Summary
One of the mechanisms proposed for antidepressant drugs is the enhancement of synaptic connections and plasticity in the hippocampus and cerebral cortex. Fibroblast growth factor 2 (FGF2), is a growth factor essential for the proper formation of synaptic connections in the cerebral cortex, maturation and survival of catecholamine neurons, and neurogenesis. In this report, we attempted to establish a correlation between antidepressant treatments and FGF2 expression in the cerebral cortex and hippocampus, two brain areas relevant for depression. Desipramine (DMI, 10 mg/kg) or fluoxetine (FLU, 5 mg/kg) were injected acutely (single injection) or chronically (daily injection for two weeks) in adult rats. Chronic, but not acute, antidepressant treatments increase FGF2 immunoreactivity in neurons of the cerebral cortex and in both astrocytes and neurons of the hippocampus. FGF2 immunoreactivity in the cortex was increased mainly in the cytoplasm of neurons of layer V. Western blot analyses of nuclear and cytosolic extracts from the cortex revealed that both antidepressants increase FGF2 isoforms in the cytosolic extracts and decrease accumulation of FGF2 immunoreactivity in the nucleus. To characterize the anatomical and cellular specificity of antidepressants, we examined FGF-binding protein (FBP), a secreted protein that acts as an extracellular chaperone for FGF2 and enhances its activity. DMI and FLU increased FBP immunoreactivity in both cortical and hippocampal neurons. Our data suggest that FGF2 and FBP may participate in the plastic responses underlying the clinical efficacy of antidepressants.
Keywords: Cerebral cortex, desipramine (DMI), fluoxetine, fibroblast growth factor 2 (FGF2), FGF-binding protein, hippocampus
Introduction
Several lines of independent investigations have implicated loss of neurons and their connections as well as alteration of glia function in the pathophysiology of depression. In particular, the hippocampus and cerebral cortex undergo loss of volume in the postmortem brains of patients with major depression (Gurvits et al. 1996; Nolan et al. 2002; Ongur et al. 1998; Sheline et al. 2003; Sheline et al. 1996). In addition, stress, a risk factor for depression in humans, evokes in animals dendritic shrinkage and cell loss within the hippocampus and cerebral cortex (Czeh et al. 2001; Gould and Tanapat 1999; McEwen 2007). Thus, it is not surprising that the ability of antidepressants to induce neurogenesis in adult hippocampus of rodents (Malberg et al. 2000; Santarelli et al. 2003) and non-human primates (Perera et al. 2007) as well as increase cortical synaptic strength in vitro (Bal-Klara and Bird 1990) has been suggested to be crucial for their therapeutic properties.
Antidepressants may reverse alterations in synaptic density that may occur in depressive disorders. However, the main mechanism of antidepressants that fully explain increased neurogenesis and synaptic strength remains controversial. Neuronal degeneration and synaptic atrophy can be prevented or limited by neurotrophic factors. An early report has shown that antidepressant treatments increase the expression of brain-derived neurotrophic factor (BDNF) in the cerebral cortex and hippocampus (Nibuya et al. 1995). BDNF is a neurotrophin crucial for neuronal plasticity. Indeed, increased BDNF expression associates with neurogenesis in the hippocampus (Ziv et al. 2006), while reduced BDNF expression in the cerebral cortex leads to a decrease in serotonergic functional transmission as well as anxiety behavior (Lyons et al. 1999). On the basis of these observations, it has been proposed that BDNF may explain the ability of antidepressants to induce hippocampal neurogenesis or other forms of synaptic plasticity. BDNF, however, may not be the only trophic factor relevant for depression. In this report, we have chosen to investigate another neurotrophic factor, basic fibroblast growth factor (FGF2). FGF2 was selected for a number of reasons, chief among them the fact that FGF2 expression decreases in the hippocampus and frontal cortex of depressed patients (Evans et al. 2004; Gaughran et al. 2006). Conversely, in rats, FGF2 expression is up-regulated in selected rat brain areas by antidepressants with different pharmacologic profiles (Mallei et al. 2002; Maragnoli et al. 2004), and by electroconvulsive shock (Follesa et al. 1994). Lastly, FGF2 has been shown to have trophic properties that are relevant for the proposed theory of antidepressants. These include the survival of catecholaminergic neurons that are impaired in major depressive disorder (Grothe and Timmer 2007; Sieber-Blum and Ren 2000), the regulation of proper formation of synaptic connections in the cerebral cortex (Korada et al. 2002), and the stimulation of neurogenesis in the hippocampus and the promotion of migration of newborn cells into the cerebral cortex (Ganat et al. 2002; Jin et al. 2005).
