Abstract
Background
The relationships between cockroach and mouse allergen exposure, anti-cockroach and anti-mouse IgE, and wheeze, rhinitis, and atopic dermatitis in children as young as age 3 years are of public health importance but have not been thoroughly evaluated.
Objective
We hypothesized that inner-city children might have anti-cockroach and anti-mouse IgE by age 3 years, and their presence would be associated with respiratory and atopic symptoms.
Methods
Children were followed prospectively from birth through age 3 years (n = 404). Residential levels of cockroach and mouse allergens, sera levels of anti-cockroach and anti-mouse IgE, and parental report of wheeze, rhinitis, and atopic dermatitis were measured.
Results
The odds of early wheeze were significantly higher among children who had IgE to cockroach (odds ratio [OR], 3.3; 95% CI, 1.8-6.2), mouse (OR, 4.6; 95% CI, 2.3-9.0), or both (OR, 9.7; 95% CI, 3.4-27.3). The odds of rhinitis or atopic dermatitis were also higher among children with IgE to cockroach, mouse, or both. Higher IgE class to cockroach and mouse was associated with wheeze and atopic dermatitis (tests for trend, P < .002).
Conclusions
Children age 2 to 3 years who have anti-cockroach and anti-mouse IgE are at increased risk of wheeze and atopy. Moreover, a dose-response relationship was found between higher IgE class and increased prevalence of wheeze, rhinitis, or atopic dermatitis. These findings indicate the importance of reducing exposure to cockroach and mouse allergens for susceptible children.
Keywords: Cockroach, mouse, allergy, wheeze, inner city, rhinitis, IgE, atopic dermatitis, eczema, asthma, sensitization
Wheeze, rhinitis, and atopic dermatitis are common in children before age 3 years and often remit. However, some children with early-onset symptoms have persistent disease and reduced lung function at school age.1 Improved predictors are needed to identify children at risk to enable appropriate interventions.
To date, several prospective studies have shown that early sensitization to aeroallergens can predict persistent asthma and atopy in children. For example, in a prospective analysis of 521 children from the Manchester cohort, the sum of anti—dust mite—, cat-, and dog-specific IgE levels assessed at age 3 years was associated with persistent wheeze at age 5 years.2 Also, the German Multicentre Allergy Study birth cohort found that the development of anti—dust mite, anti-cat, or anti-dog IgE at age 3 years was associated with decreased FEV1 values at age 7 years.3 Another analysis from the same cohort found that the development of specific IgE to any one of 9 food allergens or aeroallergens before age 1 year was associated with asthma at age 7 years but only among children with a family history of asthma.3,4
However, few studies have focused on the contribution of exposure, sensitization, or both to indoor allergens important to inner-city asthma, namely mouse and cockroach, in this young age group.5 In one study that examined mouse sensitization among urban preschoolers, Matsui et al6 found that young (mean age, 4.4 years) children with physician-diagnosed asthma and positive skin prick test responses to mice who were exposed to higher levels of mouse allergen had more symptom days, medication use, and physician’s office visits. In an analysis of 222 siblings of children in a prospective birth cohort (mean age, 2.87 years), those exposed to higher levels of cockroach allergen were more likely to have asthma. However, sensitization to cockroach was not assessed in this group.7
Despite the growing evidence that early aeroallergen sensitization can influence the risk for asthma, many questions still need to be elucidated. These include the relationship between early sensitization and the development of asthma among inner-city preschool children, the contribution of exposure and sensitization to cockroach and mouse allergens, and the effects of genetic predisposition to asthma on susceptibility to early allergen exposure. Our objective was to determine the relationships among cockroach and mouse indoor allergen exposure, the production of anti-cockroach and anti-mouse IgE levels, and the development of respiratory and atopic symptoms among inner-city children aged 2 to 3 years in a birth cohort selected independently of family history of asthma and atopy. We hypothesized that anti-cockroach and anti-mouse IgE levels by age 3 years would be associated with early wheeze, rhinitis, and atopic dermatitis in this cohort. We also hypothesized that the odds of sensitization to mouse, cockroach, or both would be heightened among those with greater levels of cockroach and mouse allergen exposure. A greater understanding of the relationship between cockroach and mouse exposure, development of allergic sensitization, and onset of symptoms in very young children from an urban birth cohort might have important implications for understanding the pathogenesis of inner-city asthma.
METHODS
Study cohort
Women were recruited during pregnancy from prenatal clinics affiliated with New York Presbyterian Hospital (Columbia campus) as part of an ongoing longitudinal birth cohort study under the auspices of the Columbia Center for Children’s Environmental Health (CCCEH), as previously described.8-11 African American and Dominican women aged 18 to 35 years who had lived in Northern Manhattan or the South Bronx for at least 1 year were recruited. Exclusion criteria included active smoking during pregnancy, drug use, diabetes, hypertension, and HIV infection. Informed consent was obtained from all participants in accordance with Columbia University’s Institutional Review Board. Serum samples and questionnaire data from 404 children were available for analysis.
Questionnaires
Detailed questionnaires were administered to the mother prenatally and every 3 months until the child reached age 2 years and subsequently every 6 months through age 3 years. The prenatal questionnaire was administered during recruitment and assessed maternal history of asthma, demographics, environmental exposures, daily activities, diet, and medications taken during pregnancy. After the birth of the child, mothers were asked about a physician’s report of asthma, probable asthma, or eczema and whether their child wheezed or experienced episodes of runny or itchy eyes, sneezing, or runny nose other than from a cold. Queries also addressed exposure to environmental tobacco smoke.
