Abstract
The composition of the transcriptome is determined by a balance between mRNA synthesis and degradation. An important route for mRNA degradation produces uncapped mRNAs, and this decay process can be initiated by decapping enzymes, endonucleases, and small RNAs. Although uncapped mRNAs are an important intermediate for mRNA decay, their identity and abundance have never been studied on a large scale until recently. Here, we present an experimental method for transcriptome-wide profiling of uncapped mRNAs that can be used in any eukaryotic system. We applied the method to study the prevalence of uncapped transcripts during the early stages of Arabidopsis thaliana flower development. Uncapped transcripts were identified for the majority of expressed genes, although at different levels. By comparing uncapped RNA levels with steady state overall transcript levels, our study provides evidence for widespread mRNA degradation control in numerous biological processes involving genes of varied molecular functions, implying that uncapped mRNA levels are dynamically regulated. Sequence analyses identified structural features of transcripts and cis-elements that were associated with different levels of uncapping. These transcriptome-wide profiles of uncapped mRNAs will aid in illuminating new regulatory mechanisms of eukaryotic transcriptional networks.
INTRODUCTION
The abundance of mRNA within cells is determined by rates of mRNA synthesis and degradation. A major research goal of the past few years has been the identification of factors controlling and participating in transcriptional regulatory networks, such as those associated with developmental processes and environmental responses (Wellmer and Riechmann, 2005; Barkoulas et al., 2007; Jiao et al., 2007). In addition to transcriptional regulation, the reconstruction of gene expression networks clearly requires data for mRNA degradation and other modes of regulation of mRNA transcript abundance (Belostotsky and Rose, 2005; Yuan et al., 2008). The molecular basis of mRNA degradation is best characterized in yeast and mammalian cells, in which general mRNA decay is initiated by deadenylation via a variety of mRNA deadenylases that shorten the 3′ poly(A) tail (Mitchell and Tollervey, 2000). Two major exoribonuclease-mediated degradation pathways have been identified in which mRNA can be digested from either the 5′ or the 3′ end following partial deadenylation (Parker and Song, 2004). In the 5′-3′ decay pathway, a decapping enzyme complex consisting of DCP1 and DCP2 removes the 5′ modified guanine nucleotide cap structure. The decapped transcripts are progressively digested by a 5′-3′ exonuclease known as XRN1 (Coller and Parker, 2004; Liu and Kiledjian, 2006). Decapping proteins accumulate in specific cytoplasmic foci referred to as P bodies (Parker and Sheth, 2007). As an alternative, deadenylated mRNAs can be degraded in a 3′-5′ direction by the cytoplasmic exosome complex (Houseley et al., 2006). In addition, nonsense-mediated mRNA decay (NMD), a quality control system, rapidly degrades mRNAs containing premature termination codons through direct decapping without deadenylation (Amrani et al., 2006; Shyu et al., 2008). The degradation of individual mRNAs can also be initiated by endonuclease cleavage, mediated either indirectly by small RNA-mediated silencing or directly by endonuclease-mediated cleavage, both of which will generate uncapped mRNA (Meyer et al., 2004; Halbeisen et al., 2008). It is largely unknown how much these endonucleases contribute to the degradation of mRNAs. Similar mRNA decay mechanisms are present in plants, and genetic analyses have highlighted their importance in the plant's life cycle (Gutierrez et al., 1999; Belostotsky and Rose, 2005; Belostotsky, 2008). One mRNA deadenylase, PARN, was found to be essential for embryogenesis in Arabidopsis thaliana (Chiba et al., 2004; Reverdatto et al., 2004; Nishimura et al., 2005). Recent work revealed that the Arabidopsis decapping complex, which includes DCP2, DCP1, and VARICOSE, with DCP2 containing the decapping activity, is similar to its human counterpart (Xu et al., 2006; Goeres et al., 2007; Iwasaki et al., 2007; Gunawardana et al., 2008). Mutagenesis studies indicated that the Arabidopsis decapping complex is essential for postembryonic development (Xu et al., 2006; Goeres et al., 2007; Iwasaki et al., 2007). In Arabidopsis, the cytoplasmic 5′-3′ exoribonuclease XRN4 is involved in the decay of specific transcripts (Kastenmayer and Green, 2000; Souret et al., 2004), and its targets include mRNAs of two regulators of the ethylene signaling pathway (Olmedo et al., 2006; Potuschak et al., 2006). The 3′-5′ degradation pathway is also present in Arabidopsis, and subunits of the exosome are functionally specialized, ranging from being dispensable to being essential for growth (Chekanova et al., 2007). Using inducible knockout lines of essential subunits, a wide range of exosome substrates were identified, including structural RNAs, a subset of mRNAs, microRNA (miRNA) intermediates, and noncoding RNAs (Chekanova et al., 2007). Similarly, NMD is also conserved in plants (Hori and Watanabe, 2005; Arciga-Reyes et al., 2006; Yoine et al., 2006; Kerenyi et al., 2008). Numerous studies indicate that mRNA decay is a determining factor for the steady state levels of mRNAs in cells. The decay of mRNA, in turn, can be affected by various developmental and environmental stimuli (Gutierrez et al., 1999; Parker and Song, 2004). Experiments using chemicals that inhibit transcription in Arabidopsis suspension cell cultures and in young seedlings have shown that mRNA half-lives can vary widely (Gutierrez et al., 2002; Narsai et al., 2007). In addition to general structural cis-acting elements that are found at the ends of virtually all mRNAs, where they affect mRNA stability, specific sequence elements have been identified that control mRNA turnover in plants (Gallie and Bailey-Serres, 1997; Gutierrez et al., 1999). Moreover, mRNA turnover affects the small RNA-mediated silencing pathway in plants (Gazzani et al., 2004; Gregory et al., 2008). The abundance of decapped mRNAs affects the levels of several classes of small interfering RNAs (siRNAs). Genome-wide profiling methods have been used to study mRNA expression and how it changes across tissues, among cell types, in developmental processes, in response to environmental stimuli, etc. However, these studies generally capture the steady state mRNA composition of the transcriptome and therefore do not reveal the layered nature of transcriptional regulation, where the abundance of a given mRNA species is determined and regulated by multiple mechanisms. Here, we present a method to capture and profile uncapped mRNAs, which are intermediates of the 5′-3′ decay pathway downstream of decapping and endonuclease cleavage. Applying this method to analyze the transcriptome during early Arabidopsis flower development, we show that levels of uncapped mRNAs are highly regulated. The prevalence of uncapping varies widely among different transcripts and can be correlated to the functions of the encoded proteins. In addition, we identify structural features that correlate with uncapping levels.
RESULTS
A Method to Profile the Decapped Transcriptome
To undertake a global study of uncapped gene transcript abundance, we developed a method based on the RNA ligase-mediated 5′ rapid amplification of cDNA ends (RLM 5′-RACE). Similarly to the original RLM 5′-RACE (Liu and Gorovsky, 1993; Llave et al., 2002), our method takes advantage of the presence of a 5′ monophosphate group at the 5′ end of the mRNAs of interest (in this case, uncapped mRNAs). This 5′ monophosphate group is used for the T4 RNA ligase-mediated ligation of the uncapped RNA to the 3′ end of an RNA adaptor, which is subsequently used to purify uncapped mRNAs. By contrast, intact mRNAs with a 5′ cap structure cannot be ligated to the RNA adaptors, since the 5′ cap efficiently blocks the reaction. Our method aims to capture all uncapped mRNAs in the population, so as to provide a transcriptome-wide profile that will not only reveal the identity but also determine the relative quantity of each uncapped mRNA species. As uncapped mRNAs are unstable, they are present in cells at very low levels compared with intact mRNAs. To efficiently isolate uncapped mRNAs free from intact, capped mRNA contamination, a two-step strategy was developed (see Supplemental Figure 1 and Supplemental Methods online), including affinity purification and selective synthesis of cDNA from decapped mRNAs (see Supplemental Figure 2 online). Following in vitro transcription, the resulting amplified and labeled RNAs were used in oligonucleotide microarray hybridizations using standard procedures (Wellmer et al., 2006). In the oligonucleotide microarrays used in the experiments, ∼25,500 Arabidopsis genes are represented (described in Wellmer et al., 2006), although noncoding RNA species are usually not among those represented. Using mock experiments without T4 RNA ligase, we confirmed that contamination from intact mRNAs with a 5′ cap was generally below the detectable level in the positive control (see Supplemental Figure 3 and Supplemental Methods online). By comparing with semiquantitative RLM 5′-RACE PCR using gene-specific primers, we found that our microarray-based method yielded similar results for the sample genes tested (see Supplemental Figure 4 online). It is possible that ex vivo decapped mRNAs could be captured by our method, although their abundance is expected to be low.