Aberrant expression of FGF2 and translocation into the nucleus may promote cell proliferation (Baguma-Nibasheka et al. 2007; Fukui et al. 2003). The scope of this study was to gain insights into the cellular localization of FGF2 after antidepressant treatments. In addition, it has been shown that the biological activity of FGF2 requires a chaperone protein or FGF binding protein 1 (FBP) (Tassi et al. 2007). Thus, we investigated whether antidepressant treatments increase FGF2 and FBP expression in the cerebral cortex and hippocampus, two brain regions that are affected in mood disorders. Defining the cellular localization of FGF2 after antidepressant treatments is essential to understanding whether FGF2 can be added to the list of potential therapies for depressive disorders.
Methods
Animals
All animal procedures were performed in strict accordance with the Laboratory Animal Welfare Act, with National Institutes of Health Guide for the Care and Use of Laboratory Animals, and after approval from the Georgetown University Animal Care and Use Committee. Adult male Sprague-Dawley rats (180-250 g; Taconic, Germantown, NY) were housed three per cage in a temperature-controlled environment with a 12 hr light/dark cycle and access to food and water ad libitum. Rats received saline, desipramine (DMI, 10 mg/kg, i.p.), or fluoxetine (FLU, 5 mg/kg, i.p.) acutely (single injection) or chronically (a daily injection for 15 days). DMI was from Sigma, St Louis, MO, FLU from Eli-Lilly, Indianapolis, IN. Rats were sacrificed either by decapitation for biochemical analyses or by transcardiac perfusion for immunohistochemistry.
Immunohistochemistry
Rats (total n=6 for each group) were anesthetized with ketamine/xylazine (80/10 mg/kg respectively, i.p.) and then sacrificed by intracardiac perfusion with 4% paraformaldehyde in 0.1M PBS, pH 7.4. The brains were removed and post-fixed in the same fixative for 2 hr, then transferred into a buffered graded sucrose (10, 20, and 30%). For FGF2 and neurons, brain sections (16 μm) were incubated overnight at 4°C with an FGF-specific antibody [1:200; Type II antibodies, (Upstate Biotechnology Inc., Lake Placid, NY)] and a cocktail containing three neurofilament antibodies [anti-neurofilament heavy, medium and light chain (1:300; Chemicon)], which stain neurons. Sections were then incubated with Alexa-Fluor 488 (1:2000; Invitrogen, Carlsbad, CA) and Alexa-Fluor 594 secondary antibodies (1:2000; Invitrogen) to visualize FGF and neurofilament, respectively. For FGF2 and astrocytes, sections were incubated overnight with an antibody against FGF2 and an antibody against glial fibrillary acidic protein (GFAP), followed by incubation with Alexa-Fluor 488 and Alexa-Fluor 594 secondary antibody to visualize FGF2 and astrocytes, respectively. FBP immunohistochemistry was carried out essentially as described (Tassi et al. 2006). In brief, antigen retrieval was done by immersing sections into 1X citrate buffer (pH 6.0; Zymed Laboratories, South San Francisco, CA) for 25 min at 95°C and then let cool for 40 min. Sections were then washed and incubated overnight with an antibody against FBP generated as previously described (Tassi et al., 2006) along with the 3 neurofilament antibodies described above. Sections were then incubated with Alexa-Fluor 488 and Alexa-Fluor 594 secondary antibodies to visualize FBP and neurons, respectively. All sections were then mounted using Vectashield mounting medium (Vector Laboratories, Burlingame, CA) with 4′,6′-diamidino-2-phenylindole as counterstaining.
Histological analysis
Sections were analyzed with a Zeiss fluorescence microscope Axioplan2 (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Positive cells were counted using a 20X objective and MetaMorph® Imaging software (Universal Imaging Corporation™, Downingtown, PA) as previously described (Bachis et al. 2006; Nosheny et al. 2004). The number of FGF2- FBP- and/or neurofilament-positive cells in the frontal cortex or hippocampus was assessed in an area of 4000 μm2 in each section. A total of 20 sections per animal (one every 100 μm) was used for counting. Cells were counted only based on staining, not shape or size.
