Abstract
Here we analyzed the light responsiveness of the mammalian target of rapamycin (mTOR) cascade, a key regulator of inducible translation, in the suprachiasmatic nuclei (SCN), the locus of the master circadian clock. Brief light exposure during the subjective night, but not during the subjective day, triggered rapid phosphorylation (a marker of catalytic activity) of the mTOR translation effectors p70 S6K, ribosomal S6 protein (S6) and 4E-BP1. In the absence of photic stimulation, marked S6 and 4E-BP1 phosphorylation was detected, indicating tonic mTOR activity in the SCN. Light stimulated the colocalized activation of p70 S6K and extracellular signal-regulated protein kinase (ERK), and pharmacological disruption of ERK signaling abolished light-induced mTOR activity, revealing that the MAPK cascade is an essential intermediate that couples light to mTOR. Together these data identify a light-responsive mTOR cascade in the SCN, and thus, raise the possibility that inducible translation contributes to the clock entrainment process.
Introduction
The inherent pacemaker activity of the mammalian circadian clock located in the suprachiasmatic nuclei (SCN) drives a vast array of biochemical, physiological and behavioral processes with 24 hr periodicity (Reppert and Weaver, 2002; Lowrey and Takahashi, 2000). As an adaptation to the ever-changing external environment, the circadian clock can be reset by multiple entrainment cues such as light (Cermakian and Sassone-Corsi, 2002; Challet et al., 2003; Hirota and Fukada, 2004). Photic input is relayed from the retina to the SCN via the retinohypothalamic tract (RHT). In response to a light stimulus, RHT axon terminals release the excitatory neurotransmitter glutamate and the neurohormone pituitary adenylate cyclase-activating peptide (PACAP) (Hannibal, 2002), which in turn bind to postsynaptic receptors on SCN neurons and evoke a series of intracellular signal transduction events that trigger clock resetting.
Transcriptional activation appears to be a key event in the entrainment process. Disruption of photically induced clock gene expression has been shown to abrogate clock entrainment (Akiyama et al., 1999; Albrecht et al., 2001). In addition, mRNA translation is also a critical event for light entrainment of the clock. Along these lines, work performed in a wide range of clock model systems has shown that the application of translation inhibitors suppresses light entrainment (Johnson and Nakashima, 1990; Raju et al., 1990; Murakami et al., 1995; Zhang et al., 1996). Several timing- and entrainment-relevant mRNA translation mechanisms have been reported. For example, in pinealocytes, heterogeneous nuclear ribonucleoprotein Q (hnRNP Q) mediates rhythmic translational regulation of arylalkylamine N-acetyltransferase (AANAT: Kim et al., 2007). In addition, nocturnin, which regulates mRNA stability through the deadenylation of the mRNA polyA tail, is rhythmically expressed in retinal photoreceptor cells and can be upregulated in response to mitogenic signals (Baggs and Green, 2003; Garbarino-Pico et al., 2007). Furthermore, our lab has recently shown that both light and the circadian clock regulate microRNA expression in the SCN (Cheng et al., 2007). microRNAs are potent negative regulators of mRNA translation, and as such may play a pivotal role in sculpting the light- and clock-regulated gene expression profile.
Given these findings, additional efforts to identify translation regulatory processes may be key to our understanding of the entrainment process. Within this context, one potential route by which mRNA translation may be regulated is the mammalian target of rapamycin (mTOR) signaling pathway (Sarbassov et al., 2005; Wullschleger et al., 2006; Proud, 2007). mTOR is a Ser/Thr kinase which is at the core of two distinct multiprotein complexes, the rapamycin-sensitive mTOR complex 1 (mTORC1) and rapamycin-insensitive mTORC2. In response to increased nutrient levels and mitogens mTORC1 activates the downstream targets p70 S6 kinase (p70 S6K) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), which in turn lead to actuation of mRNA translation machinery (Hay and Sonenberg, 2004; Tee and Blenis, 2005). Interestingly, recent work has shown that neuronal activity triggers rapid mRNA translation via an mTOR-dependent mechanism (Gong et al., 2006; Gelinas et al., 2007; Tsokas et al., 2007; Di Nardo et al.,2007) and that disruption of mTORC1 inhibits late phase long-term potentiation (LTP) in the hippocampus (Tang et al., 2002; Cammalleri et al., 2003). Furthermore, in the hypothalamus, mTOR activity has been shown to play a central role in food intake and energy balance (Cota et al., 2006). Together, these data raise the prospect that TOR signaling plays a key role in shaping activity-dependent neuronal physiology.
Here we examined the regulation of mTOR-dependent signaling in the SCN clock. The data reveal that photic stimulation triggers a phase-dependent increase in the activation state of p70 S6K and 4E-BP1. Moreover, the data reveal that mTOR is a downstream target of the p42/44 Mitogen-Activated Protein Kinase (MAPK) signaling pathway, and that light triggers coordinate cyclic AMP-response element binding protein (CREB) and mTOR activation in SCN neurons. Together these data identify mTOR as a light-activated signaling pathway.
Results
Light induces p70 S6K phosphorylation in the SCN in a phase-restricted manner
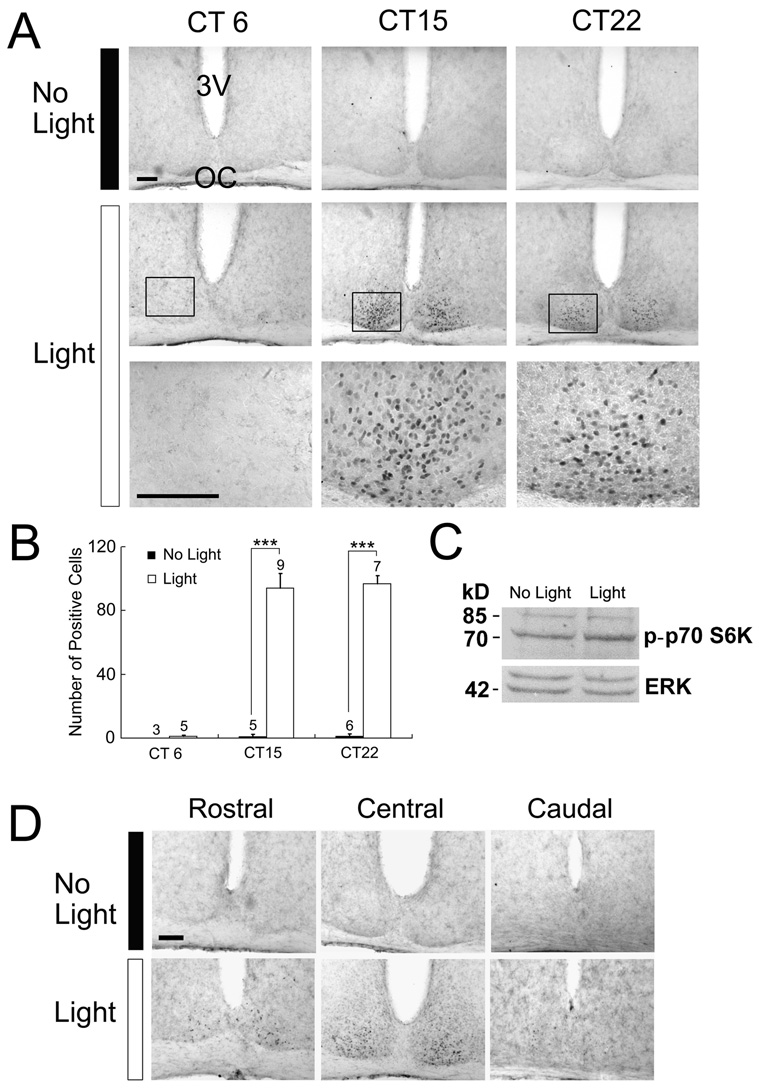
To begin to characterize the mTOR/p70 S6K pathway in the SCN, mice were entrained to a 12 h light/dark (LD) cycle, dark-adapted for 48 hrs, and then exposed to light (400 lux; 15 min) during either the subjective day or the subjective night. Animals were killed immediately after cessation of the light stimulus and the SCN-containing tissue was processed for the Thr-389 phosphorylated form of p70 S6K (p-p70 S6K). Phosphorylation at Thr-389 is associated with mTOR-dependent activation of p70 S6K, and thus can be used to monitor the relative level of pathway activity (Pearson et al., 1995; Jefferies et al., 1997; Weng Q, 1998; Burnett et al., 1998). Photic stimulation during either the early [(circadian time (CT) 15)] or late (CT 22) subjective night triggered robust p70 S6K phosphorylation in the SCN (Fig. 1A and 1B). p-p70 S6K expression was highest in the central SCN, with limited expression in the rostral and caudal regions of the SCN (Fig. 1D). In contrast to the nighttime, p70 S6K activity was not stimulated by light exposure during the middle of the subjective daytime (CT 6: Fig. 1A and 1B). The phase-restricted induction of p70 S6K activity parallels the phase-restricted light-dependent activation pattern of a number of kinases and immediate early genes as well as the phase-restricted capacity of light to entrain the clock (Meijer and Schwartz, 2003). In the absence of photic input, significant expression of the activated form of p70 S6K was not detected in the SCN at any of the three circadian time points examined (Fig 1A and 1B).