Abundance of Uncapped mRNAs Is Highly Regulated
The method described above was used to study the uncapped transcriptome during the early stages of flower development in Arabidopsis. For this, we used the 35S:AP1-GR ap1 cal floral induction system recently developed in our laboratory (Wellmer et al., 2006). In brief, flower formation in the 35S:AP1-GR ap1 cal plants is blocked, and, instead, the plants undergo a massive overproliferation of inflorescence-like meristems. Treatment of these inflorescences with the synthetic steroid hormone dexamethasone (Dex) activates the AP1-GR fusion protein and leads to a synchronized formation of large numbers of floral buds (Wellmer et al., 2006).
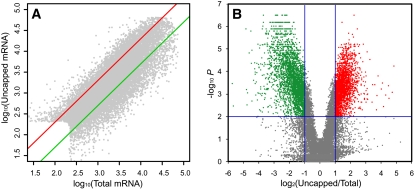
Using this system, we collected inflorescence tissues immediately before and after Dex treatment and at 1-d intervals for 5 d. At these time points, floral buds are at stages of development, 0, 2, 3, 4, 5 to 6, and 6 to 7, respectively (Wellmer et al., 2006). From those tissues, samples of purified uncapped mRNAs and of total mRNA (which mostly consists of intact mRNAs, since uncapped mRNAs are naturally present at low quantities in the cell) were prepared and used to generate labeled RNA that was cohybridized to the microarrays. During the duration of the time course, from day 0 to day 5 after the application of Dex (i.e., in early developing flowers from stages 0 to 7), we could detect expression of ∼14,000 genes (or ∼60% of annotated genes represented on the microarray). Uncapped transcripts were clearly detected for >90% of those expressed genes. In addition, a total of 1223 transcripts were detected only in the uncapped form at least at one stage (Table 1). Although the abundance of uncapped mRNAs was generally correlated with the level of total mRNA, over- and underrepresentation of uncapped transcripts was evident for many genes (Figure 1A). We used as cutoff criteria an uncapped/total mRNA ratio >2 for enriched and an uncapped/total mRNA ratio <0.5 for depleted (using a P value < 0.01 in both cases). A total of 2524 transcripts (16.1% of all expressed genes) was found as significantly enriched in the uncapped form, and a total of 3704 genes (23.6% of all expressed genes) was found as significantly depleted from uncapped transcripts (Figure 1B, Table 1). It should be noted that the ratio of uncapped/total mRNA represents a relative level of enrichment of uncapped mRNAs in comparison with total mRNA. With the average ratio of all gene transcripts at 1, larger ratios indicate higher levels of mRNA uncapping than average and vice versa. In summary, our uncapped mRNA profiling indicates that the majority of mRNAs exist in the uncapped form in vivo, though to different extents. These experiments also showed that the majority of transcripts had relatively stable levels of uncapping during the early flower development process. However we identified 315 mRNAs with significant variations of uncapping levels (time course P value < 0.05; see Supplemental Figure 5 and Supplemental Table 1 online), including a number coding for transcription factors. Taken together, these results indicate that uncapped mRNAs are prevalent and regulated during Arabidopsis early flower development. The regulation is reflected by different levels of mRNA uncapping and, to a lesser extent, developmentally regulated uncapping levels of a subset of mRNA species.
Table 1.
Relative Abundance of Total and Uncapped mRNAs in Early Developing Flowers Showing Quantities Enriched or Depleted in Uncapped Form
| Days after AP1 Induction | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Total mRNA | 14,677 | 13,633 | 14,829 | 13,171 | 14,512 | 15,604 |
| Uncapped mRNA | 13,663 (93.1%) | 12,824 (94.1%) | 14,175 (95.6%) | 11,881 (90.2%) | 13,687 (94.3%) | 14,947 (95.8%) |
| Enriched in uncapped form | 962 (6.6%) | 662 (4.9%) | 1,171 (7.9%) | 613 (4.7%) | 409 (2.8%) | 1,396 (9.0%) |
| Depleted in uncapped form | 1,374 (9.4%) | 1,176 (8.6%) | 1,583 (10.7%) | 1,622 (12.3%) | 1,256 (8.7%) | 2,219 (14.2%) |
| Only identified in uncapped form | 350 (2.4%) | 246 (1.8%) | 388 (2.6%) | 244 (1.9%) | 394 (2.7%) | 486 (3.1%) |
The indicated percentages of each category are relative to the total number of distinct transcripts detected in the total mRNA population of each time point.
Figure 1.
Expression Profiles of Uncapped mRNAs in Comparison with Total mRNAs.
Grey dots represent individual mRNAs from inflorescence tissues of 35S:AP1-GR ap1 cal plants 5 d after AP1-GR activation by Dex (floral primordia at stage 6 to 7 of development).
(A) Scatterplot of signal values from uncapped mRNAs and total mRNAs. The log10-transformed normalized expression values were plotted. Red and green lines represent the twofold change cutoff.
(B) Volcano plot where log2-transformed ratios of uncapped versus total mRNA signal are plotted against the negative log10-transformed P values for differential mRNA abundance. Transcripts with statistically different levels in the uncapped and total RNA samples (P < 0.01) and a ratio above 2 are considered as enriched in the uncapped form and are shown in red. Transcripts with statistically different levels and ratio below 0.5 were considered as depleted in the uncapped form and are shown in green.
Distinct Uncapping Profiles among Gene Functional Classes
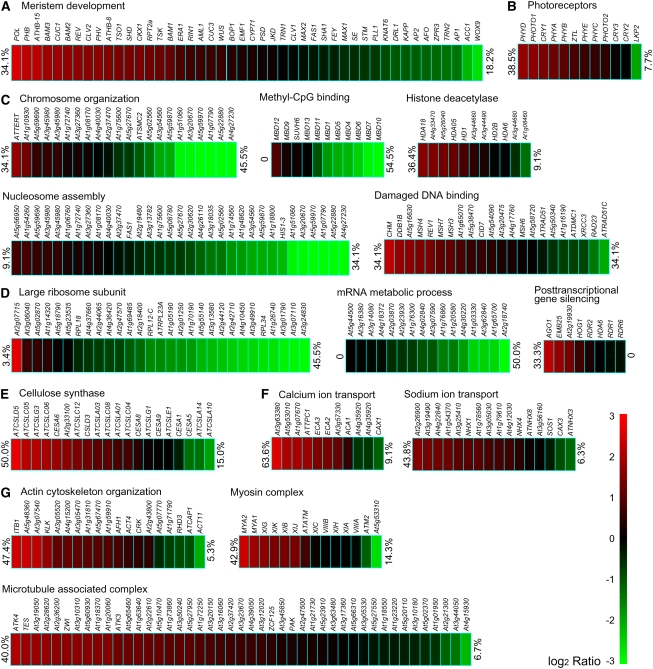
To reveal possible general relationships between functions of genes and levels of uncapping of their transcripts, we employed the Gene Ontology (GO) broad functional annotation of the Arabidopsis genome (Berardini et al., 2004). As the day 5 data showed the largest numbers of transcripts that were either enriched or depleted in uncapped forms, we focused on this time point in the subsequent analyses unless otherwise stated. Data from other time points showed similar trends as did the day 5 data. The distribution of relative uncapped/total mRNA ratios was found to be significantly biased (P < 0.001) in about half of the categories of molecular function, cellular component, and biological process (Figure 2). Among molecular function categories, genes with kinase activity, transferase activity, transporter activity, and nucleotide binding clearly had higher proportions of uncapped transcripts. On the other hand, genes with structural functions were generally less abundantly represented in the uncapped transcriptome. Genes with transcription factor activity were represented in the uncapped RNA population at an average level. With respect to the localization of the encoded proteins, gene sequences coding for proteins that are presumably targeted to the nucleus, plasma membrane, or Golgi apparatus were significantly enriched among uncapped transcripts. By contrast, uncapped transcripts were generally underrepresented from genes coding for proteins that are targeted to the ribosome, plastid, or cytosol. At the biological process level, genes encoding proteins involved in developmental processes, transport, response to abiotic or biotic stimuli, signal transduction, and DNA or RNA metabolism were all significantly enriched in the uncapped RNA population. Only genes for electron transport or energy pathways were found depleted of uncapped transcripts. A closer inspection of functional classes and levels of transcript uncapping revealed that developmental processes can vary widely in the extent to which the transcripts of genes that participate in the process are enriched, or not, in uncapped forms (Figure 3; see Supplemental Figure 6 online). For example, several RNAs involved in meristem development were found to be significantly uncapped, including POL, CLV2, and BAMs, as well as, expectedly, some miRNA target gene transcripts, such as REV, PHV, and PHB. STM and KAPP both appeared to be less represented in the 5′ uncapped transcriptome (Figure 3A). Among genes involved in flower development, cases both of uncapped-form enriched and of depleted transcripts could be identified (see Supplemental Figure 6A online). Transcripts of the floral organ identity genes (those of the ABC genetic model of organ identity determination) were all strongly capped and therefore expected to be relatively stable (see Supplemental Figure 6A online). Among the four redundant SEPALLATA genes, whose proteins form tetrameric complexes with proteins encoded by the ABC genes, SEP3 RNA was found to have a lower degree of uncapping than the others, which may be consistent with the dominating role of SEP3. Similar variable levels of uncapping were found for mRNAs of many important pathways, including cell signaling, chromatin structure, RNA metabolism and translation, cell wall construction, ion transport, cytoskeleton, photosynthesis, peroxisome, protein degradation, etc. Significant examples were selected and highlighted in Figure 3 and Supplemental Figure 6 online.
