Abstract
This article presents a detailed model for the regulation of lateral root formation in Arabidopsis thaliana seedlings grown in culture. We demonstrate that direct contact between the aerial tissues and sucrose in the growth media is necessary and sufficient to promote emergence of lateral root primordia from the parent root. Mild osmotic stress is perceived by the root, which then sends an abscisic acid–dependent signal that causes a decrease in the permeability of aerial tissues; this reduces uptake of sucrose from the culture media, which leads to a repression of lateral root formation. Osmotic repression of lateral root formation in culture can be overcome by mutations that cause the cuticle of a plant's aerial tissues to become more permeable. Indeed, we report here that the previously described lateral root development2 mutant overcomes osmotic repression of lateral root formation because of a point mutation in Long Chain Acyl-CoA Synthetase2, a gene essential for cutin biosynthesis. Together, our findings (1) impact the interpretation of experiments that use Arabidopsis grown in culture to study root system architecture; (2) identify sucrose as an unexpected regulator of lateral root formation; (3) demonstrate mechanisms by which roots communicate information to aerial tissues and receive information in turn; and (4) provide insights into the regulatory pathways that allow plants to be developmentally plastic while preserving the essential balance between aboveground and belowground organs.
INTRODUCTION
The plant root system originates from the primary root, which is established during embryogenesis. However, the bulk of the adult plant's root system is made up of lateral roots, which form postembryonically. Hence, it is the postembryonic decisions about the number and placement of lateral roots that determine the size and overall architecture of the root system.
The development of a lateral root has been well characterized at the anatomical level. In nearly all higher plants, lateral root formation initiates when lateral root founder cells are specified in the pericycle layer of a mature root (Charleton, 1991). The newly created founder cells proceed through a stereotypical pattern of cell divisions and cell expansions to produce a lateral root primordium (LRP) within the parent root. The mature LRP then emerges from the parent root through a process that involves both LRP cell expansion (Malamy and Benfey, 1997) and separation of the overlying cells of the parental root (Swarup et al., 2008). The emerged LRP becomes a lateral root when cell divisions commence at the newly activated root apical meristem. There is considerable evidence that the developmental stages of lateral root formation (initiation, LRP formation, LRP emergence, and apical meristem activation) are independently regulated, although our understanding of the regulatory mechanisms is only rudimentary (for review, see Casimiro et al., 2003; Malamy 2005, 2008).
The postembryonic formation of lateral roots allows the plant to incorporate information from the environment into decisions it makes about root system architecture. Indeed, root system architecture is profoundly affected by water and nutrient availability in the soil or culture medium (for review, see Forde and Lorenzo, 2001; Casimiro et al., 2003; Lopez-Bucio et al., 2003; Malamy 2005, 2008; Hermans et al., 2006; Osmont et al., 2007). It has been proposed that environmental response pathways allow the plant to optimize lateral root number and placement in accordance with its growth conditions, while intrinsic pathways constrain overall root system shape and size and therefore place a limit on root system plasticity (Malamy, 2005). Many components of intrinsic and environmental response pathways have been identified, but a cohesive model of the molecular pathways regulating root system development has yet to emerge. Auxin, cytokinin, and abscisic acid (ABA) have been most strongly implicated in regulating lateral root formation and in modulating lateral root formation in response to environmental cues (for review, see Casimiro et al., 2003; Lopez-Bucio et al., 2003; Malamy, 2005, 2008; De Smet et al., 2006; Fukaki et al., 2007; Osmont et al., 2007; Kuderová et al., 2008; Swarup et al., 2008), although there is also evidence for involvement of other plant hormones (for review, see Malamy, 2005, 2008; Osmont et al., 2007).
To further elucidate the molecular pathways that regulate root system development, we studied Arabidopsis thaliana seedlings growing in culture. When seedlings are grown for 12 to 14 d on media that simulates mild osmotic stress conditions, almost no lateral roots form (van der Weele et al., 2000; Deak and Malamy, 2005; Xiong et al., 2006; Qi et al., 2007). The mild osmotic stress conditions primarily inhibit lateral root formation by repressing LRP emergence (Deak and Malamy, 2005). The growth rate of the primary root is also repressed by osmotic stress. However, when plants grown in the presence or absence of osmotic stress are matched based on primary root length, obvious differences in lateral root formation are still seen (Deak and Malamy, 2005). This indicates that osmotic stress does not simply slow all aspects of root system growth, but alters the developmental program to create an unbranched root system architecture. Repression of LRP emergence by osmotic stress in culture is ABA dependent, as it does not occur in two ABA-deficient mutants (Deak and Malamy, 2005). By contrast, LRP emergence in this culture system is promoted by exogenous auxin even in the presence of osmotic stress (Deak and Malamy, 2005), suggesting that a balance between ABA and auxin signaling determines the fate of the LRP and the ultimate root system architecture and that osmotic stress in some way affects the auxin/ABA balance.
We screened mutagenized seedlings under mild osmotic stress conditions and isolated the lateral root development2 (lrd2) mutant (Deak and Malamy, 2005). As previously described, lrd2 is unable to repress LRP emergence and hence forms a highly branched root system. In this article, we report the identification of LRD2 as an allele of Long-Chain Fatty Acid Synthetase 2 (LACS2) and demonstrate that LACS2 affects lateral root formation through its role in the formation of the cuticle covering the aerial tissues. Our work further shows that cuticle integrity is essential for correct regulation of lateral root formation in culture because it limits uptake of sucrose from the media into the plant's aerial tissues. Elevated sucrose uptake and metabolism leads to increased shoot system growth and development and increased lateral root formation in lrd2 grown on mild osmotic stress conditions and in wild-type plants grown in the absence of osmotic stress. Further experiments demonstrate that osmotic stress is sensed at the root, which sends an ABA-dependent signal to the shoot to decrease aerial tissue permeability to sucrose. Together, our results define how root system architecture is regulated in Arabidopsis grown in culture, demonstrate novel communication pathways from root to shoot and shoot to root, and provide new insights into the role of sucrose in regulating lateral root formation and the maintenance of root-to-shoot ratios in plants.
RESULTS
Mutations in LRD2/LACS2 Promote Lateral Root Formation in Culture by Compromising the Formation of the Cuticle
lrd2 Is an Allele of LACS2
We previously described an lrd2 mutant that exhibits a dramatic increase in lateral root formation due to an increase in the emergence of LRP (Deak and Malamy, 2005). The lrd2 phenotype is particularly striking under mild osmotic stress conditions; few if any LRP emerge in the wild type under mild osmotic stress conditions, whereas lrd2 forms a highly branched root system (Figure 1B). Hence, this provides a good assay to examine the mechanisms that regulate lateral root formation. Complete repression of LRP emergence in the wild type but not lrd2 was seen whether salts (N salts, KCl) or mannitol was used to decrease the osmotic potential of the media (Deak and Malamy, 2005), and N salts and mannitol are therefore used interchangeably herein to simulate mild osmotic stress.
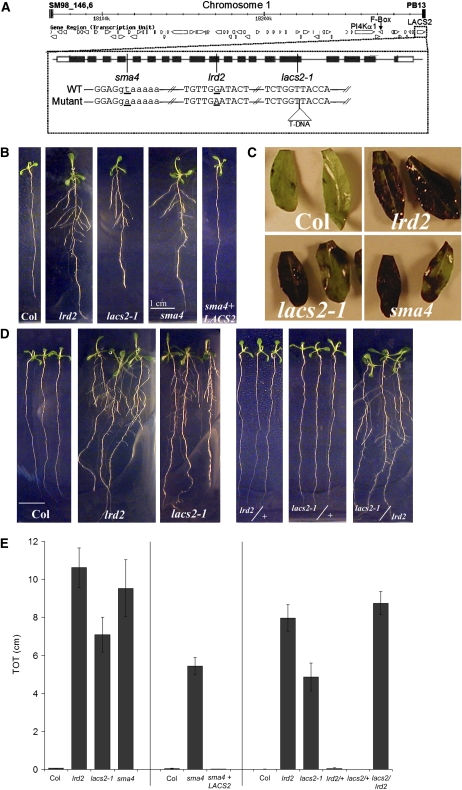
Figure 1.
lrd2 Is an Allele of LACS2.
(A) All open reading frames (open arrows) within the smallest map interval defining lrd2 are shown. The genes harboring mutations (At1g49340, PI4Kα1; At1g49360, F-box protein; At1g49430, LACS2) are noted. The gene structure of LACS2 and the locations of the mutations in lrd2 and two additional alleles of LACS2 (sma4 and lac2-1) are also shown. Black boxes represent exons, the black line represents introns, and white boxes represent untranslated regions. Positions of the mutated bases in each allele are indicated by a vertical line. Slashes indicate gaps in the sequence. Mutated bases in sma4 and lrd2 are underlined, and the position of the T-DNA insert in lacs2-1 is indicated by a labeled triangle. Uppercase letters denote exon sequence, while lowercase letters denote intron sequence.
(B) lrd2 and two additional mutant alleles of LACS2 exhibit increased lateral root formation under mild osmotic stress conditions; this phenotype can be complemented with the wild-type genomic sequence. Wild-type (Columbia [Col]), lrd2, lacs2-1, sma4, and sma4 + LACS2 (sma4 plants carrying the wild-type genomic sequence, from endogenous promoter to 3′ untranslated region [UTR] of LACS2) plants were grown for 12 d on mild osmotic stress conditions. Whereas lacs2-1 and sma4 seedlings resemble lrd2 plants, sma4 + LACS2 seedlings resemble the wild type. One representative plant for each genotype is shown.
(C) lrd2 exhibits phenotypes previously attributed to loss of LACS2 function. Three cauline leaves from each of three 3- to 6-week-old soil-grown wild-type (Col), lrd2, lacs2-1, or sma4 plants were stained with TB. Representative leaves from two plants are shown. Note variability in staining.
(D) The lacs2-1/lrd2 transheterozygote exhibits increased lateral root formation on mild osmotic stress conditions. The parental lines (wild type [Col], lrd2, and lacs2-1) and the F1 progeny of lrd2 × Col (lrd/+), lacs2-1 × Col (lacs2/+), and lacs2-1 × lrd2 (lacs2/lrd2) were grown for 12 d on mild osmotic stress conditions. Three representative plants for each genotype are shown.
(E) Total lateral root lengths (TOT) were quantified for plants shown in (B) and (D). Vertical lines separate independent experiments. Bars represent averages of 10 or more plants ± se except for sma4 in the left panel, where TOT for only three plants were averaged. All mutant TOTs are significantly different from their relevant wild-type controls (Student's t test, P value ≤ 0.0001) except for sma4 + LACS2 and the heterozygous lrd2/+ and lacs2-1/+ lines (Student's t test, P ≥ 0.21).