Figure 1. Light-induced p70 S6K phosphorylation in the SCN.
Animals were initially dark-adapted for two day and then exposed to light (400 lux, 15 min) during the mid-subjective day [circadian time (CT) 6], early night (CT 15), or late night (CT 22). A, Representative immunohistochemical images of p-p70 S6K in the SCN. Relative to control animals (No Light, top row), light exposure (middle and bottom rows) triggered a marked increase in p-p70 S6K expression at the two night time points. The highest level of antigenicity was observed in the ventral region of the SCN. Photic stimulation during the subjective day did not increase p70 S6K phosphorylation, indicating that the capacity of light to couple to p70 S6K is phase-restricted. Boxed regions are magnified below. Scale bars: 100 µm; 3V, third ventricle; OC, optic chiasm. B, Quantification of p-p70 S6K-positive cells per SCN. The number of animals used for each condition is shown above each histogram. Error bars denote the SEM. ***p < 0.001. C, Western blotting analysis of p-p70 S6K expression in the SCN. Mice received a single light pulse (15 min, 400 lux) at CT 15, and SCN tissue was dissected, pooled (n=3 for each condition) and probed for p-p70 S6K expression. Compared with the “no light” control, a modest increase in the density of the 70 kD band was detected in the light-treated condition. As a protein loading control, the blot was also probed for total ERK (erk 1 and erk 2) expression. D, Distribution of light-induced p-p70 S6K expression within the rostrocaudal axis of the SCN. A fifteen-minute light treatment (400 lux) at CT 15 triggered limited p-p70 S6K expression in the rostral and caudal regions of the SCN; the highest level of expression was observed within the central SCN. Scale bars: 100 µm
To complement the immunohistochemical analysis of p70 S6K activity, SCN tissue from control and light-treated (400 lux, 15 min, CT 15) animals was probed for p-p70 S6K expression using Western analysis. Photic stimulation triggerd a modest increase in the antigenicity of an approximate 70-kDa band (Fig. 1C). This small increase in band intensity, relative to robust increase detected using immunohistochemical labeling is the likely result of the different experimental methods. Hence, Western blotting combines responsive and none-responsive cells, thus generating an averaged response, whereas immunolabeling allows for identification of limited numbers of highly antigenic cells. Importantly, the size of the main band corresponds with the size of p70 S6K, thus supporting the results obtained using immunohistochemical detection procedures. Of note, the antibody also detected a weak band at 85-kDa. This band is likely to be p85 S6 Kinase(p85 S6K), an isoform of p70 S6K. According to the manufacturer, this antibody detects p85 S6K when phosphorylated at Thr-412, a site analogous to Thr-389 in p70 S6K. For the sake of clarity, we will only refer to p70 S6K when describing results collected with this antibody. As a protein loading control, the blot was stripped and probed for total ERK expression. Together, these data support the observation that p70 S6K is tightly regulated by photic input to the SCN.
Light-induced p70 S6K phosphorylation is mTOR-dependent
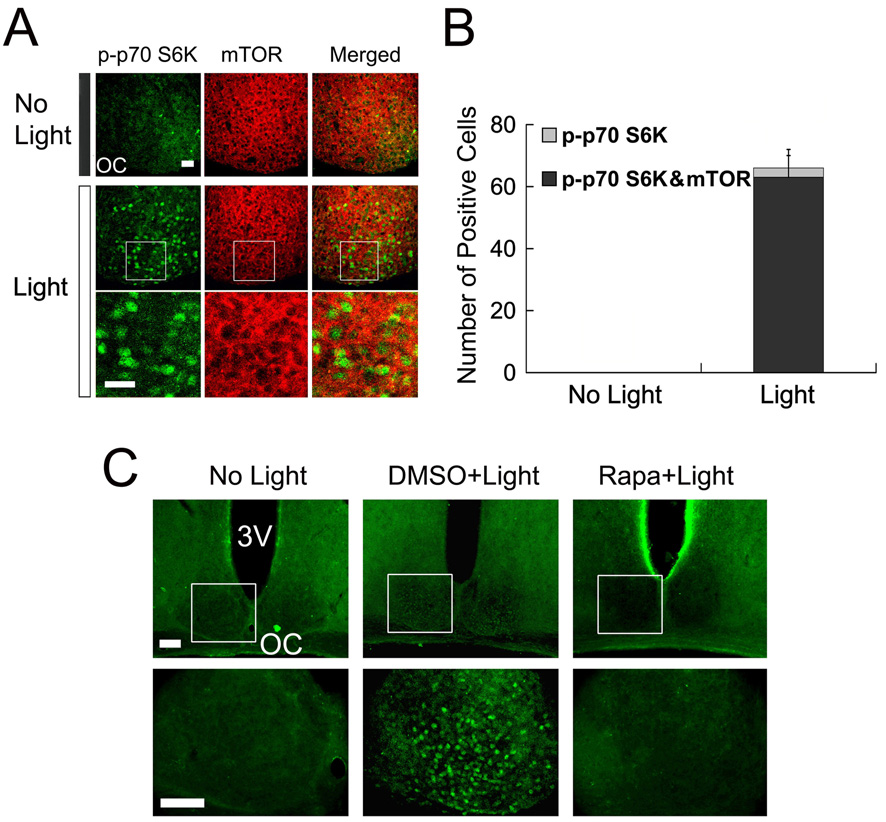
To determine whether light-induced p70 S6K phosphorylation in the SCN is mediated by mTOR signaling, we initially examined the expression of mTOR and activated p70 S6K. Immunofluorescent labeling revealed marked cytoplasm expression of mTOR in the SCN (Fig. 2A). As expected, relative to control animals (Fig. 2A top), a 15-minute light treatment (400 lux) at CT15 elicited p70 S6K activation (Fig.2A bottom). Merging the mTOR and p-p70 S6K signals revealed cellular but not spatial colocalization of the two kinases (Fig. 2A) in the SCN. Along these lines, p-p70 S6K appeared to be largely nuclear, whereas mTOR expression was largely cytoplasmic.
Figure 2. Light-induced p70 S6K activation is mTOR-dependant.
A, Representative confocal images showing colocalization of phosphorylated p70 S6K (p-p70 S6K, green) and mTOR (red) in the SCN. Compared with “no light” control animals (top), a 15-minute light treatment (400 lux) at CT 15 elicited robust p70 S6K activation in the SCN. The merged immunolabeling images (right column) reveal a largely nuclear expression pattern for p-p70 S6K and a cytoplasmic expression pattern for mTOR. OC, optic chiasm. Boxed regions are magnified below each image. Scale bars: 20 µm. B, Quantification of light-induced p-p70 S6K expression and its colocalization with mTOR in the SCN. Cellular coexpression of p-p70 S6K with mTOR was detected in 95% p-p70 S6K positive cells (315 out of 330 cells counted from five mice). Quantitation was performed on 5 control (no light) mice. Error bars denote SEM. C, Representative fluorescent micrographs showing that infusion of the mTOR inhibitor rapamycin blocks light-induced p-p70 S6K expression. Two µl of rapamycin (100 µM) or dimethylsulfoxide (DMSO, vehicle) was infused into the lateral ventricle 30 min before a 15-minute light treatment at CT 15. The boxed regions are shown below. Scale bar, low magnification: 100 µm, high magnification: 75 µm; OC, optic chiasm; 3V, third ventricle. Statistical analysis is presented in figure 5C.
To test whether mTOR activity stimulated light-induced p70 S6K phosphorylation, mice were infused in the lateral ventricle with the mTORC1 inhibitor rapamycin (100 µM, 2 µl) 30 min prior to light exposure (400 lux, 15 min) at CT 15. Relative to the robust light-induced p70 S6K phosphorylation observed in vehicle- (Dimethyl sulfoxide, DMSO) infused mice, infusion of rapamycin totally blocked light-activated p-p70 S6K expression (Fig. 2C and 5C). Together these data reveal that photic stimulation drives activation of the canonical mTOR/70 S6K signaling cassette in the SCN.