Figure 2.
Relationship between Gene Function and Level of mRNA Uncapping.
Panels show functional classifications from GO annotation organized by cellular component (A), biological process (B), and molecular function (C). In each panel, all genes detected as expressed in the total mRNA population (Total) are compared with mRNAs enriched in the uncapped form (Uncapped-enriched) and mRNAs depleted in the uncapped form (Uncapped-depleted). “Distribution” refers to the percentages of genes annotated to descriptive terms in a particular GO category divided by all (total, uncapped-enriched, or uncapped-depleted) genes. Categories with χ2 test P values < 0.001 are marked with stars.
Figure 3.
Representative Patterns of mRNA Uncapping in Genes of Various Functional Groups and Pathways.
Development (A), photoreceptor (B), chromatin (C), translation (D), cell wall (E), ion transport (F), and cytoskeleton (G). For each pathway and gene, the color of the rectangular blocks indicates the measured ratios of relative uncapped mRNA abundance versus total mRNA abundance (on a log2 scale as shown on the right side of the figure). Percentages of genes considered as enriched (red) and depleted (green) in the uncapped form are shown at the right and left sides of each group, respectively. χ2 test P values for all groups were < 0.001.
In particular, we detected a very substantial enrichment in uncapped mRNAs and a deficit of capped ones among kinase gene transcripts. This finding, together with the widely accepted important roles of kinases, led us to further dissect this group. Among the kinase families that were significantly enriched in uncapped gene transcripts were Raf kinases, casein kinases, cdc2-like kinases, receptor-like kinases (RLKs), and mitogen-activated protein kinases (see Supplemental Table 2 online). Phylogenic analyses were integrated with uncapped transcriptome profiles for the RLK family (see Supplemental Figure 7 online), which is significantly expanded in flowering plants, with >600 members in Arabidopsis (Shiu and Bleecker, 2001). High ratios of uncapped gene transcripts as well as low ratios were evident within many clades of kinases of distinct extracellular domains. For example, genes coding for leucine-rich repeat (LRR)–containing RLKs generally showed a high prevalence of uncapped transcripts. LRR subfamilies are of particular interest because they include RLKs with known developmental functions, such as BAK1, BRI1, CLV1, ERECTA, and HAESA (Torii et al., 1996; Clark et al., 1997; Li and Chory, 1997; Jinn et al., 2000; Li et al., 2002; Nam and Li, 2002). Clades spanning CLV1 and its related BAM1, 2, 3; ERECTA and its related ERL1, 2; and HAESA were all highly enriched with uncapped transcripts, although CLV1 RNA appears to be less uncapped than the RNA of BAMs. Gene transcripts for the brassinosteroid receptors BAK1 and BRI1, as well as related mRNAs to both, had intermediate levels of uncapping. Different from LRR subfamilies, various loosely related receptor-like cytoplasmic kinase subfamilies were relatively enriched with capped mRNAs. By contrast, gene transcripts coding for transcription factors showed, in general, average uncapping levels (see Supplemental Table 3 online). Except a relatively high ratio of uncapped transcripts observed among transcripts from the PHD and HB families, most families had a similar level of uncapping to the average of the entire transcriptome.
Transposable element (TE)-related genes and pseudogenes do not have obvious biological functions, although they are widespread in the Arabidopsis genome. Subsets of TE-related genes and pseudogenes are represented on our microarray, and a comparison of their mRNA uncapping profiles clearly indicated that both groups were prone to be uncapped (Figure 4A).
Figure 4.
Enhanced mRNA Uncapping Levels in TE-Related Genes, Pseudogenes, and Small RNA Targets.
(A) and (B) An mRNA uncapping level distribution of all expressed genes is shown by the histogram and the black line, while colored lines show distributions of other mRNA groups as labeled: miSVM, computational predicted miRNAs candidates; cis-NAT, overlapping cis-natural antisense transcripts. Blue vertical lines show uncapped/total ratios of 0.5 and 2, which were used as cutoffs for depletion and enrichment, respectively.
(C) Scatterplot of uncapping levels of miRNA (blue) and ta-siRNA (orange) target mRNAs versus miRNA and ta-siRNA abundance in flower tissues.
(D) Levels of uncapped and total mRNA abundance and their ratios for miRNA and ta-siRNA target genes using the color code shown at lower right. Genes are grouped according to their targeting miRNA or ta-siRNA.
Analysis of miRNA Targets Shows Variability in the Levels of Transcripts Uncapping
Plant miRNAs and trans-acting siRNAs (tasiRNAs) frequently interact with target transcripts, inducing cleavage at the tenth nucleotide of complementary sites (Llave et al., 2002). Similar to uncapped transcripts generated by uncapping enzymes, the resulting 3′ fragments have a 5′ monophosphate and can be captured by our RNA ligation-based protocol. To systematically elucidate miRNA-directed cleavage, we globally compared the relative levels of uncapped transcripts of all verified and predicted target genes of miRNAs and tasiRNAs, although our microarray-based approach could not precisely define the termini generated by cleavage. As expected, the target gene transcripts of miRNAs and tasiRNAs were enriched in uncapped forms (Figures 4B and 4C). However, a detailed review of the uncapping ratios implied that there was significant variation among target gene transcripts of distinct miRNA species and among different targets of the same miRNA family (Figures 4C and 4D). The ratio of uncapped mRNA versus total mRNA varied among different miRNA families and was not strictly correlated with miRNA abundance (Figures 4C and 4D), which was measured in separate studies of deep small RNA sequencing of inflorescences (Fahlgren et al., 2007; Kasschau et al., 2007). If similar 5′ decay rates after cleavage are assumed, such a ratio reflected the small RNA cleavage efficiency. For instance, the miR165/miR166 and miR168 family targets all showed high levels of uncapped transcripts, although the miRNAs are not among the most highly expressed. By contrast, uncapped transcripts of targets of the miR170/miR171 and miR172 families were often less abundant, although both miRNA families are expressed at higher levels, and miR172 shows one of the strongest expression levels. Target gene transcripts within the same miRNA family were usually, but not always, uncapped to similar extent, as variations existed within many families of both miRNA and tasiRNAs (Figure 4D). The variation of uncapping levels might be (partly) attributable to the mode of action of the various miRNAs. It has been shown that miR172 regulates its target gene mRNAs primarily through translational inhibition rather than mRNA cleavage (Aukerman and Sakai, 2003; Chen, 2004). Consistent with this, we found that miR172 targets were generally less uncapped than other miRNA targets (Figure 4D). Still, we were able to detect miR172 cleavage of five target transcripts AP2, TOE1-3, and SNZ by sequencing of RLM 5′-RACE products, which is an extension of a previous report (Schwab et al., 2005). Similarly, recent work on the miR156/157 target SPL3 reported that translational inhibition is the primary mechanism (Gandikota et al., 2007), which again is consistent with our finding that SPL3 RNA was weakly uncapped (Figure 4D).
