Abstract
Cell death/survival following traumatic brain injury (TBI) may be a result of alterations in the intracellular ratio of death and survival factors. Bcl-2 family genes mediate both cell survival and the initiation of cell death. Using lysate RNase protections assays, mRNA expression of the anti-cell death genes Bcl-2 and Bcl-xL, and the pro-cell death gene Bax, was evaluated following experimental brain injuries in adult male Sprague-Dawley rats. Both the lateral fluid-percussion (LFP) and the lateral controlled cortical impact (LCI) models of traumatic brain injury showed similar patterns of gene expression. Anti-cell death bcl-2 and bcl-xL mRNAs were attenuated early and tended to remain depressed for at least 3 days after injury in the cortex and hippocampus ipsilateral to injury. Pro-cell death bax mRNA was elevated in these areas, usually following the decrease in anti-cell death genes. These common patterns of gene expression suggest an important role for Bcl-2 genes in cell death and survival in the injured brain. Understanding the regulation of these genes may facilitate the development of new therapeutic strategies for a condition that currently has no proven pharmacologic treatments.
INTRODUCTION
Traumatic brain injury (TBI) in humans is a devastating condition that is a major cause of death and disability in the young, and increases the vulnerability of older individuals to neurodegenerative disease (Pentland et al., 1986; Irving et al., 1996; Graham et al., 1999; Nemetz et al., 1999; Akiyama et al., 2000; Beschorner et al., 2000; Lye and Shores, 2000; Jellinger et al., 2001; Kay et al., 2003; Liu et al., 2003). TBI results in progressive neuronal loss in the cortex, hippocampus, cerebellum and thalamus (Adams et al., 1985; Kotapka et al., 1992; Ross et al., 1993; Shiozaki et al., 2001), a pattern that has been duplicated in various rodent models of TBI (Sutton, et al., 1995; Hicks et al, 1996; Dietrich et al., 1994; Colicos et al, 1997). Changes in cellular morphology indicative of neurodegeneration are evident almost immediately following the impact in the injured cortex and hippocampus (Hicks et al, 1996; Dietrich et al., 1994; Colicos et al, 1996). Electron microscopic analysis revealed that some of these degenerating neurons appear necrotic, with swollen mitochondria, vacuolated cytoplasm and pyknotic nuclei (Dietrich et al., 1994), while others exhibit DNA fragmentation within shrunken cell bodies containing condensed chromatin and cellular structures characteristic of apoptosis (Rink et al., 1995; Conti et al., 1998; Colicos and Dash, 1996; Yakovlev et al., 1997; Newcomb et al., 1999; Fox et al., 1998). While the cellular mechanisms underlying regional cell death have yet to be elucidated, many neurochemical factors and proteins have been identified as potential canditates (Raghupathi et al., 1995; Yakovlev and Faden, 1996; McIntosh et al., 1998).
The Bcl-2 multigene superfamily include genes such as Bcl-2, Bcl-xL and Bak which mediate cell survival, and genes such as Bax, Bad and Bcl-xS which participate in the inititation of cell death (Bredesen, 1995). While Bcl-2 can protect cells from a variety of insults such as treatment with calcium ionophores, glutamate, free radicals and withdrawal of trophic factors (Reed, 1998), Bax has been suggested to be necessary for developmental cell death (Deckwerth, et al., 1996) and suppression of tumorigenesis (Yin et al., 1997). Decreases in protein and/or mRNA for Bcl-2 and Bcl-xL and a concomintant increase in protein and/or mRNA levels for Bax in injured neurons have been reported following both focal and global ischemia, nerve transection or administration of neurotoxins such as MPTP or kainic acid (Gillardon et al., 1995, 1996a, 1996b; Krajewski et al., 1995; Chen et al., 1996; Hassouna et al., 1996). Conversely, an increase in Bcl-2 immunoreactivity was observed in neurons, glia and endothelial cells surrounding the infarcted cortex following focal ischemia (Chen et al., 1995), while Bcl-2 and Bcl-xL levels were relatively unchanged in neurons that survived the ischemic insult (Gillardon et al., 1996b; Honkaniemi et al., 1996). Although these observations support the hypothesis that cell survival is dependent on the ratio of anti-apoptotic and proapoptotic Bcl-2 proteins (Korsmeyer, 1996), the specific mechanism(s) of action for Bcl-2 or Bax following CNS injury is unclear.
The role of the Bcl-2 family proteins in TBI has been evaluated to a limited extent. In a model of combined TBI and hypoxemia, Clark and coworkers observed an upregulation of Bcl-2 in cortical neurons that survived the traumatic injury (Clark et al., 1997), while increased Bax immunoreactivity was observed in apoptotic granule neurons in the dentate gyrus following controlled cortical impact injury (Kaya et al., 1999). Direct evidence for a potential role of Bcl-2 in TBI neuropathology was based on the attenuation of posttraumatic cortical neurodegeneration in transgenic mice overexpressing human Bcl-2 (Raghupathi et al., 1998; Nakamura et al., 1999). We have recently reported that in the acute post-traumatic period, injured neurons exhibited a dramatic decrease in Bcl-2 immunoreactivity, which was followed at later times by an increase in Bax mRNA and protein (Raghupathi et al., 2003). The initiation of delayed cell death requires new gene expression, so we hypothesized that certain bcl-2 homologs might be regulated at the mRNA level following traumatic brain injury. Thus, we have measured changes in bcl-2 and bcl-xL (anti-apoptotic) as well as bax (pro-apoptotic) mRNA levels, in the cortex and hippocampus of rats injured in two distinct models of traumatic brain injury. Similarities in gene modulation between the models may be an indication of more general genetic programs in the progression of secondary injuries following trauma.
MATERIALS AND METHODS
Animal Models
All protocols were approved by the appropriate Institutional Animal Care and Use Committee (IACUC). The relevance of the rat traumatic brain injury models to clinical closed head trauma has been confirmed with respect to common findings in biochemical, histopathological and functional deficits (McIntosh et al., 1989; Dixon et al., 1991; Hamm et al., 1992).