Detection of FGF2 by Western blot analysis in nuclear and cytosolic fractions
Rats (n=6 each group) were sacrificed and their brains quickly dissected on ice. Nuclear and cytosolic fractions from the cerebral cortex were prepared by detergent lysis method as described (Colangelo et al. 1998). Briefly, tissue was homogenized in lysis buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.6, 15 mM KCI, 0.1 mM EDTA, 1 mM DTT, 0.1% (v/v) Nonidet-P40, 0.5 mM phenylmethylsulphonyl fluoride, 0.1 mg/ml leupeptin, 5 mg/ml antipain, 5 mg/ml aprotinin] and incubated on ice for 10 min. Nuclei were pelleted by centrifugation at 1600 × g for 10 min at 4°C. The supernatant contains the cytosolic fraction. Nuclear proteins were then extracted in high salt buffer (420 mM NaCl, 25 mM HEPES pH 7.6, 25% glycerol, 0.1 mM EDTA, 1 mM DTT, 0.5 mM phenylmethylsulphonyl fluoride, 0. 1 mg/ml leupeptin, 5 mg/ml antipain, 5 mg/ml aprotinin) by shaking at 4°C for 20 min. Nuclear debris was removed by centrifugation at 14,000 × g for 5 min and the supernatant stored at -70°C.
Detection of FGF2 isoforms was carried out by Western blot analysis as described previously (Mallei et al. 2002; Mocchetti et al. 1996b). In brief, a fraction (1/3) of the supernatants prepared as above were added to a tube containing 50 μl of heparin-Sepharose CL-6B (Amersham Pharmacia Biotech, Piscataway, NJ) slurry (100 mg swollen in 1 ml 10 mM Tris, 1 mM EDTA containing 0.6 M NaCl) and rocked overnight at 4°C. The heparin-Sepharose was centrifuged at 13,000 × g for 5 min, and the pellet washed 3 times with 0.6 M NaCl, 10 mM Tris HCl, pH 7.4. The final pellet was boiled in loading buffer (2% SDS, 100 mM DTT, 10% glycerol, 0.25% bromophenol blue) and separated in a 15% SDS-polyacrylamide gel. Proteins were electrophoretically transferred onto nitrocellulose filter. Immunostaining of blotted proteins was carried out using a rabbit FGF2 polyclonal antibody (Chemicon International Inc., Temecula, CA). Blots were analyzed using ECL™ system (Amersham Pharmacia Biotech).
Semi-quantitative analysis of FGF2 isoforms
Relative abundance of FGF2 isoforms was estimated based on the intensity of each FGF2 positive (immunoreactive) bands using Quantity One 1-D Analysis Software (BioRad, Lab., Inc. Hercules, CA). Data are expressed as arbitrary units which consider the intensity of the immunoreactive bands, the amount of proteins loaded into the gel and the exposure time of the film.
Statistical Analysis
Biochemical and histology data were compared by Kruskal-Wallis one way ANOVA, or ANOVA and Dunnett's or Holm-Sidak tests (SigmaStat(R) software, Systat Software, Inc, Point Richard, CA).
