Abstract
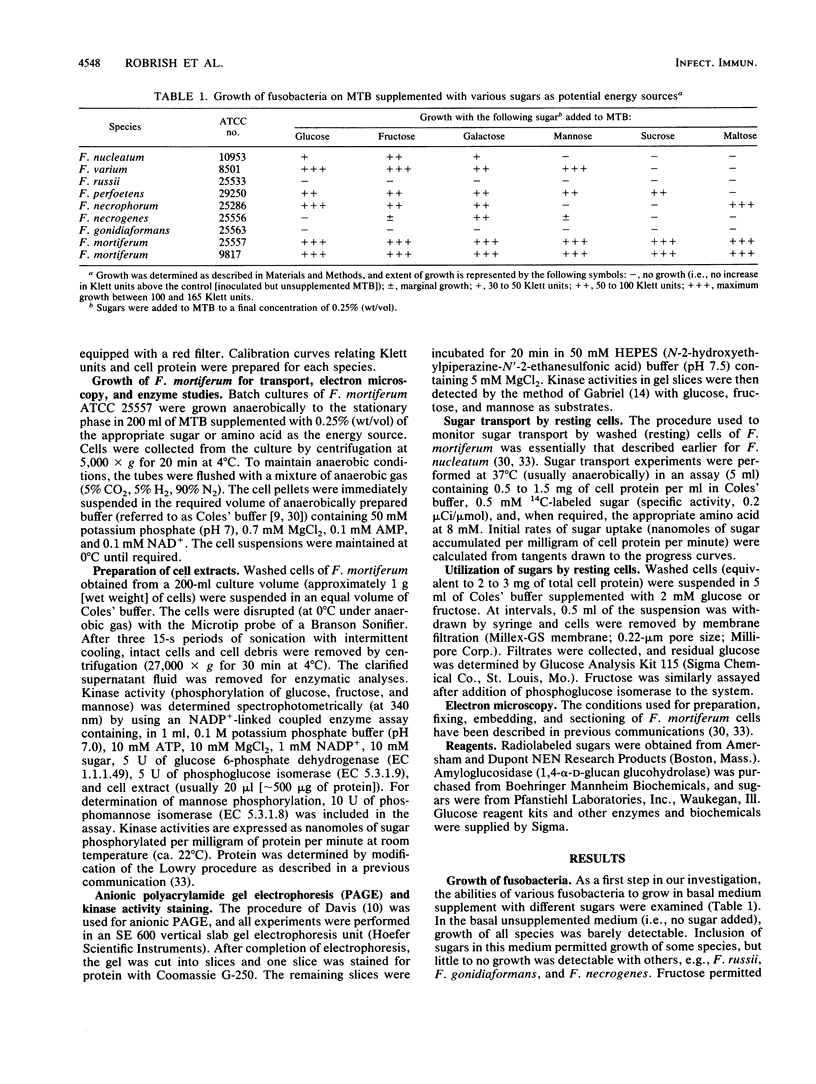
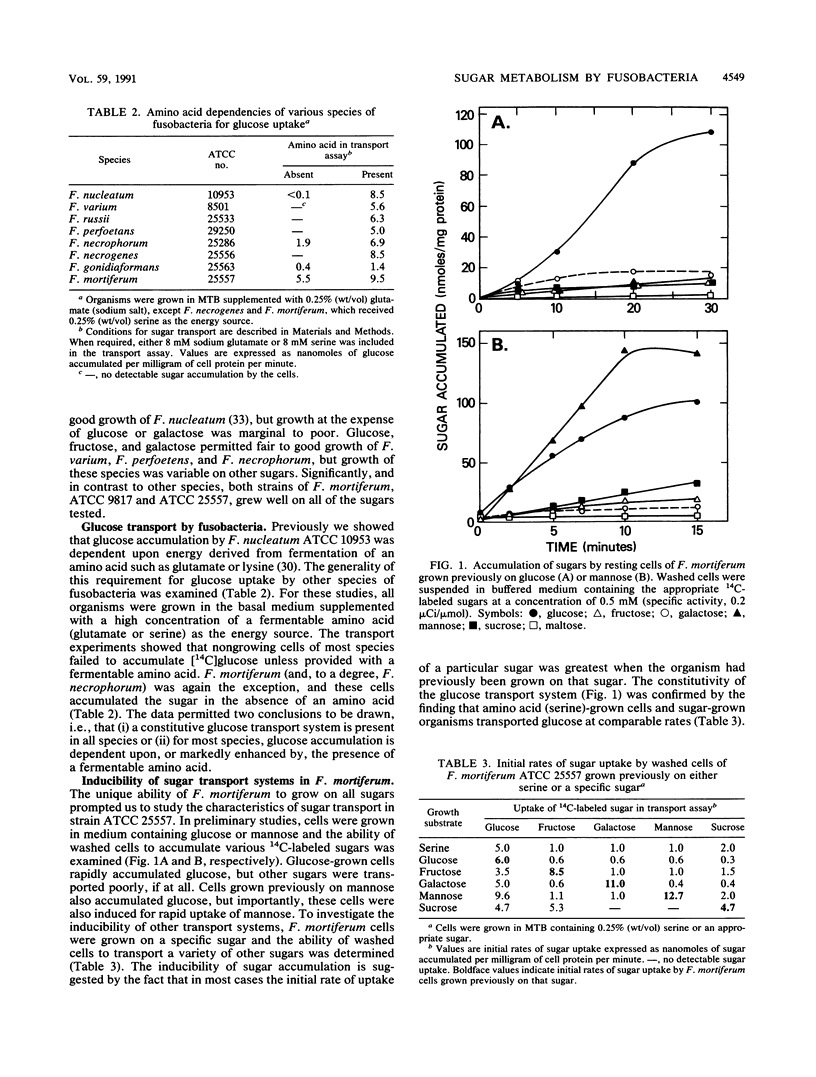
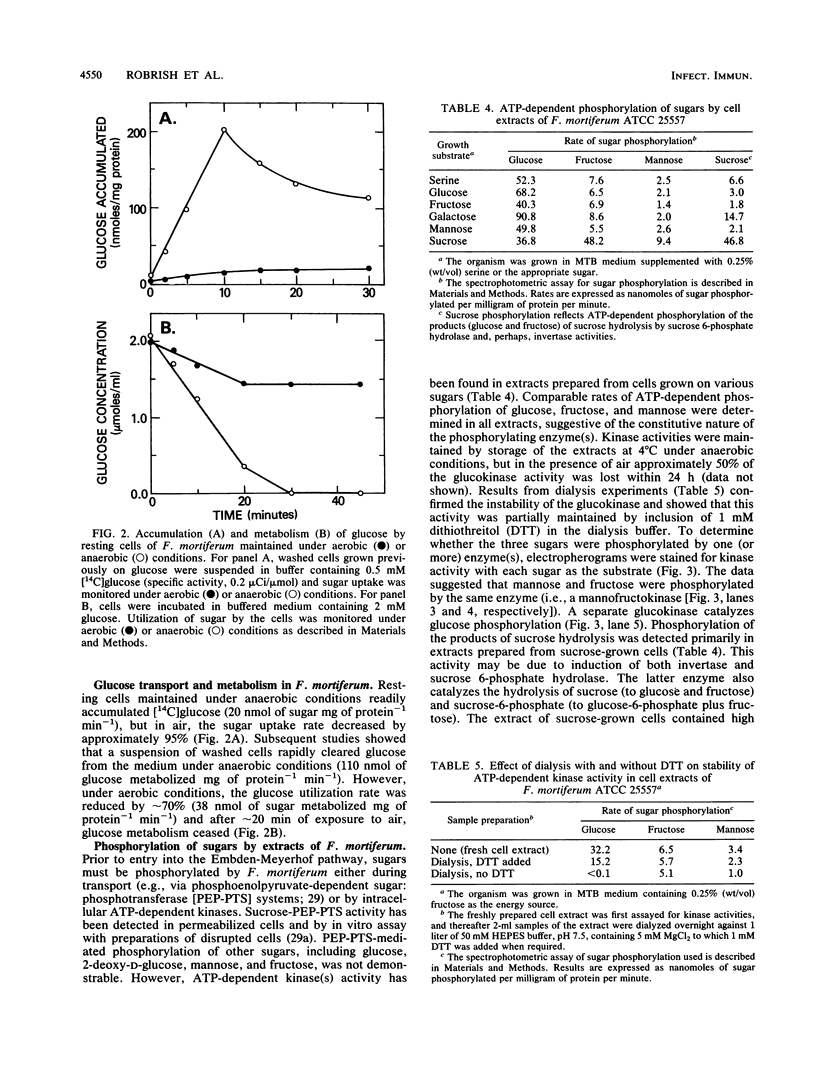
Strains of eight Fusobacterium species differed in the ability to use sugars as energy sources for growth. For Fusobacterium russii ATCC 25533, F. gonidiaformans ATCC 25563, and F. nucleatum ATCC 10953 (except for fructose), growth was marginal to poor on all of the sugars tested. Other species displayed reasonable growth on glucose, fructose, mannose, and galactose, and two strains of F. mortiferum (ATCC 25557 and ATCC 9817) grew well on six of the sugars tested, including sucrose and maltose. Glucose transport by resting cells of most of the species was dependent upon (or markedly stimulated by) the presence of a fermentable amino acid. By contrast, F. mortiferum cells rapidly accumulated glucose and other sugars in the absence of amino acids. Although these cells were constitutive for glucose uptake, accumulation of other sugars was specifically induced by growth of F. mortiferum on the appropriate sugar. Spectrophotometric analyses and in situ staining of anionic polyacrylamide gels showed that glucose and fructose (mannose) are phosphorylated by separate ATP-dependent kinases. Fructokinase was stable in air at 4 degrees C, but under these conditions, greater than 70% of the glucokinase activity was lost. After overnight dialysis of the extract, no glucokinase activity was detectable; however, 65% of the initial enzyme activity was retained by inclusion of 1 mM dithiothreitol in the dialysis buffer. Thin-section electron microscopy showed that cells of F. mortiferum produced various amounts of intracellular glycogen during growth on the following sugars (in decreasing order of formation): galactose greater than sucrose greater than glucose greater than mannose greater than fructose. Mechanisms for sugar transport regulation, phosphorylation, and polymer synthesis by F. mortiferum cells are proposed.
