Abstract
The synthesis of 22 2-aryl-1H-indoles, including 12 new compounds, has been achieved via Pd- or Rh- mediated methodologies, or selective electrophilic substitution. All three methods were based on elaborations from simple indole precursors. SAR studies on these indoles and 2-phenyl-1H-indole in S. aureus as NorA efflux pump inhibitors indicated 5-nitro-2-(3-methoxycarbonyl)phenyl-1H-indole was a slightly more potent inhibitor than the lead INF55. A promising new antibacterial lead compound against S. aureus, (2-phenyl-1H-indol-5-yl)-methanol, was also found.
Keywords: 2-Arylindoles, NorA efflux pump inhibitors, Antibacterial
Efflux pumps compromise the efficacy of a wide range of antibiotics by actively extruding them from bacterial cells.1,2 The pumps can be expressed in many different forms in both Gram-positive3 and Gram-negative bacteria4 and for some species a variety of pumps may be present with different or overlapping substrates. For the important community and nosocomially acquired human pathogen Staphylococcus aureus a number of pumps have been identified, including NorA, which has been shown to play a role in the development of clinical multidrug resistance (MDR) by this organism.5 One promising strategy for combating MDR in S. aureus is to treat infections with a combination of a NorA efflux pump inhibitor and a conventional antibiotic, with the pump inhibitor serving to restore the antibiotic’s potency by reducing its efflux from bacterial cells.6
Reported classes of NorA inhibitors include flavones and flavonolignans,7 pyrroloquinoxalines,8 4-arylpyridine-3,5-dicarboxylate esters,9 N-aryl ureas,10 and indoles.11
From the indole class, 5-nitro-2-phenylindole (2, INF55) (Scheme 1) represents a promising lead structure capable of producing a 4-fold increase in S. aureus susceptibility to ciprofloxacin when co-administered with the antibiotic at a concentration of 1.5 µg/mL.11
Scheme 1.
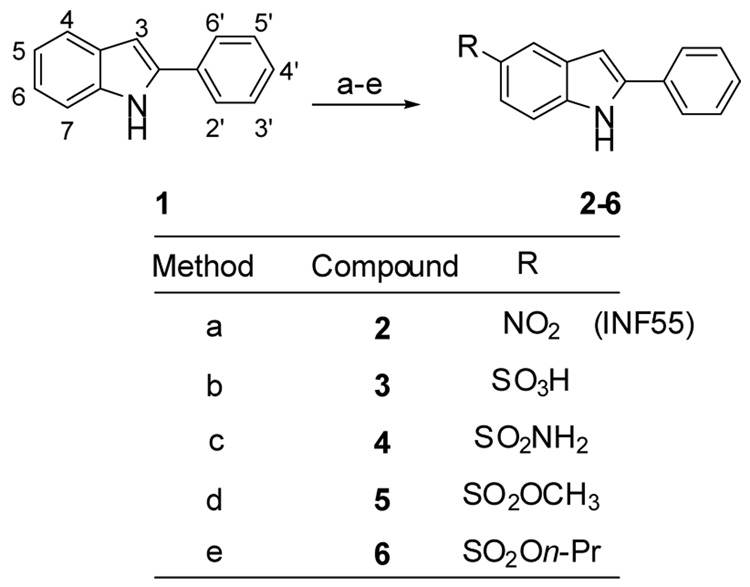
Reagents and conditions: (a) NaNO3, H2SO4 (conc.), −20 °C; 17 (b) (i) ClSO3H, 0 °C to rt, 30 mins. (ii) NaOH, H2O, rt and then 1M HCl; (c) (i) followed by (ii) NH3/THF, rt; (d) (i) followed by (ii) MeOH, pyridine, rt; (e) (i) followed by (ii) n-PrOH, pyridine rt.