Fluid-percussion brain injury
Lateral fluid percussion (LFP) is a model primarily of moderate diffuse brain injury. Adult male Sprague-Dawley rats (350−400g, n = 24) were anesthetized with sodium pentobarbital (60mg/kg, i.p.), placed in a stereotactic frame and the scalp and temporal muscle were reflected. A hollow, female Luer-Lok fitting was rigidly fixed with dental cement to a 5.0 mm craniotomy centered between bregma and lambda sutures and over the left parietal cortex. The fluid-percussion injury device was connected to the animal via the Luer-Lok fitting and brain injury of moderate (2.3−2.6 atm) severity was produced in animals as previously described (McIntosh et al., 1989). The device produces a pulse of 21−23 msec through the rapid injection of saline into the cranial cavity resulting the brief deformation of brain tissue. Animals were sacrificed by decapitation at 1d (n=6), 3d (n=5) and 7d (n=6). Sham-injured controls were surgically prepared but were not injured (n = 2 at each time point).
Controlled cortical impact brain injury
Lateral controlled cortical impact (LCI) is a model primarily of moderate focal brain injury. Adult male Sprague-Dawley rats (300−400g, n = 31) were preanesthetized using 2% isoflurane, then given oxygen with 0.75% isoflurane through a fixed face mask on a stereotaxic platform. The cranium was exposed and a 6-mm craniectomy made laterally, midway between lambda and bregma, between the central suture and the left temporal ridge. The exposed dura was subjected to a 5 mm diameter piston impact, of 3.0 mm depth, 4 m/s velocity, and 100 ms duration. The incision was closed without replacement of the bone flap. Anesthesia was discontinued and recovery was assessed by return of pinna and corneal reflexes, and righting response. Animals were sacrificed by decapitation at 0.25d (∼6h, n = 6), 1d (n=6), 3d (n=5) and 7d (n=6) postinjury. Sham-injured controls were surgically prepared but not injured (n = 2 at each time point).
Tissue preparation
Conscious animals were decapitated in a sharpened guillotine. For the LFP study, fresh cortex (inferolateral to the craniectomy site) and hippocampus (the entire vital structure) from each hemisphere were rapidly dissected on ice; tissue was immediately sonicated in 6M guanidine thiocyanate, 0.13M EDTA (6M GE) and stored at −80°C. For the LCI study, brains were rapidly dissected, frozen on powdered dry ice and stored at −80°C. Frozen brains were coronally cut into 300 μm sections in a cryostat microtome (IEC) at −8°C. Thick sections were rapidly thaw mounted on slides, refrozen on powdered dry ice, and stored at −80°C. Brain regions of interest were dissected from 300 μm frozen sections (at −10°C) using a 1000 μm micropunch cannula (Palkovits, 1973). Frozen tissue was immediately transferred to and sonicated in 40 μL of 6M GE and stored at −80°C (Strauss and Jacobowitz, 1993). Specimens for the LFP experiments were a mixture of parietal cortex or whole hippocampus, while those for the LCI experiments were microdissected from specific coronal levels anterior to the cortical impact site (Bregma −2.6 to −3.6 mm, according to the coordinates of Paxinos and Watson (Paxinos and Watson, 1986)). Therefore, LFP specimens were uniformly more concentrated (though probably less homogeneous) than the LCI micropunch specimens (e.g., 571 ± 11 μg protein per assay in LFP cortex vs. 230 ± 9 μg protein per assay in LCI cortex).
Molecular probes
The bcl-2 riboprobe was from SalI linearized pGEM-5Zf+ (Promega) containing a bcl-2 PCR fragment (639 bp) generated from rat brain cDNA (using primers at 247−259 (forward) and 885−863 (reverse) with respect to the initiation codon, Genbank accession # L14680). The bax riboprobe was from pGEM-5Zf+ (Promega) containing a bax PCR fragment (370 bp) generated from rat brain cDNA (using primers at 190−212 (forward) and 559−537 (reverse) with respect to the initiation codon, Genbank accession # U49729) and subcloned in the NcoI and SalI site of the plasmid. The bcl-xL riboprobe was from SalI linearized pGEM-5Zf+ (Promega) containing a bcl-x PCR fragment (589 bp) generated from rat brain cDNA (using primers at 21−42 (forward) and 606−628 (reverse) with respect to the initiation codon, GenBank accession# X82537). The sequence spans the regions across which alternate splicing occurs for the short (pro-apoptotic) isoform (Shiraiwa et al., 1996), yielding full length fragments only for bcl-xL. The PCR fragments were synthesized using high fidelity PCR (Boehringer-Mannheim) and rat brain cDNA (Clontech), sequenced and sequences were confirmed using a BLAST search in the GENBANK database. The cyclophilin cDNA (CYC, a “housekeeping” gene, has a constant level of expression under most circumstances) was subcloned into the pGEM-4Z vector with the PstI/NcoI 309 bp fragment of rat cyclophilin cDNA (−8 to 300, (Danielson, 1988)). Clones were also confirmed by restriction enzyme analyses. RNA probes were synthesized from linearized, purified cDNA templates, using T7 RNA polymerase in the presence of 32P-labeled ribonucleoside triphosphates (specific activity ≈ 5 × 105 dpm/ng). Full-length antisense transcripts were purified by acrylamide-urea gel electrophoresis, autoradiography (10s), excision from the gel and elution in 0.5 M ammonium acetate (pH 6.3), 1 mM EDTA, 0.2% sodium dodecylsulfate (SDS).
RNase Protection Assays (RPA)
To measure mRNA levels we used a lysate RNase protection assay, and direct counting of radionuclide activity in the protected fragments. The lysate-RPA was carried out as described (Strauss and Jacobowitz, 1993). Briefly, an excess of 32P-labeled antisense riboprobes was hybridized directly to the target mRNA in the cell or tissue lysates at 37°C overnight. A small portion of each RPA sample was set aside for a protein determination (Bradford microassay, BioRad), and the total sample protein was calculated for normalization purposes. Double-stranded RNA complexes were protected from RNase degradation, purified away from background contaminants by organic extractions, ethanol precipitation, and native polyacrylamide gel electrophoresis. Gel pieces containing full sized protected fragments were excised from the dried gel using the autoradiogram as a guide. Background was determined using specimens hybridized on dry ice, but otherwise treated the same as experimental specimens. Scintillation counting was used to detect radionuclide decay (dpm). Only dpm > 2 standard errors of the mean above background values was considered quantifiable. The signal (dpm - background) was converted to moles (by the probe specific activity) or to grams of mRNA (based on the length of the endogenous mRNA). An internal control probe (cyclophilin) was included with each assay for normalization.