Allergen measurements in the home
Dust samples were collected prenatally and postnatally from the kitchens and beds. Levels of German cockroach (Bla g 2) and mouse allergen (mouse urinary protein [MUP]) in vacuumed dust samples were assessed by means of ELISA, as described earlier.8 Bla g 2 is expressed in units per gram (1 U = 40 ng of protein), and MUP is expressed in micrograms per gram. Prenatal exposure levels were correlated with postnatal levels in this cohort.10,12 For cockroach allergen assessment, there were 302 prenatal samples from the bed and 280 from the kitchen. For mouse allergen assessment, there were 289 prenatal samples from the bed and 294 from the kitchen.
Serum antibodies
Serum samples were collected at age 2 years (n = 344) and 3 years (n = 322). Anti-cockroach, anti-mouse, and anti—dust mite IgE levels were measured by using the validated Flourescence Allergosorbent Test (Bio-Whittaker, Walkersville, Md) for the first 71 samples (cockroach: age 2 years [n = 71] and 3 years [n = 4]; mouse: age 2 years [n = 63] and 3 years [n = 0]; dust mite: age 2 years [n = 52] and 3 years [n = 0]).13,14 All subsequent samples, as well as anti-cat and anti-dog IgE levels, were measured with Immuno-CAP (Uppsala, Sweden).15-17 During the transition from one validated method to the other, a small subset of samples was analyzed by using both methods to confirm that the results are correlated (data not shown). Allergen-specific IgE levels of 0.35 IU/mL or greater (class I) were considered positive. Total IgE in cord blood was measured by using an immunoradiometric assay (total IgE immunoradiometric assay; Diagnostics Products Corp, Los Angeles, Calif). Total IgE at age 2 and 3 years was measured either by using the ImmunoCAP (age 2 years [n = 90] and age 3 years [n = 172]) or immunoradiometric assay (age 2 years [n = 257] and 3 years [n = 157]). All total and specific IgE levels were measured in duplicate; an average of the 2 values was used for analysis.
Statistical analysis
Complete data were available for 404 children for anti-cockroach and anti-mouse IgE levels, and covariates including ethnicity, sex, maternal asthma, and prenatal and postnatal environmental tobacco smoke. Sample numbers were lower for allergen exposure data (n = 280-302, depending on allergen). For all analyses not involving allergen in the dust, the sample size was 404. Early wheeze was defined as maternal report of child’s wheeze without a cold or maternal report of a physician’s diagnosis of asthma. Rhinitis was defined as maternal report of a child having episodes of runny or itchy eyes, sneezing, or runny nose other than from a cold. Children were classified as having atopic dermatitis if their mothers reported a physician’s diagnosis of eczema or if the child was using medication for eczema.
Allergen exposure levels were transformed into binary variables, which were defined in 5 different ways using cutoff points previously identified in the literature as being associated with health outcomes: grater than and less than the level of detection (LOD), 1.6, 2, median, and 8 (U/g for Bla g 2 and μg/g for MUP).18-21 Values for specific IgE that were less than the LOD were assigned a value of half the limit of detection. When children had allergen-specific IgE levels measured at age 2 and 3 years, the higher value was used. These values were correlated (Pearson’s rho: r 0.45 [P < .001] for correlation between age 2 and 3 years for anti-cockroach IgE and r = 0.5 [P < .001] for correlation between age 2 and 3 years for anti-mouse IgE). When children had only 1 measurement at age 2 or 3 years, this value was used for analysis. For logistic regression analysis with IgE as the outcome, anti-cockroach and anti-mouse IgE were transformed into binary variables (specific IgE ≥0.35 vs <0.35 IU/mL).
Binary logistic regression analysis was performed to assess associations between allergen dust levels and anti-cockroach and anti-mouse IgE levels (≥0.35 vs <0.35 IU/mL), as well as between allergen dust levels and early wheeze, rhinitis, and atopic dermatitis. Logistic regression models were built by using each of the 5 definitions for the binary variables for measuring cockroach and mouse allergen exposure levels to determine associations with anti-cockroach and anti-mouse IgE levels, wheeze, rhinitis, and atopic dermatitis. To assess the relationship between anti-cockroach and anti-mouse IgE levels and symptoms, odds ratios (ORs) were calculated. Logistic regression models were built by using the binary exposure variables of anti-cockroach and anti-mouse IgE for each of 3 binary outcome variables: wheeze, rhinitis, or eczema. Multiple logistic regression then was performed to assess for interaction and confounding by ethnicity, history of maternal asthma, sex, and environmental tobacco smoke exposure. Anti-cockroach and anti-mouse IgE values were converted into a categorical variable of IgE class (class 0, specific IgE <0.35 IU/mL; class I, specific IgE ≥0.35 and <0.70 IU/mL; and class II or greater, specific IgE 0.70 IU/mL) and investigated by using a test for trend. Specific IgE values were log-transformed to calculate geometric means, and differences in the means were analyzed by using the Mann-Whitney U test. Data were analyzed with SPSS version 15.0 (SPSS, Inc, Chicago, Ill).