To identify the gene causing the lrd2 phenotype, we used map-based cloning techniques to narrow the map interval to a 228-kb region on the south arm of chromosome 1 (Figure 1A). This region is predicted to contain 62 open reading frames. Sequencing of the complete coding region for each of these genes identified four point mutations in three genes near the south end of the map interval: At1g49340, phosphatidylinositol 4-kinase PI4Kα1; At1g49360, F-box domain protein; and At1g49430, long-chain acyl-CoA synthetase LACS2 (Figure 1A). Investigation revealed that the mutations in the first two genes were unlikely to be responsible for the lrd2 phenotype (see Supplemental Figures 1 and 2 and Supplemental Table 1 online), leading us to focus on LACS2.
lrd2 contains a G-to-A transition in the tenth exon of the LACS2 gene, which is predicted to cause a Gly-to-Glu amino acid substitution (Figure 1A). Four mutant alleles of LACS2 were previously identified, but no lateral root phenotype was described for any of them. We characterized the root system phenotype of two of these mutants in culture: lacs2-1 is null for LACS2 RNA (Schnurr et al., 2004), and sma4 contains a splice site mutation that causes an early truncation of the protein (Tang et al., 2007). Indeed, both lacs2-1 and sma4 phenocopied the lateral root formation phenotype seen in lrd2 plants when grown in culture on mild osmotic stress conditions (Figures 1B and 1E). The lrd2 plants also exhibited phenotypes previously attributed to loss of LACS2 function. For instance, an RNA null allele of LACS2 (lacs2-3) was previously shown to cause defects in the leaf cuticle, resulting in increased permeability to toluidine blue O (TB) (Bessire et al., 2007). Despite some variability, cauline leaves of lrd2, lacs2-1, and sma4 plants all showed increased permeability to TB compared with cauline leaves from wild-type plants (Figure 1C). Together, these data support the identification of lrd2 as an allele of LACS2.
To provide formal genetic proof that LRD2 is LACS2, we crossed lacs2-1 plants to lrd2 plants. The resulting F1 transheterozygote exhibited increased lateral root formation when gown on mild osmotic stress conditions, indicating a lack of complementation (Figures 1D and 1E). Finally, we examined the root systems of sma4 plants transformed with a construct containing genomic DNA 1.5 kb upstream of the predicted LACS2 translational start site, the entire coding region, and 0.8 kb of sequence downstream from the predicted LACS2 translational stop site (Tang et al., 2007). This construct was previously shown to rescue the sma4 disease-related phenotype (Tang et al., 2007). The plants carrying the transgene had dramatically reduced lateral root formation compared with sma4 (Figures 1B and 1E). Collectively, these data indicate that the mutation in LACS2 is responsible for the lrd2 phenotypes and that LRD2/LACS2 is required for repression of lateral root formation.
LACS2 Represses LR Formation in Culture through Its Role in Cutin Biosynthesis
Identifying LRD2 as an allele of LACS2 lead us to ask how mutating this gene might promote lateral root formation. RNA analysis showed that LACS2 transcripts are present throughout the plant, including the root, providing no clear insight into how LACS2 regulates lateral root formation (Shockey et al., 2002; Schnurr et al., 2004). The only previous functional characterization of LACS2 in Arabidopsis indicated that it plays a role in cutin biosynthesis in the epidermal cells of aerial tissues (Schnurr et al., 2004; Bessire et al., 2007; Tang et al., 2007). To visualize this defect, we examined the guard cells of the wild type and lrd2 after staining with Nile Red, a lipophilic dye. Staining of the cuticular ledge of guard cells was strongly reduced in gpat4 gpat8 double mutants, which have a 60 to 70% decrease in cutin aliphatic monomer content (Li et al., 2007). Indeed, in lrd2 guard cells the cuticular ledge was apparently absent, consistent with the predicted role for LRD2/LACS in cutin formation (Figure 2A).
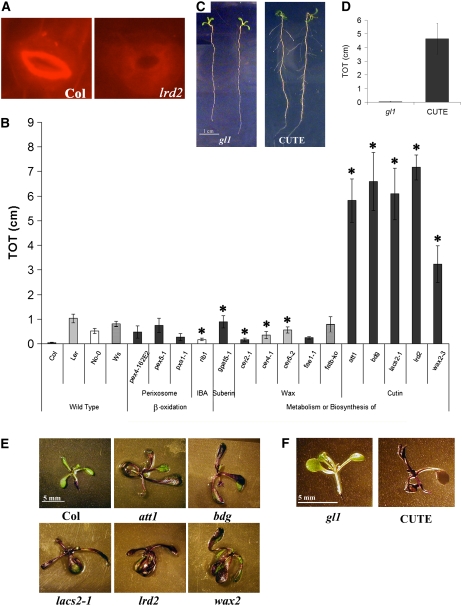
Figure 2.
Cutin-Defective Mutants Exhibit an lrd2-Like Increase in Lateral Root Formation and an Increase in Aerial Tissue Permeability on Mild Osmotic Stress Conditions.
(A) The cuticular ledge, visualized by Nile Red staining, is apparently absent in lrd2 guard cells, consistent with a role for LRD2/LACS2 in cutin formation (×40 magnification).
(B) Of a panel of fatty acid metabolism mutants, only cutin-defective mutants exhibit an lrd2-like increase in lateral root formation on mild osmotic stress conditions. Total lateral root lengths (TOT) were quantified for plants grown for 12 d on mild osmotic stress conditions. Bars represent averages of 5 to 17 plants ± se. Mutants are grouped by the biological function they alter. Three alleles of cer4 and pex4 and two alleles of gpat5 were evaluated with similar results; for simplicity, only one allele of each is shown. The appropriate wild-type plants for the mutants used are shown, and the bar's shading denotes the background: rib1 is in Nossen (No-0, white), cer2-1 and cer4-1 are in Landsberg erecta (Ler, light gray), fatB-ko is in Wassilewskija (Ws, hatched), and the remaining mutants are in Columbia (Col, dark gray). Symbols denote mutants with significantly different lateral root formation from the appropriate wild-type ecotype (Student's t test, P ≤ 0.05). Only att1, bdg, and lacs2-1 are not statistically different from lrd2 (Student's t test, P ≥ 0.20).
(C) Expression of a fungal cutinase is sufficient to cause an increase in lateral root formation. The wild type (gl1) and plants constitutively expressing a fungal cutinase from Fusarium solani f sp pisi (CUTE) were grown for 12 d on mild osmotic stress conditions. Two representative plants for each genotype are shown.
(D) Total lateral root lengths (TOT) of the plants shown in (C) were quantified. Bars represent averages of six or more plants ± se. TOT of CUTE plants is significantly different from the TOT of gl1 plants (Student's t test, P ≤ 0.010).
(E) and (F) Cutin-defective mutants, including lrd2, exhibit increased aerial tissue permeability to TB in culture. att1, bdg, lacs2-1, lrd2, wax2, CUTE plants, and appropriate background controls (gl1 for CUTE; Col for all others) were grown for 12 d on mild osmotic stress conditions and stained with TB. One representative plant from each genotype is presented.
It initially seemed unlikely that compromised cutin production would effect lateral root formation, so we looked for alternative explanations. Acyl-CoA synthetases activate free fatty acids by catalyzing their esterification to acyl-CoAs in a two-step, ATP-dependent reaction (Kornberg and Pricer, 1953). Activation is required for fatty acids to be recognized by most lipid metabolic enzymes (Watkins, 1997). LACS-dependent activation of fatty acids in mammals and yeast has been shown to be important in fatty acid desaturation and/or elongation, glycerolipid biosynthesis, protein acetylation, and the release of stored energy via β-oxidation (Watkins, 1997; Daum et al., 1998). Plants also use activated fatty acids to produce cutin in the aerial tissues, suberin in a variety of tissues (Kolattukudy, 1985; Nawrath, 2002; Graça and Santos, 2007; Li et al., 2007), and the soluble epicuticular and intracuticular waxes (Kunst and Samuels, 2003).
Because of the range of pathways that could potentially be affected in lrd2, we used a genetic approach to identify the role of LACS2 that is essential for correct regulation of lateral root formation. Mutants in β-oxidation, suberin biosynthesis, wax biosynthesis, and cutin biosynthesis were therefore all grown under mild osmotic stress conditions and their root systems were examined. β-Oxidation mutants used were previously shown to alter peroxisome biogenesis (pex4; Zolman et al., 2005) or protein import into the peroxisome (pex5, Zolman et al., 2000; pxa1, Zolman et al., 2001). A mutant with altered IBA response (rib1; Poupart et al., 2005) was also included because indole-3-butyric acid (IBA) is converted to indole-3-acetic acid (IAA) by β-oxidation in the peroxisome. A suberin mutant was tested that carries a defective copy of the acyl-CoA:glycerol-3-phosphate acyltransferase (GPAT5) and has approximately half the amount of root suberin found in the wild type (Beisson et al., 2007). Wax biosynthesis mutants were used that are compromised in a variety of steps in wax metabolism. These included ECERIFERUM mutants cer2, cer4, and cer5; a mutant allele of FATTY ACID ELONGATION1 (fae1-1); and a mutant allele of FATTY ACYL-ACP THIOESTERASE B (fatB) (Lemieux et al., 1990; Negruk et al., 1996; Bonaventure et al., 2003; Pighin et al., 2004; Rowland et al., 2006). Finally, the cutin biosynthesis mutants aberrant induction of type three genes1 (att1), bodyguard (bdg), and wax2, all of which have alterations in the amount or composition of cutin, were tested (Chen et al., 2003; Xiao et al., 2004; Kurdyukov et al., 2006).
β-Oxidation, suberin, and wax biosynthesis mutants showed little if any alteration in lateral root formation compared with their respective wild-type controls, and none phenocopied lrd2 (Figure 2B). By contrast, when the three mutants shown to have alterations in the amount or composition of leaf cutin (att1, bdg, and wax2) were grown in culture on mild osmotic stress conditions, all three closely resembled lrd2, forming significantly more lateral roots than wild-type plants (Figure 2B). The strong recapitulation of the lrd2 lateral root formation phenotype in these three cutin mutants and none of the other mutants affecting fatty acid metabolism, wax production, or suberin formation strongly suggests that, despite our initial skepticism, wild-type production and deposition of cutin is necessary to repress lateral root formation in wild-type plants.
To confirm that cutin is indeed necessary to repress lateral root formation on mild osmotic stress conditions, we examined transgenic plants that constitutively express a fungal cutinase that degrades the plant's endogenous cutin (CUTE; Sieber et al., 2000). We found that CUTE plants, like lrd2 plants, formed many lateral roots when grown in culture on mild osmotic stress conditions (Figures 2C and 2D). Because the transgene expressed in the CUTE plants specifically and uniquely degrades cutin, this result confirms that a reduction in cutin is sufficient to increase lateral root formation in culture-grown Arabidopsis seedlings.
Plants grown in culture experience extremely high humidity, which is expected to reduce the formation of the cuticle and increase its permeability (Schreiber et al., 2001; Shepherd and Wynne Griffiths, 2006). This might suggest that mutations in genes affecting cuticle/cutin biosynthesis would have little effect on plants grown in culture. However, TB staining confirmed that lrd2, lacs2-1, att1, bdg, wax2, and CUTE plants all have increased permeability to this dye in their aerial tissues compared with the wild type when grown on our mild osmotic stress culture conditions (Figures 2E and 2F). Hence, reductions in cutin correlate with increased permeability to dye in aerial tissues and increased lateral root formation in plants grown in culture.