Statistics
The mRNA results presented are mean ± standard error. To conclude that a target mRNA had changed, we required that changes be consistent (extent and direction) by both methods of normalization, and statistically significant by at least one method. Analysis of variance was performed with post hoc Dunnett test for sham comparisons, or paired Student's t-test for left vs. right side comparisons. A p<0.05 was required to reject the null hypothesis that the group means were equivalent (p<0.1 for one-sided t-test).
RESULTS
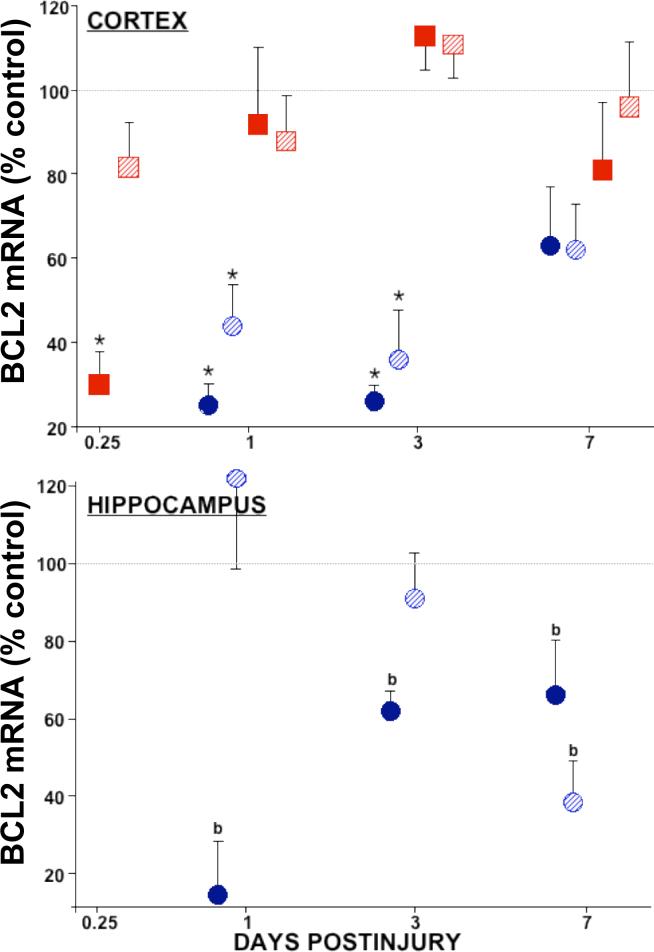
Lysate RNase protection assays were used to measure brain bcl-2, bcl-xL, and bax mRNA levels. Radionuclide decay from the protected target mRNA:riboprobe hybrids yielded absolute amounts of mRNA in the sample. Values reported in Tables I, II, and III were grams of target mRNA normalized to total protein in the sample (fg/μg protein). Relative mRNA levels presented in Figures 1, 2, and 3 were moles of target mRNA normalized to moles of cyclophilin mRNA (mole%) and further normalized to sham control levels. The anti-apoptotic bcl-2 and bcl-xL genes were downregulated in both the LFP and LCI models of traumatic brain injury. Temporal profiles in the injured cortex showed 75−80% depletion of bcl-2 mRNA early on, with the LCI levels returning to normal by 1 day (Fig. 1, top, solid squares), and the LFP bcl-2 levels remaining significantly depressed for at least 3 days postinjury (Fig. 1, top, solid circles). In the contralateral cortex, LFP showed significant decreases in bcl-2 mRNA, while LCI did not (Fig. 1, top, stripes). In the hippocampus, low levels of bcl-2 mRNA were observed in the sham animals, and while detecable, postinjury bcl-2 levels were below the level of quantitation. Using non-parametric analysis, bcl-2 levels were significantly decreased in the injured hippocampus from 1 to 7 days postinjury (data not shown). In the LCI model, bcl-2 was undetectable in micropunches of hippocampus, probably due to the low amount of tissue sampled (53 ± 3 μg protein per assay from the CA1-CA3 regions, vs. 619 ± 25 μg protein per assay in the LFP whole organ homogenates).
Table 1.
Bcl-2 mRNA Levels Decrease After Traumatic Brain Injury
| BRAIN REGION | MODEL | TIME POSTINJURY (days) | ||||
|---|---|---|---|---|---|---|
| SHAMS | 0.25 | 1 | 3 | 7 | ||
| CORTEX | bcl-2 mRNA (fg/μg protein)a | |||||
| IPSILATERAL | LFP | 0.87±0.17 | - | 0.35±0.09* | 0.29±0.10* | 0.50±0.09 |
| LCI | 1.08±0.24 | 0.33±0.08* | 1.00±0.20 | 1.22±0.11 | 0.88±0.17 | |
| CONTRALATERAL | LFP | 0.80±0.14 | - | 0.22±0.04* | 0.22±0.04* | 0.54±0.12 |
| |
LCI£ |
1.41±0.22^ |
1.16±0.17# |
1.12±0.14 |
1.08±0.16 |
0.98±0.18 |
| HIPPOCAMPUS | ||||||
| IPSILATERAL | LFP | 0.36±0.08 | - | 0.05±0.05b | 0.22±0.02b | 0.24±0.07b |
| LCI | -b | - | - | - | - | |
| CONTRALATERAL | LFP£ | 0.54±0.06# | - | 0.67±0.13# | 0.49±0.09# | 0.20±0.07b |
| LCI | -b | - | - | - | - | |
Messenger RNA levels (mean ± SEM) determined by RNase protection assay (see Methods). Sham values from multiple time points showed no differences and were combined for each anatomical area. Lateral fluid percussion (LFP) or lateral cortical impact (LCI) injury was performed as described in Methods.
Below the limit of quantitation, “-” not done (hippocampal bcl-2 mRNA levels in the LCI specimens were below the limit of quantitation).
p<0.05, ANOVA, Dunnett vs. SHAMS.
p<0.05, paired t-test, contralateral vs. ipsilateral
0.05<p<0.10
p<0.05, paired t-test, contralateral vs. ipsilateral over time groups combined.
Table 2.