RESULTS
The characteristics of the study population determined at the time of enrollment are summarized in Table I. The cohort was of roughly two thirds Dominican ethnicity and one third African American ethnicity and of predominantly low socioeconomic status. About one quarter of the cohort had a history of maternal asthma. Approximately one third of the cohort was exposed to prenatal or postnatal environmental tobacco smoke. There were no important differences between children for whom allergen exposure data were available compared with those children without such associated data (Table I). The prevalence of sensitization, early wheeze, rhinitis, atopic dermatitis, and demographic factors did not differ by more than 1% between the 2 groups; thus they were combined in analysis.
TABLE I.
Baseline characteristics of the 404 participants
| Total cohort (n = 404) |
Children with allergen dust sample (n = 332) |
|
|---|---|---|
| Sex | ||
| Male | 47% (188) | 46% (152) |
| Female | 53% (216) | 54% (180) |
| Ethnicity | ||
| Dominican | 59% (240) | 60% (199) |
| African American | 41% (164) | 40% (133) |
| Maternal history of asthma | ||
| Yes | 21% (86) | 22% (74) |
| No | 79% (318) | 78% (258) |
| Prenatal environmental tobacco smoke exposure |
||
| Yes | 37% (150) | 37% (123) |
| No | 63% (254) | 63% (209) |
| Postnatal environmental tobacco smoke exposure* |
||
| Yes | 41% (166) | 41% (135) |
| No | 59% (238) | 59% (197) |
| Wheeze | ||
| Yes | 26% (106) | 26% (87) |
| Wheeze without a cold | 19% (77) | |
| Doctor said child had asthma | 18% (73) | |
| No | 74% (298) | 74% (245) |
| Rhinitis | ||
| Yes | 60% (241) | 61% (204) |
| Sneeze or runny nose without cold | 49% (199) | |
| Runny or itchy eyes without cold | 43% (173) | |
| No | 40% (163) | 39% (128) |
| Atopic dermatitis | ||
| Yes; doctor said child had eczema | 35% (141) | 35% (117) |
| No | 65% (263) | 65% (215) |
Defined as parental report of a smoker in the home on any of the postnatal questionnaires through age 3 years.
Anti-cockroach and anti-mouse IgE and total IgE
Eleven percent (46/404) of children had anti-cockroach IgE levels of 0.35 IU/mL or greater by age 3 years. Ten percent (40/404) of children had increased anti-mouse IgE levels of 0.35 IU/mL or greater by age 3 years. In addition, 5% (20/404) of children had increased levels of both (Fig 1). The prevalence of sensitization to dust mite, cat, and dog was 5% for each by age 3 years. Among children with specific IgE levels of 0.35 IU/mL or greater, anti-cockroach IgE was closely correlated with total IgE at age 2 and 3 years, but anti-mouse IgE was not (Fig 2). Logistic regression analysis found that log-transformed total IgE levels were not associated with wheeze, rhinitis, or atopic dermatitis at age 2 or 3 years (data not shown).
FIG 1.

Frequency of anti-cockroach and anti-mouse IgE by age 3 years (n = 404). The percentage of children with anti-cockroach IgE or anti-mouse IgE of 0.35 IU/mL or greater by age 3 years is shown. Black bars represent the percentage of children in each group with IgE to both cockroach and mouse.
FIG 2.

Total IgE is linearly correlated with anti-cockroach IgE levels. Spearman’s rho for cockroach at age 2 years: r = 0.618 (P < .002, not displayed); Spearman’s rho for cockroach at age 3 years: r = 0.528 (P < .003); Spearman’s rho for mouse at age 2 years: r = 0.298 (P = .147, not displayed); Spearman’s rho for mouse at age 3 years: r = 0.220 (P = .326).
Allergen exposure and anti-cockroach and anti-mouse IgE
Cockroach allergen exposure levels ranged from 0.38 to 148 U/g for bed samples and 0.50 to 936 U/g for kitchen samples. MUP allergen levels ranged from 0.25 to 119 mg/g for bed samples and 0.50 to 1978 μg/g for kitchen samples. Statistically significant associations between levels of Bla g 2 and MUP and the development of an increased anti-cockroach and anti-mouse IgE level were not found. These included dichotomizing each allergen level at LOD, 1.6, 2, median, or 8 (U/g for Bla g 2 and μg/g for MUP) based on previously published thresholds associated with symptoms18-21 and entering Bla g 2 or MUP levels as continuous variables in logistic regression analysis. By using similar methods, no statistically significant association was found between allergen exposure and development of wheeze, rhinitis, or atopic dermatitis. There was no statistically significant interaction between allergen exposure level and specific IgE levels in these models.
Anti-cockroach and anti-mouse IgE and odds of wheeze, rhinitis, or atopic dermatitis
The odds of early wheeze by age 3 years were higher among children who had anti-cockroach IgE (OR, 3.3; 95% CI, 1.8-6.2), anti-mouse IgE (OR, 4.6; 95% CI, 2.3-9.0), or both (OR, 9.7; 95% CI, 3.4-27.3). The odds of rhinitis were greater in children with anti-mouse IgE (OR, 2.2; 95% CI, 1.03-5.6) or both anti-cockroach IgE and anti-mouse IgE (OR, 6.5; 95% CI, 1.5-28.4; Fig 3). These associations remained significant after adjustment for known covariates (Table II). In models that included sensitization to other aeroallergens, such as dust mite, cat, and dog, sensitization to cockroach was an independent predictor of wheeze (OR, 3.5; 95% CI, 1.5-8.5) and atopic dermatitis (OR, 2.9; 95% CI, 1.2-6.6), and sensitization to mouse was an independent predictor of wheeze (OR, 2.6; 95% CI, 1.1-6.0).