The MAPK pathway couples light to mTOR/p70 S6K activation
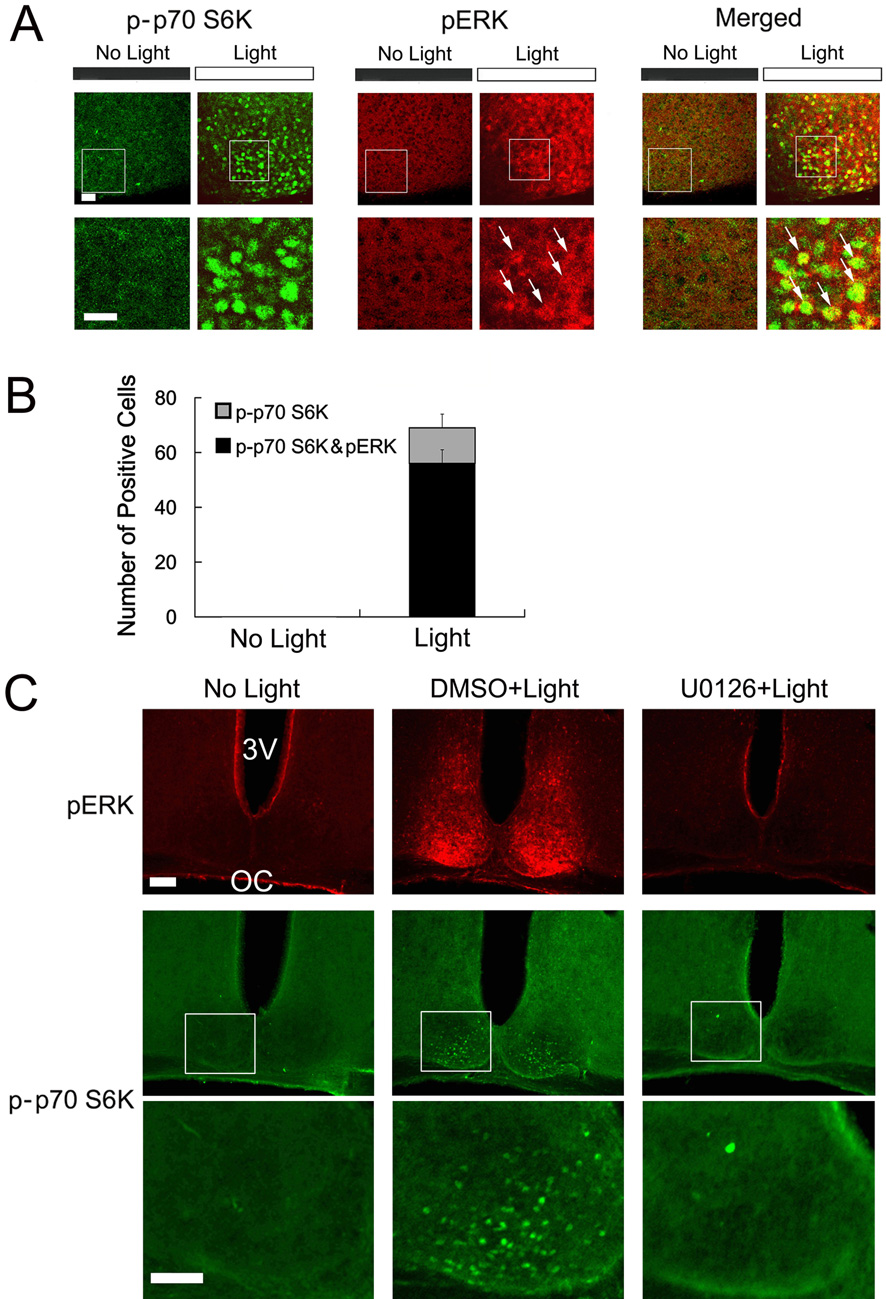
Recent reports indicate that the p42/44 MAPK pathway can function as an upstream regulator of the mTOR/p70 S6K pathway (Ma et al., 2005; Roux et al., 2004; Tsokas et al., 2007; Gelinas et al., 2007). These findings and the prominent role that MAPK signaling plays in light-entrainment of the circadian clock (Butcher et al., 2002; Coogan and Piggins, 2003), led us to examine a possible connection between MAPK signaling and the mTOR/p70 S6K pathway in the SCN. To begin to address this question, we tested whether light stimulates coordinate p70 S6K and MAPK activation. SCN tissue from control (no light) and light-treated (400 lux, 15 min, CT 15) mice was processed using immunofluorescent labeling for the phosphorylated form of p70 S6K and the dual phosphorylated (Thr-202/Tyr-204) forms of extracellular signal-regulated kinase 1 (erk 1) and erk 2 (collectively referred to as ERK). This dual phosphorylation event is required for ERK enzymatic activity and thus can be used to monitor MAPK pathway activation. Under control conditions, low levels of activated ERK and p-p70 S6K were observed (Fig. 3A). However, photic stimulation led to an increase in the phosphorylated forms of both kinases and merging the two images revealed colocalized expression of activated ERK and p70 S6K in a subset of SCN cells (Fig. 3A and 3B). Together these results identify a light-induced spatial and temporal colocalization of activated ERK and p70 S6K in the SCN.
Figure 3. The MAPK pathway couples light to mTOR/p70 S6K.
A, Compared with “no light” control animals, a 15-minute light treatment (400 lux) at CT15 elicited robust phospho-p70 S6K (p-p70 S6K: green) and phospho-ERK (pERK: red) expression. Merged confocal images revealed that light-induced p70 S6K and ERK activation occurred in the same subset of cells (arrows). Boxed regions are magnified below each image. Scale bars: 20 µm. B, Quantification of colocalized, light-induced, p-p70 S6K expression and ERK activation in the SCN. Of note, after the light flash 81% p-p70 S6K positive cells were also pERK-postive (338 out of 416 cells counted from six mice); quantitation was performed on 7 control (no light) mice. Error bars denote SEM. C, Representative fluorescent micrographs showing that light-induced p-p70 S6K is dependent on the MAPK pathway. Mice received a ventricular infusion of U0126 (10 mM, 2 µl) or dimethylsulfoxide (DMSO, 2 µl) vehicle 30 min before a 15-minute light treatment at CT 15. SCN sections were double labeled for pERK (red) and p-p70S6K (green) expression. The boxed regions are magnified and shown below. Scale bars, high magnification: 75 µm, low magnification: 100 µm; OC, optic chiasm; 3V, third ventricle. Statistical analysis is presented in figure 5C
To test the causal connection between light-induced MAPK activation and p70 S6K phosphorylation, we employed the ventricular infusion technique to disrupt MAPK signaling in the SCN. To this end, mice were infused with the specific MEK 1/2 inhibitor U0126 (10 mM, 2 µl) 30 min before photic stimulation (400 lux, 15 min) at CT 15 and tissue was immunolabed for ERK and p70 S6K activation. Consistent with our prior work (Butcher et al., 2002; Dziema et al., 2003), infusion of U0126 completely blocked light-induced ERK activation (Fig. 3C). Importantly, disruption of MAPK activation effectively suppressed light-induced p70 S6K activation (Fig. 3C). Representative data for activated ERK (pERK) and p70 S6K are from the same mouse and quantitative data are presented in figure 5C. In conclusion, these results reveal the presence of a light-responsive MAPK-mTOR/p70 S6K signaling cassette in the SCN.
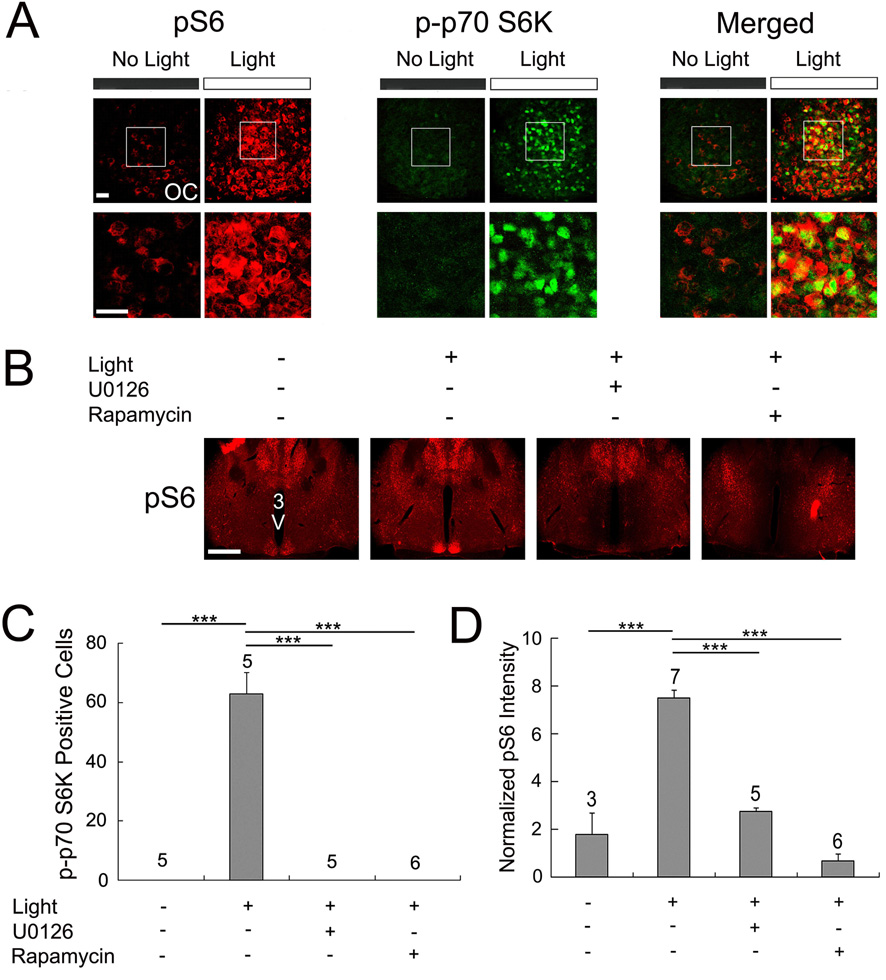
Figure 5. Light-induced S6 activation in the SCN.
