Abstract
The impact of extensively used arsenic-containing herbicides on groundwater beneath golf courses has become a topic of interest. Although currently used organoarsenicals are less toxic, their application into the environment may produce the more toxic inorganic arsenicals. The objective of this work was to understand the behavior of arsenic species in percolate water from monosodium methanearsonate (MSMA) applied golf course greens, as well as to determine the influences of root-zone media for United State Golf Association (USGA) putting green construction on arsenic retention and species conversion. The field test was established at the Fort Lauderdale Research and Education Center (FLREC), University of Florida. Percolate water was collected after MSMA application for speciation and total arsenic analyses. The results showed that the substrate composition significantly influenced arsenic mobility and arsenic species transformation in the percolate water. In comparison to uncoated sands (S) and uncoated sands and peat (S + P), naturally coated sands and peat (NS + P) showed a higher capacity of preventing arsenic from leaching into percolate water, implying that the coatings of sands with clay reduce arsenic leaching. Arsenic species transformation occurred in soil, resulting in co-occurrence of four arsenic species, arsenite (AsIII), arsenate (AsV), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) in percolate water. The results indicated that substrate composition can significantly affect both arsenic retention in soil and arsenic speciation in percolate water. The clay coatings on the soil particles and the addition of peat in the soil changed the arsenic bioavailability, which in turn controlled the microorganism-mediated arsenic transformation. To better explain and understand arsenic transformation and transport after applying MSMA in golf green, a conceptual model was proposed.
Keywords: Monosodium methanearsonate (MSMA), golf course green, arsenic transportation and transformation, arsenic speciation
INTRODUCTION
Arsenic has received increasing attention because of its potential toxicity and the considerable environmental contamination as a result of natural and anthropogenic activities. Naturally occurring arsenic is commonly found in groundwater in many countries (1). Long-term exposure to low concentrations of arsenic in drinking water can lead to skin, bladder, lung, and prostate cancers (2, 3). The U.S. Environmental Protection Agency (EPA) has lowered the maximum contaminant level (MCL) of arsenic in drinking water from 50 to 10 μg L−1 to better achieve the goal of protecting public health (4). The major anthropogenic sources of arsenic contamination in the environment include smelting of metals, burning of coal, and the use of arsenical pesticides (5–8). About 75% of the total arsenic consumption for more than 100 years in the United States was arsenical pesticides used in agriculture (9). Arsenical pesticides are applied for turf maintenance on golf courses throughout Florida. An elevated arsenic level has been found in the nearby environment and is becoming a serious concern. A survey conducted on Florida golf courses showed that about 96% of golf courses spray herbicides containing the active ingredient monosodium methylarsonate (MSMA) 2–3 times every year at an application rate of ~2 lb/acre (10). After MSMA application, a variety of chemical processes occurring in the heterogeneous environment produce several arsenic species (11). The Dade County Department of Environmental Resources Management (DERM) and Florida Department of Agriculture and Consumer Services (FDACS) conducted a collaborative study in 1999 to monitor the ground and surface water quality at five Miami-Dade County municipal golf courses (12). The study revealed that the groundwater contamination by arsenic was widespread beneath the five golf courses studied. The presence of arsenic in groundwater samples was at concentrations of potential concern, with the highest as 815 μg L−1. High concentrations of arsenic were also detected in the surrounding surface soils. The study on arsenic mobility in the soils from some selected golf courses (13) demonstrated that arsenic present in these soils was relatively mobile or mobilizable, suggesting a high potential for arsenic leaching.
Once arsenicals reach the soil, they can be subjected to various processes such as reduction/oxidation and methylation/ demethylation reactions. The environmental fate and behavior of arsenic and arsenic species transformation are of great importance for properly assessing the risk of applying arsenic-containing herbicides and have attracted much attention among scientific research units, government agencies, local communities, and MSMA manufacturers (14, 15). Although historically used inorganic arsenical pesticides have been largely replaced by the less toxic organoarsenicals, release of these compounds into the environment can still be a problem (16–18). Soil properties influence arsenic retention and mobility as well as conversion of arsenic species by biotic and abiotic processes (19, 20). These processes may result in the occurrence of more toxic inorganic arsenicals. The repeated use of organoarsenical herbicides on golf courses results in concentrated application of arsenic in the localized areas. Retention of these arsenicals in golf course soil and their subsequent leaching to percolate water and further into irrigation water may cause environmental problems with respect to human health. To understand and manage the risks posed by MSMA application on golf courses, it is essential to know the effects of soil properties on arsenical retention, mobility, and species conversion.
The objective of this work was to understand the behavior of arsenic species in percolate water from MSMA-applied golf course greens, as well as to determine the influences of root-zone media for United State Golf Association (USGA) putting green construction on arsenic retention and species conversion. The field test was established at the Fort Lauderdale Research and Education Center (FLREC), University of Florida. Percolate water was collected periodically and analyzed for arsenic speciation and total arsenic. The results indicate that arsenic retention and arsenic species leached vary with the substrate composition, providing important information on how to reduce the risk of arsenic contamination.