Lateral Root Formation in Culture Is Regulated by Aerial Tissue Permeability to Sucrose from the Media
Decreased Aerial Tissue Permeability Correlates with Osmotic Repression of Lateral Root Formation in Wild-Type Plants
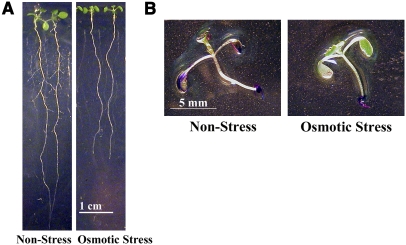
As described above, mild osmotic stress has a profoundly repressive effect on lateral root formation in wild-type plants in culture (Deak and Malamy, 2005; Figure 3A). Characterization of the lrd2 mutant suggested an unexpected role for aerial tissue permeability in regulating lateral root formation in culture. Therefore, we tested whether wild-type plants grown under mild osmotic stress conditions have decreased aerial tissue permeability compared with those grown on nonstress conditions that permit lateral root formation (media with reduced sucrose and nitrogen salts). In plants grown on nonstress conditions, TB staining of the aerial tissues was consistently observed, while essentially no TB staining was visible in the aerial tissues of seedlings grown on mild osmotic stress conditions (Figure 3B). Hence, osmotic stress decreases aerial tissue permeability to dye and represses lateral root formation in culture.
Figure 3.
Decreased Aerial Tissue Permeability Correlates with Osmotic Repression of Lateral Root Formation in Wild-Type Plants.
(A) Wild-type (Col) plants exhibit increased lateral root formation when grown on culture conditions that permit lateral root formation (nonstress) compared with those grown on mild osmotic stress culture conditions. All plants were grown for 12 d, and two representative plants from each condition are shown.
(B) Wild-type (Col) plants were grown for 12 d on osmotic stress or nonstress conditions and then the aerial tissues were stained with TB. Aerial tissues of wild-type plants grown on nonstress conditions exhibit increased permeability to TB compared with plants grown on mild osmotic stress conditions. One representative plant from each condition is shown.
Contact between the Seedling's Aerial Tissues and the Media Is Essential for Lateral Root Formation
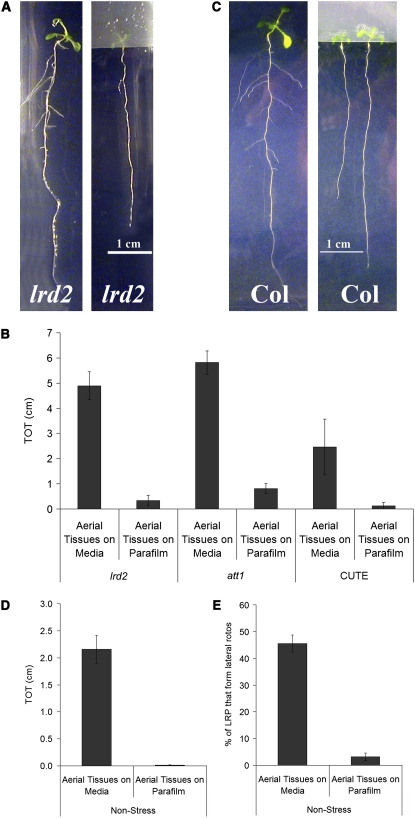
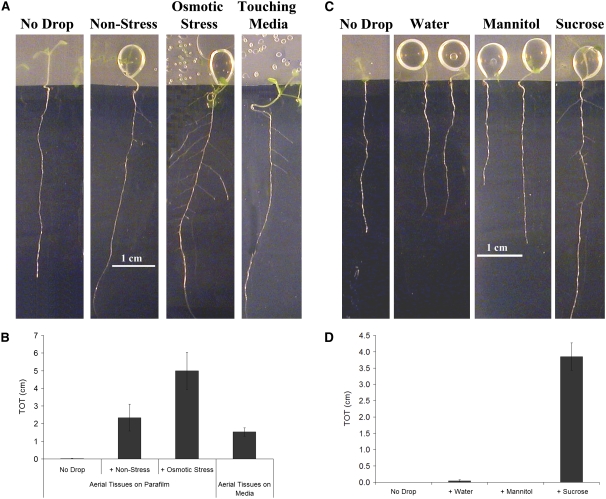
The finding that the permeability of aerial tissues to TB correlates well with lateral root formation in wild-type and mutant plants grown in culture led us to further investigate the role of the aerial tissues. In our assay, the entire seedling, including the aerial tissues, is in direct contact with the culture medium. To test whether aerial tissue contact with the media is important for lateral root formation, we first sowed lrd2 seeds on mild osmotic stress media immediately adjacent to strips of Parafilm, such that the aerial tissues grew exclusively onto the Parafilm and did not come into contact with the media at any time (Figure 4A). Indeed, this growth protocol resulted in lrd2 seedlings with dramatically reduced numbers of lateral roots (Figure 4A). Similar results were observed with other mutants with increased aerial tissue permeability due to defects in cutin biosynthesis (Figure 4B). We obtained identical results when glass cover slips were used in place of the Parafilm to block contact between aerial tissues and media (data not shown). Strikingly, we also found that when the aerial tissues of wild-type plants grown on nonstress conditions were prevented from contacting the medium, lateral root formation was completely inhibited (Figures 4C and 4D). Hence, contact between the media and aerial tissue is necessary for lateral root formation in culture.
Figure 4.
Contact between Aerial Tissues and Media Is Necessary for Lateral Root Formation.
Plants were grown for 12 d, and the aerial tissues were either allowed to contact the media (Aerial Tissues on Media) or isolated from the media by a strip of Parafilm (Aerial Tissues on Parafilm). Because the plants are photographed from the media side of the plate, the aerial tissues are only faintly visible through the strip of Parafilm.
(A) The aberrant lateral root formation of lrd2 is severely reduced or eliminated when the aerial tissues are prevented from contacting the media. One representative plant from each treatment is shown.
(B) Total lateral root lengths (TOT) were quantified for lrd2, att1, and CUTE grown for 12 d on mild osmotic stress conditions. Bars represent averages of seven or more plants ± se, except for CUTE where bars represent three plants ± se. TOTs of all genotypes grown with their aerial tissues on Parafilm are significantly reduced compared with the TOTs of similar plants with aerial tissues in contact with the media (Student's t test, P ≤ 0.01).
(C) Lateral root formation in wild-type plants is severely reduced or eliminated when the aerial tissues of plants grown for 12 d on nonstress media are prevented from contacting the media. Two representative plants from each treatment are shown.
(D) Total lateral root lengths (TOT) were quantified for plants in (C). Data presented are combined from three experimental replicates. Bars represent averages of 11 or more plants ± se. The TOT of plants grown with their aerial tissues on Parafilm is significantly reduced compared with the TOT of plants with aerial tissues in contact with the media (Student's t test, P ≤ 0.0001).
(E) The percentage of LRPs that emerge in wild-type plants grown with aerial tissues isolated on Parafilm is significantly reduced compared with plants grown with aerial tissues in contact with the media (Student's t test, P ≤ 0.0001). Plants were grown for 12 d on nonstress conditions. Data presented are combined from three experimental replicates. Bars represent averages of 11 or more plants ± se.
We examined wild-type plants with aerial tissues isolated on parafilm more closely. LRPs were formed in these plants, but few if any emerged to become lateral roots, while a significant percentage of LRPs emerged in plants grown with aerial tissues in contact with the media (Figure 4E). Hence, contact between aerial tissues and media stimulates LRP emergence.
We next asked whether contact between the aerial tissues and the media was not only necessary but also sufficient to stimulate lateral root formation. To test this idea, we added a 100-μL drop of culture medium to aerial tissues that were isolated on Parafilm. In wild-type seedlings grown on nonstress media, adding a drop of the same media restored lateral root formation; root systems resembled those of plants that had fallen off the Parafilm and were touching the nonstress media in the plate (Figures 5A and 5B). Interestingly, supplementing the aerial tissues with a drop of the media used to simulate mild osmotic stress had a similar or even greater promotive effect on lateral root formation (Figures 5A and 5B).
Figure 5.
Contact between Permeable Aerial Tissues and Sucrose Is Sufficient to Stimulate Lateral Root Formation in Wild-Type Plants.
Wild-type plants were grown for 5 d on nonstress conditions with their aerial tissues isolated from the media by a strip of Parafilm. Treatments were then applied as described and plants were photographed and analyzed 8 d later. Because the plants are photographed from the media side of the plate, the aerial tissues are only faintly visible through the strip of Parafilm; treatments are clearly visible as drops on the Parafilm.
(A) A drop of nonstress or mild osmotic stress media applied to aerial tissues is sufficient to restore lateral root formation. For comparison, a plant to which no drop was added and a plant whose aerial tissues touched the media are also shown. One representative plant from each treatment is presented.
(B) Total lateral root lengths (TOT) were quantified for plants in (A). Data presented are combined from three experimental replicates. Bars represent the average of 10 or more plants ± se. TOTs of plants to which the nonstress media or osmotic stress media was added, or that were touching the media, are significantly greater than the TOTs of plants to which no drop was added (Student's t test, P ≤ 0.01).
(C) A drop of sucrose solution, but not an equimolar mannitol solution or water alone, applied to aerial tissues is sufficient to restore lateral root formation. One representative plant from each treatment is shown. For comparison, a plant to which no drop was added is shown.
(D) Total lateral root lengths (TOT) were quantified for plants in (C). Data presented are combined from four experimental replicates. Bars represent the average of 13 or more plants ± se. TOTs of plants to which the sucrose drop was added are significantly greater than the TOTs of plants to which water had been added (Student's t test, P ≤ 0.00001), while mannitol had no significant effect (Student's t test, P ≥ 0.1).
Aerial Tissue Contact with Sucrose Is Necessary and Sufficient to Stimulate Lateral Root Formation in Wild-Type Plants
The effective stimulatory component of the culture medium may be a distinct chemical. Sucrose is a good candidate, as it has been directly implicated in stimulating lateral root formation (Takahashi et al., 2003; Karthikeyan et al., 2007). Furthermore, studies have shown that increased exposure to light (and, by inference, increased production of photosynthate) causes increased lateral root formation (Reed et al., 1998; Freixes et al., 2002, and references therein). To test whether sucrose is sufficient to promote lateral root formation in wild-type plants, a drop containing only 1% sucrose (as found in nonstress media) and agar was placed on the isolated aerial tissues of plants grown under nonstress conditions. As controls, agar drops were applied with a molar equivalent of the nonmetabolized sugar mannitol or with only water. Addition of sucrose to the seedling's aerial tissues strongly stimulated lateral root formation, whereas addition of mannitol or water alone did not (Figures 5C and 5D). By contrast, a drop of mild osmotic stress media from which the sucrose was omitted failed to promote lateral root formation (data not shown). This result demonstrates that sucrose in contact with the aerial tissues of the seedling is sufficient to promote lateral root formation in seedlings grown on nonstress conditions. These data also explain why a drop of mild osmotic stress media was at least as promotive as the drop of nonstress media when added to aerial tissues, as the osmotic stress media contains higher levels of sucrose. Together, these results demonstrate that contact between aerial tissues and sucrose in the media is necessary and sufficient to stimulate LRP emergence and, therefore, that long distance communication between the aerial tissues and the root regulates lateral root formation in culture.