FIG 3.

Increased odds of wheeze and atopy with anti-cockroach and anti-mouse IgE. B, Both anti-mouse IgE and anti-cockroach IgE of 0.35 IU/mL or greater; M, anti-mouse IgE of 0.35 IU/mL or greater; C, anti-cockroach IgE of 0.35 IU/mL or greater. *P < .05 and **P < .005 on logistic regression analysis.
TABLE II.
Anti—cockroach and anti—mouse IgE and symptoms (n = 404)
| Anti—cockroach IgE (≥0.35 IU/mL) |
Anti—mouse IgE (≥0.35 IU/mL) |
Both anti—cockroach and anti—mouse IgE (≥0.35 IU/mL) |
|
|---|---|---|---|
| Early wheeze | |||
| Bivariate OR (95% CI) | 3.31 (1.77–6.21)† | 4.58 (2.34–8.98)† | 9.66 (3.42–27.30)† |
| Multivariate OR (95% CI) | 3.25 (1.69–6.24) | 4.18 (2.07–8.42)† | 8.11 (2.77–23.74)† |
| Rhinitis | |||
| Bivariate OR (95% CI) | 1.83 (0.93–3.60) | 2.18 (1.03–4.58)* | 6.50 (1.49–28.40)* |
| Multivariate OR (95% CI) | 1.93 (0.97–3.84) | 2.18 (1.02–4.65)* | 6.64 (1.50–29.45)* |
| Atopic dermatitis | |||
| Bivariate OR (95% CI) | 3.04 (1.62–5.70)† | 2.52 (1.30–4.87)* | 4.72 (1.77–12.56)† |
| Multivariate OR (95% CI) | 2.90 (1.49–5.65)† | 2.13 (1.05–4.31)* | 3.72 (1.31–10.54)* |
Multivariate models were adjusted for maternal asthma, sex, ethnicity, and prenatal and postnatal environmental tobacco smoke exposure.
P < .05.
P < .005.
The odds of atopic dermatitis were increased among children with anti-cockroach IgE (OR, 3.0; 95% CI, 1.6-5.7), anti-mouse IgE (OR, 2.5; 95% CI, 1.3-4.9), or both (OR, 4.7; 95% CI, 1.8-12.7). However, in a multivariate logistic regression model the association is somewhat reduced, although still statistically significant, presumably because of ethnic differences in the prevalence of reported atopic dermatitis (53% of African American vs 23% of Dominican subjects).
Statistically significant associations between levels of cockroach and mouse allergen and the development of early wheeze, rhinitis, or atopic dermatitis were not detected in logistic regression models that incorporated allergen exposure levels at any of the 5 cutoff points. Associations between allergen exposure and symptoms in subgroup analyses among children with allergenspecific IgE levels of 0.35 IU/mL or greater also were not found (data not shown).
Effects of IgE class
Anti-cockroach and anti-mouse IgE classes were compared with the prevalence of early wheeze, rhinitis, or atopic dermatitis to determine the relationship between IgE level and risk for asthma or atopic symptoms. The percentage of children with early wheeze increased with increasing anti-cockroach IgE class (test for trend, P < .001) and anti-mouse IgE class (P < .001). The percentage of children with rhinitis increased with increasing anti-mouse IgE class (P = .03) but not anti-cockroach IgE class (P = .09). The percentage of children with atopic dermatitis increased with increasing anti-cockroach IgE class (P < .001) and anti-mouse IgE class (P = .002, Fig 4).
FIG 4.
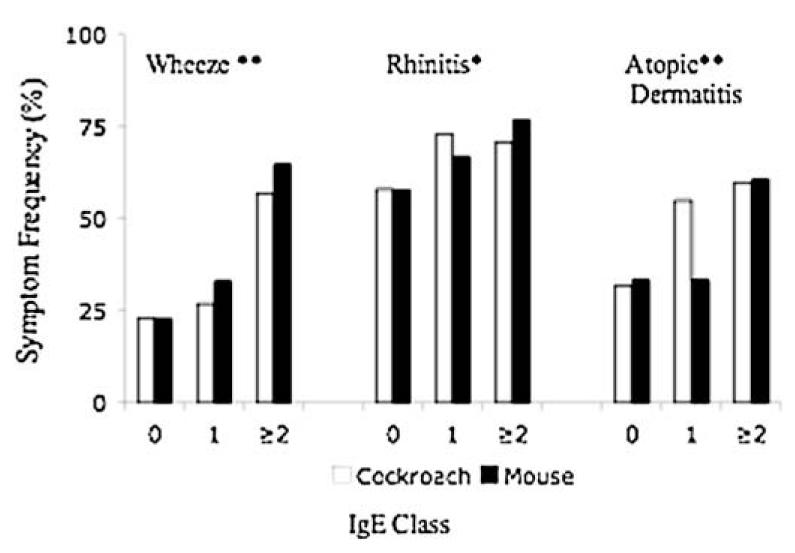
Increased prevalence of wheeze, rhinitis, and atopic dermatitis with higher IgE class (n = 404). **P < .005 on test for trend for anti-cockroach and anti-mouse IgE class. *P < 0.05 on test for trend for anti-mouse IgE class. Anti-cockroach IgE class 0, n = 358; class 1, n = 11; class 2 or greater, n = 35. Anti-mouse IgE class 0, n = 364; class 1, n = 9; class 2 or greater, n = 31.
