considered significant.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: STE14A, At5g23320 (The Arabidopsis Information Resource [TAIR] accession locus 505006585); STE14B (ICMT), At5g08335 (TAIR accession locus 2166963); ICME, At5g15860 (TAIR accession locus 2143236); PLP, At3g59380; ERA1, At5g40280; GGB, At2g39550; STE24, At4g01320; FACE-2, At2g36305.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The Effect of ICMT Overexpression on ROP Membrane Association in Arabidopsis Seedlings.
Supplemental Figure 2. ROP2 Geranylgeranylation in Vitro.
Supplemental Figure 3. Analysis of an Arabidopsis icme-1 Mutant.
Supplemental Data Set 1. Microarry Data for ICME, STE14A, and STE14B (ICMT).
Supplementary Material
Acknowledgments
This work was supported by USDA Grant 2003-02151 and National Science Foundation Grant MCB-0642957 to D.N.C.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Dring N. Crowell (crowdrin@isu.edu).
Online version contains Web-only data.
References
- Armstrong, S.A., Hannah, V.C., Goldstein, J.L., and Brown, M.S. (1995). CAAX geranylgeranyl transferase transfers farnesyl as efficiently as geranylgeranyl to RhoB. J. Biol. Chem. 270 7864–7868. [DOI] [PubMed] [Google Scholar]
- Baker, S.S., Wilhelm, K.S., and Thomashow, M.F. (1994). The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol. Biol. 24 701–713. [DOI] [PubMed] [Google Scholar]
- Barrero, J.M., Rodríguez, P.L., Quesada, V., Piqueras, P., Ponce, M.R., and Micol, J.L. (2006). Both abscisic acid (ABA)-dependent and ABA-independent pathways govern the induction of NCED3, AAO3 and ABA1 in response to salt stress. Plant Cell Environ. 29 2000–2008. [DOI] [PubMed] [Google Scholar]
- Bergo, M.O., Leung, G.K., Ambroziak, P., Otto, J.C., Casey, P.J., and Young, S.G. (2000). Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J. Biol. Chem. 275 17605–17610. [DOI] [PubMed] [Google Scholar]
- Bonetta, D., Bayliss, P., Sun, S., Sage, T., and McCourt, P. (2000). Farnesylation is involved in meristem organization in Arabidopsis. Planta 211 182–190. [DOI] [PubMed] [Google Scholar]
- Bonetta, D., and McCourt, P. (1998). Genetic analysis of ABA signal transduction pathways. Trends Plant Sci. 3 231–235. [Google Scholar]
- Boyartchuk, V.L., Ashby, M.N., and Rine, J. (1997). Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275 1796–1800. [DOI] [PubMed] [Google Scholar]
- Bracha, K., Lavy, M., and Yalovsky, S. (2002). The Arabidopsis AtSTE24 is a CAAX protease with broad substrate specificity. J. Biol. Chem. 277 29856–29864. [DOI] [PubMed] [Google Scholar]
- Bracha-Drori, K., Shichrur, K., Lubetzky, T.C., and Yalovsky, S. (2008). Functional analysis of Arabidopsis post-prenylation CaaX processing enzymes and their function in subcellular protein targeting. Plant Physiol. 148 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadiñanos, J., Varela, I., Mandel, D.A., Schmidt, W.K., Díaz-Perales, A., López-Otín, C., and Freije, J.M. (2003). AtFACE-2, a functional prenylated protein protease from Arabidopsis thaliana related to mammalian Ras-converting enzymes. J. Biol. Chem. 278 42091–42097. [DOI] [PubMed] [Google Scholar]
- Caldelari, D., Sternberg, H., Rodríguez-Concepción, M., Gruissem, W., and Yalovsky, S. (2001). Efficient prenylation by a plant geranylgeranyltransferase-I requires a functional CaaL box motif and a proximal polybasic domain. Plant Physiol. 126 1416–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary, S.N., Bultema, R.L., Packard, C.E., and Crowell, D.N. (2002). Prenylcysteine alpha-carboxyl methyltransferase expression and function in Arabidopsis thaliana. Plant J. 32 735–747. [DOI] [PubMed] [Google Scholar]
- Clarke, S. (1992). Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu. Rev. Biochem. 61 355–386. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Crowell, D.N. (2000). Functional implications of protein isoprenylation in plants. Prog. Lipid Res. 39 393–408. [DOI] [PubMed] [Google Scholar]
- Crowell, D.N., Huizinga, D.H., Deem, A.K., Trobaugh, C., Denton, R., and Sen, S.E. (2007). Arabidopsis thaliana plants possess a specific farnesylcysteine lyase that is involved in detoxification and recycling of farnesylcysteine. Plant J. 50 839–847. [DOI] [PubMed] [Google Scholar]
- Crowell, D.N., and Kennedy, M. (2001). Identification and functional expression in yeast of a prenylcysteine alpha-carboxyl methyltransferase gene from Arabidopsis thaliana. Plant Mol. Biol. 45 469–476. [DOI] [PubMed] [Google Scholar]