MATERIALS AND METHODS
Soil Selections
Three types of substrate composition have been used for simulated golf course greens, including uncoated sand (S), uncoated sand and peat (S + P), and naturally coated sand and peat (NS + P). The use of coated sands and peat can increase moisture and nutrient retention and improve turfgrass growth in sand-based putting greens without negatively influencing the putting green physical characteristics. Canadian sphagnum peat moss was added at 10% (v/v) in each mixture. Uncoated sands and naturally coated sands are the normal substrates for golf course putting greens. The uncoated sand used was common quartz sand, light gray or colorless, and did not have clay-sized coatings. Naturally coated sands, collected from Lake County, FL, had a reddish-brown hue because of oxidized Fe and Al in the clay-sized fraction of the coating. It consisted of quartz and clay fractions including hydroxy-interlayered vermiculate [(Mg,Fe2+,Al)3(Al,Si)4O10(OH)2 ·4(H2O)], kaolinite [Al2Si2O5(OH)4], geothite [FeO(OH)], and gibbsite [Al(OH)3]. The cation-exchange capacities of sands and naturally coated sands are 0.1 and 4–5 cmol/kg, respectively. Detailed information about these materials has been reported previously (21).
Field Experimental Setup and Sampling
A total of 12 experimental plots (4 for each substrate) were constructed at the University of Florida’s Fort Lauderdale Research and Education Center in Davie, FL. Details of the plot establishment can be found elsewhere (21). Briefly, plots (0.5 × 2.0 m) were constructed following the United State Golf Association (USGA) specifications with 30 cm root zone placed over 5 cm of an intermediate “choker” layer and 15 cm of quartz pea gravel with one “40 quart stock pot”, 35.6 cm inside diameter, and 40.6 cm tall lysimeter in the center. This design provides the beneficial characteristics of resistance to compaction, rapid infiltration, rapid percolation, and adequate aeration. The plots were encased with plywood along the perimeter to a depth of 30 cm to hydraulically isolate the added soil mixtures from the surrounding root-zone media. The root-zone mix, choker layer, and pea gravel in each lysimeter was suspended on a perforated-metal stainless-steel plate, as previously described (22). The surface of the plots was covered by bermudagrass turf. The lysimeters collected the percolate water as it filtered through the three layers and were pumped out approximately weekly or more often, depending on leaching events. MSMA was applied on 8/29/2002, 9/5/2002, and 9/12/2002 at 104 mg As m−2 per application. Total arsenic application was 312 mg m−2. The soil was sampled to a depth of 10 cm before MSMA applications (8/29/2002) and 1 week after the third application (9/19/2002). The percolate water was collected prior to weekly MSMA applications on 8/29/2002, 9/5/2002, and 9/12/2002 and then every 1 or 2 weeks after that. The total volume of percolate water was recorded. Water samples were kept in high-density poly-ethylene (HDPE) bottles and placed in a cooler containing ice.
Arsenic Analysis and Speciation
Arsenic speciation analysis was performed within 24 h of sampling, using high-performance liquid chromatography coupled with hydride generation atomic fluorescence spectrometry (HPLC–HG–AFS). The HPLC system consisted of a P4000 pump and an AS3000 autosampler with a 100-μL injection loop (Spectra-Physics Analytical, Inc., Fremont, CA). A Hamilton PRP-X100 anion-exchange HPLC column (250 × 4.6 mm, 10-μm particle size) was used to separate different arsenic species with a 0.015 mol L−1 phosphate mobile phase (pH 5.8) at a flow rate of 1 mL min−1. The HG–AFS instrument used was a PS Analytical Millennium Excalibur system (PSA 10.055, PS Analytical, Kent, U.K.). This system is an integrated atomic fluorescence system incorporating vapor generation, gas–liquid separation, moisture removal, and atomic fluorescence stages. Data were acquired by a real-time chromatographic control and data acquisition system. With this instrumental set up, all major species present in percolate water including arsenite (AsIII), arsenate (AsV), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) could be detected. The detection limits for arsenic speciation using HPLC–HG–AFS were about 1 μg L−1 for AsIII, MMA, and DMA and 1.5 μg L−1 for AsV, respectively, calculated based on the 3 times of the baseline noise. The relative standard deviation at 10 μg L−1 concentration level was below 7% for all species for triplicate analysis.
Total arsenic determination was carried out using inductively coupled plasma mass spectrometry (ICP/MS). Soil samples (0.5 g) were digested in 10 mL of concentrated nitric acid using an open vessel method at 110 °C for 1 h and additional 30 min after adding 1 mL of H2O2. The digested solutions were transferred to 50-mL volumetric flasks and diluted to the mark with DDI water. Further dilutions were performed to make test samples in 2% nitric acid. Water samples for total arsenic analysis by ICP/MS also contained 2% nitric acid. Internal standard (Y) was added to each sample at 50 μg L−1 prior to ICP/MS analysis. Standard check solutions and spiked samples were analyzed for QA/ QC purposes. The detection limit for total arsenic analysis was 0.05 μg L−1.
Arsenic Stability Test
To ensure that arsenic species were not altered during sample storage time, the stability of arsenic species in field samples was tested using different preservation methods. The percolate water collected from each type of substrate was spiked immediately at 20 μg L−1 in the field with AsIII, AsV, MMA, and DMA, respectively. One set of the spiked samples was frozen immediately and stored in liquid nitrogen prior to speciation analysis. Another set was stored in a cooler with ice (0–4 °C) during transportation to the lab and analyzed immediately and then stored in a refrigerator for the next day analysis. The recoveries of the spiked arsenic species were calculated to evaluate the sample preservation efficiency.
RESULTS AND DISCUSSION
Sample Preservation
Field water samples from three types of substrate, with or without standard addition, were analyzed using HPLC–HG–AFS for arsenic speciation. All sample storage methods were shown to be effective in arsenic preservation with recoveries of the spiked arsenic standards ranging from 80 to 120% (Table 1). Thus, all of the field samples were stored in an ice-cold cooler for transportation and analyzed within 24 h of sampling.