Aerial Tissues with an Increased Permeability to TB Also Show Increased Permeability to Sucrose
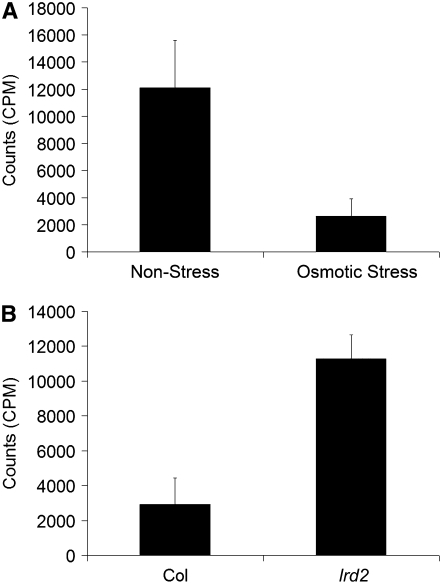
Our data show that lateral root formation is tightly correlated with aerial tissue permeability to TB and requires contact between aerial tissues and sucrose. This suggests that sucrose in the media is taken up at increased levels by permeable aerial tissues. Furthermore, contact between aerial tissues and sucrose is not sufficient to stimulate lateral root formation in wild-type plants grown on mild osmotic stress conditions, which suggests that sucrose is less able to enter the dye-impermeable aerial tissues of these plants. To test this hypothesis directly, aerial tissues of wild-type plants grown in the presence or absence of mild osmotic stress were isolated on Parafilm for 14 d and then treated with 14C-labeled sucrose. Indeed, a fourfold or greater increase in sucrose uptake was seen in plants grown on nonstress conditions compared with those grown on mild osmotic stress conditions (Figure 6A). Similarly, lrd2 plants accumulated much more 14C-sucrose when grown on osmotic stress conditions than wild-type plants grown on the same conditions (Figure 6B). Hence, quantitative measures of sucrose uptake confirm the differences in permeability that were predicted using TB staining and demonstrate that aerial tissues of plants with increased lateral root formation in culture take up more sucrose from the media.
Figure 6.
Growth Conditions and Mutations That Increase Lateral Root Formation and Permeability to TB Also Increase Aerial Tissue Uptake of Sucrose.
(A) Wild-type plants were grown on nonstress or mild osmotic stress conditions as indicated for 14 d with their aerial tissues isolated from the media by a strip of Parafilm. 14C-sucrose was then applied to the aerial tissues. Plants were harvested 1 h later, and the amount of 14C-sucrose uptake was assessed. Bars represent averages of eight or more plants ± se. Plants grown on nonstress conditions accumulate significantly more counts than plants grown on mild osmotic stress conditions (Student's t test, P ≤ 0.03).
(B) Wild-type (Col) or lrd2 plants were grown on mild osmotic stress conditions with their aerial tissues isolated from the media by a strip of Parafilm. Uptake of 14C-sucrose was assessed as in (A). Bars represent averages of nine or more plants ± se. lrd2 accumulates significantly more counts than wild-type plants grown under mild osmotic stress conditions (Student's t test, P ≤ 0.001).
Roots Sense Osmotic Stress and Alter Aerial Tissue Permeability via an ABA-Dependent Mechanism
Aerial Tissue Permeability Is Regulated through a Long-Distance Signaling Mechanism
In the above 14C sucrose uptake experiments, aerial tissues were isolated from the media by Parafilm; nevertheless, differences in uptake were clearly observed between plants grown on mild osmotic stress and nonstress conditions (Figure 6A). Therefore, the permeability of aerial tissues must be determined by the conditions experienced by the roots. Indeed, we found that in seedlings grown on mild osmotic stress conditions, the aerial tissues exhibited no TB staining whether aerial tissues were in contact with the media or prevented from contacting the media by Parafilm (Figures 3B and 7A). Similarly, in plants grown on nonstress conditions, aerial tissues showed TB staining whether aerial tissues were in contact with the media or not (Figures 3B and 7A). These observations reveal that there is a long-distance signaling pathway between the roots and aerial tissues in culture, in which roots perceive their environment and somehow modulate the barrier between the aerial tissues and their surroundings.
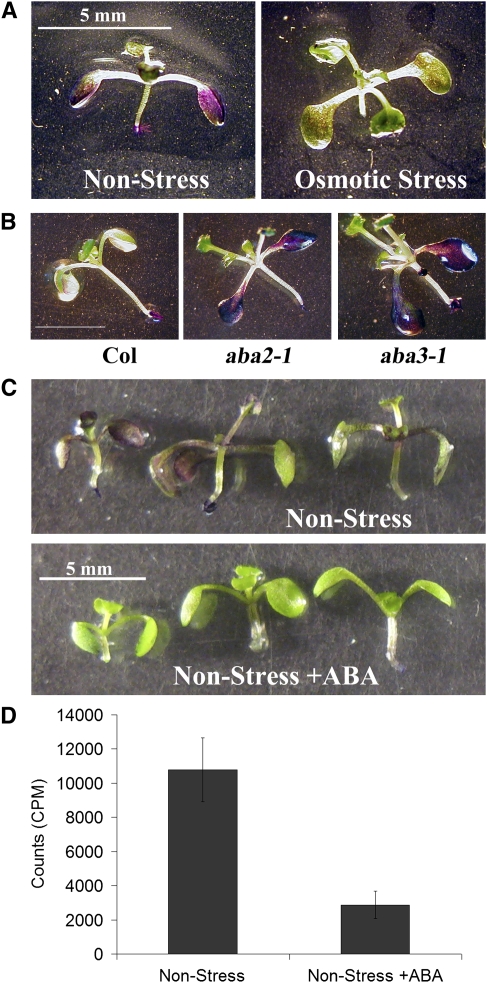
Figure 7.
ABA at the Root Is Sufficient to Alter the Permeability of Aerial Tissues.
(A) Root contact with culture media is sufficient to determine permeability of the aerial tissues to TB. Wild-type (Col) plants were grown for 12 d on nonstress or mild osmotic stress conditions as indicated with their aerial tissues isolated from the media by a strip of Parafilm. The aerial tissues of these plants were then stained with TB. One representative plant from each condition is presented.
(B) Wild-type (Col), aba2-1, and aba3-1 plants were grown for 12 d on mild osmotic stress conditions and then the aerial tissues were stained with TB. One representative plant of each genotype is shown.
(C) Wild-type (Col) plants were grown for 5 d on nonstress conditions and then transferred to nonstress media supplemented with 1 μM ABA dissolved in NaOH or with NaOH alone (control). Aerial tissues were isolated from the media by a strip of Parafilm before and after transfer. After eight additional days, the aerial tissues of these plants were stained with TB. Three representative plants from each condition are presented.
(D) Uptake of 14C-sucrose was quantified for plants grown as in (B). Bars represent averages of 14 or more plants ± se. The 14C-sucrose uptake by plants with roots in contact with ABA is significantly less than in the control (Student's t test, P ≤ 0.001).
Aerial Tissue Permeability in Wild-Type Plants Is Regulated by an ABA-Dependent Signal from the Roots
ABA is a known regulator of plant responses to osmotic stress and therefore is a good candidate for the long-distance signal coordinating osmotic stress conditions at the root and aerial tissue permeability. We previously concluded that ABA is necessary for repression of lateral root formation on mild osmotic stress conditions, based on the observation that two nonallelic mutants that are compromised in ABA biosynthesis, aba2-1 and aba3-1, demonstrate increased lateral root formation compared with the wild type (Deak and Malamy, 2005). Indeed, when aba2-1 and aba3-1 plants were grown on mild osmotic stress conditions, the aerial tissues of both mutants were more permeable to TB compared with the wild type, especially in the cotyledons (Figure 7B). Hence, ABA is required for correct regulation of aerial tissue permeability.
We next tested whether ABA regulation of aerial tissue permeability might act at a distance, as does mild osmotic stress. The addition of 1 mM ABA to nonstress media was sufficient to repress lateral root formation in wild-type plants (TOT = 0.91 ± 0.22 cm without ABA; TOT = 0.02 ± 0.01 cm with ABA, ±se, Student's t test, P < 0.001, n = 23), as previously demonstrated (DeSmet et al., 2003). Indeed, when wild-type plants were grown with their aerial tissues on Parafilm and only their roots on nonstress media supplemented with 1 mM ABA, aerial tissue permeability to TB and 14C-sucrose was decreased compared with plants grown on nonstress media alone (Figures 7C and 7D). These results indicate that (1) ABA is not only necessary but also sufficient to reduce aerial tissue permeability; and (2) ABA applied only at the roots exerts effects in the aerial tissues, confirming that ABA can precipitate a long distance signaling process that alters aerial tissue permeability.
ABA-Dependent Reduction in Aerial Tissue Permeability Does Not Depend on Stomata
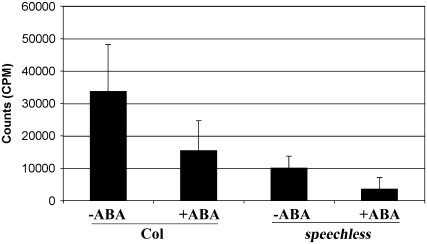
It is often difficult to distinguish between movement of molecules into the leaf through the cuticle and through the stomata (Kerstiens et al., 2006). Therefore, ABA-mediated decrease in aerial tissue permeability may be ascribed to changes in either of these structures. There is considerable literature demonstrating that osmotic stress increases cuticular wax deposition and cuticular thickness in soil grown plants, leading to decreases in water loss (reviewed in Shepherd and Wynne Griffiths, 2006), and this may be mediated by ABA. Alterntively, ABA-mediated signaling has long been known to decrease stomatal aperture. We attempted to shed light on how ABA decreases aerial tissue permeability in our assay system by examination of the speechless mutant, which lacks stomata (Serna, 2007). Indeed, there was a dramatic decrease in 14C-sucrose uptake in the aerial tissues of the speechless mutant (Figure 8), indicating that stomata are the primary route of sucrose entry. Importantly, 14C-sucrose accumulation was not completely eliminated in speechless, suggesting that sucrose can enter leaves through another method. Furthermore, ABA treatment lead to reduced 14C-sucrose uptake in both the wild-type and speechless genotypes (Figure 8). Hence, while much of the aerial tissue's permeability to external sucrose is due to the presence of stomata, and ABA is known to reduce stomatal aperture, ABA must also reduce aerial tissue permeability by another mechanism.
Figure 8.
Sucrose Enters Aerial Tissues through Both Stomata and a Second Route That Is Modified by ABA.
Wild-type and speechless mutant seedlings were germinated on nonstress media and allowed to grow for 5 d. They were then transferred to the same media with or without 1 μM ABA. After 7 d of further growth, 14C-sucrose uptake was measured. Bars represent averages of measurements on 12 to 16 cotyledons from different plants ± se. speechless mutants showed significantly less 14C-sucrose uptake than the wild type (Student's t test, P ≤ 0.0001). ABA treatment lead to reduced 14C-sucrose uptake in both wild-type (P ≤ 0.002) and speechless (P ≤ 0.00001) genotypes.