DISCUSSION
Our goal was to determine the relationship between anti-cockroach and anti-mouse IgE levels and the development of respiratory and atopic symptoms among inner-city children aged 2 to 3 years. By age 3 years, 11% had anti-cockroach IgE, 10% had anti-mouse IgE, and 5% had both types of IgE. Previously, only a few studies had reported the prevalence of allergen-specific IgE production near age 2 years and primarily to dust mite, outdoor pollens, cat, and food.22-25 Anti—dust mite IgE prevalence at age 2 years approximating 4% was measured in other cohorts of mixed risk for atopy.23,25 Prevalences of anti-cat IgE of 3.1% and 5.4% were reported for 2- and 3-year-olds, respectively, in another inner-city birth cohort.26
Importantly, the development of increased anti-cockroach IgE levels significantly increased the odds of early wheeze and atopic dermatitis, whereas the development of increased anti-mouse IgE levels significantly increased the odds of early wheeze, rhinitis, and atopic dermatitis. The odds of early wheeze, rhinitis, and atopic dermatitis were even higher among children sensitized to both cockroach and mouse. A dose-response relationship was found between higher IgE class to cockroach or mouse and increased prevalence of wheeze or atopic dermatitis. This is the first article to report such a relationship between cockroach and mouse IgE levels and respiratory symptoms in such young inner-city children. Interestingly, the Childhood Asthma Management Program (CAMP) found that sensitization to aeroallergens, such as dog, cat, and Alternaria alternata were independent predictors of lower PC20 values on methacholine challenge, whereas sensitization to cockroach was not. Conversely, this CCCEH study found that sensitizations to cockroach or mouse were independent predictors of early wheeze. Importantly, the CAMP study differed from this study in several ways: it enrolled older (age 5-12 years) asthmatic children, assessed sensitivity based on skin prick test results rather than specific IgE levels, measured Bla g 1 levels rather than Bla g 2 levels in home dust samples, and did not specifically recruit an inner-city population that might have higher cockroach allergen exposure.
This study is also the first to report an association between the early development of anti-cockroach IgE or anti-mouse IgE and the onset of atopic dermatitis. Previous studies have found associations with atopic dermatitis and sensitization to specific food allergens and aeroallergens, such as dust mite.27,28 A prospective birth cohort of 1456 children found that sensitization to aeroallergens, such as dust mite, cat, grass pollen, and A alternata, was strongly associated with parental report of asthma, atopic dermatitis, or rhinitis at age 4 years.27
This study found that anti-cockroach IgE, but not anti-mouse IgE, levels were correlated closely with total IgE levels. The result parallels recent findings in older children that high titers of anti—dust mite IgE, but not anti-cat IgE, were associated with increased total IgE levels and the prevalence of wheeze in New Zealand, where dust mite is the predominant aeroallergen.29 One interpretation of this finding is that the IgE response to arthropods, such as cockroaches, but not to mammalian allergens, such as mice, might be more allergenic, perhaps because of differences in particle size, the presence of methylated DNA, enzymatic activity, and evolutionary distance.30 Despite the differences in relationship to total IgE levels, the clinical consequences of sensitization to cockroach and mouse allergen in this cohort are similar with regard to outcomes such as wheeze, rhinitis, and atopic dermatitis. The finding that anti-cockroach IgE contributes significantly to total IgE levels in such young children is novel given the conventional understanding that total IgE in very young children is predominantly comprised of IgE against food allergens.
The finding that the prevalence of wheeze, rhinitis, and atopic dermatitis was positively associated with greater anti-cockroach or anti-mouse IgE classes complements previous literature examining sensitization to other aeroallergens and atopic outcomes. For example, in a population-based birth cohort of 4089 four-year-olds in Stockholm, higher levels of allergen-specific IgE to aeroallergens, including cat, dog, horse, birch, timothy grass, mugwort, Dermatophagoides pteronyssinus, and Cladosporium species, were associated with increased prevalence of asthma, rhinitis, and atopic dermatitis.31 In a study from the Manchester cohort, the risk of rhinitis at age 5 years increased with higher concurrent levels of specific IgE to grass, dust mite, and cat.32 A study of 2201 East German schoolchildren aged 5 to 14 years found that the prevalence of atopic dermatitis increased with increasing anti—dust mite and anti-cat IgE class.33 A prospective birth cohort of 562 unselected newborns found that the prevalence of atopic dermatitis at 18 months increased with greater IgE class to any one of 14 food or aeroallergens.34 Thus the finding in this study of increased prevalence of wheeze, rhinitis, and atopic dermatitis with higher IgE class to cockroach and mouse is consistent with previous research showing similar associations with other aeroallergens.