Table 1.
Recoveries (%) of Arsenic Species Spiked in Percolate Waters under Different Storage Conditionsa
| in liquid nitrogen
|
cooler, same day analysis
|
cooler, next day analysis
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| substrate | AsIII | DMA | MMA | AsV | AsIII | DMA | MMA | AsV | AsIII | DMA | MMA | AsV |
| S | 116 | 121 | 118 | 88 | 93 | 92 | 93 | 96 | 100 | 94 | 109 | 91 |
| S + P | 99 | 109 | 112 | 87 | 88 | 82 | 93 | 80 | 92 | 111 | 100 | 103 |
| NS + P | 94 | 106 | 105 | 113 | 89 | 91 | 96 | 97 | 97 | 109 | 96 | 93 |
S, uncoated sand; S + P, uncoated sand and peat; NS + P, naturally coated sand and peat.
Arsenic Retention and Leaching
The arsenic level in untreated substrates (0–10 cm) before MSMA application was at 0.27–0.34 mg kg−1 and increased to 0.45–0.69 mg kg−1 on 9/19/2002 after three MSMA applications (Table 2). In the meantime, some of the applied arsenic leached into percolate water. At a depth of 0–10 cm, arsenic retained in uncoated sands (S) in week 3 was significantly less than those in substrates S + P and NS + P, suggesting a higher arsenic mobility and faster leaching rate for uncoated sands.
Table 2.
Total Arsenic Concentration (mg kg−1, n = 4) in Substrates before and after MSMA Applicationsa
| time | S | S + P | NS + P |
|---|---|---|---|
| week 0 | 0.27 ± 0.02 | 0.27 ± 0.01 | 0.34 ± 0.04 |
| week 3 | 0.45 ± 0.04 | 0.58 ± 0.04 | 0.69 ± 0.08 |
S, uncoated sand; S + P, uncoated sand and peat; NS + P, naturally coated sand and peat.
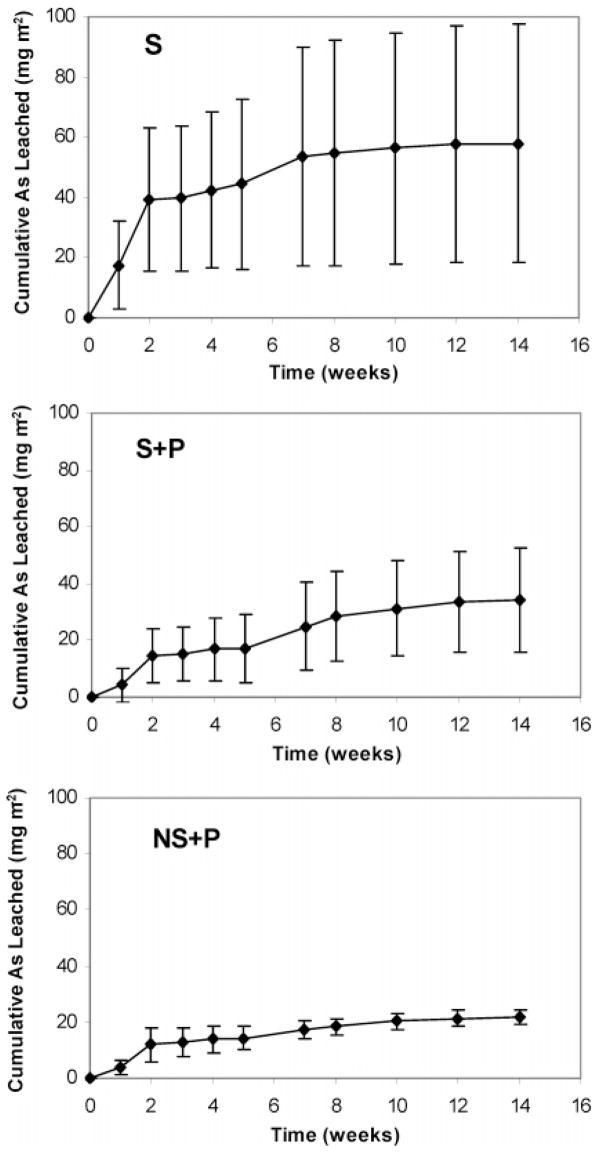
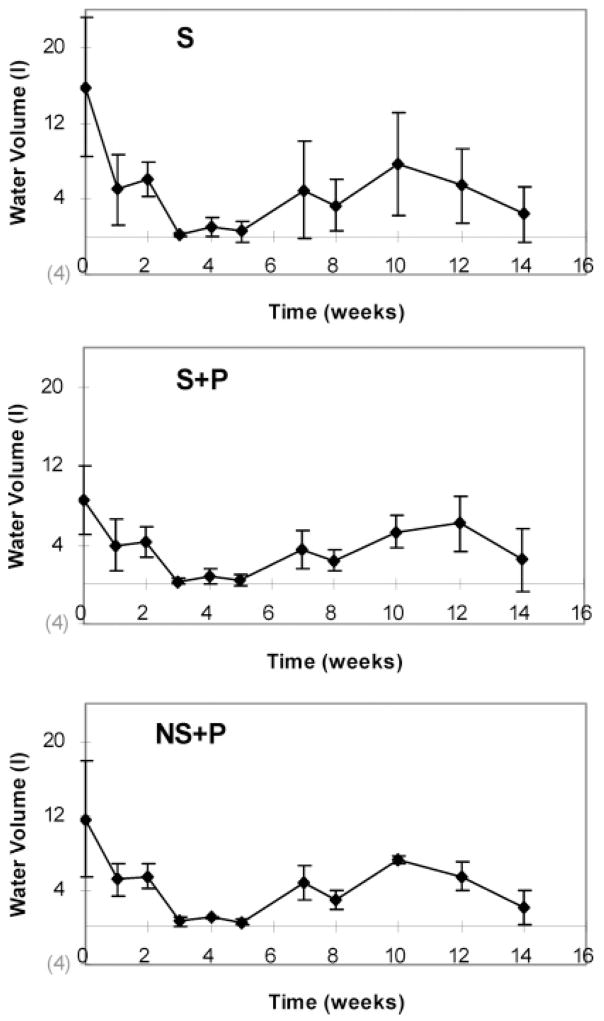
Total arsenic concentrations in percolate water vary among substrates and sampling time. Highest concentrations were obtained following the application of MSMA with an average (from four plots) of 21.83 ± 10.47, 10.29 ± 5.71, and 8.05 ± 4.84 mg m−2 for S, S + P, and NS + P, respectively, measured in week 2. Decreases in arsenic concentrations were observed with sampling time. Arsenic leaching in percolate water measured in week 14 was found to be 0.37 ± 0.54, 0.53 ± 0.63, and 0.40 ± 0.47 mg m−2, for S, S + P, and NS + P, respectively. Figure 1 illustrates the results of the average cumulative arsenic leached from all three substrates during the course of the experiment. Over the 14 week interval after the first MSMA application, the total amount of arsenic leached varied with substrate compositions. During the first 2 