- Crowell, D.N., Sen, S.E., and Randall, S.K. (1998). Prenylcysteine alpha-carboxyl methyltransferase in suspension-cultured tobacco cells. Plant Physiol. 118 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S., Ghassemian, M., Bonetta, D., Cooney, S., and McCourt, P. (1996). A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273 1239–1241. [DOI] [PubMed] [Google Scholar]
- Deem, A.K., Bultema, R.L., and Crowell, D.N. (2006). Prenylcysteine methylesterase in Arabidopsis thaliana. Gene 380 159–166. [DOI] [PubMed] [Google Scholar]
- Dykema, P.E., Sipes, P.R., Marie, A., Biermann, B.J., Crowell, D.N., and Randall, S.K. (1999). A new class of proteins capable of binding transition metals. Plant Mol. Biol. 41 139–150. [DOI] [PubMed] [Google Scholar]
- Galichet, A., Hoyerová, K., Kamínek, M., and Gruissem, W. (2008). Farnesylation directs AtIPT3 subcellular localization and modulates cytokinin biosynthesis in Arabidopsis. Plant Physiol. 146 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, J.F., Cadwallader, K., and Marshal, C.J. (1991). Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J. 10 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerlin, A., and Bach, T.J. (1998). Effects of mevinolin on cell cycle progression and viability of tobacco BY-2 cells. Plant J. 14 65–74. [DOI] [PubMed] [Google Scholar]
- Hemmerlin, A., Fischt, I., and Bach, T.J. (2000). Differential interaction of branch-specific inhibitors of isoprenoid biosynthesis with cell cycle progression in tobacco BY-2 cells. Physiol. Plant. 110 343–350. [Google Scholar]
- Hrycyna, C.A., and Clarke, S. (1990). Farnesyl cysteine C-terminal methyltransferase activity is dependent upon the STE14 gene product in Saccharomyces cerevisiae. Mol. Cell. Biol. 10 5071–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, C.D., Chary, S.N., Chernoff, E.A., Zeng, Q., Running, M.P., and Crowell, D.N. (2005). Protein geranylgeranyltransferase I is involved in specific aspects of abscisic acid and auxin signaling in Arabidopsis. Plant Physiol. 139 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, M., Inzé, D., and Depicker, A. (2002). Gateway vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7 193–195. [DOI] [PubMed] [Google Scholar]
- Koncz, C., and Schell, J. (1986). The promoter of the TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204 383–396. [Google Scholar]
- Lavy, M., Bracha-Drori, K., Sternberg, H., and Yalovsky, S. (2002). A cell-specific, prenylation-independent mechanism regulates targeting of type II RACs. Plant Cell 14 2431–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy, M., and Yalovsky, S. (2006). Association of Arabidopsis type-II ROPs with the plasma membrane requires a conserved C-terminal sequence motif and a proximal polybasic domain. Plant J. 46 934–947. [DOI] [PubMed] [Google Scholar]
- Lemichez, E., Wu, Y., Sanchez, J.P., Mettouchi, A., Mathur, J., and Chua, N.H. (2001). Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 15 1808–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Shen, J.J., Zheng, Z.L., Lin, Y., and Yang, Z. (2001). The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 126 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loraine, A.E., Yalovsky, S., Fabry, S., and Gruissem, W. (1996). Tomato Rab1A homologs as molecular tools for studying rab geranylgeranyl transferase in plant cells. Plant Physiol. 110 1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish, C.A., and Rando, R.R. (1996). Isoprenylation/methylation of proteins enhances membrane association by a hydrophobic mechanism. Biochemistry 35 8473–8477. [DOI] [PubMed] [Google Scholar]
- Parish, C.A., Smrcka, A.V., and Rando, R.R. (1996). The role of G protein methylation in the function of a geranylgeranylated beta gamma isoform. Biochemistry 35 7499–7505. [DOI] [PubMed] [Google Scholar]
- Pei, Z.M., Ghassemian, M., Kwak, C.M., McCourt, P., and Schroeder, J.I. (1998). Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282 287–290. [DOI] [PubMed] [Google Scholar]
- Qian, D., Zhou, D., Ju, R., Cramer, C.L., and Yang, Z. (1996). Protein farnesyltransferase in plants: Molecular characterization and involvement in cell cycle control. Plant Cell 8 2381–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Concepción, M., Toledo-Ortiz, G., Yalovsky, S., Caldelari, D., and Gruissem, W. (2000). Carboxyl-methylation of prenylated calmodulin CaM53 is required for efficient plasma membrane targeting of the protein. Plant J. 24 775–784. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Concepción, M., Yalovsky, S., Zik, M., Fromm, H., and Gruissem, W. (1999). The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO J. 18 1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running, M.P., Fletcher, J.C., and Meyerowitz, E.M. (1998). The WIGGUM gene is required for proper regulation of floral meristem size in Arabidopsis. Development 125 2545–2553. [DOI] [PubMed] [Google Scholar]