A, Confocal images showing immunolabeling for S6 phosphorylation (pS6, red) and p-p70 S6K (green) in the SCN. Compared with control animals (no light), a 15-minute light treatment (400 lux) at CT 15 elicited an increase in pS6 and p-p70 S6K. Merging the two signals revealed colocalization of light-induced p-p70 S6K and pS6 expression in a subset of the cells. OC, optic chiasm. Boxed regions are magnified below each image. Scale bars: 20 µm. B, Infusion of U0126 and rapamycin blocked light-induced p-p70 S6K expression in the SCN. U0126 (10 mM, 2 µl), rapamycin (100 µM, 2 µl) or dimethylsulfoxide (DMSO, 2 µl) was infused into the lateral ventricle 30 min before a 15-minute light treatment at CT 15. Also, note that infusion of U0126 and rapamycin led to a decrease in pS6 expression within the brain regions surrounding the third ventricle. Scale bar: 750 µm; 3V, third ventricle. C and D, Quantitative analysis of light-induced p-p70 S6K (C) and pS6 (D) expression in the SCN. For (D), the Y-axis denotes normalized fluorescent intensity (0-255 scale). Please see the Methods section for a description of the quantitation procedure. The treatment conditions are shown below the histograms. Error bars denote SEM. The numbers above the bars indicate the number of animals used for each condition. ***p < 0.001
Light-induced 4E-BP1 phosphorylation
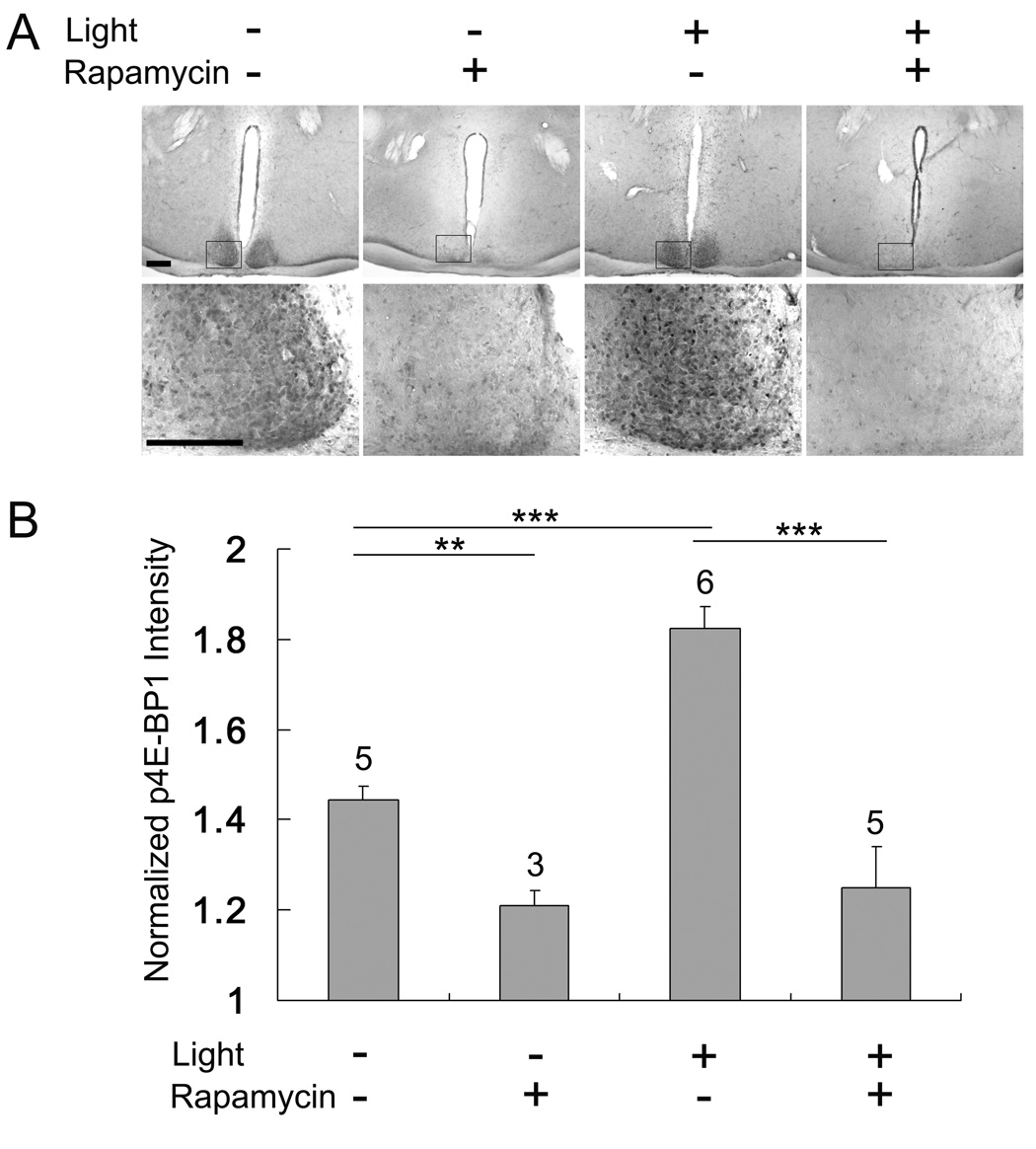
In addition to p70 S6K, 4E-BP1 is another direct downstream effector of mTOR that has been shown to regulate mRNA translation (Sarbassov et al., 2005; Wullschleger et al., 2006; Proud, 2007). In the absence of stimulation, 4E-BP1 binds to eIF4E, thereby blocking its ability to stimulate cap-dependent mRNA translation initiation (Gingras et al., 1999; Haghighat et al., 1995; Lin et al., 1995). A number of studies have shown that this association can be disrupted by mTOR-dependent phosphorylation of 4E-BP1 (Lin et al., 1994; Pause et al., 1994). To gain insight into the functional status of 4E-BP1, SCN tissue was probed with an antibody against 4E-BP1 phosphorylated at Thr-37 and Thr-46, two sites that regulate 4E-BP1 binding to eIF4E (Burnett et al., 1998; Gingras et al.,1999; Mothe-Satney et al., 2000). Interestingly, under control conditions (CT 15-no light) marked 4E-BP1 phosphorylation was detected throughout the SCN (Fig. 4A), whereas little immunoreactivity was detected outside of the SCN. To test whether 4E-BP1 phosphorylation was dependent on mTOR, mice were sacrificed 30 min after rapamycin (100 µM, 2 µl) infusion. Disruption of mTOR signaling led to a dramatic decrease in 4E-BP1 phosphorylation, indicating that tonic mTOR activity drives a relatively high basal level of 4E-BP1 phosphorylation in the SCN. To test whether 4E-BP1 is stimulated by photic input, mice were exposed to light (400 lux, 15 min) at CT 15, and SCN tissue was processed for 4E-BP1 phosphorylation. Representative data and quantitative analysis revealed that photic stimulation led to a modest, but significant, increase in 4E-BP1 phosphorylation (Fig. 4A and 4B).
Figure 4. Phosphorylated 4E-BP1 (Thr-37/46) in the SCN.
A, Representative immunohistochemical images of phosphorylated p4E-BP1 (p4E-BP1) expression in the SCN. The treatment conditions are shown above the images. Under control conditions (no light) marked p4E-BP1expression was detected in the SCN at CT15. Pretreatment with rapamycin (100 µM, 2 µl) 30 min prior to sacrificing triggered a significant reduction in p4E-BP1 expression. A 15-minute light treatment (400 lux) at CT 15 stimulated a moderate increase of p4E-BP1 expression in the SCN. Rapamycin (100µM, 2 µl) infusion (30 min before light) blocked light-induced p4E-BP1 expression. Mice not infused with rapamycin were infused with dimethylsulfoxide (DMSO, 2 µl). Scale bars: low magnification 250 µm, high magnification 150 µm. B, Quantitative analysis of 4E-BP1 phosphorylation under the four experimental conditions. The treatment conditions are shown below the histograms. Values are normalized against p4E-BP1 levels in the lateral hypothalamus. Please see the Methods section for a description of the quantitation procedures. Error bars denote SEM. The numbers above the bars indicate the number of animals tested for each condition. ***p < 0.001; ** p < 0.01.
p-p70 S6K phosphorylates ribosomal S6 protein in the SCN
Next, we turned to potential downstream targets of p70 S6K in the SCN. To this end, we examined the regulation of the ribosomal S6 protein (S6). S6 is a downstream target of p70 S6K and has been suggested to play a role in regulation of TOP-dependent translation (Meyuhas, 2000). To monitor the activation state of S6, tissue was immunolabeled for phosphorylation of S6 (pS6) at Ser-240 and Ser-244. Importantly, p70 S6K targets these sites, and thus, changes in its phosphoryalation state can be used to infer levels of both S6 and p70 S6K activity. At CT 15, low levels of S6 phosphorylation were detected in the SCN (Fig. 5A). However, photic stimulation (400 lux, 15 min) triggered a robust increase of S6 phosphorylation (Fig.5A) and double labeling revealed colocalized expression of activated p70 S6K and S6 (Fig. 5A). To confirm that light-induced S6 phosphorylation was dependent on the mTOR/p70 S6K pathway, mice were infused with rapamycin (100 µm, 2 µl) 30 min prior to photic stimulation. Light-induced S6 phosphorylation was markedly attenuated by rapamycin (Fig. 5B and 5D). Interestingly, the low magnification images shown in figure 4B reveal that rapamycin infusion decreased S6 phosphorylation throughout the periventricular hypothalamic region, which includes the SCN. As expected, the infusion of U0126 also led to a significant decrease in light-induced S6 phosphorylation within the SCN (Fig. 5B and 5D). Together, these data indicate that light triggers p70 S6K enzymatic activity (as assessed by S6 phosphorylation).