weeks, the greatest leaching was observed for all three substrates. This was associated with the three MSMA applications during the time period. After the first 2 weeks, arsenic leaching declined in each plot, showing a decrease in the slope of the cumulative leaching curve. This can be explained by the lack of rainfall during this time period, which caused a sharp decrease in the volume of percolate water (Figure 2), and by the termination of MSMA application after 2 weeks. The transformation of arsenic species that occurred during this time period could affect arsenic mobility and leachability (see below on arsenic speciation studies). Starting from week 5 (10/3/2003), the total arsenic leached in each plot again increased, most likely because of the increase in percolate (Figure 2).
Figure 1.
Cumulative arsenic leaching curves for S, S + P, and NS + P. Total arsenic was the sum of all of the arsenic species detected by HPLC–HG–AFS. Data were the average of four replicates for each type of substrate.
Figure 2.
Changes of percolate water volume with sampling time. Data were the average of four replicates for each type of substrate.
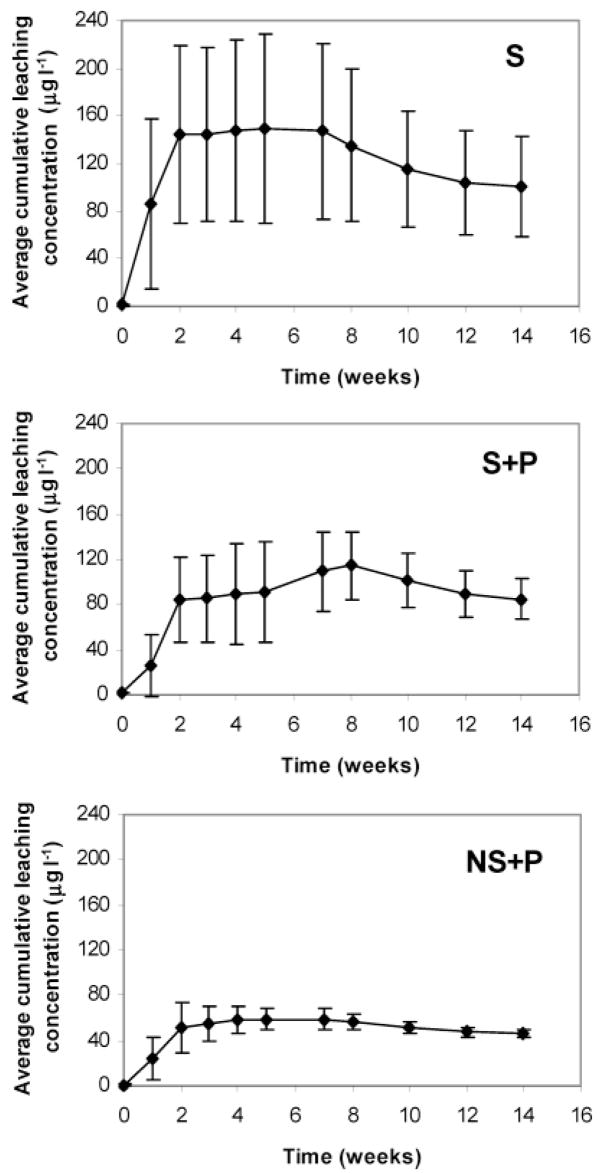
Large standard deviations of the accumulative arsenic leached were observed among the four plots for each substrate, especially for S and S + P (Figure 1). The cumulative arsenic leached from each substrate was determined by several factors, including substrate composition, water amounts from rain, and sprinkler system used. It can be seen from Figure 2 that the percolate varied with sampling time. For a specific substrate, the differences in accumulatively leached arsenic most likely resulted from the sprinkler system, which may not have watered the plots uniformly. Therefore, the results shown in Figure 1 may not provide fully representative information on arsenic leaching ability for a specific substrate. To eliminate the effects from variations in the amount of percolate in different plots and to compare the results from different substrates, the accumulative arsenic leached was normalized to the accumulated water volume collected at each plot for the 14-week experimental period (Figure 3) and termed “cumulative leaching concentration”. The deviations between the 4 replicates were reduced compared with the cumulative total arsenic leaching shown in Figure 1, while the similar leaching trends still remain.