A recent computational approach to identify miRNAs in plant genomes predicted ∼1200 miRNA candidates (termed miSVM) using a supervised computational learning method (Lindow et al., 2007). A comparison of the uncapping profiles of the putative targets of these newly predicted miSVMs with the uncapping profiles of the entire transcriptome showed that the miSVM targets were slightly more prone to uncapping (Figure 4B), implying that a subset of these miSVMs may be real miRNAs. In particular, among these miSVM putative targets, we identified 372 that showed significant levels of uncapping (see Supplemental Table 4 online). However, since there are non-miRNA targets that show high levels of uncapping, as well as known miRNA targets that show limited levels of uncapping, it is not possible to conclude from these data that they are truly miRNA targets. A significant portion of the genes in Arabidopsis (∼7%) have overlapping cis-natural antisense transcripts (cis-NAT; Jen et al., 2005; Wang et al., 2005). Indeed, a few overlapping cis-NATs in Arabidopsis have been found to form a regulatory circuit in which siRNAs derived from one transcript regulate stability of the other transcript (Borsani et al., 2005; Katiyar-Agarwal et al., 2006; Katiyar-Agarwal et al., 2007). However, it is still under debate to what extent those cis-NATs are associated with siRNAs (Henz et al., 2007). Still, a subset of 646 pairs (with redundancy) of cis-NAT genes was identified as being under potential siRNA regulation, having opposite expression patterns for sense and antisense transcripts (Jin et al., 2008). The uncapped mRNA profiles of these selected cis-NAT genes were not clearly different from other genes (Figure 4B).
The 5′ Untranslated Region Structure and Transcript Length Associated with Uncapped Transcript Level
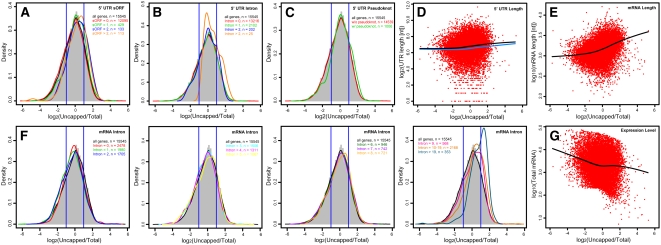
The integration of signals from mRNA binding regulatory proteins coordinately regulates selective mRNA decay as well as transcription (Gallie, 2002; Belostotsky and Rose, 2005; Keene, 2007). To understand features of mRNAs that may regulate their interaction with other regulatory molecules and/or may affect turnover through other means, we explored various structural properties of mRNAs with respect to their relationship with the mRNA uncapping profiles detected in our experiments. To reveal features of the untranslated region (UTR) that are related to mRNA uncapping, we tested UTR length, GC content, minimal free energy of a secondary structure, introns, short open reading frames (sORFs), and pseudoknots. We found that some characteristics of the 5′ UTR, including the presence of sORFs, introns, or pseudoknots, were associated with higher levels of mRNA uncapping. Among these structural features, the presence of sORFs had the most obvious effect in that mRNAs with sORFs in their 5′ UTR were clearly enriched in uncapped transcripts (Figure 5A). Such enrichment became stronger for mRNAs with more than one 5′ UTR sORF.
Figure 5.
Correlation of Gene Transcript Features and Relative mRNA Uncapping Levels.
Number of 5′ UTR small ORFs (A), number of 5′ UTR introns (B), number of 5′ UTR pseudoknots (C), 5′ UTR length (D), mRNA total length (E), number of mRNA introns (F), and total mRNA abundance (G). In (A) to (C) and (F), the distribution of mRNA uncapping levels of all expressed genes is shown by the histogram and the black line, and colored lines indicate the distribution of other mRNA groups as labeled. Blue vertical lines show uncapped/total ratios of 0.5 and 2, which were used as cutoffs for depletion and enrichment, respectively. In (D), (E), and (G), each measurement of a sequence feature is plotted against log2-transformed ratios of relative uncapped versus total mRNA abundance, and the black line of each panel is the locally weighted scatterplot smoothing (lowess) fit of the data. In (D), the blue line is the lowess fit of mRNAs without 5′ UTR sORF, intron, or pseudoknot.
Similarly, the existence of introns and pseudoknots in the 5′ UTR correlated with higher uncapping levels. Whereas the presence of one intron in the 5′ UTR of the gene has a rather limited effect, the presence of two or more introns in the same UTR correlated with enrichment of uncapped mRNAs (Figure 5B). A pseudoknot is an RNA tertiary structure containing two stem-loop structures in which the first stem's loop forms part of the second stem (Staple and Butcher, 2005). For pseudoknot structures in the 5′ UTR, having only one pseudoknot is correlated with elevated levels of uncapped mRNAs (Figure 5C). The association of these structural features with higher levels of uncapped transcripts cannot be explained simply by the additional 5′ UTR length, as such an association was not found in randomly selected mRNAs with similar 5′ UTR lengths. The 5′ UTR length in general appeared to be poorly correlated with levels of uncapped transcripts (Figure 5D). GC content and minimal free energy for secondary structure were not found to be associated with uncapping levels (see Supplemental Figures 8A and 8B online). In contrast with these 5′ UTR features, we did not find features of the 3′ UTR that were associated with levels of uncapping. The NMD pathway of mRNA quality control employs 3′ UTR features as signals for mRNA decay, which results in both decapping and deadenylation. Our data indicated that neither the existence of 3′ UTR introns, nor intron location (beyond 50 to 55 bp downstream of the stop codon), nor intron length (over 300 nucleotides) was associated with enhanced uncapping of endogenous genes (see Supplemental Figures 8C and 8D online). Note that the latter two of these features are hallmarks for NMD as revealed by reporter transgenes (Kertesz et al., 2006; Kerenyi et al., 2008). Other 3′ UTR structural features, namely, pseudoknots, GC contents, minimal free energy, and length, were also not clearly associated with enhanced uncapping (see Supplemental Figures 8E to 8H online). However, the total length of an mRNA was found to be correlated with its level of uncapping. It is evident that longer transcripts were generally more enriched in the uncapped form than were shorter ones, and this is most evident for transcripts longer than 1000 nucleotides (Figure 5E). Consistent with this, mRNAs with greater numbers of introns tended to have higher ratios of the uncapped form (Figure 5F). mRNAs without introns did not follow this trend, as they were not enriched in the uncapped pool. In addition, we found that uncapping level is associated with total mRNA abundance, such that weakly expressed mRNAs had higher ratios of uncapped transcripts (Figure 5G).
Sequence Elements in 5′ and 3′ UTR Correlate with mRNA Uncapping
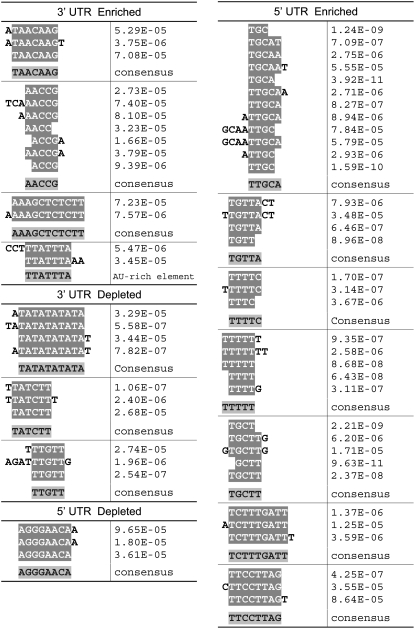
Transcript decay can be affected by conserved sequence elements in the 3′ and 5′ UTR regions. Thus, genes that show enrichment or depletion for uncapped transcripts could share common regulatory elements or motifs in their UTR regions. Using a modified enumerative method that statistically analyzes the frequency of exactly matched overrepresented motifs, we examined all possible motifs of up to 12 nucleotides in both the 3′ and the 5′ UTR of mRNAs with high and with low uncapping levels. By comparing with the group of all the genes represented in the microarray, we were able to identify around a dozen consensus motifs that were statistically overrepresented by both raw counting and on a per-UTR basis (Figure 6). We found more motifs associated with enhanced uncapping levels than with reduced uncapping in both the 5′ and the 3′ UTRs, although the same number of input genes was used for both analyses. Among these motifs, one version of the well characterized AU-rich element was found in the 3′ UTR of mRNAs enriched for uncapped transcripts. Notably, seven 5′ UTR elements were identified from highly uncapped transcripts, all of which were thymine (or uracil for RNA) rich.