Light-induced p-p70 S6K and pCREB colocalize in the SCN
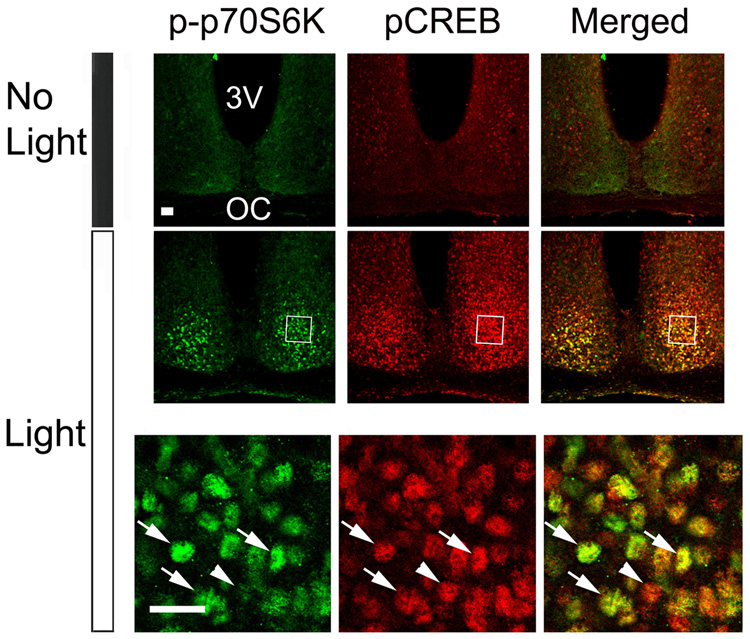
Finally, to gain insight into whether mTOR/p70 S6K activity occurs in neurons in which rapid transcriptional activation occurs, we examined the spatial and temporal correlation between p-p70 S6K expression and expression of the Ser-133 phosphorylated form of CREB (pCREB). Ser-133 phosphorylation is necessary for CREB to stimulate transcription activation (Gonzalez and Montminy, 1989). CREB activity was the focus of this examination, since a large number of studies have shown that CREB is a light-responsive transcription factor (Obrietan et al., 1998; Dziema et al., 2003) that stimulates clock gene expression (Travnickova-Bendova et al., 2002; Butcher et al., 2005). To study the relationship between p70 S6K and CREB activation, SCN tissue from control (no light) and light-treated (400 Lux, 15 min, CT 15) mice was double-labeled for p-p70 S6K and pCREB. Representative confocal images show a spatial and temporal correlation between light-induced p-p70 S6K and pCREB (Fig. 6). Along these lines, nearly all pCREB immunoreactive SCN cells within the central SCN were also immunopositive for p-p70 S6K. These data raise the possibility that light stimulates inducible transcription (as assessed by pCREB immunolabeling) and translation (as assessed by p-p70 S6K immunolabeling) in the same light responsive SCN cells.
Figure 6. Light-induced p70 S6K phosphorylation and CREB phosphorylation in the SCN.
Compared with the control mice (No Light), photic stimulation (400 lux, 15 min) at CT 15 increased the number of p-p70 S6K-positive (green) and pCREB-positive (red) cells. Merging of p-p70 S6K and pCREB signals (right) revealed that the expression of the activated kinase and transcription factor were colocalized in a subset of the cells (arrows); arrowhead denotes a cell where colocalization was not detected. The boxed regions are magnified and shown below. Scale bar: 20 µm.
Discussion
The goal of this line of inquiry was to begin to identify inducible translation control pathways in the SCN. To this end, we focused on the mTOR signaling cassette. We report that light evokes phase-dependent activation of the mTOR pathway and that mTOR is a downstream target of the MAPK cascade. Together, these data reveal a new light-actuated signaling cassette in the SCN.
mTOR signaling is a key regulator of inducible gene transcription, ribosome biogenesis, and mRNA translation (Sarbassov et al., 2005; Wullschleger et al., 2006; Proud, 2007). In one of the best-characterized routes to its activation, growth factors, including insulin, signal to mTOR through a phosphatidylinositol-3-kinase (PI3K)/AKT -dependent process. AKT activation leads to phosphorylation of tuberous sclerosis protein2 (TSC2), which, in turn, blocks its GTPase-activating protein (GAP) activity for the small G protein Rheb, thus allowing the GTP-loaded form of Rheb to activate mTOR (as part of the mTORC1 complex) (Gao et al., 2002; Inoki et al., 2002; Long et al., 2005; Li et al., 2004). In this study, we examined two principal downstream effectors of mTORC1 which have been implicated in inducible translation control: p70 S6K and 4E-BP1.
The activation of p70 S6K is coordinately regulated by a complex series of phosphorylation steps. A key step in this process is mTOR-mediated phosphorylation of Thr-389 (Weng et al., 1998). In turn, this event creates a docking site for phosphoinositide-dependent kinase 1 (PDK1), which then phosphorylates p70 S6K within the catalytic domain at Thr-229 (Pullen et al., 1998; Frödin et al., 2002), thus leading to enzymatic activation of p70 S6K (Pullen and Thomas, 1997). In our study, we found that a brief photic stimulus triggered p70 S6K phosphorylation at Thr-389. Interestingly, in the absence of photic stimulation, immunohistochemical labeling was not able to detect the Thr-389 phosphorylated form of p70 S6K in the SCN. This lack of a signal raised the possibility that the SCN exhibits low basal levels of mTOR activity. However, Western analysis was able to detect phosphorylated p70 S6K under control conditions, thus suggesting that our immunohistochemical staining approach lacked the necessary sensitivity to detect a baseline level of phosphorylation. Furthermore, data from S6 and 4E-BP1 (discussed below) supports the idea that the SCN exhibits a relatively high level of mTOR activity.
Although most SCN neurons express mTOR1, light-induced p-p70 S6K expression was only detected in a subset of cells. There are a number of potential explanations for this. Along these lines, limited p-p70 S6K expression may be due to the low sensitivity of the antibody (noted above). Another possibility is that we may be identifying only a subset of the “activated cells’; hence activation may be rapid and transient in some cells whereas others cells may show delayed activation and rapid inactivation. Thus, data collected from a single time point may only capture a subset of the responsive cells. Finally, at a cellular level, the degree of RHT innervation may also likely contribute to the efficiency of activation.
To test whether light-induced phosphorylation of p70 S6K and 4E-BP1 were dependent on mTOR, we employed an intraventricular infusion method to deliver the mTORC1 inhibitor rapamycin to the ventricular system. Similar rapamycin infusion approaches have been used by other groups to study the effects of mTOR in the brain (Tischmeyer et al., 2003; Narita et al., 2005). The infusion of rapamycin led to a complete inhibition of p70 S6K activity, its downstream target, S6, as well as 4E-BP1. Together, these data strongly support the idea that light triggers activation of mTOR-dependent signaling. It should be noted that rapamycin does not affect mTOR as part of the mTORC2 complex (Wullschleger et al., 2006). Interestingly, mTORC2 is regulated by the same uspstream TSC2/Rheb2 signaling cassette, but actuates a distinct set of signaling events from mTORC1, such as actin polymerization (Jacinto et al., 2004). Additional work will be required to assess inducible activation and function of mTORC2 in the SCN.
Interestingly, in contrast to p70 S6K, we detected relatively high levels of 4E-BP1 phosphorylation under control conditions and light treatment led to a relatively modest increase in 4E-BP1 phosphorylation. The activation of 4E-BP1 is mediated by a sequential set of phosphorylation events. Activation appears to be initiated by the phosphorylation of Thr-37 and Thr-46 (the two sites examined in this study). This dual phosphorylation is a priming event that allows for phosphorylation at Ser-65 and Thr-70 to occur (Gingras et al., 1999; Gingras et al., 2001). Ser-65 and Thr-70 phosphorylation are thought to be the trigger that initiates dissociation of 4E-BP1 from eIF4E, thereby allowing cap-dependent mRNA translation to occur (Lin et al., 1994; Pause et al., 1994). A number of studies have shown that the phosphorylation of Thr-37 and Thr-46 is mediated by mTOR, whereas Ser-65 and Thr-70 phosphorylation is regulated by both mTOR-dependent and -independent signaling (Gingras et al., 1999; Nojima et al., 2003). Of note, the finding that rapamycin infusion triggered a decrease in 4E-BP1 phosphorylation supports the idea that the SCN exhibits a relatively high tonic level of mTOR activity, thus raising the possibility that the mTOR pathway plays a broader role in the SCN than simply conveying photic information.