Figure 3.
Normalized cumulative arsenic concentration (NCAC) in percolate water versus sampling time. The NCAC was calculated by using the cumulative arsenic leached (mg m−2) at a certain time divided by the cumulative volume (L) of the percolated water collected at the same time.
The large deviations in the normalized arsenic leaching rate (Table 3) observed from substrates S and S + P likely resulted from the large variations in the volumes of percolate water collected among the four plots (Figure 2). Compared to S and S + P, NS + P showed a higher capacity of preventing arsenic from leaching into percolate water, with leaching rate at 46 ± 4 μg L−1, implying that the coatings of sands with clay reduce arsenic leaching. It appears that the clay fractions in the coated sands play an important role in adsorbing arsenic because of their high surface area and high iron and aluminum contents. In general, soils with higher clay content retain more arsenic than soils with lower clay content. The adsorption of arsenic on soil is also dependent on the type of clays, which follows the order for arsenic sorption as kaolinite > vermiculite > montmorillonite (23, 24). The greater the sorption of arsenic onto soil, the less that is available for transport through substrate pores.
Table 3.
Normalized Arsenic Leaching Rate (μg L−1) during a 14-Week Perioda
| S | S + P | NS + P |
|---|---|---|
| 100 ± 42 | 85 ± 17 | 46 ± 4 |
S, uncoated sand; S + P, uncoated sand and peat; NS + P, naturally coated sand and peat.
Arsenic Species Transformation and Leachability
The determination of arsenic leachability through measuring the total arsenic concentration does not provide information on transformation of arsenic species during their transport through the substrate. Speciation studies can provide an insight into arsenic distribution patterns, identify its toxicity in ecosystems, and explain arsenic transformation and mobility.
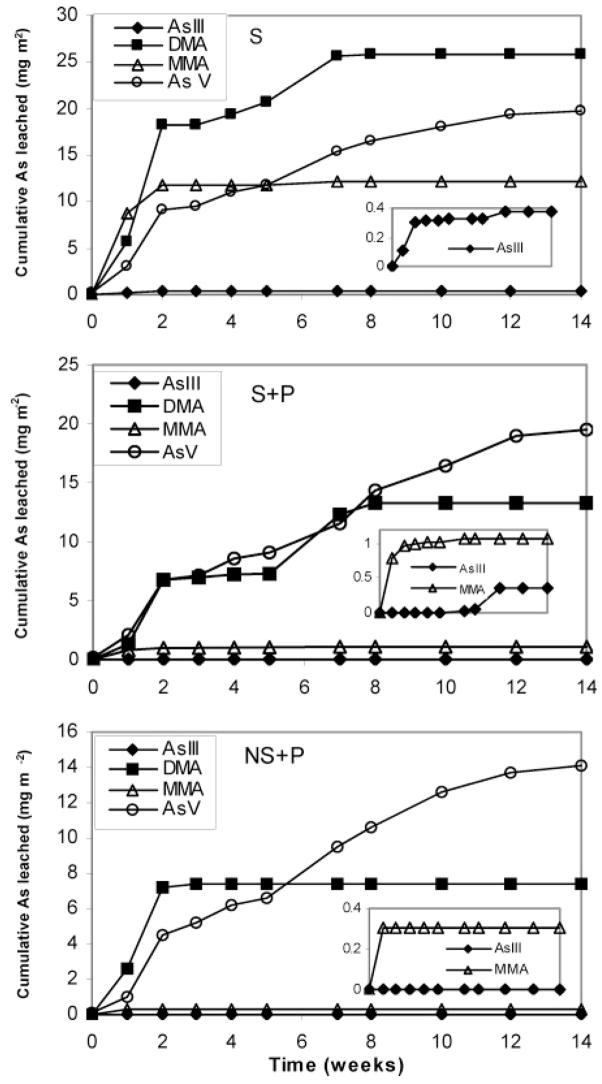
It should be pointed out that the analytical technique employed in this study, HPLC–HG–AFS, determines the hydride-forming arsenic species, i.e., AsIII, AsV, MMA, and DMA, which are reported to be the major arsenic forms in agricultural soils and waters (25). The total arsenic concentration in percolate waters was also measured using ICP/MS, and the results were in accordance with the sum of concentrations of all arsenic species detected using HPLC–HG–AFS. This confirmed that the four hydride-forming arsenic species, AsIII, AsV, MMA, and DMA composed of the majority of arsenic present in the percolate water (data and chromatogram not shown). Thus, the results from HPLC–HG–AFS analysis are used throughout the discussion. It can be seen that arsenic species transformation clearly occurred after MSMA application and during its transport through the substrates (Figure 4). The extent and magnitude of species transformation was dependent on substrate compositions.
Figure 4.
Cumulative leaching of the four arsenic species in different types of substrate. The data show the average of four replicates for each type of substrate.