Figure 6.
Sequence Motifs Overrepresented in 3′ or 5′ UTRs of mRNAs That Are Enriched or Depleted in the Uncapped Form.
Each sequence shown was individually identified as a statistically significant overrepresented sequence. The sequences in each aligned group were those we determined to represent the same motif. The P value was determined using the binomial distribution to find the likelihood of the observed number of particular elements occurring in a randomly chosen set of promoters.
DISCUSSION
Transcriptome-Wide Profiling Reveals Widespread Uncapped mRNAs in Vivo
In a eukaryotic cell, the dynamic life of an mRNA begins with its transcription in the nucleus. Once completely processed, including 5′ cap addition, splicing, editing, and polyadenylation, it is transported to the cytoplasm and translated by the ribosome. At the end of its life, the mRNA is degraded. Although mRNA steady state levels have been extensively studied at the genome scale in various organisms, work on other major stages in the life of an mRNA life are mostly limited to selected genes. The systems perspective emerging in biology promises to explain the transcriptional regulation based on modular networks of transcription, regulation, and degradation (Belostotsky and Rose, 2005; Yuan et al., 2008). It is therefore essential that biological systems data include RNA dynamics. Using chemical inhibition of transcription in cultured cells and young seedlings, it has been shown that mRNA decay rates vary widely in Arabidopsis (Gutierrez et al., 2002; Narsai et al., 2007). These experiments, however, do not distinguish between the (at least) two parallel mechanisms that coexist after deadenylation: decapping plus 5′-3′ degradation and 3′-5′degradation. In addition, it is important to avoid the use of chemical inhibitors of transcription since they induce stress responses (Narsai et al., 2007). The experimental approach we developed in this work captures all uncapped mRNAs based on their 5′ monophosphate and 3′ poly(A) tail (see Supplemental Figure 1 online), which are found following 5′ decapping or endonuclease cleavage. In addition, this protocol may capture a small number of intermediates before capping and in splicing due to lariats being debranched. The identity and abundance of uncapped mRNAs were then analyzed using microarrays. Using this strategy, we were able to identify uncapped mRNAs for most of the expressed genes (Table 1). It should be noted that because poly(A) tails of uncapped mRNAs, as well as capped mRNAs, have a range in size (Couttet et al., 1997), our protocol selecting for poly(A)+ RNAs potentially excludes uncapped mRNAs with extremely short poly(A) tails.
A recent study of Arabidopsis exosome mutants suggested that only ∼200 mRNAs are affected by defects in exosome subunits (Chekanova et al., 2007). Our results are consistent with this observation, together suggesting that 5′ decapping and subsequent 5′-3′ degradation plays the most significant role in mRNA decay in Arabidopsis. This is an extension of the prediction based on the degradation profile of unstable oat (Avena sativa) phytochrome A mRNAs (Higgs and Colbert, 1994). Alternatively, this observation can also be explained by most mRNA decay being initiated by an unidentified endonuclease cleavage independent of 5′ decapping.
Among all uncapped transcripts, this method also has the potential to identify small RNA cleavage targets at the genome scale. Indeed, a similar RNA ligation-based method has recently been developed to identify endogenous miRNA and tasiRNA targets when combined with deep sequencing (Addo-Quaye et al., 2008; German et al., 2008; Gregory et al., 2008). In addition to taking an inventory of all uncapped mRNAs, we were able to compare the relative levels of uncapped mRNA with total mRNA, which is predominantly composed of intact processed mRNA, to reveal the relative degree of uncapping for each gene transcript. This is important to understand the uncapping profiles, as uncapped mRNA abundance and mRNA steady state level are largely correlated (Figure 1). This finding indicates that the uncapping process is, to some extent, a passive process and that the abundance of total mRNA is a major factor affecting the levels of uncapped mRNA. However, in spite of the general positive correlation, variations between the abundance of uncapped mRNAs and total mRNAs were evident (Figure 1, Table 1), which indicates that mRNA decay, which leads to uncapped mRNAs, is likely to be regulated. The potential for uncapping to be, at least partially, a regulated process is suggested by our results. First, it appears that, at any given level of mRNA abundance, different transcripts show various levels of uncapping; second, for a given transcript, the level of uncapping relative to the level of that gene's expression across a developmental process is not always constant.
Relationship between Gene Function and Uncapped Transcripts
Our profiling analysis suggests that levels of uncapped mRNAs vary significantly among genes encoding proteins of distinct functions or with different subcellular localization. Detailed analysis of the uncapping profile revealed two general trends: first, mRNAs encoding essential biological functions for cellular viability are usually weakly uncapped; second, regulatory gene transcripts are more likely to be uncapped. The first trend is suggested by the general negative correlation between relative uncapping levels (instead of absolute uncapped mRNA abundance) and total mRNA levels (Figure 5F). In addition, mRNAs of TE-related genes and pseudogenes, which are thought to have no biological function for the host genome, tend to show higher levels of uncapping (Figure 4). The second trend is derived from the general observation that genes encoding proteins with regulatory functions, such as kinases and other signaling proteins, were usually more uncapped than those encoding structural proteins. This trend is also observed within various biological pathways (Figure 3; see Supplemental Figure 6 online).
Uncapped mRNA regulation is surely more complicated than this, though. First, several housekeeping function pathways were found to have a high proportion of transcript uncapping (Figure 3; see Supplemental Figure 6 online). Second, there are numerous variations within pathways. By integration of uncapping profiles with the phylogenetic relationship of RLKs, the predominant kinase family in plants, we found that sequence-related genes usually share similar levels of transcript uncapping as well (see Supplemental Figure 7 online), although exceptions were also found. Evolution of mRNA stability/instability signals after sequence divergence may explain this finding to some extent.
Gene Transcript Features Correlate with Uncapping Profiles
As can be expected, cis- and trans-acting factors correlate with the uncapping levels of gene transcripts. For trans-acting factors, small RNAs would be expected to be uncapping promoters. We indeed found that known and predicted miRNA and tasiRNA target mRNAs were proportionately more uncapped than the average (Figure 4B). Similarly, the high ratios of uncapped transcripts for TE-related genes and pseudogenes could be contributed to their significant levels of small RNA-generated endonuclease cleavage (Figure 4A). We also found a slight enrichment toward the uncapped form in targets of novel computationally predicted miRNAs (Lindow et al., 2007; Figure 4B), which would suggest that at least a small portion of them are real miRNAs. In addition, our data indicate that the uncapping levels of miRNA and tasiRNA targets are not well correlated with abundance of the corresponding small RNAs. Such variation is seen both among different miRNA and tasiRNA families as well as among targets within the same family. These differences could be a result of the interplay between the two modes of regulation by small RNAs: target cleavage and translational inhibition. Indeed, a recent report reveals that translational inhibition is widely used by plant miRNAs and siRNAs (Brodersen et al., 2008). Our uncapped versus total mRNA ratios could serve as quantitative indicators for different balancing points between transcript cleavage and translation inhibition. Still, additive effects of decapping enzymes or endonucleases on selected target mRNAs may complicate some cases. Our attempt to identify cis-acting signals associated with enhanced or reduced uncapping levels led to the finding that they are predominantly located in the 5′ UTR. Single-strand RNA molecules tend to form secondary structures, which make cis-acting signals for RNA different from DNA cis-acting elements. In addition to short sequence motifs, RNA secondary structures are good candidates for interaction with other factors, such as proteins. Our analysis indicated that sORFs, introns, and pseudoknots in the 5′ UTR, as well as 5′ UTR length, could all render the transcript more susceptible to uncapping (Figures 5A to 5D). Consistent with our results, it has been previously reported that secondary structures in the 5′ UTR accelerated decay of yeast PGK1 mRNA (Muhlrad et al., 1995). Alternatively, the positive correlation between 5′ UTR and relative uncapping levels may be explained by the downstream removal kinetics of uncapped transcripts. These secondary structures and length of 5′ UTR may attenuate uncapped transcripts by slowing down 5′-3′ exonuclease degradation rate. In addition to features of the 5′ UTR, we found that the length of mRNA also correlates positively with uncapping (Figure 5E). Although a relationship between transcript length and mRNA decay has not been reported, a reanalysis of Arabidopsis mRNA half-life data in cultured cells (Narsai et al., 2007) revealed a similar trend that shorter mRNAs usually have a longer half-life, which is evident for mRNAs less than 2 kb (see Supplemental Figure 9 online). Notably, an inverse correlation between the transcript length and the ribosome density has been found in yeasts and fruit fly (Arava et al., 2003; Lackner et al., 2007; Qin et al., 2007). As there is likely a competition between translation and mRNA decay, low ribosome density and high uncapping levels may be associated. The enrichment of uncapped long mRNAs could be a result of an accumulation of 5′ to 3′ exonuclease decay intermediates, at least in part. Using computational methods to search for consensus oligomer sequences, we identified a few enriched motifs from highly uncapped gene transcripts within either the 3′ UTR or the 5′ UTR (Figure 6). Most of these elements were identified from relatively uncapped transcript classes, suggesting a wider recruitment of cis-elements to promote mRNA degradation. Given the statistical overrepresentation by both raw counting and on a per-promoter basis, these novel elements warrant further experimental characterization. Notably, a novel GU-rich element, which was identified through enumerative search from short-lived mRNAs in cultured human T cells, has been experimentally verified in a recent work (Vlasova et al., 2008).