The absence of a direct relationship between cockroach and mouse residential dust allergen levels and asthma and atopic symptoms is compatible with emerging research that the relationship between allergen exposure and sensitization is much more complex than a straightforward dose-response curve. The complex interactions between genetic predisposition and environmental co-exposures, such as CD14 polymorphisms and endotoxin, are emblematic of this emerging paradigm.35 The findings here resemble those from the CAMP study, which reported that among children sensitized to dog or A alternata there was no difference on methacholine challenge in PC20 values between those exposed and unexposed to these allergens.36 Our finding contrasts with other studies that have demonstrated a positive relationship between allergen residential exposure and sensitization,5,37-39 but most of these studies were cross-sectional comparisons among older and predominantly asthmatic children. Furthermore, the National Inner-City Asthma Study did report an association between mouse allergen exposure and sensitization, but differences in the allergens measured in the assays (Mus m 1 vs MUP), in the mouse allergen levels found in the homes (0.52 μg/g Mus m 1), and in participant inclusion characteristics (age and all asthmatic subjects)40 might explain the discrepancies as well. The result in this article also contrasts with recently published cockroach data from a New York City Head Start cohort. Important differences in the age of the children (CCCEH, age 2-3 years; Head Start, age 4 years), the prevalence of anti-cockroach IgE (CCCEH cohort, 11%; Head Start cohort, 22%), and measured cockroach allergen levels (CCCEH cohort, geometric mean Bla g 2 from bed samples = 1.4 U/g; Head Start cohort, 4.8 U/g) might be the explanations.21 In other published work examining the effects of birth order on respiratory symptoms,11 the 2 New York City cohorts demonstrated variant findings as well, suggesting that some patterns of asthma development might vary, even among low-income populations within the same city.12
There are several limitations of this study. Allergy skin prick testing for cockroach and mouse was not conducted at this age. Anti-cockroach IgE correlates relatively well with skin prick test results in this age group,41 but Matsui et al42 found a significant discrepancy between skin testing and anti-mouse IgE levels. Although this is likely due to testing different extracts (derived from mouse urine vs mouse epithelium), it does raise the possibility of misclassification or underrepresentation of all children truly sensitized to mouse. However, the children in this study were tested with mouse urine protein that is generally more allergenic than mouse epithelium and corresponds better to the dust samples. Another limitation was the need to merge data from age 2 and 3 years to improve statistical power. This approach precluded analysis of new-incident IgE at age 3 years. Indeed, a Danish birth cohort of 562 infants found a dominant pattern of transient low-level sensitization.34 Importantly, in this same cohort they found that persistent sensitization, high titers of allergen-specific IgE, or sensitization to multiple aeroallergens correlated best with atopic dermatitis, suggesting potential bias toward the null in our cohort. Kulig et al43 found that aeroallergen-specific IgE levels tend to increase in the great majority of children as they approach school age, but noted that 19% of their cohort followed a different pattern and had transient sensitization to aeroallergens in the first 6 years of life. Kulig et al acknowledged that whether the predictive probabilities of atopy differ between these 2 groups is unknown. Unfortunately, the statistical necessity of merging the age 2 and 3 year data in this cohort precludes our investigation of this important question. The change in methodology for assessment of specific IgE from the Flourescence Allergosorbent Test to the ImmunoCAP is an additional limitation, although one that would tend to bias our results toward the null.44 Another limitation was the use of parental report of wheeze, rhinitis, and atopic dermatitis rather than standardized direct physician assessment of these outcomes. Finally, differences in host susceptibility caused by genetic influences and other environmental exposures might have added variability to our results.
In conclusion, the development of anti-cockroach and anti-mouse IgE by age 3 years is associated with increased risk of wheeze, rhinitis, and atopic dermatitis. The repeated finding that an increased specific IgE level in the presence of respiratory or atopic symptoms by this age might predict persistent asthma and atopy lends further potential clinical significance to the results.3,34,45 Prospective follow-up of this cohort will help determine whether the development of anti-cockroach and anti-mouse IgE by age 3 years is associated with impairment in lung function, persistent asthma, or both. Despite the lack of a direct association with measured allergen levels, interventions directed toward cockroach and mouse allergen reduction might have long-term benefit to inner-city children who are susceptible to these exposures.
Acknowledgments
We thank the participating mothers and children. This work would not have been possible without the hard work and dedication of the research workers and field technicians.
Supported by the National Institute of Environmental Health Sciences (grant nos. 5 P01 ES009600, 5 R01 ES008977, and P30 ES009089), the US Environmental Protection Agency (grant nos. R827027 and RD-832141), the Irving General Clinical Research Center (grant no. RR00645), the Educational Foundation of America, the Horace W. Goldsmith Foundation, the Gladys & Roland Harriman Foundation, The John Merck Fund, The New York Community Trust, and the Trustees of the Blanchette Hooker Rockefeller Fund.
Abbreviations used
- CAMP
Childhood Asthma Management Program
- CCCEH
Columbia Center for Children’s Environmental Health
- LOD
Level of detection
- MUP
Mouse urinary protein
- OR
Odds ratio
Footnotes
Disclosure of potential conflict of interest: M. S. Perzanowski has received grant from the National Institutes of Health (NIH). G. L. Chew has received grants from the National Institute of Environmental Health Sciences. R. L. Miller has received grants or other research funding from the NIH, the Environmental Protection Agency, and the Sandler Program for Asthma Research. The rest of the authors have declared that they have no conflict of interest.
Clinical implications: IgE to cockroach or mouse is associated with wheeze and atopy by age 3 years in an inner-city cohort.