Sucrose Coordinates Growth of the Root and Shoot Systems
Only Metabolized Sugars Stimulate LRP Emergence
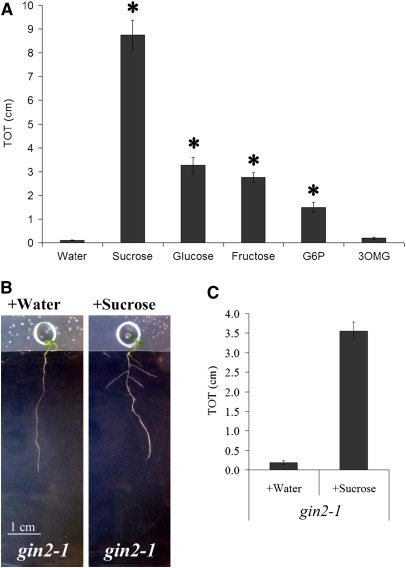
In the absence of osmotic stress, exogenous sucrose enters permeable leaves in culture, leading to dramatic stimulation of LRP emergence. Sucrose accumulation in leaves is normally the result of photosynthesis. Sucrose metabolism results in energy production and creates the building blocks for many carbon compounds necessary for growth. Sucrose and its immediate metabolite glucose can also act as signals; nonmetabolized analogs can regulate certain downstream processes, indicating that they act independent of metabolism (for review, see Gupta and Kaur, 2005). Glucose phosphorylation by hexokinase is a key step in metabolism-independent glucose signaling (Gupta and Kaur, 2005). To further investigate the mode of action of sucrose in altering root system architecture, we examined the effect of downstream metabolites. Glucose, fructose, and glucose-6-phosphate all induced lateral root formation (Figure 9A), although to a lesser extent than sucrose. By contrast, the nonmetabolized, phosphorylated glucose analog 3-O-methyl glucose (Gupta and Kaur, 2005) failed to induce lateral root formation (Figure 9A). Furthermore, in the hexokinase mutant gin2, which abrogates glucose signaling but is capable of glucose metabolism (Moore et al., 2003), exogenous sucrose was still able to induce LRP emergence (Figures 9B and 9C). Taken together, these results strongly suggest that sucrose metabolism rather than signaling is the regulatory factor involved in LRP emergence.
Figure 9.
Increases in Sugar Metabolism, Not Sugar Signaling, Stimulate Lateral Root Formation.
(A) Wild-type plants were grown for 5 d on nonstress conditions with their aerial tissues isolated from the media by a strip of Parafilm. A drop of water containing equimolar amounts of sucrose, glucose, fructose, glucose-6-phosphate (G6P), or 3-O-methylglucose (3OMG) or water alone was then applied as described, and total lateral root lengths (TOT) were quantified 8 d later. Data presented are combined from two experimental replicates. Bars represent the average of 25 to 70 plants ± se. Symbols indicate a significantly greater TOT when compared with water (Student's t test, P ≤ 0.0001). 3-o-methylglucose does not stimulate lateral root formation significantly when compared with water (Student's t test, P ≥ 0.05).
(B) Stimulation of lateral root formation by sucrose does not require the major hexokinase GIN2. gin2-1 plants were grown for 5 d on nonstress conditions with their aerial tissues isolated from the media by a strip of Parafilm. A drop of sucrose, but not water alone, applied to aerial tissues is sufficient to restore lateral root formation in both genotypes.
(C) Total lateral root lengths (TOT) of the plants shown in (B) were quantified. Bars represent averages of 23 or more plants ± se. Sucrose caused a significantly increased TOT in gin2-1 (Student's t test, P ≤ 0.00001).
Sucrose-Mediated Changes in Root System Architecture Can Be Separated from Some but Not All Other Sucrose Effects on Growth and Development
Uptake of sucrose by permeable aerial tissues leads to several physiological changes in addition to increased LRP emergence. In wild-type plants grown on nonstress conditions with aerial tissues isolated on Parafilm in the presence of a drop of sucrose, lateral root formation was stimulated, primary roots were longer, and the overall mass of the aerial tissues was increased (Table 1). Close examination of the aerial tissues revealed that the sucrose-induced increase in mass is due both to an increased number of leaves and an increase in the size of the cotyledons and leaves (Table 1). Therefore, we considered the possibility that increases in LRP emergence, primary root length, and shoot system development are inextricably linked and that sucrose causes a coordinated increase in all aspects of plant growth rather than having a specific effect on root architecture. However, primary roots of plants grown on osmotic stress conditions for 20 d are longer than the primary roots of 12-d-old plants grown on nonstress conditions; nevertheless, 20-d-old plants on osmotic stress conditions still show little or no LRP emergence, while 12-d-old plants on nonstress conditions have highly branched root system architectures, as evidenced by the strong difference in TOT values (Table 2; previously shown in Deak and Malamy, 2005). Hence, increased sucrose uptake by the aerial tissues does not simply hasten an invariant program of growth and development, but alters the developmental program by selectively increasing LRP emergence.
Table 1.
Sucrose Contacting Permeable Aerial Tissues Increases Both Shoot and Root Development
| TOT (cm)ab | PRL (cm)bc | FW of Aerial Tissues (mg)bd | No. of Visible Leavesb | Surface Area of Cotyledons (mm2)b | Total Surface Area (mm2)b | |
|---|---|---|---|---|---|---|
| +Watere | 0.06 ± 0.04 | 4.93 ± 0.48 | 2.39 ± 0.33 | 5.5 ± 0.27 | 82.3 ± 9.4 | 114.1 ± 14.6 |
| +Sucrosee | 7.44 ± 0.81 | 7.62 ± 0.16 | 6.84 ± 0.49 | 7.8 ± 0.13 | 148.6 ± 12.7 | 354.9 ± 17.6 |
| P valuef | <0.0001 | <0.001 | <0.0001 | <0.0001 | <0.001 | <0.0001 |
Total lateral root length.
Values are presented as the average values taken from 10 plants ± se.
Primary root length.
Aerial tissues were excised at the hypocotyl:root junction and weighed. FW, fresh weight.
Plants were grown with aerial tissues isolated on Parafilm for 5 d, at which point a 100-μL drop containing water or 1% sucrose in 0.7% agar was added. Plants were analyzed 8 d later.
Significance of difference between sucrose and water treatments evaluated by Student's t test.
Table 2.
The Relationship between Lateral Root Formation, Primary Root Length, and Aerial Tissue Mass Differs Quantitatively under Different Growth Conditions
| Days after Planting | Conditions | TOT (cm)ab | PRL (cm)bc | FW of Aerial Tissues (mg)bd |
|---|---|---|---|---|
| 12 | Nonstress | 5.09 ± 0.77 | 6.99 ± 0.13 | 5.19 ± 0.47 |
| 12 | Osmotic stress | 0.00 ± 0.00 | 4.86 ± 0.35 | 2.51 ± 0.12 |
| P valuee | <0.0001 | <0.0001 | <0.0001 | |
| 20 | Osmotic stress | 0.20 ± 0.07 | 7.99 ± 0.28 | 5.54 ± 0.39 |
| P valuef | <0.0001 | <0.005 | 0.5800 |
TOT, total lateral root length.
PRL, primary root length.
Aerial tissues from were excised at the hypocotyl:root junction. FW, fresh weight.
Data are presented as the average of 15 or more plants ± se.
Significance of the difference between 12-d-old plants grown in the presence or absence of osmotic stress was evaluated by a Student's t test. Osmotic stress leads to a significant decrease in TOT, PRL, and aerial tissue FW.
Significance of the difference between the 20-d-old plants grown on osmotic stress media and the 12-d-old plants grown on nonstress conditions was evaluated by a Student's t test. Aerial tissue FW were not statistically different, while PRL was significantly greater in the 20-d-old plants. By contrast, TOT was significantly lower in 20-d-old plants grown on osmotic stress conditions than in 12-d-old plants grown on nonstress conditions. Hence, increased PRL and aerial tissue FW fail to correlate with increases in TOT.
The above result shows that reaching a specific primary root length cannot be causal for LRP emergence and therefore effectively separates primary root length from lateral root formation. To test whether increases in shoot system growth can be separated from LRP emergence, we compared plants grown for 20 d under mild osmotic stress (little or no LRP emergence) to those grown for 12 d under nonstress conditions (extensive LRP emergence). The aerial tissues were approximately equal in mass (Table 2), indicating that the overall size of the shoot system cannot be causal for LRP emergence. However, leaf size and leaf number differed between these plants in some experiments, leaving the possibility that either parameter is inextricably correlated or even causal for LRP emergence.
From these data, it is not possible to determine whether sucrose metabolism stimulates lateral root formation directly or indirectly. The most likely scenarios based on the above observation are that (1) a sucrose metabolism-induced increase in leaf size or number leads to increased LRP emergence, or (2) sucrose metabolism stimulates coordinated but independent increases in LRP emergence and shoot system development.
DISCUSSION
A Model for Regulation of LRP Emergence in Culture
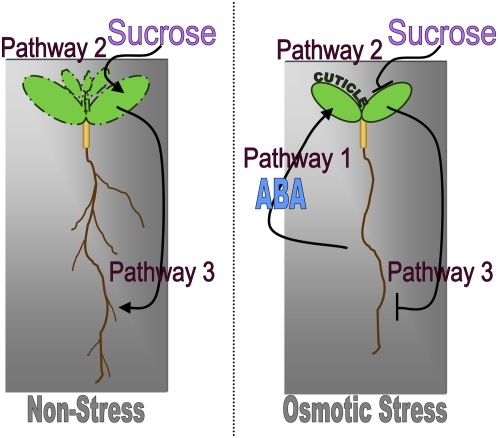
We initially set out to understand how mild osmotic stress, simulated by the addition of various osmotica to culture media, represses lateral root formation. Our data indicate that the connection between osmotic stress conditions and lateral root formation in culture is indirect and requires three separate but interacting pathways (Figure 10).
Figure 10.
Regulation of Lateral Root Formation in Culture.
Pathway 1: The root senses the osmotic potential of the growth media. In plants grown on mild osmotic stress conditions, the root sends an ABA signal that leads to decreased permeability of the aerial tissues. This pathway is not initiated, or operates to a lesser degree, in wild-type plants grown on nonstress conditions. No decrease in aerial tissue permeability is seen in mutants compromised in cuticle formation or biosynthesis of ABA. Pathway 2: Aerial tissue permeability determines the plant's ability to take up sucrose from the media. Plants grown on conditions that lead to permeable aerial tissues (i.e., nonstress) take up more sucrose than plants grown on conditions that lead to relatively impermeable aerial tissues (i.e., mild osmotic stress). Pathway 3: Sucrose in the aerial tissues is metabolized, leading to increased aerial tissue development and lateral root formation. Plants with impermeable aerial tissues cannot take up sufficient sucrose to induce these changes.