Bcl-XL mRNA Levels Decrease After Lateral Traumatic Brain Injury
| BRAIN REGION | MODEL | TIME POSTINJURY (days) | ||||
|---|---|---|---|---|---|---|
| SHAMS | 0.25 | 1 | 3 | 7 | ||
| CORTEX | bcl-xL mRNA (fg/μg protein)a | |||||
| IPSILATERAL | LFP | 5.69±0.57 | - | 3.80±0.51* | 5.75±0.72 | 6.90±0.91 |
| LCI | 17.5±1.3 | 12.4±1.6* | 12.6±1.2* | 10.5±1.4* | 12.0±1.3* | |
| CONTRALATERAL | LFP | - | - | - | - | - |
| |
LCI£ |
13.5±1.6 |
11.7±1.2 |
13.2±2.8# |
16.1±1.3# |
13.6±1.5 |
| HIPPOCAMPUS | ||||||
| IPSILATERAL | LFP | 3.31±0.56 | - | 3.48±0.86 | 3.77±0.20 | 4.00±0.61 |
| LCI | 14.4±1.8 | 7.43±1.24* | 18.0±3.2 | 5.79±1.47* | 8.76±1.59 | |
| CONTRALATERAL | LFP | 3.98±0.87 | - | 3.79±0.65 | 4.25±1.04 | 4.11±0.77 |
| LCI | 13.2±1.1 | 7.13±1.14 | 9.50±2.07 | 9.78±2.35^ | 9.32±1.99 | |
Messenger RNA levels (mean ± SEM) determined by RNase protection assay (see Methods). Sham values from multiple time points showed no differences and were combined for each anatomical area. Lateral fluid percussion (LFP) or lateral cortical impact (LCI) injury was performed as described in Methods. “-” not done.
p<0.05, ANOVA, Dunnett vs. SHAMS.
p<0.05, paired t-test, contralateral vs. ipsilateral
0.05<p<0.10
p<0.05, paired t-test, contralateral vs. ipsilateral over time groups combined.
Table 3.
Bax mRNA Levels Increase After Traumatic Brain Injury
| BRAIN REGION | MODEL | TIME POSTINJURY (days) | ||||
|---|---|---|---|---|---|---|
| SHAMS | 0.25 | 1 | 3 | 7 | ||
| CORTEX | bcl-xL mRNA (fg/μg protein)a | |||||
| IPSILATERAL | LFP | 5.27±0.51 | - | 6.67±0.49 | 7.45±0.85* | 7.44±0.46* |
| LCI | 2.04±0.25 | 2.81±0.32§ | 1.92±0.35 | 1.57±0.38 | 1.28±0.17 | |
| CONTRALATERAL | LFP£ | 4.39±0.42 | - | 5.76±0.67# | 5.55±0.14^ | 3.21±0.44# |
| |
LCI£ |
2.91±0.56^ |
2.40±0.38 |
2.35±0.29 |
2.43±0.65^ |
1.71±0.26 |
| HIPPOCAMPUS | ||||||
| IPSILATERAL | LFP | 1.68±0.27 | - | 1.86±0.17 | 2.59±0.30* | 2.40±0.20* |
| LCI | 3.45±0.86 | 2.74±0.27 | 2.76±0.80 | 5.69±0.62* | 7.25±1.35* | |
| CONTRALATERAL | LFP | 1.69±0.20 | - | 1.62±0.25 | 2.18±0.57 | 2.13±0.12 |
| LCI | 5.28±0.71 | 5.65±1.06# | 4.86±0.91 | 4.38±0.95# | 6.18±1.43 | |
Messenger RNA levels (mean ± SEM) determined by RNase protection assay (see Methods). Sham controls from multiple time points showed no differences and were combined for each anatomical area. Lateral fluid percussion (LFP) or lateral cortical impact (LCI) injury was performed as described in Methods. “-” not done.
p<0.05, ANOVA, Dunnett vs. shams
p<0.05, ANOVA, Fisher PLSD vs. shams.
p<0.05, paired t-test, contralateral vs. ipsilateral
0.05<p<0.10
p<0.05, paired t-test, contralateral vs. ipsilateral over time groups combined.
Figure 1. Relative levels of bcl-2 mRNA in cortex and hippocampus after traumatic brain injury.
In the injured cortex (top), bcl-2 mRNA decreased 70−80% at 0.25 d after LCI (solid squares) and from 1 to 3 d after LFP (solid circles) TBI. Contralateral cortex showed no changes (LCI, striped squares), or 50−60% decreases in bcl-2 mRNA from 1 to 3 d postinjury (LFP, striped circles). Hippocampal bcl-2 levels (bottom) were constitutively below (LCI, not shown) or dropped below the limit of quantitation (b) after LFP TBI. Relative mRNA levels (mean ± SEM) are the mole percent of cyclophilin mRNA, normalized to sham controls. Ipsilateral: solids, Contralateral: stripes. *p<0.05 vs. shams, ANOVA, Dunnett.
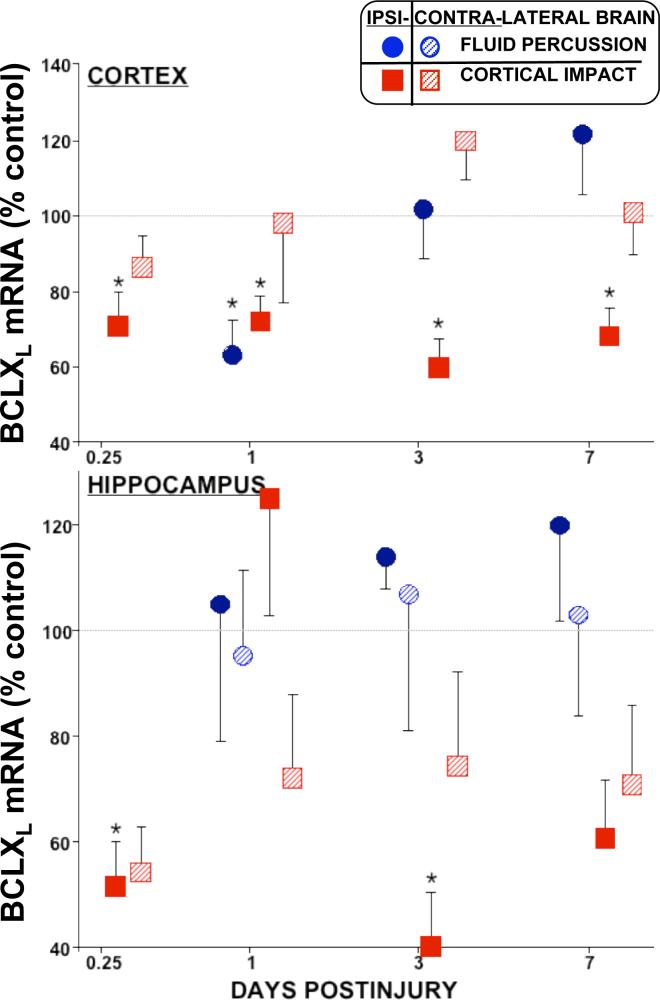
Figure 2. Relative Levels of bcl-xL mRNA in cortex and hippocampus decreased early on in both models of TBI.