Full text
PDF


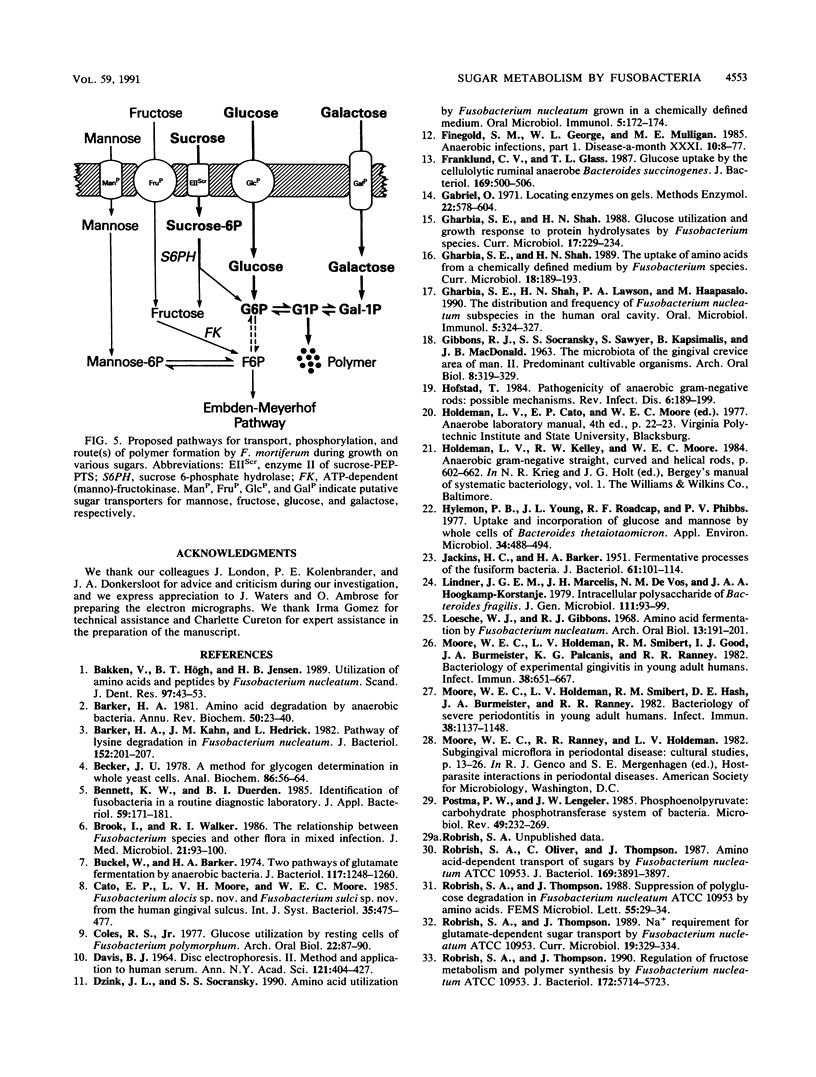

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakken V., Högh B. T., Jensen H. B. Utilization of amino acids and peptides by Fusobacterium nucleatum. Scand J Dent Res. 1989 Feb;97(1):43–53. doi: 10.1111/j.1600-0722.1989.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Barker H. A. Amino acid degradation by anaerobic bacteria. Annu Rev Biochem. 1981;50:23–40. doi: 10.1146/annurev.bi.50.070181.000323. [DOI] [PubMed] [Google Scholar]
- Barker H. A., Kahn J. M., Hedrick L. Pathway of lysine degradation in Fusobacterium nucleatum. J Bacteriol. 1982 Oct;152(1):201–207. doi: 10.1128/jb.152.1.201-207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. U. A method for glycogen determination in whole yeast cells. Anal Biochem. 1978 May;86(1):56–64. doi: 10.1016/0003-2697(78)90318-4. [DOI] [PubMed] [Google Scholar]
- Bennett K. W., Duerden B. I. Identification of fusobacteria in a routine diagnostic laboratory. J Appl Bacteriol. 1985 Aug;59(2):171–181. doi: 10.1111/j.1365-2672.1985.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Brook I., Walker R. I. The relationship between Fusobacterium species and other flora in mixed infection. J Med Microbiol. 1986 Mar;21(2):93–100. doi: 10.1099/00222615-21-2-93. [DOI] [PubMed] [Google Scholar]
- Buckel W., Barker H. A. Two pathways of glutamate fermentation by anaerobic bacteria. J Bacteriol. 1974 Mar;117(3):1248–1260. doi: 10.1128/jb.117.3.1248-1260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles R. S., Jr Glucose utilization by resting cells of Fusobacterium polymorphum. Arch Oral Biol. 1977;22(2):87–90. doi: 10.1016/0003-9969(77)90083-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dzink J. L., Socransky S. S. Amino acid utilization by Fusobacterium nucleatum grown in a chemically defined medium. Oral Microbiol Immunol. 1990 Jun;5(3):172–174. doi: 10.1111/j.1399-302x.1990.tb00418.x. [DOI] [PubMed] [Google Scholar]
- Finegold S. M., George W. L., Mulligan M. E. Anaerobic infections. Part I. Dis Mon. 1985 Oct;31(10):1–77. [PubMed] [Google Scholar]
- Franklund C. V., Glass T. L. Glucose uptake by the cellulolytic ruminal anaerobe Bacteroides succinogenes. J Bacteriol. 1987 Feb;169(2):500–506. doi: 10.1128/jb.169.2.500-506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., KAPSIMALIS B. Synthesis of intracellular iodophilic polysaccharide by Streptococcus mitis. Arch Oral Biol. 1963 May-Jun;8:319–329. doi: 10.1016/0003-9969(63)90024-4. [DOI] [PubMed] [Google Scholar]