Pathway 1
The root perceives the osmotic potential of the growth media. When plants are grown in culture on mild osmotic stress conditions, roots direct the formation of relatively impermeable aerial tissues (Figure 10, Pathway 1). Since the ABA-deficient mutants aba2-1 and aba3-1 have increased aerial tissue permeability compared with the wild type on mild osmotic stress conditions, and exogenous ABA added to the roots is sufficient to reduce aerial tissue permeability, we conclude that ABA is an essential component of this pathway. The mechanism by which osmotic stress and ABA reduce aerial tissue permeability is unclear, but at least some proportion of ABA effects are independent of stomata. In the absence of osmotic stress, this pathway is negligible and aerial tissues are relatively permeable.
Pathway 2
The aerial tissues are in direct contact with the media. If these tissues are permeable, as they are under nonstress conditions, they take up sucrose from the media (Figure 10, Pathway 2). If the aerial tissues are relatively impermeable due to mild osmotic stress conditions experienced at the root, there is greatly reduced sucrose uptake. When genetic mutations are present that prevent proper formation of the cuticle (i.e., in lrd2), aerial tissues remain permeable to sucrose even under osmotic stress conditions.
Pathway 3
Under nonstress conditions, or in plants with defects in cutin formation, sucrose is taken up by aerial tissues and metabolized. This leads to an increase in LRP emergence through an unknown mechanism (Figure 10, Pathway 3). If the aerial tissues are impermeable, the presence of exogenous sucrose cannot induce lateral root formation, explaining the repressed phenotype of wild-type plants grown on mild osmotic stress conditions.
The Mechanism for Root-Mediated Regulation of Aerial Tissue Permeability
Our data indicate that osmotic regulation of aerial tissue permeability in culture requires ABA and that ABA applied to the root is sufficient to reduce sucrose uptake. It is tempting to conclude, based on this data, that osmotic stress in our system is communicated to the shoot via mobile ABA produced by the root, as has long been postulated for other long distance osmotic stress responses (Sauter et al., 2001; Wilkinson and Davies, 2002). However, in Arabidopsis and tomato (Solanum lycopersicum), certain root-directed osmotic stress responses in the aerial tissue, including stomatal closure, do not require ABA production in the root (Holbrook et al., 2002; Christmann et al., 2007). Therefore, it is possible that osmotic stress induces transport of some other signal that alters aerial tissue permeability in an ABA-dependent manner or that exogenously applied ABA bypasses the endogenous mechanism. Further experiments will therefore need to be done to definitively distinguish whether ABA acts as a mobile signal in this response pathway.
The likeliest targets of the ABA-mediated pathway that reduces aerial tissue permeability are the cuticle and the stomata (Kerstiens et al., 2006). There is considerable literature demonstrating that osmotic stress increases cuticular wax deposition and cuticular thickness in soil-grown plants (Shepherd and Wynne Griffiths, 2006, and references therein), while stomata are well known to have decreased aperture size in response to osmotic stress and ABA. The primary defect of the lrd2/lacs2 mutant is in the cuticle, but it has been demonstrated that disruption of cutin formation can lead to a loss of the guard cell cuticular ledge (Li et al., 2007; Figure 2A); this may compromise the ability of the stomata to close. Our experiments with the stomataless mutant speechless demonstrate that in plants with wild-type cuticles, the stomata are the major route by which exogenous sucrose enters the leaves. However, it is clear that ABA still causes a significant reduction in permeability in the speechless mutant. Our final model is therefore that osmotic stress leads to ABA increases in the root and that ABA signaling reduces aerial tissue permeability at least in part through a stomata-independent mechanism.
The Mechanism for Sucrose-Mediated Stimulation of LRP Emergence
Our finding that sucrose uptake by permeable aerial tissues is necessary and sufficient to stimulate lateral root formation in culture was unanticipated because roots of plants grown in culture are in contact with sucrose at all times; therefore, one would not expect sucrose to be limited by reduced access through the aerial tissues. Nevertheless, when aerial tissues could not take up sucrose, either because of an impermeable cuticle or an artificial barrier between the tissues and the media (i.e., Parafilm), lateral root formation was completely repressed in our assay system. This suggests that exogenous sucrose acts differently at the aerial tissues than at the root. Similarly, Takahashi et al. (2003) previously showed that adventitious roots were stimulated by sucrose in culture but that the hypocotyl had to be in direct contact with the sucrose medium for this stimulation to take place, leading them to conclude that the stimulatory sucrose was not derived from the primary root. It is possible that exogenous sucrose at the root is taken up with water through the xylem, while sucrose may enter the aerial tissues through the apoplast and be actively loaded into the phloem, as is endogenous sucrose (Lalonde et al., 2004). The fate of these pools of sucrose may be very different, and root and shoot uptake of exogenous sucrose might therefore have very different downstream effects.
Once taken up by the aerial tissues, it seems likely that exogenous sucrose augments the endogenous apoplastic sucrose pool that is derived from photosynthesis. Although soil-grown plants would not be expected to obtain sucrose by uptake from solutions surrounding their aerial tissues, it is probable that the root systems do respond to increases in photosynthate. Light stimulates lateral root formation (Reed et al., 1998), and a recent report showed that phytochrome is a mediator of root system architecture as well (Salisbury et al., 2007). Similarly, Crookshanks et al. (1998) showed that increased ambient CO2 concentrations lead to increased lateral root formation, presumably via the production of increased photosynthate. Furthermore, nutrient deficiencies (phosphorus and nitrogen) that increase the root-to-shoot ratio and alter root system architecture in many plants are accompanied by accumulation of sugars and starches in leaves and altered carbon allocation to the root (Hermans et al., 2006). Hence, photosynthesis and sugar accumulation in the aerial tissues is likely to be an important determinant of lateral root formation both in the context of cell culture and under more physiological conditions.
We found that sucrose metabolites were active in increasing LRP emergence, and a mutant in glucose signaling was still able to increase LRP emergence in response to sucrose, strongly suggesting that sucrose does not act as a signaling molecule in this process. Sucrose metabolites were less effective than sucrose in stimulating lateral root formation. This could be due to differences in uptake, transport, or metabolism; however, their promotive effect is clear. In light of our finding that sugar metabolism stimulates lateral root formation, it is particularly interesting that a cytoplasmic invertase gene was recently identified in a screen for genes essential for repression of lateral root formation on mild osmotic stress conditions (Qi et al., 2007). Lateral root formation was presumably repressed by the reduced sucrose uptake at the aerial tissues in this screen, as in our own screen. Invertases can affect the local levels of sucrose and other hexoses and can provide energy via sucrose cleavage at the site of activity (Koch, 2004; Lalonde et al., 2004). Invertase activity also affects the partitioning of sugars between source and sink tissues. This and other reports of the effects of invertase expression on root development further strengthen the link between sucrose and its metabolites and the regulation of lateral root formation (Sonnewald et al., 1991; Tang et al., 1999; Koch, 2004; Lalonde et al., 2004; Sergeeva et al., 2006).
How does increased sucrose metabolism stimulate LRP emergence and switch root system development to form a more highly branched architecture? Sucrose may be translocated to the root to be metabolized, providing additional energy and carbohydrates to the root system; the increase in root sucrose may be read as a signal to preferentially promote lateral roots. Indeed, the sucrose may be delivered directly to the LRP. Alternatively, the relevant metabolism of sucrose may occur in the aerial tissues. In this case, lateral root formation may be promoted indirectly by stimulating cotyledon and leaf growth. Increased leaf size was tightly correlated with increased LRP emergence in all experiments, wild-type plants grown under nonstress conditions have larger cotyledons and leaves than those grown on mild osmotic stress conditions, and mutants with cuticle defects, including lrd2/lacs2 and aba2-1 and aba3-1, have larger aerial organs in culture (Deak and Malamy, 2005; Figures 1 to 3). A final possibility is that metabolism of sucrose in the leaf may stimulate transport of auxin to the root. Shoot-derived auxin has been strongly implicated in regulating lateral root formation and presents an alternative to shoot-derived carbohydrate as a potential stimulator for LRP emergence (Bhalerao et al. (2002). Work is ongoing to distinguish between these possibilities.
In summary, our results clearly show that lateral root formation in culture is strongly influenced by whether or not leaves take up sucrose from the media. Environmental and genetic changes can alter aerial tissue permeability to media sucrose; therefore, this is an important finding because of its impact on the interpretation of experiments that use Arabidopsis grown in culture to study root system architecture. Our data also provide new insights that are applicable to plants grown in more natural settings than the Petri dish. First, we have shown that roots sense osmotic stress and send an ABA-mediated signal to the shoots to regulate aerial tissue permeability. This is an important and clear demonstration of a signaling mechanism connecting environment cues at the root to a shoot response, and it is reasonable to expect that a similar response occurs in nature when root experience osmotic stress. The ABA-mediated pathway is at least partially independent of stomata, suggesting that signals from the root may alter the cuticle. The regulation of a functional cuticle is important for many aspects of plant growth and development, especially water exchange, and has received little attention. Secondly, we have shown that increased sucrose levels in the aerial tissues and subsequent sucrose metabolism stimulates LRP emergence. This demonstrates that sucrose metabolism is stimulatory to that specific stage of lateral root formation and that increased sucrose metabolism leads to a developmental switch to create a more highly branched root system architecture. Hence, this provides a second example of long-distance communication, with sucrose at the aerial tissues determining the growth and development of the root system. It is tempting to speculate that sucrose, as the major photosynthetic product, provides the root with a readout of shoot system size and thereby allows the plant to coordinate root system and shoot system development. Together, although our in vitro studies of Arabidopsis root system development on mild osmotic stress conditions have taken an unexpected turn, they have (1) identified sucrose as an unexpected regulator of lateral root formation; (2) provided important new insights into the mechanisms by which roots communicate information to aerial tissues and receive information in turn; and (3) shed light on the regulatory pathways that allow plants to be developmentally plastic while preserving the essential balance between aboveground and belowground organs.
METHODS
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use in noncommercial research purposes.
Plant Growth Conditions
Seeds were surface sterilized in 100% bleach plus Tween-20 for 3 min and then rinsed four times with distilled water. The seeds were allowed to vernalize and imbibe for 2 to 3 d at 4°C. For all root branching assays, 5 to 10 seeds were sown on Petri dishes containing various media as described below and in the text. Plates were wrapped with Parafilm and oriented vertically to allow roots to grow on the surface of the media. Plates were placed in a growth chamber set with 16-h-light/8-h-dark cycles at 22°C.
To prevent contact between aerial tissues and media, a strip of ethanol-sterilized Parafilm was laid on the solidified media and the seeds were sown directly below the strip. Similar results were obtained when autoclaved glass cover slips were used instead, demonstrating that the repression of lateral root formation was not due to either the Parafilm or the ethanol sterilization step. Agar drops with or without various media components, as indicated in the text, contained 0.7% agar. The agar was cooled until it started to solidify slightly. One hundred microliters per plant was added to the parafilm, just above the top of the aerial tissues, and then pushed into contact with the aerial tissues.
