Lead poisoning, which is especially prevalent in children, is still the most common environmentally caused disease nationally and worldwide.1 Development of extractants and fluorescent chemosensors2 for Pb(II) is of importance for monitoring EPA mandated levels and for a variety of applications related to lead toxicity, biodistribution, and removal. For such applications, it is essential to achieve high selectivity for Pb(II) against both alkaline earth and other common metal ions present in biological systems, such as Cu(II) and Zn(II).3 Exploiting the unique coordination properties of Pb(II)3,4 provides opportunities for the design of selective sensors5 and extractants.6 Successful fluorescent Pb(II) sensor designs are based on complexation by peptides,7 ionizable chelates,8 DNAzymes,9 or macrocycles10 with covalently linked fluorophores. Macrocycles can be tedious to synthesize and typically exhibit a wide variety of polydentate Pb(II) coordination patterns. On the other hand, ion exchange extraction from water to an organic solvent by simpler chelates can lead to improved selectivity by taking advantage of the unique preference of Pb(II) for low-coordinate hemidirected3 geometries with a stereochemically expressed lone pair. Moreover, simpler chelates tend to be easier to synthesize, an advantage that could potentially be exploited for sensor discovery by using rapid screening methods.11 As part of our effort to design low-coordinate Pb(II) extractants and sensors, we now wish to report efficient and selective ion-exchange extraction of Pb(II) from water into 1,2-dichloroethane (DCE) with concurrent fluorescence quenching using as an ion-exchanger, the sulfonamide fluorophore 1. This simple system does not require a secondary co-ligand in order to extract Pb and shows remarkable extraction selectivity against other metals with DPb > 130 DCu and DPb > 1400 DZn.
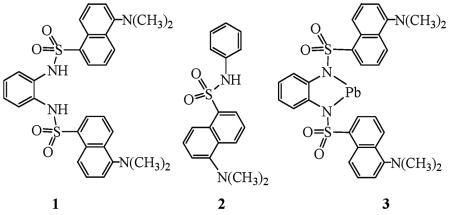
Sulfonamide 1 is available in good yields from o-phenylenediamine and 1-(dimethylamino)-5-naphthalene sulfonyl chloride (dansyl chloride).12 The dansyl group is a well-known fluorophore that is sensitive to its chemical environment.13 Godwin et al.7 and Leray et al.10a have reported elegant examples of Pb(II) fluorescence detection in aqueous matrices based on the dansyl fluorophore. Reinhoudt et al.8b have reported quenching of the dansyl fluorescence by Pb(II) on a self-assembled monolayer (SAM)-modified glass surface. The X-ray crystal structure of 1 shows the S=O groups located in an anti conformation with self-association via N–H···O=S hydrogen bonds, giving dimers, instead of the 1D chains observed before for analogous disulfonamides.14
Ligand 1 was shown to extract Pb(II) from water into DCE via ion-exchange when used together with NH(i-Pr)2. One clear advantage of 1 over the analogous sulfonamide extractants, 1,2-C6H4(NH2SO2C6H5)2 and 1,2-C6H4(NH2SO2C6H4-p-But)215, is that the formed binary complex, Pb[1,2-C6H4(NSO2C10H6-5-N(CH3)2)2] (3), is soluble in DCE, and therefore, there is no need for the use of a co-ligand. 1H NMR of the organic phases after extraction indicated the presence of 3. The negative mode electrospray ionization mass spectrum of a methanol solution of 3 showed the formation of the 3·CH3O− ion at m/z = 811.
The distribution ratios (DPb = [Pb]o/[Pb]aq) were determined by inductively coupled plasma mass spectrometry (ICP–MS) of the aqueous phases after extraction (for [Pb]aq) and after stripping the organic phases with 0.1 M HNO3 (for [Pb]o). Double stripping of the organic phases showed no increase in [Pb]o. Specifically, when 1 (3.5 mM) was used together with 2.2 equiv of NH(i-Pr)2, it extracted 99.5% of Pb from a 3.5 mM Pb(NO3)2 solution. A plot of [Pb]o versus [1]t showed saturation for [1]t > 3.5 mM, indicating 1:1 complexation. Control experiments, using 1 or NH(i-Pr)2 only, showed that the presence of both components is essential for extracted. The monodansylamide 216 did not extract any Pb(II) under the same conditions. A comparison of 1 with the ion-pair extractant 18-crown-6 under identical conditions gave a DPb = 0.950 for extraction by 1 versus DPb = 1.26 × 10−3 by the crown ether, which is also higher than the extraction by synergistic combinations of 2,2′-bipy and analogous disulfonamide ion-exchangers.15 Ligand 1 was also shown to be effective in the extraction of micromolar Pb(II) concentrations, with [1]t = 2.97 mM, [NH(i-Pr)2]t = 6.53 mM, and [Pb(II)]t between 3.75 and 284 μM ([1]t/[Pb(II)]t ranging from 10.5 to 792); ligand 1 extracted 100% of Pb(II) ([Pb]aq < 0.01 μM).