Structure-activity relationships for INF55 are only just starting to emerge. In one theoretical COMFA (3D QSAR) analysis it was suggested that replacement of the indole 5-nitro group with other electron withdrawing substituents should be favorable for activity.10 A series of 2-arylbenzo[b]thiophenes related to INF55 were recently reported as NorA inhibitors12 suggesting that the indole-NH is not essential for activity. In 2006 we reported preliminary structure-activity data showing that various substituents around the 2-aryl ring of INF55 improved NorA inhibitory potency.13 For example, 5′-benzyloxy-2′-hydroxymethyl-5-nitroindole was found to potentiate the activity of the mild antibacterial agent berberine (a known substrate of NorA) more than 15-fold against the NorA overexpressing S. aureus strain K2361 at a concentration of 0.8 µg/mL (c.f. INF55 potentiates berberine activity to the same degree at the higher concentration of 3.0 µg/mL).
The current work discloses our latest systematic SAR exploration around the 2-aryl ring of INF55 and details the first experimental description of the effects of substituting the indole 5-nitro group. The goal of this study was to obtain a deeper understanding of the SAR of INF55 and analogues with a view to increasing potency against NorA in S. aureus. Furthermore, we sought to broaden our knowledge of substituent tolerance around the INF55 nucleus in order to advance our long-term goals of developing potent dual-action antimicrobials,14 including both non-cleavable hybrid molecules15 and cleavable “mutual” prodrugs16 which combine antibacterial agents and NorA-inhibiting moieties into single molecules.
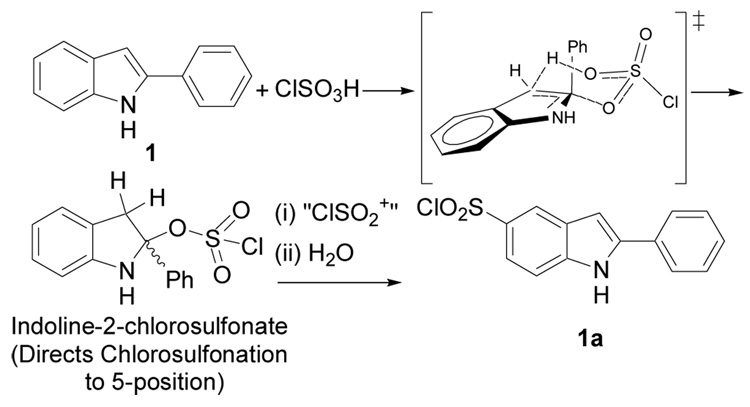
INF55 2 was synthesized in one step (Scheme 1) from commercially available 2-phenylindole 1 using the known regioselective nitration procedure.17 Compounds 3–6 were each synthesized in two steps starting from 1 and proceeding via a 5-chlorosulfonyl-2-phenylindole intermediate 1a (Scheme 2). The intermediate was cleanly accessed using a previously unreported, highly regioselective 5-chlorosulfonation of 2-phenylindole. Briefly, 1 was stirred with neat chlorosulfonic acid at 0 °C and allowed to warm to rt with stirring over 30 mins. The reaction mixture was then poured slowly onto crushed ice and the product filtered, washed with water and dried under vacuum (80%). As 1a degraded when left at rt over a period of several days, the freshly prepared crude 5-chlorosulfonyl-2-phenylindole (1a) was then reacted with appropriate nucleophiles to furnish the 5-sulfonyl-2-phenylindole derivatives 3–6 (Scheme 1) in good yields (3 = 72%; 4 = 70%; 5 = 57%; 6 = 76%).
Scheme 2.
Proposed mechanism for the regioselective 5-chlorosulfonation of 2-phenylindole in neat chlorosulfonic acid.
We propose that the regioselective 5-chlorosulfonation of 2-phenylindole proceeds via the electrophilic aromatic substitution mechanism shown in Scheme 2. Under strongly acidic conditions (e.g. neat ClSO3H) indoles are known to protonate at the indole C3 position which serves to protect this normally reactive site from electrophiles. C3 protonation of 2-phenylindole would yield a 2-phenylindolinium cation that could potentially react directly with a weak nucleophile like ClSO3− to form an indoline-2-chlorosulfonate adduct.