- Running, M.P., Lavy, M., Sternberg, H., Galichet, A., Gruissem, W., Hake, S., Ori, N., and Yalovsky, S. (2004). Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc. Natl. Acad. Sci. USA 101 7815–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein, S., Berkower, C., and Michaelis, S. (1994). Nucleotide sequence of the yeast STE14 gene, which encodes farnesylcysteine carboxyl methyltransferase, and demonstration of its essential role in a-factor export. Mol. Cell. Biol. 14 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, N., Yamaguchi, Y., Koizumi, N., and Sano, H. (2002). Functional characterization of a heavy metal binding protein CdI19 from Arabidopsis. Plant J. 32 165–173. [DOI] [PubMed] [Google Scholar]
- Trueblood, C.E., Ohya, Y., and Rine, J. (1993). Genetic evidence for in vivo cross-specificity of the CaaX-box protein prenyltransferases farnesyltransferase and geranylgeranyltransferase-I in Saccharomyces cerevisiae. Mol. Cell. Biol. 13 4260–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov, Y., Rookes, J.E., Tilbrook, K., Chakravorty, D., Mason, M.G., Anderson, D., Chen, J.G., Jones, A.M., and Botella, J.R. (2007). Heterotrimeric G protein gamma subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell 19 1235–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt, R.E., Ainley, W.M., Nagao, R.T., Conner, T.W., and Key, J.L. (1993). Expression of the Arabidopsis AtAux2-11 auxin-responsive gene in transgenic plants. Plant Mol. Biol. 22 731–749. [DOI] [PubMed] [Google Scholar]
- Xiao, C., Xin, H., Dong, A., Sun, C., and Cao, K. (1999). A novel calmodulin-like protein gene in rice which has an unusual prolonged C-terminal sequence carrying a putative prenylation site. DNA Res. 6 179–181. [DOI] [PubMed] [Google Scholar]
- Xin, Z., Zhao, Y., and Zheng, Z.L. (2005). Transcriptome analysis reveals specific modulation of abscisic acid signaling by ROP10 small GTPase in Arabidopsis. Plant Physiol. 139 1350–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, L., Lee, H., Ishitani, M., and Zhu, J.K. (2002). Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J. Biol. Chem. 277 8588–8596. [DOI] [PubMed] [Google Scholar]
- Yalovsky, S., Kulukian, A., Rodríguez-Concepción, M., Young, C.A., and Gruissem, W. (2000). Functional requirement of plant farnesyltransferase during development in Arabidopsis. Plant Cell 12 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky, S., Loraine, A.E., and Gruissem, W. (1996). Specific prenylation of tomato Rab proteins by geranylgeranyl type-II transferase requires a conserved cysteine-cysteine motif. Plant Physiol. 110 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki, K., and Shinozaki, K. (1993). Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol. Gen. Genet. 236 331–340. [DOI] [PubMed] [Google Scholar]
- Yamamoto, Y.Y., Deng, X., and Matsui, M. (2001). Cip4, a new COP1 target, is a nucleus-localized positive regulator of Arabidopsis photomorphogenesis. Plant Cell 13 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., Cramer, C.L., and Watson, J.C. (1993). Protein farnesyltransferase in plants. Molecular cloning and expression of a homolog of the beta subunit from the garden pea. Plant Physiol. 101 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F.L., and Casey, P.J. (1996). Protein prenylation: Molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65 241–269. [DOI] [PubMed] [Google Scholar]
- Zheng, Z.L., Nafisi, M., Tam, A., Li, H., Crowell, D.N., Chary, S.N., Schroeder, J.I., Shen, J., and Yang, Z. (2002). Plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 14 2787–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J.K., Bressan, R.A., and Hasegawa, P.M. (1993). Isoprenylation of the plant molecular chaperone ANJ1 facilitates membrane association and function at high temperature. Proc. Natl. Acad. Sci. USA 90 8557–8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelhoffer, E.C., Medrano, L.J., and Meyerowitz, E.M. (2000). Cloning of the Arabidopsis WIGGUM gene identifies a role for farnesylation in meristem development. Proc. Natl. Acad. Sci. USA 97 7633–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.