The extraction of various dicationic metal nitrates was investigated at constant total metal concentration ([M(II)]t) and varying ligand concentrations (0.60–2.99 mM). Ligand 1 was found to extract Pb(II) selectively over Zn(II), Co(II), Cd(II), Ni(II), and Cu(II) with DPb(II)/DM(II) = 1410 (for Zn), 1380 (for Co), 829 (for Cd), 794 (for Ni), and 133 (for Cu) (Figure 1). Neither Ca(II) nor Na(I) were extracted to any appreciable extent. We ascribe the observed selectivity to the formation of the low-coordinate complex 3, which is apparently disfavored for other competing metals. The crystal structure of the binary Pb complex of the analogous bis-(phenylsulfonyl) derivative of phenylenediamine, which is insoluble in DCE, has showed the formation of a coordination polymer via S=O–Pb axial coordination and a stereochemically active lone pair.15 The fact that the dansyl complex 3 is soluble in DCE indicates that the S=O–Pb axial coordination is no longer significant and is most likely replaced by weak coordination from solvent molecules or water. The formation of a low-coordinate complex with a stereochemically active lone pair could possibly explain the high extraction selectivity for Pb(II) over other dicationic metals.
Figure 1.
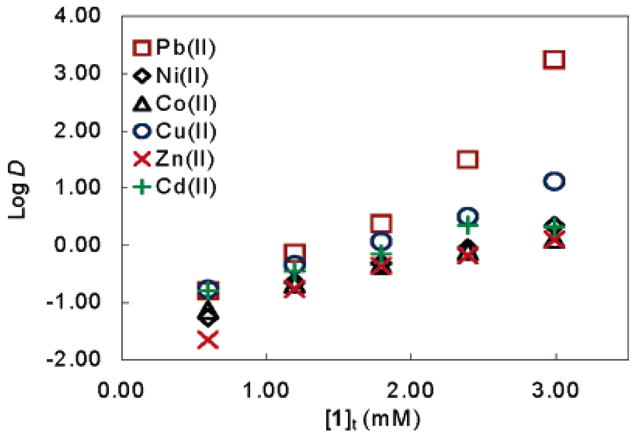
Plot of log [M(II)]t versus [1]t: [Pb]t = 2.72 mM, [Ni]t = 2.72 mM, [Co]t = 2.67 mM, [Cu]t =2.80 mM, [Zn]t = 2.53 mM, [Cd]t = 2.59 mM.
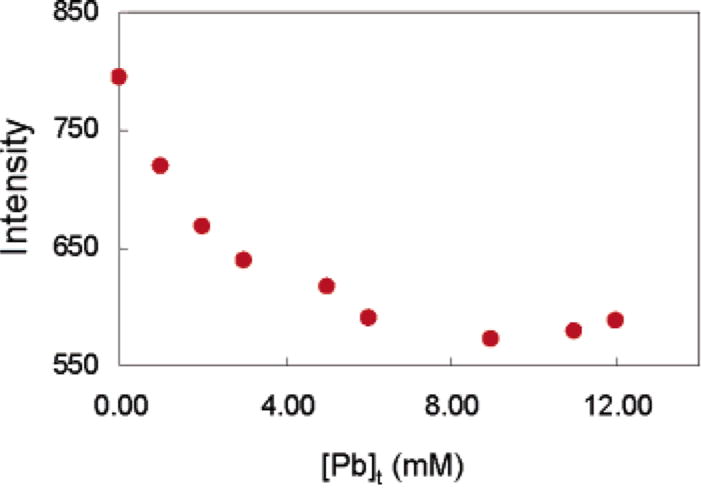
The extraction of Pb(II) from water into DCE by 1 or the control 2 was also investigated by fluorescence spectroscopy. Emission spectra of the organic phases were collected before and after contact with Pb(NO3)2(aq) under identical conditions to the ICP–MS distribution experiments. Fluorescence quenching was observed at 516 nm after contact of a solution of 1 and NH(i-Pr)2 in DCE with Pb(NO3)2(aq). Specifically, the fluorescence intensity was reduced by as much as 29% upon contact of 1 (10 mM) and NH(i-Pr)2 (22 mM) with 5.5 mM Pb(NO3)2 (Figure 2). On the other hand, a solution of 2 and NH(i-Pr)2 in DCE showed no changes in its fluorescence upon contact with Pb(NO3)2 (Figure 3). This is consistent with the ICP–MS results, which showed no extraction by 2, and indicates that bidentate coordination is necessary for Pb(II) extraction, and that the observed quenching is a direct result of Pb(II) complexation by 1. Overall, the Pb selectivity for this remarkably simple extraction-based system could be considered comparable, or even superior to previously reported designs.7,10a However, in our case, Pb sensing is based on quenching and not on ratiometric fluorescence enhancement.