A related indoline-2-sulfonate adduct has been reported as a participant in the regioselective formation of 5-substituted indoles.18 What is perhaps more likely is that the indoline-2-chlorosulfonate is formed in a concerted process that proceeds via the cyclic 6-membered transition state shown in Scheme 2. The resulting indoline-2-chlorosulfonate, which contains an ortho-substituted aniline ring, should direct a ClSO2+ electrophile to the indole C5 position (i.e. para to the aniline nitrogen), with indole 1a returned after quenching with water.
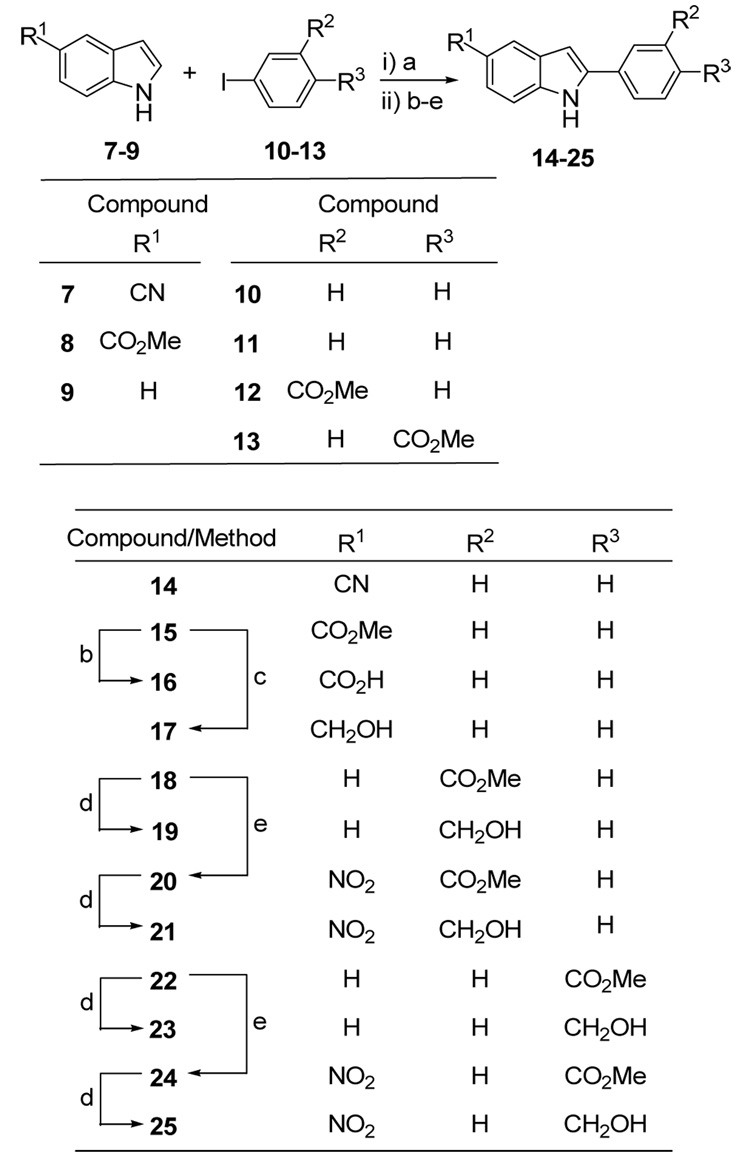
Compounds 14, 15, 18 and 22 were prepared by direct C2 arylation of commercially available indoles 7–9 with the respective aryl iodides 10–13 using the recently reported Rh-catalyzed coupling procedure (Scheme 3).19 Yields for the couplings were disappointing (14–43%) but nevertheless provided sufficient quantities of pure materials for use in our study. 2D NOESY spectra of compounds 18 and 22 confirmed that indole C2 arylation had occurred in preference to arylation at C3 (i.e. NOEs were observed between the indole H3 and H4 signals for both 18 and 22).