Media Composition
Media simulating mild osmotic stress was made using mannitol or nitrogen salts as the osmotica. When mannitol was used, the media was composed of 100 mL/L Macronutrients (0.3322 g/L CaCl2·6H2O, 0.1807 g/L MgSO4, and 0.17 g/L KH2PO4), 100 mL/L Murashige and Skoog basal salt micronutrient solution (10× stock from Sigma-Aldrich), 45 g/L sucrose, 0.5 g/L MES, 5 mL/L 1 M NH4NO3, 5 mL/L 1 M KNO3, and 10.93 g/L mannitol. When nitrogen salts were used as the osmotica, media were prepared as above except that mannitol was omitted and nitrogen salts were raised to 20 mL/L 1 M NH4NO3 and 20 mL/L 1 M KNO3. Nonstress media were composed of 100 mL/L macronutrients (0.3322 g/L CaCl2·6H2O, 0.1807 g/L MgSO4, and 0.17 g/L KH2PO4), 100 mL/L Murashige and Skoog basal salt micronutrient solution (10× stock from Sigma-Aldrich), 10 g/L sucrose, 0.5 g/L MES, 5 mL/L 1 M NH4NO3, and 5 mL/L 1 M KNO3. For all media, the pH was adjusted to 5.7 using 1 n KOH, and 0.7% Difco agar, granulated (Fisher) was added before autoclaving.
ABA (Sigma-Aldrich) was dissolved in 0.1 n sodium hydroxide (NaOH) and added to cooled autoclaved media to a final concentration of 1 μM. Glucose (Sigma-Aldrich), fructose (Sigma-Aldrich), glucose 6- phosphate (Sigma-Aldrich), and 3-O-methylglucose (Sigma-Aldrich) were dissolved in water and added to a final concentration of 29.2 mM (molar equivalent of 1% sucrose) either to cooled autocloaved media after being filter sterilized or before autoclaving.
Genetic Analysis and Mapping
To define the region containing the LRD2 gene, the lrd2 mutants (Col-0 background) were crossed to wild-type Ws plants. The ecotype Ws was chosen instead of the more standard Ler because Ler seedlings resemble the lrd2 seedlings under our assay conditions. The resulting F1 progeny were allowed to self-pollinate, and the F2 progeny were screened for individuals with the lrd2 phenotype. Genomic DNA from 1880 lrd2-like plants was isolated and used for mapping using standard positional mapping techniques. Map positions of all simple sequence length polymorphic and cleaved-amplified polymorphic sequence markers used for rough mapping were taken from the public chromosome maps (http://www.Arabidopsis.org). For fine mapping, markers that were polymorphic between Col and Ws ecotypes were identified. Breakpoint analysis of recombinant F2 plants from an lrd2 × Ws cross delimited the lrd2 mutation to an ∼228-kb region flanked by SM98_146.6 and PB13. These markers exploit polymorphisms at restriction enzyme recognition sites that are also identified for other ecotypes (PERL1055317 and PERL0157438, respectively). The PCR primers used to identify the SM98_146.6 polymorphism (where Ws contains an MspI site) were F, 5′-TCAGAGAGAGAGAACTCACG-3′; R, 5′-TGGTAGGTGGAGAATTTTGC-3′. For the PB13 polymorphism (where Col contains an RsaI site), primers used were F, 5′-TATGCCATGGACCTCTTTGC-3′; R, 5′-CCAACATTACAAGGTGATCC-3′. Fine mapping narrowed the region to 62 open reading frames. For each of these, the complete genomic region from 5′ UTR to 3′ UTR was sequenced.
Creation and Verification of the lacs2-1/lrd2 Transheterozygote
Pollen from plants homozygous for the lacs2-1 T-DNA was used to fertilize emasculated homozygous lrd2 flowers, and PCR was performed to verify the presence of both the lacs2-1 and the lrd2 chromosomes. For lacs2-1, the T-DNA insert was amplified using F, 5′-CGGTGTTCCACGAGTTTACG-3′, and R, 5′-TGGGAAAACCTGGCGTTACCCAACTTAAT-3′. The lrd2 chromosome was verified as described above.
Assessment of Aerial Tissue Permeability to TB
Aerial tissues were submerged in an aqueous solution of 0.05% (w/v) TB [3-amino-7-(dimethylamino)-2-methylphenazathionium chloride; JT Baker] for 10 or 15 min and then thoroughly washed with distilled water to remove excess TB from plants. Aerial tissues were photographed with the roots removed.
Assessment of Aerial Tissue Permeability to Sucrose
Plants were grown with their aerial tissues isolated from the media by the strip of Parafilm. After 12 or 14 d (see figure legends), a 1-μL drop composed of 50% 14C-sucrose (Sigma-Aldrich) and 50% aqueous solution of 0.05% (w/v) TB (JT Baker) was applied to the abaxial side of one cotyledon. The TB helped to identify the application site. After 1 h of incubation, the application site was washed extensively with water and the whole plant was submerged in ScintiVerse* E Cocktail (Fisher Scientific) for analysis using a Beckman scintillation counter (model 6000IC).
Determination of Total Lateral Root Length, Primary Root Length, and Leaf Surface Area
Digital images of plants were traced by hand using ImageJ 1.29j (NIH) and converted from pixels to the appropriate metric equivalent. The total lateral root length (TOT) equals the sum of the lengths of all individual lateral roots on a given plant. The primary root length was determined by tracing from the root-shoot junction to the root tip of each plant. Leaf surface area was calculated from digital images of aerial tissues that had been dissected apart. Surface area was determined by tracing the outline of the adaxial surface of each cotyledon or leaf.
Microscopy Analyses
To assess lateral root emergence, all tissues were cleared by incubating sequentially, 15 min each, in (1) 20% methanol acidified with 4% concentrated hydrochloric acid, 55°C; (2) 7% NaOH in 60% ethanol. Tissues were then rehydrated by 10-min incubations in 40, 20, and 10% ethanol and then infiltrated for 10 min in 25% glycerol/5% ethanol. Cleared tissues were then mounted in 50% glycerol and visualized using differential interference contrast optics on a Leica DMR microscope. Even very early lateral root primordia can be easily visualized using this method. Emerged LRP are defined as any LRP that show signs of growth from the lateral root apical meristem (determined by observing the increased number of small cells near the lateral root tip; Malamy and Benfey, 1997) or that have become autonomous lateral roots.
Nile Red (Fluka 72485) staining was performed as described by Li et al. (2007). The cuticular ledge of the guard cells were imaged using a rhodamine filter set (Leica 41002B HQ:R/D11) on a Leica DMR microscope equipped with a UV light source.
Plant Material
aba2-1, aba3-1 (Leon-Kloosterziel et al., 1996), bdg1 (Kurdyukov et al., 2006), cer2-1 (Negruk et al., 1996), cer4-1, cer4-3, cer4-4 (Rowland et al., 2006), cer5-2 (Pighin et al., 2004), fae1-1 (Lemieux et al., 1990), gin2-1 (Moore et al., 2003), pex4-162E2, pex4-169D3, pex4-191D3 (Zolman et al., 2005), pex5-1 (Zolman et al., 2000), and pxa1 (Zolman et al., 2001) were obtained from The Arabidopsis Information Resource (www.Arabidopsis.org). The remaining mutants described were gifts from Dominique Bergman (spch-3; MacAlister et al., 2007), John Browse (lacs2-1; Schnurr et al., 2004), Roger Innes (sma4, sma4 + LACS2 [EP:LACS2]; Tang et al., 2007; and att1; Xiao et al., 2004), Matthew Jenks (wax2; Chen et al., 2003), Gloria Muday (rib1; Poupart et al., 2005), Christiane Nawrath (CUTE; Sieber et al., 2000), and John Ohlrogge (fatB-ko; Bonaventure et al., 2003; and gpat5; Beisson et al., 2007).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: aba2-1, At1g52340; aba3-1, At1g16540; att1-2 (also known as CYP86A2), At4g00360; bdg1, At1g64670; cer2-1, At4g24510; cer4-1, cer4-3, and cer4-4, At4g33790; cer5-2, At1g51500; fae1-1, At4g34520; fatB-ko, At1g08510; gin2-1, At4g29130; gpat5-1 and gpat5-2, At3g11430; lacs2-1, lrd2, and sma4, At1g49430; slr-1, At4g14550; speechless-3, At5g53210; pex4-162E2, pex4-169D3, and pex4-191D3, At5g25760; pex5-1, At5g56290; pxa1-1, At4g39850; rib1, unknown; and wax2-2 and wax2-3, At5g57800.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. lrd2 Plants Carry Additional EMS-Induced Mutations.
Supplemental Figure 2. The Mutations in PI4Ka1 Do Not Contribute to the lrd2 Phenotype.
Supplemental Table 1. Expression of the Wild-Type PI4Kα1 cDNA Sequences Fail to Rescue the lrd2 Phenotype.
Supplemental Methods.
Supplementary Material
Acknowledgments
We thank the many people who contributed plant material (see above). We also thank Jean Greenberg, Gloria Muday, and Peter Roycewicz for their valuable scientific input throughout the course of this research, Jee Jung and Paul Brannon for their work on mapping the LRD2 gene, Laura Felleye for help with data collection for all experiments, and the University of Chicago greenhouse staff and sequencing center staff for their technical support. This work has been supported by National Science Foundation CAREER Grant 0238529 and USDA-CSREES Grant 2007-030206 to J.E.M. and National Institutes of Health training grants to D.R.M., P.A.I., and K.I.D.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jocelyn E. Malamy (jmalamy@bsd.uchicago.edu).
Online version contains Web-only data.
References
- Beisson, F., Li, Y., Bonaventure, G., Pollard, M., and Ohlrogge, J.B. (2007). The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19 351–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessire, M., Chassot, C., Jacquat, A.C., Humphry, M., Borel, S., Petétot, J.M., Métraux, J.P., and Nawrath, C. (2007). A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 26 2158–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao, R.P., Eklof, J., Ljung, K., Marchant, A., Bennett, M., and Sandberg, G. (2002). Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 29 325–332. [DOI] [PubMed] [Google Scholar]
- Bonaventure, G., Salas, J.J., Pollard, M.R., and Ohlrogge, J.B. (2003). Disruption of the FATB gene in Arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 15 1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro, I., Beeckman, T., Graham, N., Bhalerao, R., Zhang, H., Casero, P., Sandberg, G., and Bennett, M.J. (2003). Dissecting Arabidopsis lateral root development. Trends Plant Sci. 8 165–171. [DOI] [PubMed] [Google Scholar]
- Charleton, W.A. (1991). Lateral root initiation. In Plant Roots: The Hidden Half, Y. Waisel, A. Eshel, and U. Kafkafi, eds (New York: Marcel Dekker), pp. 103–128.