METHODS
Plant Materials and Experimental Design
The Arabidopsis thaliana 35S:AP1-GR ap1-1 cal-1 line (in the Landsberg erecta background) was used in this study. This line allows for the synchronization of early developing flowers (Wellmer et al., 2006). Plants were grown on a soil:vermiculite:perlite mixture under constant illumination with a light intensity range of 80 to 100 μmol·m−2·s−1 at 20°C. Inflorescence tissue was collected under a dissecting microscope 1, 2, 3, 4, or 5 d after Dex treatment or before treatment (Wellmer et al., 2006). Four independent sets of biological samples were used for the experiments. Uncapped and total RNA samples derived from each time point were cohybridized. The dyes used for labeling RNA from a given time point were switched in the replicate experiments to reduce dye-related artifacts.
Ligation-Mediated Isolation of Uncapped mRNA
Briefly, uncapped mRNAs were isolated by adapting a modified RLM 5′-RACE protocol to globally sample RNAs with a 5′ monophosphate and a 3′ poly(A)+ tail. An RNA adaptor was ligated directly to poly(A)+ RNA having a free 5′ monophosphate. This adaptor was subsequently used for affinity purification and selective double-strand cDNA synthesis from uncapped mRNA. For more detailed methodology, see the Supplemental Methods and Supplemental Figure 1 online.
Microarray Analysis
Microarrays were based on the Arabidopsis Genome Oligo Set Version 1.0 and Version 1 Upgrade (Operon). These sets consist of a total of 30,194 70-mer oligonucleotides that correspond to 25,521 annotated genes according to The Arabidopsis Information Resource (TAIR) genome annotation version 6. The oligonucleotide probes in these sets were preferentially designed to correspond to the 3′ end of transcripts, when possible. Microarrays were printed and processed as previously described (Wellmer et al., 2004, 2006). Labeled antisense RNA from uncapped mRNA and from total mRNA from the same biological sample were cohybridized. Microarrays were scanned with a GenePix 4200A scanner, and raw images were analyzed using the GenePix Pro 5.0 software (Molecular Devices). GenePix Pro output data were normalized using eCADS to remove intensity-dependent biases (Dabney and Storey, 2007). A reproducible significant intensity above that 90% of negative controls in three out of four replicate experiments was used as the threshold to classify a probe as detecting a positive signal of gene expression (Ma et al., 2005). To identify enrichment or depletion of uncapped transcripts, significance analysis was performed using EDGE for each time point and for the developmental time course, which address the multiple testing errors using the false discovery rate (Leek et al., 2006).
Assignment of Gene Functions
GOSlim annotation developed by TAIR was used to organize sets of genes into broad ontology categories (Berardini et al., 2004). Further classification of kinases was according to the KinG database (Krupa et al., 2004). Further classification within the RLK family followed Shiu and Bleecker (2001). Classification of transcription factors was according to the Database of Arabidopsis Transcription Factors (Guo et al., 2005). TE-related genes and pseudogenes were according to TAIR annotation version 7. Annotations of miRNA and tasiRNA as well as their targets were according to the Arabidopsis Small RNA Project database (Gustafson et al., 2005). Computationally predicted miSVMs were from Lindow et al. (2007). Putative nat-siRNA targets were following Jin et al. (2008). Further classification of all other functional groups combined GO annotation by TAIR and TIGR, gene annotation by TAIR, and manual curation according to the literature.
Analysis of Sequence Features
Transcript sequences and the associated annotation were downloaded from TAIR (Arabidopsis genome annotation version 7). The minimal energy for secondary structures was calculated using RNAfold (Hofacker et al., 1994), and pseudoknot structures were predicted using RNABOB (ftp://selab.janelia.org/pub/software/rnabob/). All other pattern search and calculations were performed using custom scripts in Perl and R.
Accession Number
All raw microarray data from our analyses have been deposited in the Gene Expression Omnibus under accession number GSE12043.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Overview of the T4 RNA Ligase-Mediated Method for the Isolation of Uncapped mRNA.
Supplemental Figure 2. Comparison of PCR Conditions for Second Strand Synthesis and Amplification.
Supplemental Figure 3. Bioanalyzer Electropherograms of Amplified aRNA Samples.
Supplemental Figure 4. RLM-RACE PCR Verification of Uncapping Profiles of Sample Genes.
Supplemental Figure 5. Overview of Genes with Developmentally Regulated Transcript Uncapping Levels.
Supplemental Figure 6. Representative Patterns of mRNA Uncapping in Various Functional Groups and Pathways.
Supplemental Figure 7. Degrees of Lineage-Specific Uncapping in the RLK Family.
Supplemental Figure 8. Relationship between Gene Transcript Features and Relative mRNA Uncapping Levels.
Supplemental Figure 9. Comparison of mRNA Half-Lives with Uncapping Levels and mRNA Length.
Supplemental Table 1. Genes with Developmentally Regulated Transcript Uncapping Levels during Early Flower Development.
Supplemental Table 2. Uncapping Levels of All Kinase Families.
Supplemental Table 3. Uncapping Levels of All Transcription Factor Families.
Supplemental Table 4. Significantly Uncapped Predicted miRNA Targets.
Supplemental Methods.
Supplementary Material
Acknowledgments
We thank Vijaya Kumar, Lorian Schaeffer, and Joanne Tan-Cabugao for assistance with microarray manufacture, David Mathog for advice on sequence analysis, and Zachary Nimchuk, Adrienne Roeder, and Kathrin Schrick for comments on the manuscript. This work was supported by National Science Foundation 2010 Project Grant 0520193 to J.L.R. and E.M.M. and by the Millard and Muriel Jacobs Genetics and Genomics Laboratory at the California Institute of Technology.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) are: Yuling Jiao (jiao@caltech.edu) and Elliot M. Meyerowitz (meyerow@caltech.edu).