Scheme 3.
Reagents and conditions: (a) [Rh(coe)2Cl2]2, [p-CF3-C6H4]3P, Cs(OPiv)2, 1,4-dioxane, 120 °C;19 (b) 1M LiOH, THF, 75 °C; (c) LiAlH4, THF, rt; (d) LiBH4, THF, rt; (e) NaNO3, H2SO4 (conc.), −20 °C.17
The methyl ester 15 was smoothly hydrolyzed to the carboxylic acid 16 under standard conditions using LiOH/THF, and 15 was also reduced without incident to the hydroxymethyl derivative 17 using LiAlH4 (Scheme 3). Esters 18 and 22 were reduced in essentially quantitative yield to their respective hydroxylmethyl derivatives 19 and 23 using LiBH4. The same esters 18 and 22 could be regioselectively nitrated at −20 °C in NaNO3/H2SO4 mixtures17 to provide the 5-nitro-2-arylindole derivatives 20 and 24 in 80% and 81% yields respectively. Compounds 22 and 24 were comprehensively characterized by 2D NMR spectroscopy, with NOESY spectra confirming that mononitration had occurred selectively at the indole C5 position for both compounds (i.e. observed NOEs included NH/H7, H6/H7 and H3/H4). Esters 20 and 24 were subsequently reduced to their respective hydroxymethyl derivatives 23 and 25 in high yields (90% and 79% respectively) using LiBH4 (Scheme 3).
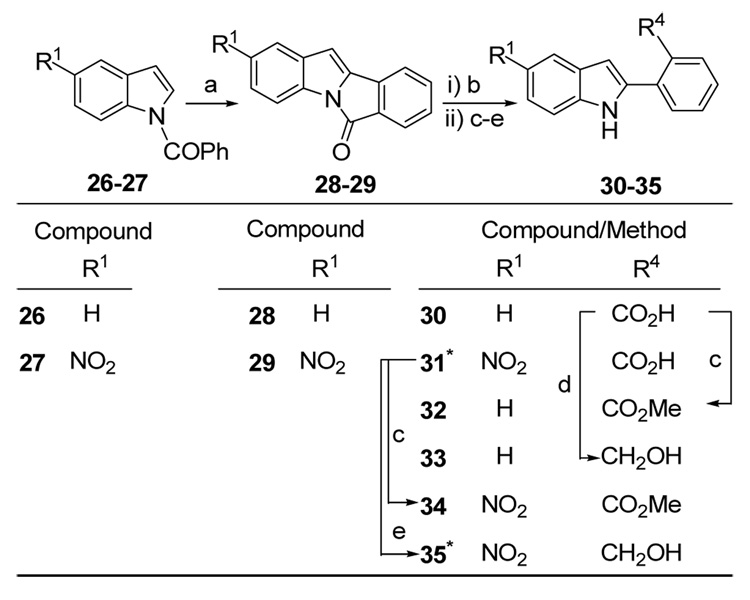
A Pd-mediated intramolecular cyclization approach was used to access the 2′-substituted-2-arylindoles 31–35. All attempts to access these analogues via Rh-catalyzed 2-arylation of indoles with ortho-substituted aryl iodides failed. N-benzoylindoles 26 and 27 (Scheme 4) were converted to their respective 6-oxo-6Hisoindolo[ 2,1-α]indoles 28 and 29 using the palladium (II) acetate promoted oxidative intramolecular cyclization procedure reported by Itahara.20 Ring-opening of compounds 28 and 29 by lactam hydrolysis (t-BuOK, t-BuOH, H2O)20 yielded the 2′-carboxylic acids 3020 and 3113 respectively. Compound 30 was converted to the methyl ester 32 under standard conditions (AcCl/MeOH) and to the hydroxymethyl derivative 33 using LiAlH4. The acid 2-(2′-carboxyphenyl)-5-nitroindole 31 was converted to its methyl ester 34 using AcCl/MeOH and to its hydroxymethyl derivative 3513 using BH3.THF.