REFERENCES
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A. IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol. 2005;116:744–9. doi: 10.1016/j.jaci.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Illi S, von Mutius E, Lau S, Nickel R, Niggemann B, Sommerfeld C, et al. The pattern of atopic sensitization is associated with the development of asthma in childhood. J Allergy Clin Immunol. 2001;108:709–14. doi: 10.1067/mai.2001.118786. [DOI] [PubMed] [Google Scholar]
- 4.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 5.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 6.Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97:514–20. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 7.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J Allergy Clin Immunol. 2001;107:41–7. doi: 10.1067/mai.2001.111143. [DOI] [PubMed] [Google Scholar]
- 8.Chew GL, Perzanowski MS, Miller RL, Correa JC, Hoepner LA, Jusino CM, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. 2003;111:1348–51. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller RL, Chew GL, Bell CA, Biedermann SA, Aggarwal M, Kinney PL, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med. 2001;164:995–1001. doi: 10.1164/ajrccm.164.6.2011107. [DOI] [PubMed] [Google Scholar]
- 10.Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006;117:1082–9. doi: 10.1016/j.jaci.2005.12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein IF, Perzanowski MS, Lendor C, Garfinkel RS, Hoepner LA, Chew GL, et al. Prevalence of allergy symptoms and total IgE in a New York City cohort and their association with birth order. Int Arch Allergy Immunol. 2005;137:249–57. doi: 10.1159/000086338. [DOI] [PubMed] [Google Scholar]
- 12.Perzanowski MS, Canfield SM, Chew GL, Mellins RB, Hoepner LA, Jacobson JS, et al. Birth order, atopy, and symptoms of allergy and asthma among inner-city children attending Head Start in New York City. Clin Exp Allergy. 2008;38:968–76. doi: 10.1111/j.1365-2222.2008.02967.x. [DOI] [PubMed] [Google Scholar]
- 13.Choudhry S, Avila PC, Nazario S, Ung N, Kho J, Rodriguez-Santana JR, et al. CD14 tobacco gene-environment interaction modifies asthma severity and immunoglobulin E levels in Latinos with asthma. Am J Respir Crit Care Med. 2005;172:173–82. doi: 10.1164/rccm.200409-1232OC. [DOI] [PubMed] [Google Scholar]
- 14.Wu CH, Wang NM, Lee MF, Kao CY, Luo SF. Cloning of the American cockroach Cr-PII allergens: evidence for the existence of cross-reactive allergens between species. J Allergy Clin Immunol. 1998;101:832–40. doi: 10.1016/S0091-6749(98)70312-4. [DOI] [PubMed] [Google Scholar]
- 15.Wood RA, Segall N, Ahlstedt S, Williams PB. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol. 2007;99:34–41. doi: 10.1016/S1081-1206(10)60618-7. [DOI] [PubMed] [Google Scholar]
- 16.Leimgruber A, Mosimann B, Claeys M, Seppey M, Jaccard Y, Aubert V, et al. Clinical evaluation of a new in-vitro assay for specific IgE, the immuno CAP system. Clin Exp Allergy. 1991;21:127–31. doi: 10.1111/j.1365-2222.1991.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 17.Kam KL, Hsieh KH. Comparison of three in vitro assays for serum IgE with skin testing in asthmatic children. Ann Allergy. 1994;73:329–36. [PubMed] [Google Scholar]
- 18.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106:1075–80. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 19.Call RS, Smith TF, Morris E, Chapman MD, Platts-Mills TA. Risk factors for asthma in inner city children. J Pediatr. 1992;121:862–6. doi: 10.1016/s0022-3476(05)80329-4. [DOI] [PubMed] [Google Scholar]
- 20.Sarpong SB, Hamilton RG, Eggleston PA, Adkinson NF., Jr Socioeconomic status and race as risk factors for cockroach allergen exposure and sensitization in children with asthma. J Allergy Clin Immunol. 1996;97:1393–401. doi: 10.1016/s0091-6749(96)70209-9. [DOI] [PubMed] [Google Scholar]
- 21.Chew GL, Perzanowski MS, Canfield SM, Goldstein IF, Mellins RB, Hoepner LA, et al. Cockroach allergen levels and associations with cockroach-specific IgE. J Allergy Clin Immunol. 2008;121:240–5. doi: 10.1016/j.jaci.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Sigurs N, Hattevig G, Kjellman B, Kjellman NI, Nilsson L, Bjorksten B. Appearance of atopic disease in relation to serum IgE antibodies in children followed up from birth for 4 to 15 years. J Allergy Clin Immunol. 1994;94:757–63. doi: 10.1016/0091-6749(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 23.Wahn U, Lau S, Bergmann R, Kulig M, Forster J, Bergmann K, et al. Indoor allergen exposure is a risk factor for sensitization during the first three years of life. J Allergy Clin Immunol. 1997;99:763–9. doi: 10.1016/s0091-6749(97)80009-7. [DOI] [PubMed] [Google Scholar]
- 24.Hagendorens MM, Ebo DG, Bridts CH, Van de Water L, De Clerck LS, Stevens WJ. Prenatal exposure to house dust mite allergen (Der p 1), cord blood T cell phenotype and cytokine production and atopic dermatitis during the first year of life. Pediatr Allergy Immunol. 2004;15:308–15. doi: 10.1111/j.1399-3038.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CC, Peterson EL, Ownby DR. Gender differences in total and allergenspecific immunoglobulin E (IgE) concentrations in a population-based cohort from birth to age four years. Am J Epidemiol. 1998;147:1145–52. doi: 10.1093/oxfordjournals.aje.a009413. [DOI] [PubMed] [Google Scholar]