Figure 2.
Fluorescence emission at 516 nm upon Pb coordination and extraction by 1: [1]t = 7.40 mM, [NH(i-Pr)2] = 16.30 mM; λexc = 340 nm.
Figure 3.
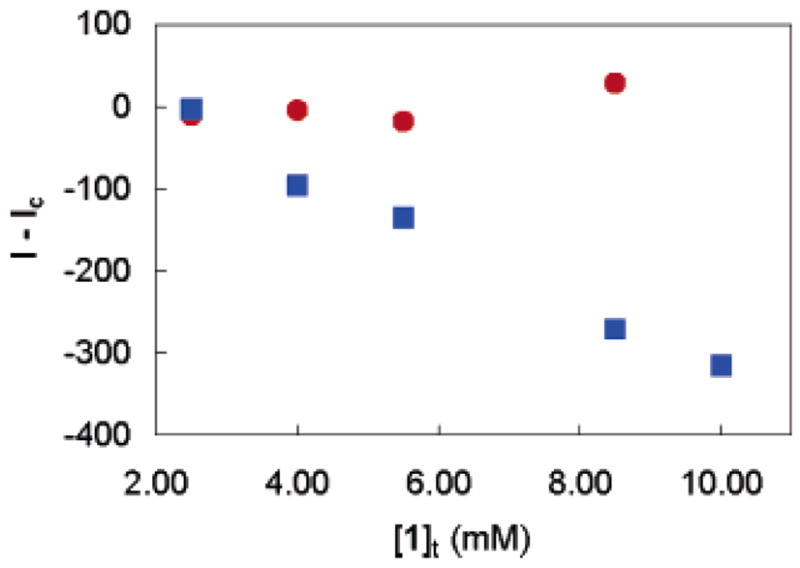
Fluorescence intensity difference at 516 nm for 1/diisopropylamine (■) and 2/diisopropylamine (●) after contact with Pb(NO3)2(aq) (I), compared to contact with blank (Ic) versus [L]t. [Pb]t = 5.5 mM; λexc = 340 nm.
In conclusion, it has been demonstrated that a simple (bis)-dansylamide ion-exchanger selectively extracts Pb(II) from water into DCE, with concurrent fluorescence quenching. We are planning extensive structural and spectroscopic studies with 1 and analogous chelates, as well as application of the ion-exchange extraction methodology for rapid screening of potential extractants and sensors for Pb(II) and other toxic metals.
Acknowledgments
We thank Drs. R. Alvarado and W. Zhang for experimental help. This project was supported in part by NIH-NIEHS/ARCH (S11 ES11181), NIH-MBRS/RISE (R25 GM061347), and the University of Miami (diffractometer fund).
Footnotes
Supporting Information Available: Synthesis, procedures, extraction plots, and X-ray crystallographic details of 1. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lin-Fu JS. Lead Poisoning, A Century of Discovery and Rediscovery. In: Needleman HL, editor. Human Lead Exposure. Lewis Publishing; Boca Raton, FL: 1992. [Google Scholar]
- 2.(a) EV Anslyn. Synthetic Receptors as Sensors. Tetrahedron. 2004;60:11051. [Google Scholar]; (b) Czarnik AW, editor. Fluorescent Chemosensors for Ion and Molecule Recognition. American Chemical Society; Washington, DC: 1993. ACS Symposium Series 358. [Google Scholar]; (c) de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, Mccoy CP, Rademacher JT, Rice TE. Chem Rev. 1997;97:1515. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]; (d) Fabbrizzi L, Poggi A. Chem Soc Rev. 1995;24:197. [Google Scholar]
- 3.Claudio ES, Godwin HA, Magyar JS. Prog Inorg Chem. 2003;51:1. [Google Scholar]
- 4.(a) Harrison PG. In: Comprehensive Coordination Chemistry. Wilkinson G, Grillard RD, McCleverty JA, editors. Vol. 3. Pergamon Press; Oxford, U.K.: 1987. p. 183. [Google Scholar]; (b) Parr J. Polyhedron. 1997;16:551. [Google Scholar]; (c) Powers RE, Fuller WL, Raymond KN. Comp Supramol Chem. 1996;10:537. [Google Scholar]
- 5.(a) Valeur B, Leray I. Coord Chem Rev. 2000;205:3. [Google Scholar]; (b) de Silva AP, Fox DB, Huxley AJM, Moody TS. Coord Chem Rev. 2000;205:41. [Google Scholar]; (c) Liu J, Lu Y. J Am Chem Soc. 2004;126:12298. doi: 10.1021/ja046628h. [DOI] [PubMed] [Google Scholar]; (d) Sun Y, Wong MD, Rosen BP. J Biol Chem. 2001;276:14955. doi: 10.1074/jbc.M010595200. [DOI] [PubMed] [Google Scholar]