Scheme 4.
Reagents and conditions: (a) Pd(OAc)2, AcOH, 110 °C;20 (b) tBuOK, tBuOH/H2O, 82 °C;20 (c) AcCl, MeOH, reflux; (d) LiAlH4, THF, rt; (e) BH3-THF, rt.13 * Denotes compounds synthesized previously.13
The potentiation activities of compounds 1–6 and 14–17 (Table 1) were determined against the wild type S. aureus strain (8325-4), a mutant strain deleted in NorA (K1758) and a NorA overexpressing strain (K2378) with the antibacterial agent berberine using a checkerboard assay which involved co-administration of varying concentrations of the inhibitor and berberine.21 This assay provides access to combinatorial interactions (synergistic, antagonistic, indifferent, or additive) between the two molecules. The inhibitor ‘MIC’ is defined as the lowest concentration of inhibitor required to potentiate berberine’s MIC by at least a factor of two. The MIC values for berberine (as the chloride salt) alone against S. aureus were 269 µM (8325-4) and 67 µM (K1758)), and 2152 µM (K2378). The activities of compounds 18–25 and 32–35 (Table 2) were determined against the same three strains of S. aureus but using our previously published methodology13 (even though not as complete as the full checkerboard assay), in order to enable a better comparison with the prior results on compounds with other 2-aryl substituents. Briefly, for this methodology, cells were grown in the presence of a sub-inhibitory concentration of berberine [81 µM (8325-4); 20 µM (K1758); and 161 µM (K2378)] and varying concentrations of the test compounds. Bacterial growth was monitored by measuring the absorption at 600 nm and MIC values were determined. MICs represent the minimum concentrations of the test compounds (in combination with the fixed concentration of berberine) that completely inhibited cell growth during an 18 h incubation at 37 °C. Differences in the values for INF55 (2) in Table 1 and Table 2 reflect the difference in the potentiation assay used, although the different result with the NorA knockout mutant K1758 (Table 1) was not resolved.
Table 1.
Bacterial inhibition and potentiation results for C5-substituted analogues of INF55 (2) with berberine in S. aureus. NP denotes no antibacterial potentiation; IA denotes intrinsic antibacterial activity. Potentiation values are shown in brackets and are the ratios of berberine MICs with and without inhibitor present.
 |
MIC (µM) of inhibitor | |||
|---|---|---|---|---|
| Δ norA K1758 plus berberine | WT 8325-4 plus berberine | NorA++ K2378 plus berberine | ||
| Compd | R | |||
| 1 | H | NP | 65 (4) | 259.0 (4) |
| 2 | NO2 | NP | 3.3 (2) | 6.5 (2) |
| 3 | SO3H | NP | NP | NP |
| 4 | SO2NH2 | NP | NP | NP |
| 5 | SO2OCH3 | IA | IA | IA |
| 6 | SO2On-Pr | IA | IA | IA |
| 14 | CN | NP | 3.6 (2) | 7.1 (2) |
| 15 | CO2Me | NP | NP | NP |
| 16 | CO2H | NP | NP | NP |
| 17 | CH2OH | IA | IA | IA |
Table 2.
Bacterial inhibition and potentiation results for 2-aryl analogues of INF55 (2) with berberine in S. aureus. NP denotes no antibacterial potentiation. The inhibitor concentrations are the minimum for potentiation of berberine MIC by factors of 3.3 (K1758), 3.3 (8325-4), and 13.3 (K2378).