In the injured cortex (top), bcl-xL decreased 30−40% in both models. In the hippocampus (bottom), bcl-xL mRNA decreased in a biphasic manner after LCI (solid squares), while high variation after LFP (circles) obscured possible changes. Relative mRNA levels (mean ± SEM) are the mole percent of cyclophilin mRNA, normalized to sham controls. Ipsilateral: solids, Contralateral: stripes. *p<0.05 vs. shams, ANOVA, Dunnett.
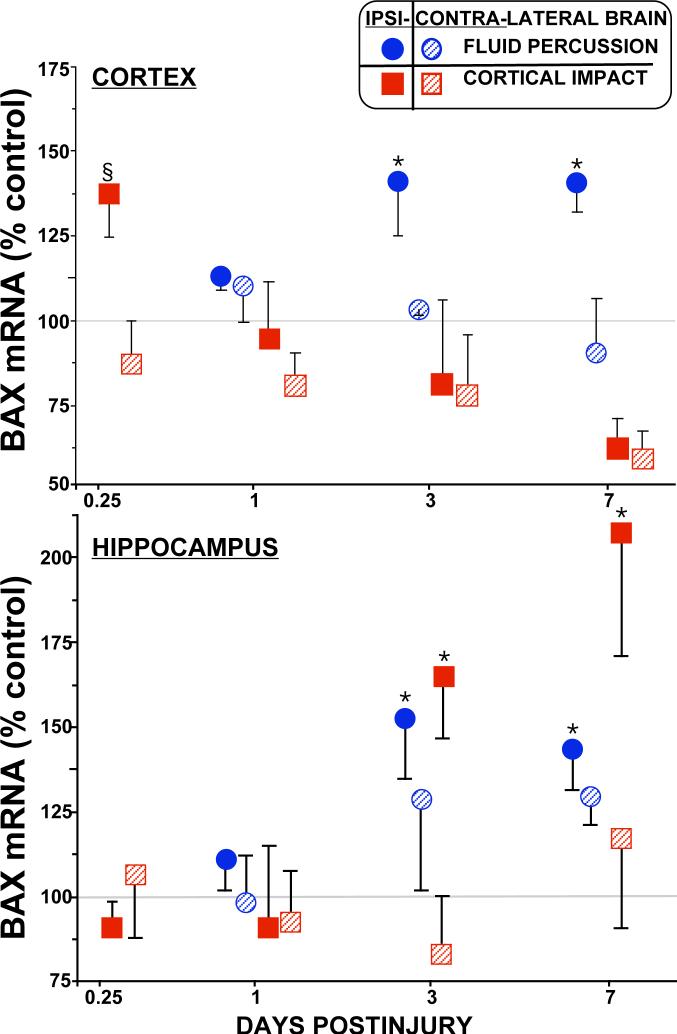
Figure 3. Relative Levels of bax mRNA in cortex and hippocampus after traumatic brain injury.
Levels of bax mRNA are increased in both models of traumatic brain injury. In the injured cortex (top), bax mRNA increased ∼40% by 0.25 d after LCI (solid squares), and from 3 to 7 d after LFP (solid circles). In the hippocampus (bottom), bax levels increased in both TBI models from 3 to 7 d after injury by ∼50% (LFP, solid circles), up to ∼200% (LCI, solid squares). Relative mRNA levels (mean ± SEM) are the mole percent of cyclophilin mRNA, normalized to sham controls. Ipsilateral: solids, Contralateral: stripes. *p<0.05 vs. shams, ANOVA, Dunnett (§p<0.05 by Fisher PLSD).
Levels of bcl-xL mRNA in the injured cortex also decreased (∼30%) early on in both LFP and LCI models of traumatic brain injury (Fig. 2). While the LFP levels returned to normal by 3 days, in the LCI injured cortex bcl-xL mRNA remained depressed for at least 7 days postinjury (Fig. 2, solid squares). Hippocampal bcl-xL mRNA levels decreased 40−60% in the LCI model, but not at all in the LFP model. Measurements of bcl-xL mRNA in the hippocampus showed large errors in most groups in both models, indicating the likelihood of some ongoing dynamic process (rather than a problem with sample collection). This was further supported by the biphasic decreases observed in the LCI model at 0.25 and 3 days, but not 1 day postinjury (Fig. 2). There were no significant differences in contralateral hippocampal bcl-xL mRNA levels. In the LFP model, bcl-xL mRNA levels in the ipsilateral diencephalon decreased (∼50%, p<0.05, Dunnett) at 1d postinjury; there were no changes at other times or contralateral to injury (data not shown). Apparent decreases contralateral to injury (Table 2) were not statistically significant by either means of normalization.
Pro-apoptotic bax mRNA levels increased in both traumatic brain injury models (Fig. 3). Injured cortex showed ∼40% increases in bax mRNA at 0.25 days in the LCI model, and at 3 and 7 days in the LFP model. In the injured hippocampus, bax mRNA increased by 50−100% at 3 and 7 days postinjury in both models (Fig. 2, bottom). In the LFP model, bax mRNA levels decreased in the ipsilateral diencephalon (∼60%, p<0.05, Dunnett) only at 1d postinjury (data not shown). No other changes were observed contralaterally, or in ventral cortex (distant from the injury site), in either model.