MMA was leached from all three substrates but mainly in the first few weeks when MSMA was applied. For substrate S that showed less arsenic sorption capacity, a large quantity of MMA leached out unaltered at 12.1 mg m−2, which consisted of 20.8% of the total arsenic leached in 14 weeks. MMA leached from S + P and NS + P were 1.1 and 0.3 mg m−2, corresponding to 3.2 and 1.4% of the total arsenic, respectively (Figure 4). These results suggest that, compared to substrate S, MMA underwent a faster transformation in and/or stronger adsorption on S + P and NS + P.
Much larger amounts of DMA were leached from all three substrates compared to MMA. Significant differences in arsenic leaching amount and time among the substrates were observed, with higher DMA concentrations leached continuously in the first 7 or 8 weeks for S and S + P after MSMA application and lower DMA concentration only in the first 2 or 3 weeks for NS + P (Figure 4). Average cumulated DMA leaching were 25.8, 13.3, and 7.4 mg m−2 for S, S + P, and NS + P, respectively.
Small amounts of AsIII were found in the percolate water only for S and S + P at about 0.4 mg m−2. Arsenic leached in the form of AsIII represented a minor fraction of the total arsenic in percolate. AsIII was not detectable in the coated (NS + P) substrate. AsV was the only arsenic species that leached out continuously over the entire experimental period for all substrates. The average AsV leached from S, S + P, and NS + P substrates were 19.7, 19.5, and 14.1 mg m−2, respectively. The longer the arsenic remained in the substrate, the more likely it was transformed to AsV. After 8 weeks for uncoated sand substrates (S and S + P) and 3 weeks for coated sand substrate (NS + P), AsV was the only species detected in the percolate (Figure 4).
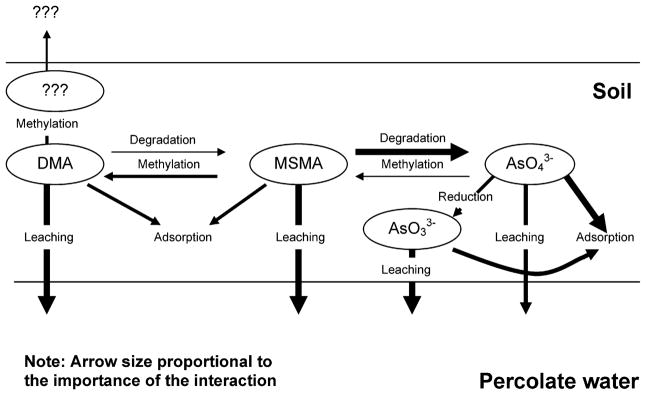
On the basis of the results obtained in this study, a conceptual model is proposed to help explain and understand arsenic transformation and transport after applying MSMA to a golf green (Figure 5). Note that the arrow size is proportional to the importance of the process. After MSMA application, a variety of chemical and biological processes occurring in the heterogeneous environment may alter the arsenic speciation. MMA was detected soon after application of MSMA and disappeared immediately after stopping application of MSMA, suggesting that MSMA was efficiently transformed via methylation and demethylation to other arsenic species, most likely DMA and AsV in this case. Many soil and water microorganisms are capable of mediating arsenic methylation (26, 27). Once DMA was formed, it could undergo several processes including adsorption to soil particles, leaching with percolate water, demethylation back to MMA, and further methylation to volatile arsenic species (e.g., trimethylarsine). High concentrations of DMA observed in percolate indicate that leaching is an important pathway of arsenic biogeochemical cycling in these soils. Adsorption of MMA and DMA to soils has been correlated to soil clay content, showing much more adsorption in soils with greater contents of clay and iron oxides (28). This was clearly demonstrated by the leaching behavior of both MMA and DMA in the three substrates, where their leaching capability was lower in the coated sand substrate (Figure 4). Leaching of MMA and DMA seemed to be limited by the presence of peat in the sand root-zone medium. The leachability/mobility of MMA and DMA has been found to be higher than that of arsenate (AsV) because they are much less particle-reactive compared to arsenate (28). Formation of volatile methylated arsine from DMA is possible. However, the magnitude of producing these compounds seemed to be minor because large amounts of DMA were rapidly leached into percolate water.
Figure 5.
Conceptual model showing arsenic transformation and transport after MSMA application in a golf green.
Degradation of MMA to AsV appeared to be the major transformation pathway. It can be seen from Figure 4 that AsV was the predominate species, with DMA and MMA having disappeared completely after 8 weeks of MSMA application. The arsenic–carbon bond in MMA, like most metal/metalloid–carbon bonds, lies in the normal range of chemical-bond energies (26). The practical stability of the As–C bond does not preclude the transformation of this compound into others. Indeed, intense UV irradiation of alkylarsenic under oxidizing conditions results in quantitative formation of arsenate. The photochemical cleavage could be an important degradation pathway on the grass leaf and in surface soil environments. The most significant MMA demethylation process in the soil is likely mediated by a variety of microorganisms (11, 25, 26). The type of micro-organisms, their populations, and the chemical and physical factors of the soils affect the rate of degradation/demethylation. Woolson et al. reported that the estimated half-life of MMA and DMA applied to a field was 20–22 days (29).
Transport of AsV in the substrates is affected strongly by the soil properties. Many studies have suggested that AsV has a low potential for leaching and can be very persistent in soil. This is thought to be due to the low solubility of arsenic–soil cation complexes (30). As a result, arsenic introduced into the environment may accumulate in soil layers near the surface (11, 31, 32). AsV transport through the substrates utilized in this study, however, was fairly rapid. The lower arsenic retentions in uncoated sands (S and S + P) compared to naturally coated sands (NS + P) were likely caused by the lack of strong interactions between arsenic and the sand surfaces. The presence of organic matter (peat) did not significantly change the arsenic retention in soil (Figure 4).