Online version contains Web-only data
References
- Addo-Quaye, C., Eshoo, T.W., Bartel, D.P., and Axtell, M.J. (2008). Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Curr. Biol. 18 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrani, N., Sachs, M.S., and Jacobson, A. (2006). Early nonsense: mRNA decay solves a translational problem. Nat. Rev. Mol. Cell Biol. 7 415–425. [DOI] [PubMed] [Google Scholar]
- Arava, Y., Wang, Y., Storey, J.D., Liu, C.L., Brown, P.O., and Herschlag, D. (2003). Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 100 3889–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciga-Reyes, L., Wootton, L., Kieffer, M., and Davies, B. (2006). UPF1 is required for nonsense-mediated mRNA decay (NMD) and RNAi in Arabidopsis. Plant J. 47 480–489. [DOI] [PubMed] [Google Scholar]
- Aukerman, M.J., and Sakai, H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoulas, M., Galinha, C., Grigg, S.P., and Tsiantis, M. (2007). From genes to shape: Regulatory interactions in leaf development. Curr. Opin. Plant Biol. 10 660–666. [DOI] [PubMed] [Google Scholar]
- Belostotsky, D.A. (2008). State of decay: An update on plant mRNA turnover. Curr. Top. Microbiol. Immunol. 326 179–199. [DOI] [PubMed] [Google Scholar]
- Belostotsky, D.A., and Rose, A.B. (2005). Plant gene expression in the age of systems biology: Integrating transcriptional and post-transcriptional events. Trends Plant Sci. 10 347–353. [DOI] [PubMed] [Google Scholar]
- Berardini, T.Z., et al. (2004). Functional annotation of the Arabidopsis genome using controlled vocabularies. Plant Physiol. 135 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsani, O., Zhu, J., Verslues, P.E., Sunkar, R., and Zhu, J.K. (2005). Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell 123 1279–1291. [DOI] [PMC free article] [PubMed]
- Brodersen, P., Sakvarelidze-Achard, L., Bruun-Rasmussen, M., Dunoyer, P., Yamamoto, Y.Y., Sieburth, L., and Voinnet, O. (2008). Widespread translational inhibition by plant miRNAs and siRNAs. Science 320 1185–1190. [DOI] [PubMed] [Google Scholar]
- Chekanova, J.A., et al. (2007). Genome-wide high-resolution mapping of exosome substrates reveals hidden features in the Arabidopsis transcriptome. Cell 131 1340–1353. [DOI] [PubMed] [Google Scholar]
- Chen, X. (2004). A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303 2022–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba, Y., Johnson, M.A., Lidder, P., Vogel, J.T., van Erp, H., and Green, P.J. (2004). AtPARN is an essential poly(A) ribonuclease in Arabidopsis. Gene 328 95–102. [DOI] [PubMed] [Google Scholar]
- Clark, S.E., Williams, R.W., and Meyerowitz, E.M. (1997). The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89 575–585. [DOI] [PubMed] [Google Scholar]
- Coller, J., and Parker, R. (2004). Eukaryotic mRNA decapping. Annu. Rev. Biochem. 73 861–890. [DOI] [PubMed] [Google Scholar]
- Couttet, P., Fromont-Racine, M., Steel, D., Pictet, R., and Grange, T. (1997). Messenger RNA deadenylylation precedes decapping in mammalian cells. Proc. Natl. Acad. Sci. USA 94 5628–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabney, A.R., and Storey, J.D. (2007). Normalization of two-channel microarrays accounting for experimental design and intensity-dependent relationships. Genome Biol. 8 R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlgren, N., Howell, M.D., Kasschau, K.D., Chapman, E.J., Sullivan, C.M., Cumbie, J.S., Givan, S.A., Law, T.F., Grant, S.R., Dangl, J.L., and Carrington, J.C. (2007). High-throughput sequencing of Arabidopsis microRNAs: Evidence for frequent birth and death of MIRNA genes. PLoS ONE 2 e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie, D.R. (2002). Protein-protein interactions required during translation. Plant Mol. Biol. 50 949–970. [DOI] [PubMed] [Google Scholar]
- Gallie, D.R., and Bailey-Serres, J. (1997). Eyes off transcription! The wonderful world of post-transcriptional regulation. Plant Cell 9 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandikota, M., Birkenbihl, R.P., Hohmann, S., Cardon, G.H., Saedler, H., and Huijser, P. (2007). The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. 49 683–693. [DOI] [PubMed] [Google Scholar]
- Gazzani, S., Lawrenson, T., Woodward, C., Headon, D., and Sablowski, R. (2004). A link between mRNA turnover and RNA interference in Arabidopsis. Science 306 1046–1048. [DOI] [PubMed] [Google Scholar]
- German, M.A., et al. (2008). Global identification of microRNA-target RNA pairs by parallel analysis of RNA ends. Nat. Biotechnol. 26 941–946. [DOI] [PubMed] [Google Scholar]
- Goeres, D.C., Van Norman, J.M., Zhang, W., Fauver, N.A., Spencer, M.L., and Sieburth, L.E. (2007). Components of the Arabidopsis mRNA decapping complex are required for early seedling development. Plant Cell 19 1549–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory, B.D., O'Malley, R.C., Lister, R., Urich, M.A., Tonti-Filippini, J., Chen, H., Millar, A.H., and Ecker, J.R. (2008). A link between RNA metabolism and silencing affecting Arabidopsis development. Dev. Cell 14 854–866. [DOI] [PubMed] [Google Scholar]
- Gunawardana, D., Cheng, H.C., and Gayler, K.R. (2008). Identification of functional domains in Arabidopsis thaliana mRNA decapping enzyme (AtDcp2). Nucleic Acids Res. 36 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, A., He, K., Liu, D., Bai, S., Gu, X., Wei, L., and Luo, J. (2005). DATF: A database of Arabidopsis transcription factors. Bioinformatics 21 2568–2569. [DOI] [PubMed] [Google Scholar]
- Gustafson, A.M., Allen, E., Givan, S., Smith, D., Carrington, J.C., and Kasschau, K.D. (2005). ASRP: The Arabidopsis Small RNA Project Database. Nucleic Acids Res. 33 D637–D640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, R.A., Ewing, R.M., Cherry, J.M., and Green, P.J. (2002). Identification of unstable transcripts in Arabidopsis by cDNA microarray analysis: Rapid decay is associated with a group of touch- and specific clock-controlled genes. Proc. Natl. Acad. Sci. USA 99 11513–11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, R.A., MacIntosh, G.C., and Green, P.J. (1999). Current perspectives on mRNA stability in plants: Multiple levels and mechanisms of control. Trends Plant Sci. 4 429–438. [DOI] [PubMed] [Google Scholar]
- Halbeisen, R.E., Galgano, A., Scherrer, T., and Gerber, A.P. (2008). Post-transcriptional gene regulation: From genome-wide studies to principles. Cell. Mol. Life Sci. 65 798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henz, S.R., Cumbie, J.S., Kasschau, K.D., Lohmann, J.U., Carrington, J.C., Weigel, D., and Schmid, M. (2007). Distinct expression patterns of natural antisense transcripts in Arabidopsis. Plant Physiol. 144 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs, D.C., and Colbert, J.T. (1994). Oat phytochrome A mRNA degradation appears to occur via two distinct pathways. Plant Cell 6 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacker, I.L., Fontana, W., Stadler, P.F., Bonhoeffer, L.S., Tacker, M., and Schuster, P. (1994). Fast folding and comparison of RNA secondary structures. Monatsh. Chem. 125 167–188. [Google Scholar]
- Hori, K., and Watanabe, Y. (2005). UPF3 suppresses aberrant spliced mRNA in Arabidopsis. Plant J. 43 530–540. [DOI] [PubMed] [Google Scholar]
- Houseley, J., LaCava, J., and Tollervey, D. (2006). RNA-quality control by the exosome. Nat. Rev. Mol. Cell Biol. 7 529–539. [DOI] [PubMed] [Google Scholar]
- Iwasaki, S., Takeda, A., Motose, H., and Watanabe, Y. (2007). Characterization of Arabidopsis decapping proteins AtDCP1 and AtDCP2, which are essential for post-embryonic development. FEBS Lett. 581 2455–2459. [DOI] [PubMed] [Google Scholar]
- Jen, C.H., Michalopoulos, I., Westhead, D.R., and Meyer, P. (2005). Natural antisense transcripts with coding capacity in Arabidopsis may have a regulatory role that is not linked to double-stranded RNA degradation. Genome Biol. 6 R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, Y., Lau, O.S., and Deng, X.W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8 217–230. [DOI] [PubMed] [Google Scholar]
- Jin, H., Vacic, V., Girke, T., Lonardi, S., and Zhu, J.K. (2008). Small RNAs and the regulation of cis-natural antisense transcripts in Arabidopsis. BMC Mol. Biol. 9 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn, T.L., Stone, J.M., and Walker, J.C. (2000). HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 14 108–117. [PMC free article] [PubMed] [Google Scholar]