- Chen, X., Goodwin, S.M., Boroff, V.L., Liu, X., and Jenks, M.A. (2003). Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 15 1170–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann, A., Weiler, E.W., Steudle, E., and Grill, E. (2007). A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 52 167–174. [DOI] [PubMed] [Google Scholar]
- Crookshanks, M., Taylor, G., and Dolan, L. (1998). A model system to study the effects of elevated CO2 on the developmental physiology of roots: The use of Arabidopsis thaliana. J. Exp. Bot. 49 593–597. [Google Scholar]
- Daum, G., Lees, N.D., Bard, M., and Dickson, R. (1998). Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast 14 1471–1510. [DOI] [PubMed] [Google Scholar]
- Deak, K.I., and Malamy, J. (2005). Osmotic regulation of root system architecture. Plant J. 43 17–28. [DOI] [PubMed] [Google Scholar]
- De Smet, I., Signora, L., Beeckman, T., Foyer, C.H., and Zhang, H. (2003). An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 33 543–555. [DOI] [PubMed] [Google Scholar]
- De Smet, I., Zhang, H., Inze, D., and Beeckman, T. (2006). A novel role for abscisic acid emerges from underground. Trends Plant Sci. 11 434–439. [DOI] [PubMed] [Google Scholar]
- Forde, B., and Lorenzo, H. (2001). The nutritional control of root development. Plant Soil 232 51–68. [Google Scholar]
- Freixes, S., Thibaud, M.-C., Tardieu, F., and Muller, B. (2002). Root elongation and branching is related to local hexose concentration in Arabidopsis thaliana seedlings. Plant Cell Environ. 25 1357–1366. [Google Scholar]
- Fukaki, H., Okushima, Y., and Tasaka, M. (2007). Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 256 111–137. [DOI] [PubMed] [Google Scholar]
- Graça, J., and Santos, S. (2007). Suberin: A biopolyester of plants. Macromol Biosci. 7 128–135. [DOI] [PubMed] [Google Scholar]
- Gupta, A., and Kaur, N. (2005). Sugar signaling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 30 761–776. [DOI] [PubMed] [Google Scholar]
- Hermans, C., Hammond, J.P., White, P.J., and Verbruggen, N. (2006). How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 11 1360–1385. [DOI] [PubMed] [Google Scholar]
- Holbrook, N.M., Shashidhar, V.R., James, R.A., and Munns, R. (2002). Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. J. Exp. Bot. 53 1503–1514. [PubMed] [Google Scholar]
- Karthikeyan, A.S., Varadarajan, D.K., Jain, A., Held, M.A., Carpita, N.C., and Raghothama, K.G. (2007). Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta 225 907–918. [DOI] [PubMed] [Google Scholar]
- Kerstiens, G., Schreiber, L., and Lendzian, K.J. (2006). Quantification of cuticular permeability in genetically modified plants. J. Exp. Bot. 57 2547–2552. [DOI] [PubMed] [Google Scholar]
- Koch, K. (2004). Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 7 235–246. [DOI] [PubMed] [Google Scholar]
- Kolattukudy, P.E. (1985). Enzymatic penetration of the plant cuticle by fungal pathogens. Annu. Rev. Phytopathol. 23 223–250. [Google Scholar]
- Kornberg, A., and Pricer, W.E., Jr. (1953). Enzymatic synthesis of the coenzyme A derivatives of long chain fatty acids. J. Biol. Chem. 204 329–343. [PubMed] [Google Scholar]
- Kuderová, A., Urbánková, I., Válková, M., Malbeck, J., Brzobohaty, B., Némethová, D., and Hejátko, J. (2008). Effects of conditional IPT-dependent cytokinin overproduction on root architecture of Arabidopsis seedlings. Plant Cell Physiol. 49 570–582. [DOI] [PubMed] [Google Scholar]
- Kunst, L., and Samuels, A.L. (2003). Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 42 51–80. [DOI] [PubMed] [Google Scholar]
- Kurdyukov, S., Faust, A., Nawrath, C., Ba, S., Voisin, D., Efremova, N., Franke, R., Schreiber, L., Saedler, H., Métraux, J.-P., and Yephremova, A. (2006). The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 18 321–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde, S., Wipf, D., and Frommer, W.B. (2004). Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu. Rev. Plant Biol. 55 341–372. [DOI] [PubMed] [Google Scholar]
- Lemieux, B., Miquel, M., Somerville, C., and Browse, J. (1990). Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. Theor. Appl. Genet. 80 234–240. [DOI] [PubMed] [Google Scholar]
- Leon-Kloosterziel, K.M., Gil, M.A., Ruijs, G.J., Jacobsen, S.E., Olszewski, N.E., Schwartz, S.H., Zeevaart, J.A.D., and Koornneef, M. (1996). Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J. 10 655–661. [DOI] [PubMed] [Google Scholar]
- Li, Y., Beisson, F., Koo, A.J., Molina, I., Pollard, M., and Ohlrogge, J. (2007). Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc. Natl. Acad. Sci. USA 104 18339–18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bucio, J., Cruz-Ramirez, A., and Herrera-Estrella, L. (2003). The role of nutrient availability in regulating root architecture. Curr. Opin Plant Biol. 6 280–287. [DOI] [PubMed] [Google Scholar]
- MacAlister, C.A., Ohashi-Ito, K., and Bergmann, D.C. (2007). Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 445 537–540. [DOI] [PubMed] [Google Scholar]
- Malamy, J.E. (2005). Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 28 67–77. [DOI] [PubMed] [Google Scholar]
- Malamy, J.E. (2008). Lateral root development. In Root Development, T. Beeckman, ed (Oxford, UK: Blackwell Publishing Limited), in press.
- Malamy, J.E., and Benfey, P.N. (1997). Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124 33–44. [DOI] [PubMed] [Google Scholar]
- Moore, B., Zhou, L., Rolland, F., Hall, Q., Cheng, W.H., Liu, Y.X., Hwang, I., Jones, T., and Sheen, J. (2003). Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300 332–336. [DOI] [PubMed] [Google Scholar]
- Nawrath, C. (2002). The biopolymers cutin and suberin. In The Arabidopsis Book, C.R. Somerville and E.M. Meyerowitz, eds (Rockville, MD: American Society of Plant Biologists), doi/http://www.aspb.org/publications/arabidopsis/. [DOI] [PMC free article] [PubMed]
- Negruk, V., Yang, P., Subramanian, M., McNevin, J.P., and Lemieux, B. (1996). Molecular cloning and characterization of the CER2 gene of Arabidopsis thaliana. Plant J. 9 137–145. [DOI] [PubMed] [Google Scholar]
- Osmont, K.S., Sibout, R., and Hardtke, C.S. (2007). Hidden branches: Developments in root system architecture. Annu. Rev. Plant Biol. 58 93–113. [DOI] [PubMed] [Google Scholar]
- Pighin, J.A., Zheng, H., Balakshin, L.J., Goodman, I.P., Western, T.L., Jetter, R., Kunst, L., and Samuels, A.L. (2004). Plant cuticular lipid export requires an ABC transporter. Science 306 702–704. [DOI] [PubMed] [Google Scholar]
- Poupart, J., Rashotte, A.M., Muday, G.K., and Waddell, C.S. (2005). The rib1 mutant of Arabidopsis has alterations in indole-3-butyric acid transport, hypocotyl elongation, and root architecture. Plant Physiol. 139 1460–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, X., Wu, Z., Li, J., Mo, X., Wu, S., Chu, J., and Wu, P. (2007). AtCYT-INV1, a neutral invertase, is involved in osmotic stress-induced inhibition on lateral root growth in Arabidopsis. Plant Mol. Biol. 64 575–587. [DOI] [PubMed] [Google Scholar]
- Reed, R.C., Brady, S.R., and Muday, G.K. (1998). Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 118 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland, O., Zheng, H., Hepworth, S.R., Lam, P., Jetter, R., and Kunst, L. (2006). CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiol. 142 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury, F.J., Hall, A., Grierson, C.S., and Halliday, K.J. (2007). Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 50 429–438. [DOI] [PubMed] [Google Scholar]
- Sauter, A., Davies, W.J., and Hartung, W. (2001). The long-distance abscisic acid signal in the droughted plant: The fate of the hormone on its way from root to shoot. J. Exp. Bot. 52 1991–1997. [DOI] [PubMed] [Google Scholar]
- Schnurr, J., Shockey, J., and Browse, J. (2004). The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 16 629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, L., Skrabs, M., Hartmann, K.D., Diamantopoulos, P., Simanova, E., and Santrucek, J. (2001). Effect of humidity on cuticular water permeability of isolated cuticular membranes and leaf disks. Planta 214 274–282. [DOI] [PubMed] [Google Scholar]
- Sergeeva, L.I., Keurentjes, J.J.B., Bentsink, L., Vonk, J., van der Plas, L.H.W., Koorneef, M., and Vreugdenhil, D. (2006). Vacuolar invertase regulates elongation of Arabidopsis thaliana roots as revealed by QTL and mutant analysis. Proc. Natl. Acad. Sci. USA 103 2994–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna, L. (2007). bHLH proteins know when to make a stoma. Trends Plant Sci. 12 483–485. [DOI] [PubMed] [Google Scholar]
- Shepherd, T., and Wynne Griffiths, D. (2006). The effects of stress on plant cuticular waxes. New Phytol. 171 469–499. [DOI] [PubMed] [Google Scholar]
- Shockey, J.M., Fulda, M.S., and Browse, J.A. (2002). Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol. 129 1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber, P., Schorderet, M., Ryser, U., Buchala, A., Kolattukudy, P., Métrauz, J.-P., and Nawrath, C. (2000). Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure an properties of the cuticle and postgenital organ fusions. Plant Cell 12 721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald, U., Brauer, M., von Schaewen, A., Stitt, M., and Willmitzer, L. (1991). Transgenic plants expressing yeast-derived invertase in either the cytosol, vacuole or the apoplast: A powerful tool for studying sucrose metabolism and sink/source interactions. Plant J. 1 95–106. [DOI] [PubMed] [Google Scholar]
- Swarup, K., et al. (2008). The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10 946–954. [DOI] [PubMed] [Google Scholar]
- Takahashi, F., Sato-Nara, K., Kobayashi, K., Suzuki, M., and Suzuki, H. (2003). Sugar induced adventitious roots in Arabidopsis seedlings. J. Plant Res. 116 83–91. [DOI] [PubMed] [Google Scholar]
- Tang, D., Simonich, M.T., and Innes, R.W. (2007). Mutations in LACS2, a long chain acyl-CoA synthetase, enhance susceptibility to avirulent Pseudomonas syringae, but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiol. 114 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, G.-Q., Luscher, M., and Sturm, A. (1999). Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell 11 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weele, C.M., Spollen, W.G., Sharo, R.E., and Baskin, T.I. (2000). Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 51 1555–1562. [DOI] [PubMed] [Google Scholar]
- Watkins, P.A. (1997). Fatty acid activation. Prog. Lipid Res. 36 55–83. [DOI] [PubMed] [Google Scholar]
- Wilkinson, S., and Davies, W.J. (2002). ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Environ. 25 195–210. [DOI] [PubMed] [Google Scholar]
- Xiao, F., Goodwin, S.M., Xiao, Y., Sun, Z., Baker, D., Tang, X., Jenks, M.A., and Zhou, J.M. (2004). Arabidopsis CYP86A2 represses Pseudomonas syringae type III genes and is required for cuticle development. EMBO J. 23 2903–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, L., Wang, R.G., Mao, G., and Koczan, J.M. (2006). Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 142 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman, B.K., Monroe-Augustus, M., Silva, I.D., and Bartel, B. (2005). Identification and functional characterization of Arabidopsis PEROXIN4 and the interacting protein PEROXIN22. Plant Cell 17 3422–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman, B.K., Silva, I.D., and Bartel, B. (2001). The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid beta-oxidation. Plant Physiol. 127 1266–1278. [PMC free article] [PubMed] [Google Scholar]
- Zolman, B.K., Yoder, A., and Bartel, B. (2000). Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.