- 6.(a) Hancock RD, Maumela H, De Sousa AS. Coord Chem Rev. 1996;148:315. [Google Scholar]; (b) Yordanov AT, Roundhill DM. Coord Chem Rev. 1998;170:93. [Google Scholar]; (c) Talanova GG, Hwang HS, Talanov VS, Bartsch RA. Chem Commun. 1998:419. [Google Scholar]
- 7.Deo S, Godwin H. J Am Chem Soc. 2000;122:174. [Google Scholar]
- 8.(a) Chae MY, Yoon J, Czarnik AD. J Mol Recognit. 1996;9:297. doi: 10.1002/(SICI)1099-1352(199607)9:4%3C297::AID-JMR335%3E3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]; (b) Crego-Calama M, Reinhoudt DN. Adv Mater. 2001;13:1171. [Google Scholar]
- 9.(a) Li J, Lu Y. J Am Chem Soc. 2000;122:10466. [Google Scholar]; (b) Liu J, Lu Y. Anal Chem. 2003;75:6666. doi: 10.1021/ac034924r. [DOI] [PubMed] [Google Scholar]
- 10.(a) Métivier R, Leray I, Valeur B. Chem Commun. 2003:996. doi: 10.1039/b301323e. [DOI] [PubMed] [Google Scholar]; Chem Eur J. 2004;10:4480. [Google Scholar]; (b) Hayashita T, Qing D, Minagawa M, Lee JC, Ku CH, Teramae N. Chem Commun. 2003:2160. doi: 10.1039/b305758e. [DOI] [PubMed] [Google Scholar]; (c) Chen CT, Huang WP. J Am Chem Soc. 2002;124:6246. doi: 10.1021/ja025710e. [DOI] [PubMed] [Google Scholar]; (d) Xia WS, Schmehl RH, Li CJ, Mague JT, Luo CP, Guldi DM. J Phys Chem B. 2002;106:833. [Google Scholar]; (e) Beeby A, Parker JD, Williams JAG. J Chem Soc, Perkin Trans 2. 1996:1565. [Google Scholar]; (f) Addleman RS, Bennnett J, Tweedy SH, Elshani S, Wai CM. Talanta. 1998;46:573. doi: 10.1016/s0039-9140(97)00319-6. [DOI] [PubMed] [Google Scholar]; (g) Padilla-Tosta ME, Lloris JM, Martinez-Mañez R, Marcos MD, Miranda MA, Pardo T, Sancenon F, Soto J. Eur J Inorg Chem. 2001:1475. [Google Scholar]
- 11.Choudhary S, Morrow JR. Angew Chem, Int Ed. 2002;41:4096. doi: 10.1002/1521-3773(20021104)41:21<4096::AID-ANIE4096>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Amundsen LH. J Am Chem Soc. 1937;59:146. [Google Scholar]
- 13.(a) Walkup GK, Imperiali B. J Am Chem Soc. 1998;120:609. [Google Scholar]; (b) Li YH, Chan LM, Tyer L, Moody RT, Himel CM, Hercules DM. J Am Chem Soc. 1975;97:3118. [Google Scholar]; (c) Zheng Y, Orbulescu J, Ji X, Andreopoulos F, Pham SM, Leblanc RM. J Am Chem Soc. 2003;125:2680. doi: 10.1021/ja0293610. [DOI] [PubMed] [Google Scholar]; (d) Prodi L, Bolletta F, Montalti M, Zaccheroni N. Eur J Inorg Chem. 1999:455. [Google Scholar]
- 14.(a) Bryan JC, Rosenberg JM, Kavallieratos K. Acta Crystallogr. 2005;E61:o396. [Google Scholar]; (b) Eagle CT, Kavallieratos K, Bryan JC. J Chem Crystallogr. 2002;32:165. [Google Scholar]
- 15.Kavallieratos K, Rosenberg JM, Bryan JC. Inorg Chem. 2005;44:2573. doi: 10.1021/ic050047r. [DOI] [PubMed] [Google Scholar]
- 16.Webr G, Teale FWS. Trans Faraday Soc. 1958;54:640. [Google Scholar]