Microorganism-mediated arsenic transformation, including methylation, demethylation, oxidation, and reduction are dependent on the bioavailability of arsenic species (27). Strong adsorption of arsenic on soil particles reduces arsenic uptake by microorganisms, consequently limiting the rate of transformation. This seems to be the case for DMA production in S + P and NS + P (Figure 4). The possibly strong interactions between colloids derived from substrates S + P and NS + P and MMA may also contribute to the limited bioavailability of MMA, resulting in a reduced formation of DMA. On the other hand, the lack of strong interactions between MMA and uncoated sands enhanced the methylation of MMA (S in Figure 4). The formation of AsIII in some substrates was likely from the reduction of AsV via microorganism-mediated processes. Microbial reduction of AsV to AsIII may occur by at least two principal mechanisms: dissimilatory reduction, where AsV is utilized as a terminal electron acceptor during anaerobic respiration, and detoxification activity, which involves an AsV reductase and an AsIII efflux pump (33). The AsV reduction via detoxification can occur under both anaerobic and aerobic conditions. Reduction of AsV via either of these two mechanisms requires the presence of bioavailable AsV in soil solutions. A relatively high concentration of AsIII found in substrate S resulted mainly from the highly available AsV present in this substrate (Figure 4). According to previous reports, several natural clays such as kaolinite and illite have been found to enhance the oxidation of AsIII (34, 35). Therefore, the lack of AsIII in the percolate water in substrate NS + P was possibly related to its mineral-clay coatings, which may produce an oxidizing environment on the coated sands.
In summary, three substrates, all used in Florida golf courses, were studied on arsenic transport and transformation associated with MSMA application on a golf course green. The results showed that the substrate composition significantly influenced arsenic mobility and arsenic species transformation in the substrate and in the percolate water. After 14 weeks of MSMA application, the total arsenic leached into the percolate water was 18.6, 10.8, and 7.0% for S, S + P, and NS + P, respectively (Table 4). In comparison to S and S + P, NS + P showed a higher capacity of preventing arsenic from leaching into percolate water, implying that the coatings of sands with clay reduce arsenic leaching. Arsenic species transformation clearly occurred after MSMA application and during its transport through the substrates. The extent and magnitude of species transformation was dependent on substrate compositions. Microorganism-mediated processes were likely involved in arsenic transformation. The soil composition, including clay coating on the soil particles, and the addition of peat in the soil affected not only the transport of arsenic in the soil but also its bioavailability, which in turn controls the microorganism-mediated arsenic transformation. A conceptual model was proposed to help explain and understand arsenic transformation and transport after applying MSMA in golf course green.
Table 4.
Total Arsenic Leaching Rate in 14 Weeks (~8/29/2002–12/4/2002)a
| S | S + P | NS + P | |
|---|---|---|---|
| mean rate (%) | 18.6 | 10.8 | 7.0 |
| rate range (%) | ~5.0–32.4 | ~5.6–16.8 | ~6.3–8.2 |
S, uncoated sand; S + P, uncoated sand and peat; NS + P, naturally coated sand and peat.
Acknowledgments
We thank the Advanced Mass Spectrometry Facility (AMSF) at FIU for the access to ICP/MS. Technical assistance by Karen Williams, Dara Park, and David Rich is greatly acknowledged. This is SERC contribution number 256.
This study was partially supported by a grant from the United States Golf Association (USGA) and by the National Institute of Environmental Health Science (S11 ES11181).
LITERATURE CITED
- 1.Jain CK, Ali I. Arsenic: Occurrence, toxicity, and speciation techniques. Water Res. 2000;34:4304. [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Arsenic. U.S. Department of Health and Human Services; Atlanta, GA: 1999. [Google Scholar]
- 3.National Research Council. Arsenic in Drinking Water. National Academy Press; Washington, DC: 2001. [Google Scholar]
- 4.EPA headquarters press release. Washington, DC: 10312001. EPA announces arsenic standard for drinking water of 10 parts per billion. [Google Scholar]
- 5.Peterson PJ, et al. Effect of Heavy Metal Pollution on Plants. 1. In: Lepp NW, editor. Effect of Trace Metals on Plant Function. Applied Science Publishers; London, U.K: 1981. p. 279. [Google Scholar]
- 6.Hallberg KB, Sehlin HM, Lindstroem EB. Toxicity of arsenic during high-temperature bioleaching of gold-bearing arsenical pyrite. Appl Microbiol Biotechnol. 1996;45:212–216. [Google Scholar]
- 7.Amonoo-Neizer EH, Nyamah D, Bakiamoh SB. Mercury and arsenic pollution in soil and biological samples around the mining town of Obuasi, Ghana. Water, Air, Soil Pollut. 1996;91:363–373. [Google Scholar]