 |
MIC (µM) of inhibitor | ||||
|---|---|---|---|---|---|
| Δ norA K1758 plus berberine | WT 8325-4 plus berberine | NorA++ K2378 plus berberine | |||
| Compd | R1 | R2 | |||
| 32 | H | 2′-CO2Me | NP | 20 | 20 |
| 33 | H | 2′-CH2OH | NP | 45 | 45 |
| 18 | H | 3′-CO2Me | 40 | 5 | 10 |
| 19 | H | 3′-CH2OH | NP | 45 | 45 |
| 22 | H | 4′-CO2Me | NP | 20 | 40 |
| 23 | H | 4′-CH2OH | NP | 22 | 45 |
| 225 | NO2 | H | 0.63 | 1.3 | 1.3 |
| 34 | NO2 | 2′-CO2Me | NP | 34 | NP |
| 3513 | NO2 | 2′-CH2OH | 24 | 47 | 47 |
| 20 | NO2 | 3′-CO2Me | 0.51 | 1.0 | 1.0 |
| 21 | NO2 | 3′-CH2OH | 9 | 5 | 9 |
| 24 | NO2 | 4′-CO2Me | NP | 17 | NP |
| 25 | NO2 | 4′-CH2OH | 5 | 2 | 2 |
The inherent antibacterial activity of each compound in the absence of berberine was > 1000 µM (the testing limit employed in this assay), except for the sulfonate esters 5 and 6 (MIC values of 174 µM and 79.3 µM respectively against all three S. aureus strains), and 17. The alcohol 17 showed an MIC of 14.0 µM against the NorA knockout strain, and 28.0 µM against both the wild type and NorA overexpressing strains.22 Interestingly, in further testing, 17 was shown to be inactive as an antibacterial against a panel of other Gram-negative and Gram-positive pathogens.23
Compounds 1–6 and 14–17 (Table 1) varied only with respect to substituents at the indole-C5 position allowing for direct conclusions to be drawn regarding the importance of the 5-NO2 group of INF55. Three of the compounds tested (5, 6, and 17) showed intrinsic antibacterial activity and thus the potentiation of the MIC value could not be accurately determined for these analogues.
Structure-activity conclusions regarding the importance of the C5 position of INF55 for inhibitory activity against the NorA pump include:
Removing the C5 substituent (1) is deleterious to activity.
Carbonyl based electron-withdrawing groups at C5 (15, 16) abolish all activity.
Inhibitors with sulfonic acids/esters/amides at C5 (compounds 3–6) show no potentiation. Compounds 5 and 6 display mild intrinsic antibacterial activity.
Substitution with a nitrile group leads to retention of potency with 14 showing similar potentiation activity to INF55.
Compounds 20, 21, 24, 25, 34 and 35 (Table 2)24 systematically explore the effects of methyl ester and hydroxymethyl substituents at the 2′, 3′ and 4′ positions of the 2-aryl ring of INF55. Compounds 18, 19, 22, 23, 32 and 33 explore these same effects in compounds lacking the 5-NO2 of INF55. One of the goals of this study was to identify analogues of INF55 that retained or improved NorA inhibitory potency while containing functional groups useful for covalent attachment to antimicrobial agents. We were particularly interested in analogues bearing hydroxymethyl groups since these could be incorporated into dual-action NorA inhibitor-antibacterial “mutual” prodrugs bearing labile ester linkages. Success of this strategy of course requires that the alcohol-bearing INF55 analogue released from such prodrugs is a potent NorA inhibitor. The methyl ester analogues were intermediates in the synthesis of the hydroxymethyl analogues and were tested here to add depth to the SAR. The analogous series lacking the 5-NO2 group was explored in an attempt to identify inhibitors which might avoid the known toxicity problems of nitroaromatic compounds.