- Gharbia S. E., Shah H. N., Lawson P. A., Haapasalo M. Distribution and frequency of Fusobacterium nucleatum subspecies in the human oral cavity. Oral Microbiol Immunol. 1990 Dec;5(6):324–327. doi: 10.1111/j.1399-302x.1990.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Hofstad T. Pathogenicity of anaerobic gram-negative rods: possible mechanisms. Rev Infect Dis. 1984 Mar-Apr;6(2):189–199. doi: 10.1093/clinids/6.2.189. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Young J. L., Roadcap R. F., Phibbs P. V., Jr Uptake and incorporation of glucose and mannose by whole cells of Bacteroides thetaiotaomicron. Appl Environ Microbiol. 1977 Nov;34(5):488–494. doi: 10.1128/aem.34.5.488-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKINS H. C., BARKER H. A. Fermentative processes of the fusiform bacteria. J Bacteriol. 1951 Feb;61(2):101–114. doi: 10.1128/jb.61.2.101-114.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner J. G., Marcelis J. H., de Vos N. M., Hoogkamp-Korstanje J. A. Intracellular polysaccharide of Bacteroides fragilis. J Gen Microbiol. 1979 Mar;111(1):93–99. doi: 10.1099/00221287-111-1-93. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Gibbons R. J. Amino acid fermentation by Fusobacterium nucleatum. Arch Oral Biol. 1968 Feb;13(2):191–202. doi: 10.1016/0003-9969(68)90051-4. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Good I. J., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in young adult humans. Infect Immun. 1982 Nov;38(2):651–667. doi: 10.1128/iai.38.2.651-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Oliver C., Thompson J. Amino acid-dependent transport of sugars by Fusobacterium nucleatum ATCC 10953. J Bacteriol. 1987 Sep;169(9):3891–3897. doi: 10.1128/jb.169.9.3891-3897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Thompson J. Regulation of fructose metabolism and polymer synthesis by Fusobacterium nucleatum ATCC 10953. J Bacteriol. 1990 Oct;172(10):5714–5723. doi: 10.1128/jb.172.10.5714-5723.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H., Zilm P. S., Gully N. J., Pfennig A. L., Marsh P. D. Aspects of the growth and metabolism of Fusobacterium nucleatum ATCC 10953 in continuous culture. Oral Microbiol Immunol. 1991 Aug;6(4):250–255. doi: 10.1111/j.1399-302x.1991.tb00486.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Wahren A. Polysaccharide accumulation in Fusiformis necrophorus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Oct;82B(5):635–643. doi: 10.1111/j.1699-0463.1974.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Walker C. B., Ratliff D., Muller D., Mandell R., Socransky S. S. Medium for selective isolation of Fusobacterium nucleatum from human periodontal pockets. J Clin Microbiol. 1979 Dec;10(6):844–849. doi: 10.1128/jcm.10.6.844-849.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H., Neuhaus F., Hussels H. A biochemical study of fusiform anaerobes. Med Microbiol Immunol. 1971;157(1):10–16. doi: 10.1007/BF02121286. [DOI] [PubMed] [Google Scholar]