Next, our attention turned to potential effectors of p70 S6K in the SCN. To this end, we focused on S6. The rationale for examining S6 was two-fold: first, its phosphorylation can be used as a functional read-out for p70 S6K activity, and second, several studies have shown that it regulates the translation of a subset of mRNAs which contain a 5' tract of oligopyrimidine (TOP) (Jefferies et al.,1994; Terada et al.,1994; Jefferies et al.,1997; Kawasome et al., 1998; Schwab et al., 1999). Interestingly, a number of 5'TOP mRNAs are components of the translation machinery and thus may influence the overall translational potential of the cell. However, the precise role of S6 in this process is not clear, and several studies have reported that S6 does not directly regulate translation of 5'TOP mRNAs (Tang et al., 2001; Ruvinsky et al., 2005). Our results show that light induced a marked increase in S6 phosphorylation at Ser-240 and Ser-244 and that rapamycin potently blocked this process. Interestingly, pS6 was detected in the absence of photic input, further supporting the idea that the SCN exhibits tonic mTOR activity.
The phase-restricted capacity of light to activate mTOR-dependent signaling is consistent with a large body of work showing that photic stimulation exerts a dominant effect during the night time domain. Along these lines, phase-restricted light responses have been characterized at both a behavioral and molecular level. For example, light exposure during the early night causes a phase delay in clock timing, whereas light exposure during the late night causes a phase advance in clock timing (Daan and Pittendrigh, 1976). At the molecular level, the capacity of light to trigger the expression of the immediate early genes such as Fos, JunB and early growth response factor 1 (EGR-1) and core clock timing genes period 1-and period-2 is restricted to the night (Aronin et al., 1990; Kornhauser et al., 1990, 1992; Rusak et al., 1990, Albrecht et al., 1997; Zylka et al., 1998; Mendoza et al., 2007). Likewise, light activation of the CREB/CRE transcription pathaway and kinases such as protein kinase C and the MAPK cascade is phase-restricted to the night time domain (Ginty et al., 1993; Obrietan et al., 1998, 1999; Lee et al., 2007). The data reported here add the mTOR signaling pathway to this phase-restricted photic response network.
One key question for this study relates to the upstream signaling cascade that couples light to rapid activation of mTOR. To this end, we examined the MAPK cascade, a key signaling intermediate in light-mediated SCN entrainment (Obrietan et al., 1998; Butcher et al., 2002; Coogan and Piggins, 2003). As an initial assessment of the potential role of MAPK signaling in light-induced mTOR activity, SCN sections were double labeled for activated ERK and p70 S6K. These assays detected a light-induced temporal and cellular correlation between MAPK pathway activity and p70 S6K phosphorylation. Furthermore, infusion of the MEK1/2 inhibitor U0126 potently repressed p70 S6K activity, thus indicating that light-induced MAPK signaling is an upstream activator of mTOR in the SCN.
There are several potential mechanisms by which the MAPK pathway could stimulate mTOR activity. First, recent work has revealed that ERK triggers phosphorylation-dependent inactivation of TSC2 GAP activity (Ma et al., 2005, 2007). Second, ERK-regulated 90 kDa ribosomal kinase 1 (RSK1) has been shown to block TSC2 activity (Roux et al., 2004), and thereby stimulate mTOR1 signaling. Interestingly, we recently reported that RSK1 is activated by light in the SCN (Butcher et al., 2004). Additional work will be required to delineate the precise mechanism by which MAPK signaling regulates mTOR activity in the SCN.
In the SCN, MAPK signaling is thought to couple light to the core clock timing mechanism via a process that elicits transcription factor activation and, in turn, clock gene expression. Along these lines, the MAPK cascade has been shown to facilitate CRE-dependent transcription and period1 transcription (Obrietan et al., 1999; Dziema et al., 2003; Travnickova-Bendova et al., 2002; Butcher et al., 2005). Interestingly, the data presented here reveal that light triggers coordinate activation of CREB and mTOR-mediated signaling in the SCN. Given that MAPK signaling regulates both of these processes, these data raise the possiblity that the MAPK cascade serves coordinate roles as a regulator of gene transcription and mRNA translation (Fig. 7). Further studies aimed at addressing inducible translation regulation will shed new light on the key cellular signaling events that shape circadian clock timing and entrainment.
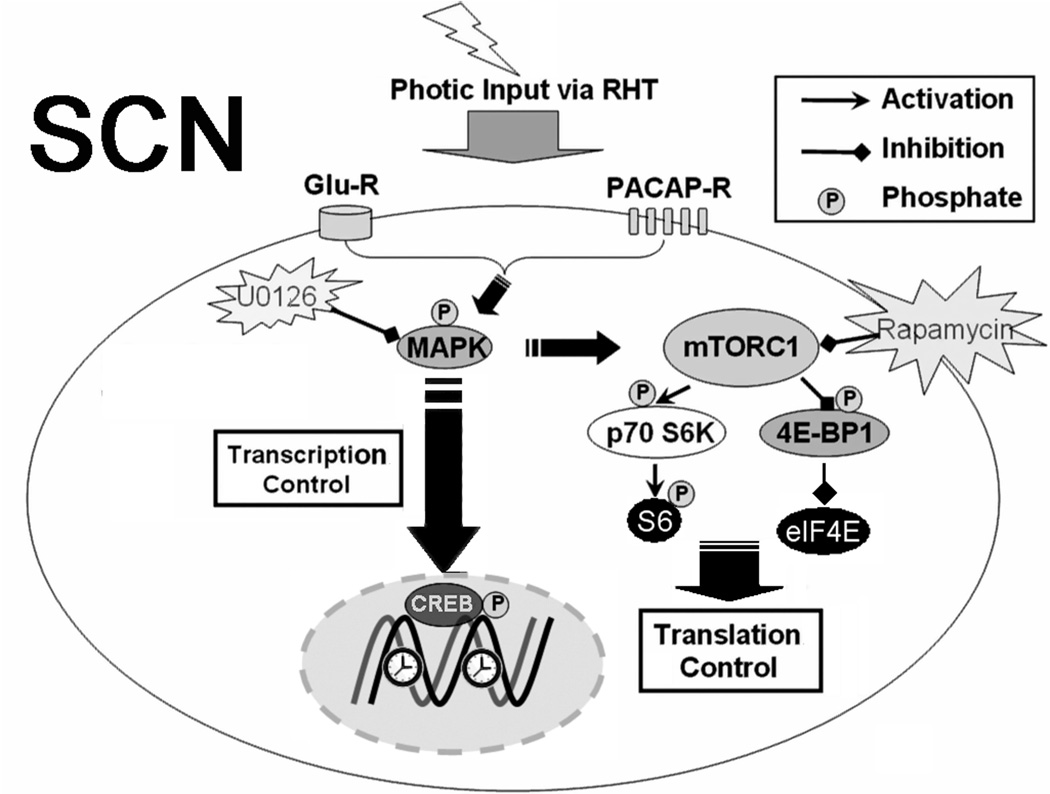
Figure 7. Schematic of proposed mTOR signaling pathway in the SCN.
Photic input from the retina induces the release of glutamate and PACAP from RHT terminals. Transmitter binding to postsynaptic receptors on SCN neurons evokes a series of intracellular signal transduction events, including MAPK pathway activation. One downstream target of the MAPK cascade is CREB, which, when phosphorylated on Ser-133, drives clock gene transcription. Light-induced activation of the MAPK cascade also stimulates mTORC1, which in turn targets the translation regulators p70 S6K and 4E-BP1. Activated p70 S6K stimulates S6 phosphorylation, whereas 4E-BP1 phosphorylation has been shown to lead to its dissociation from eIF4E, thereby allowing cap-dependent mRNA translation to occur. U0126 is a specific MEK 1/2 inhibitor and rapamycin inhibits mTORC1.
Experimental Methods
Photic stimulation and tissue processing
Initially, adult (8~10-week-old) C57BL/6 mice were entrained to a 12 h LD cycle for at least 2 weeks and then transferred to total darkness for two consecutive 24 h cycles. After dark adaptation, animals received a single light exposure (400 lux, 15 min) at one of three time points: the middle of the subjective day (CT 6), early subjective night (CT 15), or late subjective night (CT 22). CTs were calculated based on Zeitgeber time (ZT) and the tau of C57BL/6 mice (approximately 23 h 45 min) under free running conditions, with ZT 0 denoting light on and ZT 12 denoting light off. Immediately after light treatment, animals were anesthetized via an intraperitoneal (ip) injection of ketamine (95.2 mg/kg) and xylazine (30.8 mg/kg) under dim red light (Kodak series 2 filter <10 lux at cage level; Eastman Kodak, Rochester, NY) and both eyes were covered with an opaque black tape. The mice were then transcardially perfused with cold saline followed by perfusion with 4% paraformaldehyde (w/v in 10 mM phosphate-buffered saline, PBS, pH 7.4). From the end of light exposure, it took 5~8 min to begin paraformaldehyde perfusion. Next, brains were harvested, cut into 1.5 mm coronal slices with an oscillating tissue slicer (OTS 2000; Electron Microscopy Sciences, Fort Washington, PA), post-fixed in 4 % paraformaldehyde for 4~6 hr at room temperature and then transferred into 30% sucrose (w/v, with 2 mM sodium azide and 3 mM NaF) overnight at 4 °C. The “no light” control animal groups underwent the same handling conditions at the same time points. All procedures were in accordance with Ohio State University animal welfare guidelines and approved by the Institutional Animal Care and Use Committee.