Despite differences in injury phenotype (LFP is somewhat diffuse vs. LCI which is more focal) and sample collection (fresh macrodissection vs. frozen microdissection) between the models, common patterns of bcl-2 family gene expression were observed following traumatic brain injury (Table 4). All of these comparisons were between injured brain areas at various postinjury time points and the corresponding sham brain area (combined for all time points). There were also many cases of differences in gene expression between ipsilateral and contralateral regions (Tables 1, 2 and 3). These differences did not always correspond to injury-related changes. For example, in LCI animals bcl-2, bcl-xL and bax mRNA levels were all significantly higher in the contralateral cortex . In LFP animals, levels of bcl-2 and bax mRNAs were higher in the contralateral cortex and hippocampus, respectively. Left-right differences in gene expression in certain sham groups were most likely the effect of surgical manipulations, however, it is possible these findings indicate lateralization (no naïve animals were examined in these studies to test this). Nevertheless, when normalized to the proper control group (i.e., comparable brain area from the sham group, Figs. 1-3), the temporal and neuroanatomical changes in gene expression described above were independent of these left-right differences.
Table 4.
Patterns of Bcl-2 gene expression in LFP and LCI models of traumatic brain injurya
DISCUSSION
The present study quantifies the common patterns of Bcl-2, Bcl-xL and Bax gene expression in two rat models of traumatic brain injury. An early decrease (6−24h) in bcl-2 and bcl-xL mRNA levels is followed by a delayed phase (3−7d) increase in bax mRNA levels. This bipartite/biphasic pattern (decline in anti-apoptotic activity followed by augmented proapoptotic activity) was seen in ipsilateral cortical and subcortical (hippocampus) structures proximal to the injury site, but not in distal areas (ventral cortex or contralateral parietal cortex, contralateral hippocampus, diencephalon, brain stem, cerebellum).
In the cortex, bcl-2 and bcl-xL mRNA levels decreased early, with one or both of these complementary genes diminished for at least 3 days postinjury (and out through 7 days in the LCI model). In the hippocampus, bcl-2 and bcl-xL mRNA levels also decreased early on. In fact, hippocampal bcl-2 levels were undetectable in the LCI model, and became unmeasurable following injury in the LFP model. Large variances were evident in hippocampal bcl-xL mRNA levels in both models over the observation period. In the LCI model, bcl-xL mRNA levels appeared to decrease 50−60% at 6 hours and 3 days, but not at 1 day postinjury.
The level of bax mRNA expression was consistently elevated by 40−100% at 3 and 7 days postinjury in the cortex (LFP only) and hippocampus (both models). Early elevation of bax mRNA was observed in the cortex (LCI) at 6 hours postinjury. Thus, Bax showed more acute changes in LCI cortex, with more extended changes in LFP, conistent with the temporal course of cavitation in LCI vs LFP. Bax expression in the hippocampus was similar, consistent with the temporal course of delayed cell death in this region.
Clearly, in both models the pro-apoptotic bax gene was up-regulated in hippocampus, a region distal to the injury site. However, variation in mRNA levels was greater for the LCI experiments. This was most likely due to the method of specimen collection. Anatomical variability between rats lead to subtle differences in injury localization, changes in gene expression and subsequent cellular responses. Despite these variations, apoptosis-related bcl-2 family gene expression measured in these animals showed fairly consistent patterns that reflect moderate injury to the somatosensory cortex and underlying neocortex.
Differences in absolute mRNA levels between the models might also be attributable to the method of collection. Homogenization of gross brain regions (LFP specimens) would tend to dilute changes in the target mRNA among less affected tissue, while the micropunched regions (LCI samples) were chosen using the in situ hybridization histochemistry analyses (Raghupathi et al., 2003) as a guide. In the case of bcl-2 in the hippocampus, the low level of expression favored detection in the LFP samples which were more concentrated (tissue per unit volume). Decreased mRNA levels in either model could reflect cell loss, however cyclophilin and protein levels, also measured in these studies did not change significantly with time or injury.
The Bcl-2 gene family members participate in apoptosis, either tipping the balance toward cell survival (bcl-2, bcl-xL, etc.) or toward programmed cell death (bax, bcl-xS, etc.). After experimental brain injuries, delayed apoptotic cell death has been observed in the cortex, hippocampus and other brain areas. Neuronal apoptosis predominates in the cortex and hippocampus, with astrocytic and monocytic apoptosis being scarce or absent (Chen et al., 1997; Conti et al., 1998; Wennersten et al., 2003). In the present study, the regional and temporal changes in Bcl-2 family gene expression suggest an association with delayed cell death following lateral fluid-percussion (LFP) and lateral cortical impact (LCI) traumatic brain injury. The results are consistent with the “apostat” mechanism of cell death, which suggests that a shift in the cellular ratio of cell death activator (Bax, Bad, Bcl-xs, c-Jun N-terminal kinase, etc.) and suppressor (Bcl-2, Bcl-xl, extracellular signal regulated kinase, etc.) proteins regulates the fate of a cell (Oltvai et al., 1993; Xia et al., 1995). Thus, cell (neuronal) survival may be compromised due to decreased expression of Bcl-2, and a concomitant increase in Bax levels.
Delayed cell death following TBI can occur due to the activation of multiple pathways which include the differential expression of the Bcl-2 family of genes. Overexpression of bcl-2 in vivo leads to neuroprotection following axotomy (de Bilbao and Dubois-Dauphin, 1996); and mice overexpressing human bcl-2 in neurons have a markedly reduced cortical contusion following experimental brain injury (Raghupathi et al., 1998; Nakamura et al., 1999). It is tempting to speculate that the acute disappearance of bcl-2 and the overexpression of bax in the injured brain exacerbates neuronal vulnerability to trauma (Raghupathi et al., 2003).
Alternatively, the parallel findings in different brain injury models of time- and brain region-specific decreases in apoptosis suppressor genes, with subsequent increases in apoptosis promoter genes support the existence of a conserved pattern of apoptosis-related gene expression. The evolution of this biphasic pattern may represent a culling process to remove irreparably damaged cells from the injured brain. An initial phase of reduced anti-apoptotic activity may allow cell death promoters to kill damaged cells. Subsequently, cells that survive but are not fully functional (for example, cells that cannot repair DNA or produce adequate survival factors) may be eliminated by the wave of increased Bax expression. Therapeutic strategies to modulate the expression of these genes may confer resistance to trauma-induced delayed cell death, but careful timing and targeting of these treatments will be necessary to attenuate functional deficits after TBI.