- Kasschau, K.D., Fahlgren, N., Chapman, E.J., Sullivan, C.M., Cumbie, J.S., Givan, S.A., and Carrington, J.C. (2007). Genome-wide profiling and analysis of Arabidopsis siRNAs. PLoS Biol. 5 e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmayer, J.P., and Green, P.J. (2000). Novel features of the XRN-family in Arabidopsis: Evidence that AtXRN4, one of several orthologs of nuclear Xrn2p/Rat1p, functions in the cytoplasm. Proc. Natl. Acad. Sci. USA 97 13985–13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar-Agarwal, S., Gao, S., Vivian-Smith, A., and Jin, H. (2007). A novel class of bacteria-induced small RNAs in Arabidopsis. Genes Dev. 21 3123–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar-Agarwal, S., Morgan, R., Dahlbeck, D., Borsani, O., Villegas, A., Jr., Zhu, J.K., Staskawicz, B.J., and Jin, H. (2006). A pathogen-inducible endogenous siRNA in plant immunity. Proc. Natl. Acad. Sci. USA 103 18002–18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene, J.D. (2007). RNA regulons: Coordination of post-transcriptional events. Nat. Rev. Genet. 8 533–543. [DOI] [PubMed] [Google Scholar]
- Kerenyi, Z., Merai, Z., Hiripi, L., Benkovics, A., Gyula, P., Lacomme, C., Barta, E., Nagy, F., and Silhavy, D. (2008). Inter-kingdom conservation of mechanism of nonsense-mediated mRNA decay. EMBO J. 27 1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz, S., Kerenyi, Z., Merai, Z., Bartos, I., Palfy, T., Barta, E., and Silhavy, D. (2006). Both introns and long 3′-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 34 6147–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa, A., Abhinandan, K.R., and Srinivasan, N. (2004). KinG: A database of protein kinases in genomes. Nucleic Acids Res. 32 D153–D155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner, D.H., Beilharz, T.H., Marguerat, S., Mata, J., Watt, S., Schubert, F., Preiss, T., and Bahler, J. (2007). A network of multiple regulatory layers shapes gene expression in fission yeast. Mol. Cell 26 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek, J.T., Monsen, E., Dabney, A.R., and Storey, J.D. (2006). EDGE: Extraction and analysis of differential gene expression. Bioinformatics 22 507–508. [DOI] [PubMed] [Google Scholar]
- Li, J., and Chory, J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90 929–938. [DOI] [PubMed] [Google Scholar]
- Li, J., Wen, J., Lease, K.A., Doke, J.T., Tax, F.E., and Walker, J.C. (2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110 213–222. [DOI] [PubMed] [Google Scholar]
- Lindow, M., Jacobsen, A., Nygaard, S., Mang, Y., and Krogh, A. (2007). Intragenomic matching reveals a huge potential for miRNA-mediated regulation in plants. PLoS Comput. Biol. 3 e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., and Kiledjian, M. (2006). Decapping the message: A beginning or an end. Biochem. Soc. Trans. 34 35–38. [DOI] [PubMed] [Google Scholar]
- Liu, X., and Gorovsky, M.A. (1993). Mapping the 5′ and 3′ ends of Tetrahymena thermophila mRNAs using RNA ligase mediated amplification of cDNA ends (RLM-RACE). Nucleic Acids Res. 21 4954–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave, C., Xie, Z., Kasschau, K.D., and Carrington, J.C. (2002). Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297 2053–2056. [DOI] [PubMed] [Google Scholar]
- Ma, L., Sun, N., Liu, X., Jiao, Y., Zhao, H., and Deng, X.W. (2005). Organ-specific expression of Arabidopsis genome during development. Plant Physiol. 138 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, S., Temme, C., and Wahle, E. (2004). Messenger RNA turnover in eukaryotes: Pathways and enzymes. Crit. Rev. Biochem. Mol. Biol. 39 197–216. [DOI] [PubMed] [Google Scholar]
- Mitchell, P., and Tollervey, D. (2000). Musing on the structural organization of the exosome complex. Nat. Struct. Biol. 7 843–846. [DOI] [PubMed] [Google Scholar]
- Muhlrad, D., Decker, C.J., and Parker, R. (1995). Turnover mechanisms of the stable yeast PGK1 mRNA. Mol. Cell. Biol. 15 2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, K.H., and Li, J. (2002). BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110 203–212. [DOI] [PubMed] [Google Scholar]
- Narsai, R., Howell, K.A., Millar, A.H., O'Toole, N., Small, I., and Whelan, J. (2007). Genome-wide analysis of mRNA decay rates and their determinants in Arabidopsis thaliana. Plant Cell 19 3418–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, N., Kitahata, N., Seki, M., Narusaka, Y., Narusaka, M., Kuromori, T., Asami, T., Shinozaki, K., and Hirayama, T. (2005). Analysis of ABA hypersensitive germination2 revealed the pivotal functions of PARN in stress response in Arabidopsis. Plant J. 44 972–984. [DOI] [PubMed] [Google Scholar]
- Olmedo, G., Guo, H., Gregory, B.D., Nourizadeh, S.D., Aguilar-Henonin, L., Li, H., An, F., Guzman, P., and Ecker, J.R. (2006). ETHYLENE-INSENSITIVE5 encodes a 5′→3′ exoribonuclease required for regulation of the EIN3-targeting F-box proteins EBF1/2. Proc. Natl. Acad. Sci. USA 103 13286–13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, R., and Sheth, U. (2007). P bodies and the control of mRNA translation and degradation. Mol. Cell 25 635–646. [DOI] [PubMed] [Google Scholar]
- Parker, R., and Song, H. (2004). The enzymes and control of eukaryotic mRNA turnover. Nat. Struct. Mol. Biol. 11 121–127. [DOI] [PubMed] [Google Scholar]
- Potuschak, T., Vansiri, A., Binder, B.M., Lechner, E., Vierstra, R.D., and Genschik, P. (2006). The exoribonuclease XRN4 is a component of the ethylene response pathway in Arabidopsis. Plant Cell 18 3047–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, X., Ahn, S., Speed, T.P., and Rubin, G.M. (2007). Global analyses of mRNA translational control during early Drosophila embryogenesis. Genome Biol. 8 R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverdatto, S.V., Dutko, J.A., Chekanova, J.A., Hamilton, D.A., and Belostotsky, D.A. (2004). mRNA deadenylation by PARN is essential for embryogenesis in higher plants. RNA 10 1200–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab, R., Palatnik, J.F., Riester, M., Schommer, C., Schmid, M., and Weigel, D. (2005). Specific effects of microRNAs on the plant transcriptome. Dev. Cell 8 517–527. [DOI] [PubMed] [Google Scholar]
- Shiu, S.H., and Bleecker, A.B. (2001). Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 98 10763–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu, A.B., Wilkinson, M.F., and van Hoof, A. (2008). Messenger RNA regulation: to translate or to degrade. EMBO J. 27 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souret, F.F., Kastenmayer, J.P., and Green, P.J. (2004). AtXRN4 degrades mRNA in Arabidopsis and its substrates include selected miRNA targets. Mol. Cell 15 173–183. [DOI] [PubMed] [Google Scholar]
- Staple, D.W., and Butcher, S.E. (2005). Pseudoknots: RNA structures with diverse functions. PLoS Biol. 3 e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii, K.U., Mitsukawa, N., Oosumi, T., Matsuura, Y., Yokoyama, R., Whittier, R.F., and Komeda, Y. (1996). The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova, I.A., Tahoe, N.M., Fan, D., Larsson, O., Rattenbacher, B., Sternjohn, J.R., Vasdewani, J., Karypis, G., Reilly, C.S., Bitterman, P.B., and Bohjanen, P.R. (2008). Conserved GU-rich elements mediate mRNA decay by binding to CUG-binding protein 1. Mol. Cell 29 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.J., Gaasterland, T., and Chua, N.H. (2005). Genome-wide prediction and identification of cis-natural antisense transcripts in Arabidopsis thaliana. Genome Biol. 6 R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer, F., Alves-Ferreira, M., Dubois, A., Riechmann, J.L., and Meyerowitz, E.M. (2006). Genome-wide analysis of gene expression during early Arabidopsis flower development. PLoS Genet. 2 e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer, F., and Riechmann, J.L. (2005). Gene network analysis in plant development by genomic technologies. Int. J. Dev. Biol. 49 745–759. [DOI] [PubMed] [Google Scholar]
- Wellmer, F., Riechmann, J.L., Alves-Ferreira, M., and Meyerowitz, E.M. (2004). Genome-wide analysis of spatial gene expression in Arabidopsis flowers. Plant Cell 16 1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., Yang, J.Y., Niu, Q.W., and Chua, N.H. (2006). Arabidopsis DCP2, DCP1, and VARICOSE form a decapping complex required for postembryonic development. Plant Cell 18 3386–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoine, M., Nishii, T., and Nakamura, K. (2006). Arabidopsis UPF1 RNA helicase for nonsense-mediated mRNA decay is involved in seed size control and is essential for growth. Plant Cell Physiol. 47 572–580. [DOI] [PubMed] [Google Scholar]
- Yuan, J.S., Galbraith, D.W., Dai, S.Y., Griffin, P., and Stewart, C.N., Jr. (2008). Plant systems biology comes of age. Trends Plant Sci. 13 165–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.