- 26.Perzanowski MS, Chew GL, Divjan A, Johnson A, Goldstein IF, Garfinkel RS, et al. Cat ownership is a risk factor for the development of anti-cat IgE but not current wheeze at age 5 years in an inner-city cohort. J Allergy Clin Immunol. 2008;121:1047–52. doi: 10.1016/j.jaci.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108:E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 28.Kulig M, Bergmann R, Niggemann B, Burow G, Wahn U. Prediction of sensitization to inhalant allergens in childhood: evaluating family history, atopic dermatitis and sensitization to food allergens. The MAS Study Group. Multicentre Allergy Study. Clin Exp Allergy. 1998;28:1397–403. doi: 10.1046/j.1365-2222.1998.00439.x. [DOI] [PubMed] [Google Scholar]
- 29.Erwin EA, Ronmark E, Wickens K, Perzanowski MS, Barry D, Lundback B, et al. Contribution of dust mite and cat specific IgE to total IgE: relevance to asthma prevalence. J Allergy Clin Immunol. 2007;119:359–65. doi: 10.1016/j.jaci.2006.12.648. [DOI] [PubMed] [Google Scholar]
- 30.Platts-Mills TA, Satinover SM, Naccara L, Litonjua AA, Phipatanakul W, Carter MC, et al. Prevalence and titer of IgE antibodies to mouse allergens. J Allergy Clin Immunol. 2007;120:1058–64. doi: 10.1016/j.jaci.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Wickman M, Ahlstedt S, Lilja G, van Hage Hamsten M. Quantification of IgE antibodies simplifies the classification of allergic diseases in 4-year-old children. A report from the prospective birth cohort study—BAMSE. Pediatr Allergy Immunol. 2003;14:441–7. doi: 10.1046/j.0905-6157.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 32.Marinho S, Simpson A, Soderstrom L, Woodcock A, Ahlstedt S, Custovic A. Quantification of atopy and the probability of rhinitis in preschool children: a population-based birth cohort study. Allergy. 2007;62:1379–86. doi: 10.1111/j.1398-9995.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- 33.Schafer T, Heinrich J, Wjst M, Adam H, Ring J, Wichmann HE. Association between severity of atopic eczema and degree of sensitization to aeroallergens in schoolchildren. J Allergy Clin Immunol. 1999;104:1280–4. doi: 10.1016/s0091-6749(99)70025-4. [DOI] [PubMed] [Google Scholar]
- 34.Johnke H, Norberg LA, Vach W, Host A, Andersen KE. Patterns of sensitization in infants and its relation to atopic dermatitis. Pediatr Allergy Immunol. 2006;17:591–600. doi: 10.1111/j.1399-3038.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 35.Smit LA, Bongers SI, Ruven HJ, Rijkers GT, Wouters IM, Heederik D, et al. Atopy and new-onset asthma in young Danish farmers and CD14, TLR2, and TLR4 genetic polymorphisms: a nested case-control study. Clin Exp Allergy. 2007;37:1602–8. doi: 10.1111/j.1365-2222.2007.02831.x. [DOI] [PubMed] [Google Scholar]
- 36.Nelson HS, Szefler SJ, Jacobs J, Huss K, Shapiro G, Sternberg AL. The relationships among environmental allergen sensitization, allergen exposure, pulmonary function, and bronchial hyperresponsiveness in the Childhood Asthma Management Program. J Allergy Clin Immunol. 1999;104:775–85. doi: 10.1016/s0091-6749(99)70287-3. [DOI] [PubMed] [Google Scholar]
- 37.Warner AM, Bjorksten B, Munir AK, Moller C, Schou C, Kjellman NI. Childhood asthma and exposure to indoor allergens: low mite levels are associated with sensitivity. Pediatr Allergy Immunol. 1996;7:61–7. doi: 10.1111/j.1399-3038.1996.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 38.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 39.Marks GB. House dust mite exposure as a risk factor for asthma: benefits of avoidance. Allergy. 1998;53:108–14. doi: 10.1111/j.1398-9995.1998.tb05010.x. [DOI] [PubMed] [Google Scholar]
- 40.Kattan M, Mitchell H, Eggleston P, Gergen P, Crain E, Redline S, et al. Characteristics of inner-city children with asthma: the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:253–62. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 41.Lopes MI, Miranda PJ, Sarinho E. Use of the skin prick test and specific immunoglobulin E for the diagnosis of cockroach allergy. J Pediatr (Rio J) 2006;82:204–9. doi: 10.2223/JPED.1487. [DOI] [PubMed] [Google Scholar]
- 42.Matsui EC, Eggleston PA, Breysse PN, Rand CS, Diette GB. Mouse allergen-specific antibody responses in inner-city children with asthma. J Allergy Clin Immunol. 2007;119:910–5. doi: 10.1016/j.jaci.2006.12.663. [DOI] [PubMed] [Google Scholar]
- 43.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 44.Szymanski W, Chyrek-Borowska S. CAP system methods versus FAST methods in immunologic monitoring of specific immunotherapy in pollen allergy. Pneumonol Alergol Pol. 1995;63(Suppl 2):52–9. [PubMed] [Google Scholar]
- 45.Sherrill DL, Stein R, Halonen M, Holberg CJ, Wright A, Martinez FD. Total serum IgE and its association with asthma symptoms and allergic sensitization among children. J Allergy Clin Immunol. 1999;104:28–36. doi: 10.1016/s0091-6749(99)70110-7. [DOI] [PubMed] [Google Scholar]