Table 2 shows that all compounds lacking 5-NO2 groups (i.e. 18, 19, 22, 23, 32, 33) were significantly less potent than INF55. Another trend to emerge was that, apart from 24 with respect to 25, each of the methyl ester analogues was more potent than its corresponding hydroxymethyl derivative. The 3′-CO2Me derivative 20 proved to be the most active compound identified in this study, displaying slightly higher potency than INF55 against all three S. aureus strains. Another important finding was that analogue 25 bearing a 4′-CH2OH group was almost equipotent with INF55 against the wild type and NorA overexpressing strain. This suggests that 25 is the best compound to progress into our studies of mutual prodrugs employing labile ester linkages. It should be mentioned that there is a possibility that non-covalent complex formation between berberine and the indole-based inhibitors reported here may play a role in the potentiation observed.26
In conclusion, the SAR data for indole-based NorA efflux pump inhibitors has been deepened by this study and three new potent inhibitors, 14, 20 and 25, have been uncovered. Inhibitor 25 represents a promising candidate for incorporation into dual action mutual prodrugs targeting the NorA pump. In addition, a new indole derivative 17 was identified with specific antibacterial activity against S. aureus. The mode of action of this compound and the reason for its selective activity against this organism remains to be investigated.
Acknowledgments
We wish to thank the University of Wollongong, Australia and Northeastern University, Boston, for supporting this work. The award of an APA scholarship (Joseph Ambrus) and NHMRC C.J.Martin Fellowship (Michael Kelso) are also gratefully acknowledged. Kim Lewis, Gabriele Casadei and Anthony Ball were supported by Grant R21 AI59483-01 from the NIH. We thank Glenn Kaatz for kindly providing S. aureus strains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Marquez B. Biochimie. 2005;87:1137. doi: 10.1016/j.biochi.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Lomovskaya O, Zgurskaya HI, Totrov M, Watkins WJ. Nature Rev. Drug Discovery. 2007;6:56. doi: 10.1038/nrd2200. [DOI] [PubMed] [Google Scholar]
- 3.Kaatz GW. In: Frontiers in Antimicrobial Resistance. White DG, Alekshun MN, McDermott PF, editors. Washington, DC: ASM Press; 2005. p. 275. [Google Scholar]
- 4.Poole K. Clin. Microbiol. Infect. 2004;10:12. doi: 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaatz GW, Seo SM, Ruble CA. Antimicrob. Agents Chemother. 1993;37:1086. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bremner JB. Pure Appl. Chem. 2007;79:2143. [Google Scholar]
- 7.Guz NR, Stermitz FR, Johnson JB, Beeson TD, Willen S, Hsiang J-F, Lewis K. J. Med. Chem. 2001;44:261. doi: 10.1021/jm0004190. [DOI] [PubMed] [Google Scholar]
- 8.Vidaillac C, Guillon J, Moreau S, Arpin C, Lagardere A, Larrouture S, Dallemagne P, Caignard D-H, Quentin C, Jarry C. J. Enzyme Inhib. Med. Chem. 2007;22:620. doi: 10.1080/14756360701485406. [DOI] [PubMed] [Google Scholar]
- 9.Marquez B, Neuville L, Moreau NJ, Genet J-P, dos Santos AF, Cano de Andrade MC, Goulart Sant'Ana AE. Phytochem. 2005;66:1804. doi: 10.1016/j.phytochem.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Markham PN, Mulhearn DC, Neyfakh AA, Crich D, Jaber M-R, Johnson ME. Application: WO. USA: Influx, Inc.; 2000. p. 119. 32196. [Google Scholar]
- 11.Markham PN, Westhaus E, Klyachko K, Johnson ME, Neyfakh AA. Antimicrob. Agents Chemother. 1999;43:2404. doi: 10.1128/aac.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier dit Chabert J, Marquez B, Neville L, Joucla L, Broussous S, Bouhours P, David E, Pellet-Rostaing S, Marquet B, Moreau N, Lemaire M. Bioorg. Med. Chem. 2007;15:4482. doi: 10.1016/j.bmc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Samosorn S, Bremner JB, Ball A, Lewis K. Bioorg. Med. Chem. 2006;14:857. doi: 10.1016/j.bmc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Bremner JB, Ambrus JI, Samosorn S. Curr. Med. Chem. 2007;14:1459. doi: 10.2174/092986707780831168. [DOI] [PubMed] [Google Scholar]