- 8.Trepka MJ, Heinrich J, Schulz C, Krause C, Popescu M, Wjst M, Heinz-Erich Wichmann H. Arsenic burden among children in industrial areas of eastern Germany. Sci Total Environ. 1996;180:95–105. doi: 10.1016/0048-9697(95)04945-2. [DOI] [PubMed] [Google Scholar]
- 9.Onken BM, Hossner LR. Determination of arsenic species in soil solution under flooded conditions. Soil Sci Soc Am J. 1996;60:1385–1392. [Google Scholar]
- 10.Chen M, Ma LQ, Daroub SH, Snyder GH, Cisar JL, Cai Y. Use and fate of arsenic herbicide in Florida golf courses. ASA/CSSA/SSSA Annual Meetings Abstracts; Denver, CO. 2003. CD-ROM, S-11-chen952427-Oral.pdf. [Google Scholar]
- 11.Tonner-Navarro LE, Halmes NC, Roberts SM. Technical Report Prepared for the Division of Waste Management: CEHT/ TR-98-01. Florida Department of Environmental and Human Toxicology (CEHT); University of Florida: 1998. Risk Assessment of Organic versus Inorganic Arsenic. [Google Scholar]
- 12.Wiegand GE. Preliminary Report: Environmental Quality Monitoring for Pesticides and Arsenic at Five Municipal Golf Courses in Miami-Dade County; Florida. 1999. [Google Scholar]
- 13.Cai Y, Cabrera JC, Georgiadis M, Jayachandran K. Assessment of arsenic mobility in South Florida golf courses. Sci Total Environ. 2002;291:123–134. doi: 10.1016/s0048-9697(01)01081-6. [DOI] [PubMed] [Google Scholar]
- 14.Busey P. The Florida Green. 2003. winter issue. MSMA a loaded gun to your head; pp. 36–39. [Google Scholar]
- 15.Eldan M. MSMA makers dispute hazard potential. Florida Turf Digest. 2004;21 [Google Scholar]
- 16.Bednar AJ, Garbarino JR, Ranville JF, Wildeman TR. Presence of organoarsenicals used in cotton production in agricultural water and soil of the southern United States. J Agric Food Chem. 2002;50:7340–7344. doi: 10.1021/jf025672i. [DOI] [PubMed] [Google Scholar]
- 17.Hiltbold AE, Hajek BF, Buchanan GA. Distribution of arsenic in soil profiles after repeated applications of MSMA (monosodium methanearsonate) Weed Sci. 1974;22:272–275. [Google Scholar]
- 18.Tamaki S, Frankenberger WT. Environmental biochemistry of arsenic. Rev Environ Contam Toxicol. 1992;124:79–110. doi: 10.1007/978-1-4612-2864-6_4. [DOI] [PubMed] [Google Scholar]
- 19.Cox MS, Bell PF, Kovar JL. Arsenic supply characteristics of four cotton-producing soils. Plant Soil. 1996;180:11–17. [Google Scholar]
- 20.Marin AR, Masscheleyn PH, Patrick WH. Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant Soil. 1993;152:245–253. [Google Scholar]
- 21.Snyder RH. PhD Dissertation. University of Florida; Gainesville, FL: 2003. Investigation of coated sands and peat for use in golf course putting green constriction; p. 208. [Google Scholar]
- 22.Cisar JL, Snyder GH. Mobility and persistence of pesticides in a USGA-type green I. Putting green facility for monitoring pesticides. Int Turfgrass Soc Res J. 1993;7:971–977. [Google Scholar]
- 23.Dickens R, Hiltbold AE. Movement and persistence of methanearsonates in soil. Weed Sci. 1967;15:299–304. [Google Scholar]
- 24.Onken BM, Adriano DC. Arsenic availability in soil with time under saturated and subsaturated conditions. Soil Sci Soc Am. 1997;61:746–752. [Google Scholar]
- 25.Frankenberger WT Jr, editor. Environmental Chemistry of Arsenic. Marcel Dekker; New York: 2002. p. 391. [Google Scholar]
- 26.Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev. 1989;89:713–764. [Google Scholar]
- 27.Frankenberger WT, Jr, Arshad M. Volatization of arsenic, chapter 16. In: Frankenberger WT Jr, editor. Environmental Chemistry of Arsenic. Marcel Dekker; New York: 2002. [Google Scholar]
- 28.Aurillo A, Mason RP, Hemond HF. Speciation and fate of arsenic in three lakes of the Aberjona watershed. Environ Sci Technol. 1994;28:577–585. doi: 10.1021/es00053a008. [DOI] [PubMed] [Google Scholar]
- 29.Woolson EA, Aharonson N, Iadevaia R. J Agric Food Chem. 1982;30:580–584. [Google Scholar]
- 30.Bhumbla DK, Keefer RF. Arsenic mobilization and bioavailability in soils. In: Nriagu JO, editor. Arsenic in the Environment, Part I: Cycling and Characterization. John Wiley and Sons; New York: 1994. pp. 51–82. [Google Scholar]
- 31.Corwin DL, David A, Goldberg S. Mobility of arsenic in soil from the Rocky Mountain Arsenal area. J Contam Hydrol. 1999;39:35–58. [Google Scholar]
- 32.Peters GR, McCurdy RF, Hindmarsh JT. Environmental aspects of arsenic toxicity. Cri Rev Clin Lab Sci. 1996;33:457–493. doi: 10.3109/10408369609080055. [DOI] [PubMed] [Google Scholar]
- 33.Inskeep WP, McDermott TR, Fendorf S. Arsenic(V)/(III) cycling in soils and natural waters: Chemical and microbiological processes, chapter 8. In: Frankenberger WT Jr, editor. Environmental Chemistry of Arsenic. Marcel Dekker; New York: 2002. [Google Scholar]
- 34.Manning BA, Goldberg S. Adsorption and stability of arsenic-(III) at the clay mineral–water interface. Environ Sci Technol. 1997;31:2005. [Google Scholar]
- 35.Lin Z, Puls RW. Adsorption, desorption, and oxidation of arsenic affected by clay minerals and aging process. Environ Geol. 2000;39:753–759. [Google Scholar]