Cannulation and infusion paradigms
Mice were anesthetized via an ip injection of ketamine (95.2 mg/kg) and xylazine (30.8 mg/kg) and placed in a stereotaxic apparatus (Cartesian Research). The coordinates (posterior, 0.34 mm from bregma; lateral, 0.90 mm from the midline; and dorsoventral, −2.15 mm from bregma) were used to place the tip of a 24-gauge guide cannula into the lateral ventricle. Cannulae were held in place with dental cement and a 30-gauge stylus was secured in the cannula to ensure patentcy. After surgery animals were housed individually and allowed to recover for at least 2 weeks under a standard 12 h LD cycle. For the infusion, animals were restrained by hand under dim red light, and the infusate was delivered at a rate of 1 µl/min. To disrupt the MAPK cascade, 2 µl of 1,4-diamino-2,3-dicyano-1,4-bis o-aminophenylmercapto butadiene (U0126, 10 mM; Calbiochem, La Jolla, CA) was infused 30 min before photic stimulation. To inhibit the activity of mTOR, 2 µl of rapamycin (100 µM, Cell Signaling Technology: Kunz et al., 1993; Brown et al., 1994) was infused 30 min before light treatment. Control animals were infused with an equivalent volume of vehicle (DMSO).
Immunohistochemistry
Coronal brain sections (1.5 mm) containing the SCN were thin cut (40 µm) using a freezing microtome and placed in PBS containing 2 mM sodium azide and 3 mM NaF, pH 7.4. For the immunohistochemical staining, sections were first treated with 0.3 % H2O2 and 20 % methanol in PBS for 10 min to deactivate endogenous peroxidases and permeabilize the tissue and then blocked for 1 h in 10% goat serum/PBS and incubated (overnight, 4°C) in mouse monoclonal anti-phospho-p70 S6 kinase (Thr-389) antibody (1:1000 final dilution; Cell Signaling Technology, Beverly, MA) or rabbit monoclonal anti-phospho-4E-BP1 (Thr-37/46)(236B4) antibody (1:1000 final dilution; Cell Signaling Technology). Next, tissue was incubated for 1.5 hr at room temperature in biotinylated anti-mouse IgG (1:200;Vector Laboratories, Burlingame, CA) and then placed in an avidin/biotin HRP complex for 1 h (prepared according to instructions of the manufacturer; Vector Laboratories). Sections were washed in PBS (three times, 10 min per wash) between each labeling step. The signal was visualized using nickel-intensified DAB substrate (Vector Laboratories) and sections were mounted on gelatin-coated slides with Permount media (Fisher Scientific, Houston, TX).
For immunofluorescent labeling, tissue was permeabilized with PBST (PBS with 1 % Triton X-100) for 30 min, blocked as described above and then incubated (overnight, 4° C) in 5 % goat serum/PBS with one or more of the following antibodies: mouse monoclonal anti-phospho-p70 S6 kinase (Thr-389) (1:300; Cell Signaling Technology), rabbit monoclonal anti-mTOR (1:300; Cell Signaling Technology), rabbit polyclonal anti-phosphorylated ERK (Thr-202, Tyr-204) (1:300; Cell Signaling Technology), rabbit polyclonal anti-phospho-S6 ribosomal protein (Ser-240/244) (1:300; Cell Signaling Technology) or rabbit polyclonal phospho-CREB (Ser-133) (1:300; Cell Signaling Technology). The following day, sections were incubated (3 h, room temperature) in Alexa Fluor-594-conjugated goat anti-rabbit IgG antibody (1:500; Molecular Probes, Eugene, OR) and/or Alexa Fluor-488-conjugated goat anti-mouse IgG antibody (1:500; Molecular Probes). Brain sections were washed in PBS (three times, 10 min per wash) between each labeling step. Sections were mounted on slides with Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI).
Bright-field or dark-field fluorescent photomicrographs were captured using a 16 bit digital camera (Micromax YHS 1300; Princeton Instruments, Trenton, NJ) mounted on an inverted Leica microscope (DM IRB; Nussloch, Germany). Images were acquired with Metamoph software (Molecular Devices, Sunnyvale CA). Confocal fluorescent images were captured using a Zeiss 510 Meta confocal microscope (Oberkochen, Germany). All confocal parameters (pinhole, contrast, brightness, etc.) were held constant for all data sets from the same experiment.
Western blotting
Light-treated mice were transcardially perfused (as described above) with cold saline for 1~2 min and their brains were removed rapidly. Brains were then immersed in chilled, oxygenated artificial cerebrospinal fluid (ACSF, in mM: NaCl 117, KCl 4.7, NaH2PO4 1.2, MgCl2 1.2, CaCl2 2.5, NaHCO3 25, and d-glucose 11: 299 ± 4 mOsm), blocked and cut into 500 µm coronal sections with the oscillating tissue slicer. The tissue sections were then frozen on dry ice, placed on a dissecting microscope and SCN were isolated using a razor blade. Tissue was lysed in 100 µl RIPA buffer [50 mm Tris-HCl, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate, 1 mm sodium vanadate, 1 mm NaF and 1× protease inhibitor cocktail (Roche)]. Extracts (24 µl/lane) were electrophoresed into an 8% SDS-PAGE gel, then transblotted onto polyvinylidene difluoride membranes (Immobilon-P, Millipore). Membranes were blocked in 10% bovine serum albumin (BSA, Fisher scientific, Fair Lawn, NJ) and then incubated (overnight, 4 °C) in PBST (with 5 % BSA) with mouse monoclonal anti-phospho-p70 S6 kinase (Thr-389, Cell Signaling Technology) antibody (1:1000). Next, membranes were incubated in PBST (with 5 % milk) with a goat anti-mouse IgG alkaline phosphatase-conjugated antibody (1:4000, PerkinElmer Life Sciences). Immunoreactivity was developed using the Western-star alkaline phosphatase detection system (Tropix). As a protein loading control, membranes were probed for total ERK expression using a goat polyclonal anti-ERK antibody (1:1000 final dilution, Santa Cruz Biotechnology) followed by a donkey anti-goat IgG antibody conjugated to horseradish peroxidase. The signal was visualized using Renaissance chemiluminescent horseradish peroxidase substrate (PerkinElmer Life Sciences). Between each antibody treatment, membranes were washed a minimum of three times (10 min/wash) in PBST. SCN tissue was pooled from three mice for each condition and the experiment was repeated three times.
Materials
Unless otherwise indicated, all reagents were obtained from Sigma.
Data analysis
All photomicrographic data sets were statistically analyzed using Adobe Photoshop software (Adobe Systems Incorporated, San Jose, CA). For cell counting, an intensity threshold filter was initially applied to eliminate nonspecific background labeling, and then the number of detectable signals above threshold (now defined as positive cells) were counted for each SCN. A mean value for each animal was generated from 3 central SCN sections per animal; this value was then used to generate the group mean. For the pS6 intensity analysis, SCN were digitally outlined and the mean pixel values determined. Next, a digital oval (150×200 pixels) was placed on the adjacent lateral hypothalamus and this mean value was subtracted from the adjacent SCN signal to provide a normalized SCN intensity value. For the p4E-BP1 intensity analysis, a digital circle (Φ1000 pixels) was placed in the ventral SCN and the mean labeling intensity was determined. A digital oval (150×200 pixels) was then placed over the adjacent lateral hypothalamus and the ratio of the SCN signal to mean lateral hypothalamic signal was generated and used to normalize SCN intensity values. The lateral hypothalamic 4E-BP1 values were not altered by light or rapamycin infusion. Mean data from different animals were pooled into treatment groups and compared by one-way ANOVA followed by SNK post-tests. P < 0.05 was accepted as statistically significant. The values are presented as the mean ± standard error of mean (SEM). All statistical analysis was performed using SPSS software (SPSS Inc, Chicago, IL).