Supplementary Material
REFERENCES
- Adams JH, Doyle D, Graham DI, Lawrence AE, McLellan DR, Gennarelli TA, Pastuszko M, Sakamoto T. The contusion index: a reappraisal in human and experimental non-missile head injury. Neuropathology & Applied Neurobiology. 1985;11:299–308. doi: 10.1111/j.1365-2990.1985.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiology of Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beschorner R, Adjodah D, Schwab JM, Mittelbronn M, Pedal I, Mattern R, Schluesener HJ, Meyermann R. Long-term expression of heme oxygenase-1 (HO-1, HSP-32) following focal cerebral infarctions and traumatic brain injury in humans. Acta Neuropathologica. 2000;100:377–384. doi: 10.1007/s004010000202. [DOI] [PubMed] [Google Scholar]
- Bredesen DE. Neural Apoptosis. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- Chen J, Graham SH, Chan PH, Lan P, Zhou RL, Simon RP. Bcl-2 is expressed in neurons that survive focal ischemia in the rat. NeuroReport. 1995;6:394–398. doi: 10.1097/00001756-199501000-00040. [DOI] [PubMed] [Google Scholar]
- Chen J, Graham SH, Nakayama M, Zhu RL, Jin K, Stetler RA, Simon RP. Apoptosis repressor genes Bcl-2 and Bcl-x-long are expressed in the rat brain following global ischemia. Journal of Cerebral Blood Flow & Metabolism. 1997;17:2–10. doi: 10.1097/00004647-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhu RL, Nakayama M, Kawaguchi K, Jin K, Stetler RA, Simon RP, Graham SH. Expression of the apoptosis-effector gene, Bax, is up-regulated in vulnerable hippocampal CA1 neurons following global ischemia. Journal of Neurochemistry. 1996;67:64–71. doi: 10.1046/j.1471-4159.1996.67010064.x. [DOI] [PubMed] [Google Scholar]
- Clark RS, Chen J, Watkins SC, Kochanek PM, Chen M, Stetler RA, Loeffert JE, Graham SH. Apoptosis-suppressor gene bcl-2 expression after traumatic brain injury in rats. Journal of Neuroscience. 1997;17:9172–9182. doi: 10.1523/JNEUROSCI.17-23-09172.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicos MA, Dash PK. Apoptotic morphology of dentate gyrus granule cells following experimental cortical impact injury in rats: possible role in spatial memory deficits. Brain Res. 1996a;739:120–131. doi: 10.1016/s0006-8993(96)00824-4. [DOI] [PubMed] [Google Scholar]
- Colicos MA, Dixon CE, Dash PK. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 1996b;739:111–119. doi: 10.1016/s0006-8993(96)00819-0. [DOI] [PubMed] [Google Scholar]
- Conti AC, Raghupathi R, Trojanowski JQ, McIntosh TK. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. Journal of Neuroscience. 1998;18:5663–5672. doi: 10.1523/JNEUROSCI.18-15-05663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson P, Forss-Petter S, Brow MA, Calavetta L, Douglass J, Milner RH, Sutcliffe JG. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA & Cell Biology. 1988;7:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- de Bilbao F, Dubois-Dauphin M. Time course of axotomy-induced apoptotic cell death in facial motoneurons of neonatal wild type and bcl-2 transgenic mice. Neuroscience. 1996;71:1111–1119. doi: 10.1016/0306-4522(95)00505-6. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Jr, Snider WD, Korsmeyer SJ. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Halley M. Early Microvascular and Neuronal Consequences of Traumatic Brain Injury: A Light and Electron Microscopic Study in Rats. J Neurotrauma. 1994;11:289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- Dixon C, Clifton G, Lighthall J, Yaghmai A, Hayes R. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Fox GB, Fan L, Levasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- Gillardon F, Klimaschewski L, Wickert H, Krajewski S, Reed JC, Zimmerman M. Expression pattern of candidate cell death effector proteins Bax, Bcl-2, Bcl-x and c-Jun in sensory and motor neurons following sciatic nerve transection in the rat. Brain Res. 1996a;739:244–250. doi: 10.1016/s0006-8993(96)00829-3. [DOI] [PubMed] [Google Scholar]
- Gillardon F, Lenz C, Waschke KF, Krajewski S, Reed JC, Zimmermann M, Kuschinsky W. Altered Expression of Bcl-2, Bcl-X, Bax, and c-Fos colocalizes with DNA fragmentation and ischemic cell damage following middle cerebral artery occlusion in rats. Mol Brain Res. 1996b;40:254–260. doi: 10.1016/0169-328x(96)00059-9. [DOI] [PubMed] [Google Scholar]
- Gillardon F, Wickert H, Zimmermann M. Up-regulation of bax and down-regulation of bcl-2 is associated with kainate-induced apoptosis in mouse brain. Neurosci Lett. 1995;192:85–88. doi: 10.1016/0304-3940(95)11619-8. [DOI] [PubMed] [Google Scholar]
- Graham DI, Horsburgh K, Nicoll JA, Teasdale GM. Apolipoprotein E and the response of the brain to injury. Acta Neurochirurgica - Supplementum. 1999;73:89–92. doi: 10.1007/978-3-7091-6391-7_15. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Hassouna I, Wickert H, Zimmerman M, Gillardon F. Increase in bax expression in substantia nigra following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment of mice. Neurosci Lett. 1996;204:85–88. doi: 10.1016/0304-3940(96)12323-5. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Soares HD, Smith DH, Mcintosh TK. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 1996;91:236–246. doi: 10.1007/s004010050421. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J, Massa SM, Breckinridge M, Sharp FR. Global ischemia induces apoptosis-associated genes in hippocampus. Mol Brain Res. 1996;42:79–88. doi: 10.1016/s0169-328x(96)00121-0. [DOI] [PubMed] [Google Scholar]
- Irving EA, Nicoll J, Graham DI, Dewar D. Increased tau immunoreactivity in oligodendrocytes following human stroke and head injury. Neuroscience Letters. 1996;213:189–192. doi: 10.1016/0304-3940(96)12856-1. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Paulus W, Wrocklage C, Litvan I. Effects of closed traumatic brain injury and genetic factors on the development of Alzheimer's disease. European Journal of Neurology. 2001;8:707–710. doi: 10.1046/j.1468-1331.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Kay AD, Petzold A, Kerr M, Keir G, Thompson E, Nicoll JA. Alterations in cerebrospinal fluid apolipoprotein E and amyloid beta-protein after traumatic brain injury. Journal of Neurotrauma. 2003;20:943–952. doi: 10.1089/089771503770195795. [DOI] [PubMed] [Google Scholar]