- 15.Ball AR, Casadei G, Samosorn S, Bremner JB, Ausubel FM, Moy TI, Lewis K. ACS Chem. Biol. 2006;1:594. doi: 10.1021/cb600238x. [DOI] [PubMed] [Google Scholar]
- 16.Singh G, Dev Sharma P. Indian J. Pharm. Sci. 1994;56:69. [Google Scholar]
- 17.Noland WE, Rush KR, Smith LR. J. Org. Chem. 1966;31:65. [Google Scholar]
- 18.Russell HF, Harris BJ, Hood DB, Thompson EG, Watkins AD, Williams RD. Org. Prep. Proced. Int. 1985;17:391. [Google Scholar]
- 19.Wang X, Lane BS, Sames D. J. Am. Chem. Soc. 2005;127:4996. doi: 10.1021/ja050279p. [DOI] [PubMed] [Google Scholar]
- 20.Itahara T. Bull. Chem. Soc. Jpn. 1981;54:305. [Google Scholar]
- 21.A checkerboard assay was conducted to specify the degree of potentiation of berberine by the test compounds and to determine the specificity of these compounds for the NorA efflux pump. Serial 2-fold dilutions of berberine and a test compound were mixed in each well of a 96-well microtiter plate so that each row (and column) contained a fixed amount of one agent and increasing amounts of the second agent. The resulting plate presents a pattern in which every well contains a unique combination of concentrations between the two molecules. The concentrations of berberine ranged from 100 to 12.5 µg/mL (S. aureus 8325-4) and 200 to 800 µg/mL (S. aureus K2378), while inhibitor concentrations ranged from 1.56 to 100 µg/mL. Each plate also contained a row and column in which a serial dilution of each agent was present alone. Cells were added to each well at a final concentration of 5 10^5 CFU/mL, and plates incubated at 37 °C for 20 h. Growth was assayed by absorption at 600nm with a microtiter plate reader (Spectramax PLUS384, Molecular Devices). An OD less than 0.05 was considered to reveal no growth.
- 22.For the antimicrobial susceptibility assay, cells (10^5 CFU/mL) were inoculated into broth and dispensed at 0.2 mL/well in 96-well microtiter plates. MICs were determined by serial 2-fold dilution of the test compound. The MIC was defined as the concentration of an antimicrobial that completely inhibited cell growth during an 18–20 h incubation at 37 °C Growth was assayed with a microtiter plate reader (Spectramax PLUS384; Molecular Devices) by monitoring absorption at 600 nm.
- 23.Compound 17 was tested against the following panel of Gram-negative pathogens with MICs given in brackets in µg/mL; the highest concentration tested was 200 µg/mL: Escherichia coli O157:H7, BW25113, and UT189 (all >200); Salmonella typhimurium CS132 (>200); Pseudomonas aeruginosa PA01 and PA14 (both > 200). This compound was also tested against a number of Gram-positive pathogens as follows with MICs in brackets in µg/mL; the highest concentration tested was 200 µg/mL for all species apart from the Clostridium species, where the highest concentration tested was 100 µg/mL: Enterococcus faecalis MMH594, V583, and OG1RF (all >200); Clostridium difficile VPI10463, CD196, and CD2001 (all>100), and Clostridium perfringens (>100).
- 24.Biological testing of compounds 30 and 31 was not undertaken as the latter compound with an orthocarboxylic acid group had been tested previously13 and showed poor potentiation of berberine, while the former compound was only made as a precursor for the corresponding methyl ester 32 in order to obtain a complete set of methyl esters for SAR purposes.
- 25.The MIC of this compound with berberine was also determined, for comparison purposes, against S. aureus K2361 and found to be 1.56 µg/mL, broadly commensurate with the previously obtained value (3 µg/mL)13 in this NorA pump overexpressing strain.
- 26.Zloh M, Gibbons S. Theor. Chem. Acc. 2007;117:231. [Google Scholar]