Acknowledgments
We are grateful to Yunsik Choi and Mary Cheng for technical assistance and helpful discussion. This work was supported by an NSF grant (IBN-0090974) a grant from the NIMH (MH62335) and by the Ohio State Neuroscience Center Core grant (5P30NS045758).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J. Neurosci. 1999;19:1115–1121. doi: 10.1523/JNEUROSCI.19-03-01115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. mPer1 and mPer2 are essential for normal resetting of the circadian clock. J. Biol. Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- Aronin N, Sagar SM, Sharp FR, Schwartz WJ. Light regulates expression of a Fos-related protein in rat suprachiasmatic nuclei. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5959–5962. doi: 10.1073/pnas.87.15.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggs J, Green CB. Nocturnin, a deadenylase in Xenopus laevis retina, a mechanism for posttranscriptional control of circadian-related mRNA. Curr. Biol. 2003;13:189–198. doi: 10.1016/s0960-9822(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Burnett P, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher G, Dziema H, Collamore M, Burgoon PW, Obrietan K. The p42/44 mitogen-activated protein kinase pathway couples photic input to circadian clock entrainment. J Biol Chem. 2002;277:29519–29525. doi: 10.1074/jbc.M203301200. [DOI] [PubMed] [Google Scholar]
- Butcher G, Lee B, Hsieh F, Obrietan K. Light- and clock-dependent regulation of ribosomal S6 kinase activity in the suprachiasmatic nucleus. Eur. J. Neurosci. 2004;19:907–915. doi: 10.1111/j.0953-816x.2004.03155.x. [DOI] [PubMed] [Google Scholar]
- Butcher G, Lee B, Cheng HY, Obrietan K. Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism. J. Neurosci. 2005;25:5305–5313. doi: 10.1523/JNEUROSCI.4361-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammalleri M, Lütjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian N, Sassone-Corsi P. Environmental stimulus perception and control of circadian clocks. Curr. Opin. Neurobiol. 2002;12:359–365. doi: 10.1016/s0959-4388(02)00347-1. [DOI] [PubMed] [Google Scholar]
- Challet E, Caldelas I, Graff C, Pévet P. Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol. Chem. 2003;384:711–719. doi: 10.1515/BC.2003.079. [DOI] [PubMed] [Google Scholar]
- Cheng H, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan A, Piggins HD. Circadian and photic regulation of phosphorylation of ERK1/2 and Elk-1 in the suprachiasmatic nuclei of the Syrian hamster. J. Neurosci. 2003;23:3085–3093. doi: 10.1523/JNEUROSCI.23-07-03085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents, II The variability of phase response curves. J. Comp. Physiol. 1976;106:253–266. [Google Scholar]
- Di Nardo A, Nedelec S, Trembleau A, Volovitch M, Prochiantz A, Montesinos ML. Dendritic localization and activity-dependent translation of Engrailed 1 transcription factor. Mol. Cell. Neurosci. 2007;35:230–236. doi: 10.1016/j.mcn.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K. The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur. J. Neurosci. 2003;17:1617–1627. doi: 10.1046/j.1460-9568.2003.02592.x. [DOI] [PubMed] [Google Scholar]
- Frödin M, Antal TL, Dümmler BA, Jensen CJ, Deak M, Gammeltoft S, Biondi RM. A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J. 2002;21:5396–5407. doi: 10.1093/emboj/cdf551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat. Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- Garbarino-Pico E, Niu S, Rollag MD, Strayer CA, Besharse JC, Green CB. Immediate early response of the circadian polyA ribonuclease nocturnin to two extracellular stimuli. RNA. 2007;13:745–755. doi: 10.1261/rna.286507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas J, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J. Biol. Chem. 2007;282:27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Gingras A, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation, a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, yslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty D, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J. Biol. Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic-AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Haghighat A, Mader S, Pause A, Sonenberg N. Repression of cap-dependent translation by 4E-binding protein 1, competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 1995;14:5701–5709. doi: 10.1002/j.1460-2075.1995.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res. 2002;309:73–88. doi: 10.1007/s00441-002-0574-3. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog. Sci. 2004;21:359–368. doi: 10.2108/zsj.21.359. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell. Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell. Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Jefferies H, Reinhard C, Kozma SC, Thomas G. Rapamycin selectively represses translation of the "polypyrimidine tract" mRNA family. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies H, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Nakashima H. Cycloheximide inhibits light-induced phase shifting of the circadian clock in Neurospora. J. Biol. Rhythms. 1990;5:159–167. doi: 10.1177/074873049000500207. [DOI] [PubMed] [Google Scholar]
- Kawasome H, Papst P, Webb S, Keller GM, Johnson GL, Gelfand EW, Terada N. Targeted disruption of p70, s6k. defines its role in protein synthesis and rapamycin sensitivity. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5033–5038. doi: 10.1073/pnas.95.9.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Woo KC, Cho S, Ha DC, Jang SK, Kim KT. Rhythmic control of AANAT translation by hnRNP Q in circadian melatonin production. Genes Dev. 2007;21:797–810. doi: 10.1101/gad.1519507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhauser J, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- Kornhauser J, Nelson DE, Mayo KE, Takahashi JS. Regulation of jun-B messenger RNA and AP-1 activity by light and a circadian clock. Science. 1992;255:1581–1584. doi: 10.1126/science.1549784. [DOI] [PubMed] [Google Scholar]
- Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73:585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Lee B, Almad A, Butcher GQ, Obrietan K. Protein kinase C modulates the phase-delaying effects of light in the mammalian circadian clock. Eur J Neurosci. 2007;26:451–462. doi: 10.1111/j.1460-9568.2007.05664.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Corradetti MN, Inoki K, Guan KL. TSC2, filling the GAP in the mTOR signaling pathway. Trends Biochem. Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Lin T, Kong X, Haystead TA, Pause A, Belsham G, Sonenberg N, Lawrence JC., Jr PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science. 1994;266:653–656. doi: 10.1126/science.7939721. [DOI] [PubMed] [Google Scholar]
- Lin T, Kong X, Saltiel AR, Blackshear PJ, Lawrence JC., Jr Control of PHAS-I by insulin in 3T3-L1 adipocytes. Synthesis, degradation, and phosphorylation by a rapamycin-sensitive and mitogen-activated protein kinase-independent pathway. J. Biol. Chem. 1995;270:18531–18538. doi: 10.1074/jbc.270.31.18531. [DOI] [PubMed] [Google Scholar]
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr. Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Genetics of the mammalian circadian system: Photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet. 2000;34:533–562. doi: 10.1146/annurev.genet.34.1.533. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Bonner P, Bernardi R, Franz DN, Witte D, Cordon-Cardo C, Pandolfi PP. Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res. 2007;15:7106–7112. doi: 10.1158/0008-5472.CAN-06-4798. [DOI] [PubMed] [Google Scholar]
- Meijer J, Schwartz WJ. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J. Biol. Rhythms. 2003;18:235–249. doi: 10.1177/0748730403018003006. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Pévet P, Challet E. Circadian and photic regulation of clock and clock-controlled proteins in the suprachiasmatic nuclei of calorie-restricted mice. Eur. J. Neurosci. 2007;25:3691–3701. doi: 10.1111/j.1460-9568.2007.05626.x. [DOI] [PubMed] [Google Scholar]
- Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur. J. Biochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- Mothe-Satney I, Yang D, Fadden P, Haystead TA, Lawrence JC., Jr Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol. Cell. Biol. 2000;20:3558–3567. doi: 10.1128/mcb.20.10.3558-3567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N, Nishi R, Katayama T, Nasu T. Inhibitor of protein synthesis phase-shifts the circadian oscillator and inhibits the light induced-phase shift of the melatonin rhythm in pigeon pineal cells. Brain Res. 1995;693:1–7. doi: 10.1016/0006-8993(95)00633-2. [DOI] [PubMed] [Google Scholar]
- Narita M, Akai H, Kita T, Nagumo Y, Narita M, Sunagawa N, Hara C, Hasebe K, Nagase H, Suzukiq T. Involvement of mitogen-stimulated p70-S6 kinase in the development of sensitization to the methamphetamine-induced rewarding effect in rats. Neuroscience. 2005;132:553–560. doi: 10.1016/j.neuroscience.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mTOR partner, raptor, binds the mTOR substrates, p70 S6 kinase and 4E-BP1, through their TOS, TOR signaling. motifs. J. Biol. Chem. 2003;278:15461–15464. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat. Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Smith D, Athos J, Storm DR. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J. Biol. Chem. 1999;274:17748–17756. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Pearson R, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud C. Signalling to translation, how signal transduction pathways control the protein synthetic machinery. Biochem. J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- Raju U, Yeung SJ, Eskin A. Involvement of proteins in light resetting ocular circadian oscillators of Aplysia. Am. J. Physiol. 1990;258:R256–R262. doi: 10.1152/ajpregu.1990.258.1.R256. [DOI] [PubMed] [Google Scholar]
- Reppert S, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schwab M, Kim SH, Terada N, Edfjall C, Kozma SC, Thomas G, Maller JL. p70 S6K controls selective mRNA translation during oocyte maturation and early embryogenesis in Xenopus laevis. Mol. Cell. Biol. 1999;19:2485–2494. doi: 10.1128/mcb.19.4.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hornstein E, Stolovich M, Levy G, Livingstone M, Templeton D, Avruch J, Meyuhas O. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol. Cell Biol. 2001;21:8671–8683. doi: 10.1128/MCB.21.24.8671-8683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee A, Blenis J. mTOR, translational control and human disease. Semin. Cell Dev. Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischmeyer W, Schicknick H, Kraus M, Seidenbecher CI, Staak S, Scheich H, Gundelfinger ED. Rapamycin-sensitive signalling in long-term consolidation of auditory cortex-dependent memory. Eur. J. Neurosci. 2003;18:942–950. doi: 10.1046/j.1460-9568.2003.02820.x. [DOI] [PubMed] [Google Scholar]
- Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7728–7733. doi: 10.1073/pnas.102075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J. Neurosci. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Q, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J. Biol. Chem. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Takahashi JS, Turek FW. Critical period for cycloheximide blockade of light-induced phase advances of the circadian locomotor activity rhythm in golden hamsters. Brain Res. 1996;740:285–290. doi: 10.1016/s0006-8993(96)00900-6. [DOI] [PubMed] [Google Scholar]
- Zylka M, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals, differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]