- Kaya SS, Mahmood A, Li Y, Yavuz E, Goksel M, Chopp M. Apoptosis and expression of p53 response proteins and cyclin D1 after cortical impact in rat brain. Brain Research. 1999;818:23–33. doi: 10.1016/s0006-8993(98)01204-9. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ. Regulators of cell death. Trends Genet. 1995;11:101–105. doi: 10.1016/S0168-9525(00)89010-1. [DOI] [PubMed] [Google Scholar]
- Kotapka MJ, Graham DI, Adams JH, Gennarelli TA. Hippocampal pathology in fatal non-missile human head injury. Acta Neuropathol. 1992;83:530–534. doi: 10.1007/BF00310031. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Mai JK, Krajewska M, Sikorska M, Mossakowski MJ, Reed JC. Upregulation of Bax Protein Levels in Neurons Following Cerebral Ischemia. J Neurosci. 1995;15:6364–6376. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Gao HM, Hong JS. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environmental Health Perspectives. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye TC, Shores EA. Traumatic brain injury as a risk factor for Alzheimer's disease: a review. Neuropsychology Review. 2000;10:115–129. doi: 10.1023/a:1009068804787. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Juhler M, Wieloch T. Novel pharmacological strategies in the treatment of experimental brain injury. J Neurotrauma. 1998;15:731–769. doi: 10.1089/neu.1998.15.731. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Raghupathi R, Merry DE, Scherbel U, Saatman KE, McIntosh TK. Overexpression of Bcl-2 is neuroprotective after experimental brain injury in transgenic mice. Journal of Comparative Neurology. 1999;412:681–692. doi: 10.1002/(sici)1096-9861(19991004)412:4<681::aid-cne9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, Kurland LT. Traumatic brain injury and time to onset of Alzheimer's disease: a population-based study. American Journal of Epidemiology. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- Newcomb JK, Zhao X, Pike BR, Hayes RL. Temporal profile of apoptotic-like changes in neurons and astrocytes following controlled cortical impact injury in rats. Exp Neurol. 1999;158:76–88. doi: 10.1006/exnr.1999.7071. [DOI] [PubMed] [Google Scholar]
- Oltvai Z, Milliman C, Korsmeyer S. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Research. 1973;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Pres; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- Pentland B, Jones PA, Roy CW, Miller JD. Head injury in the elderly. Age & Ageing. 1986;15:193–202. doi: 10.1093/ageing/15.4.193. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Fernandez SC, Murai H, Trusko SP, Scott RW, Nishioka WK, McIntosh TK. BCL-2 overexpression attenuates cortical cell loss after traumatic brain injury in transgenic mice. Journal of Cerebral Blood Flow & Metabolism. 1998;18:1259–1269. doi: 10.1097/00004647-199811000-00013. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, McIntosh TK, Smith DH. Cellular Responses to Brain Injury. Brain Pathol. 1995;5:437–442. doi: 10.1111/j.1750-3639.1995.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Zhang C, Strauss KI, Krajewski S, Reed JC, McIntosh TK. Temporal alterations in cellular Bax:Bcl-2 ratio following traumatic brain injury in the rat. J Neurotrauma. 2003;20:421–435. doi: 10.1089/089771503765355504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- Rink AD, Fung KM, Trojanowski JQ, Lee VM-Y, Neugebauer E, McIntosh TK. Evidence of Apoptotic Cell Death After Experimental Traumatic Brain Injury in the Rat. Amer J Pathol. 1995;147:1575–1583. [PMC free article] [PubMed] [Google Scholar]
- Ross DT, Graham DI, Adams JH. Selective loss of neurons from the thalamic reticular nucleus following severe human head injury. J Neurotrauma. 1993;10:151–165. doi: 10.1089/neu.1993.10.151. [DOI] [PubMed] [Google Scholar]
- Shiozaki T, Akai H, Taneda M, Hayakata T, Aoki M, Oda J, Tanaka H, Hiraide A, Shimazu T, Sugimoto H. Delayed hemispheric neuronal loss in severely head-injured patients. Journal of Neurotrauma. 2001;18:665–674. doi: 10.1089/089771501750357618. [DOI] [PubMed] [Google Scholar]
- Shiraiwa N, Inohara N, Okada S, Yuzaki M, Shoji S, Ohta S. An additional form of rat Bcl-x, Bcl-xbeta, generated by an unspliced RNA, promotes apoptosis in promyeloid cells. J Biol Chem. 1996;271:13258–13265. doi: 10.1074/jbc.271.22.13258. [DOI] [PubMed] [Google Scholar]
- Strauss KI, Jacobowitz DM. Quantitative measurement of calretinin and β-actin mRNA in rat brain micropunches without prior isolation of RNA. Molecular Brain Res. 1993;20:229–239. doi: 10.1016/0169-328x(93)90045-q. [DOI] [PubMed] [Google Scholar]
- Sutton RL, Lescaudron L, Stein DG. Unilateral cortical contusion injury in the rat: Vascular disruption and temporal development of cortical necrosis. J Neurotrauma. 1993;10:135–149. doi: 10.1089/neu.1993.10.135. [DOI] [PubMed] [Google Scholar]
- Wennersten A, Holmin S, Mathiesen T. Characterization of Bax and Bcl-2 in apoptosis after experimental traumatic brain injury in the rat. Acta Neuropathologica. 2003;105:281–288. doi: 10.1007/s00401-002-0649-y. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Faden AI. Molecular strategies in CNS injury. J Neurotrauma. 1995;12:767–777. doi: 10.1089/neu.1995.12.767. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Knoblach SM, Fan L, Fox GB, Goodnight R, Faden AI. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurlogical dysfunction after traumatic brain injury. J Neurosci. 1997;17:7415–7424. doi: 10.1523/JNEUROSCI.17-19-07415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.