Results
DMI and FLU increase the levels of FGF2 in cortical neurons
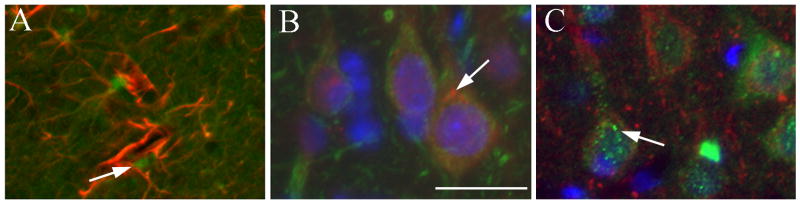
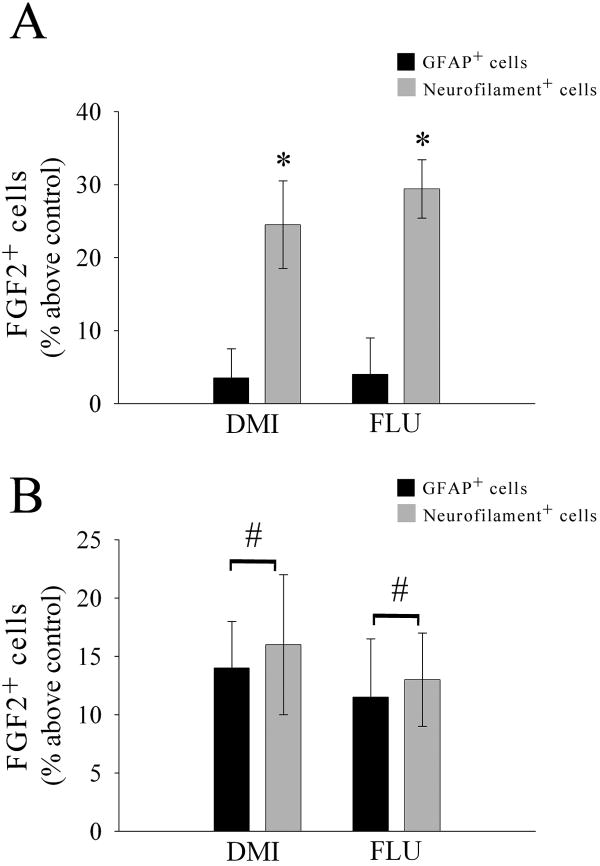
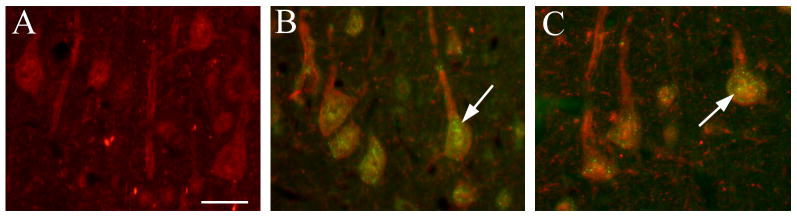
In previous studies we have shown that DMI or FLU causes an increase in FGF2 expression in various portions of the cerebral cortex and hippocampus but not in other brain areas (Mallei et al. 2002). Because cell distribution of FGF2 can dictate its biological action (Leadbeater et al. 2006), it is crucial to define the cellular localization of FGF2 after antidepressant treatments. Rats received an injection of saline, DMI (10 mg/kg, i.p.) or FLU (5 mg/kg, i.p.), for 15 days and were sacrificed 24 hr after the last injection. Sections (16 μm) were prepared throughout the frontal cortex and hippocampus and stained for FGF2 and neurofilament or FGF2 and GFAP. In the cortex of saline-treated animals FGF2 immunoreactivity was confined mostly to nuclei of GFAP-positive cells (Fig. 1A), supporting previous data that in the cerebral cortex FGF2 is typically synthesized in astrocytes (Gomez-Pinilla et al. 1992; Hayes et al. 1995). In contrast, in chronically DMI (Fig. 1B) or FLU-treated animals (Fig. 1C), FGF2 immunoreactivity was also evident in neurofilament positive cells, and its distribution within neurons was perinuclear (see arrows in Figs. 1B and C) as well as nuclear. Semi-quantitative analysis of FGF2 immunoreactivity revealed that chronic DMI and FLU increase the number of FGF2 positive neurons but not FGF2 positive astrocytes in the frontal cortex [ANOVA and Dunnett's test, degrees of freedom (DF) = 3; p<0.01; Fig. 2A]. Thus, it appears that prolonged antidepressant treatments induce accumulation of FGF2 in cortical neurons.
Figure 1. Chronic antidepressant treatments increase FGF2 immunoreactivity in cortical neurons.
Photomicrographs are showing FGF2 immunoreactivity in coronal sections of the cerebral cortex from rats treated chronically with saline (A), DMI (B) or FLU (C). In A, red=GFAP, green=FGF2. In B, red=FGF2, green=neurofilaments. In C, red=neurofilaments, green=FGF2. Blue=DAPI. Note that in saline-treated rats FGF2 immunoreactivity is mainly in nuclei of GFAP positive cells (arrow). In DMI and FLU-treated rats, FGF2 immunoreactivity is seen perinuclearly in neurofilament positive cells (arrows). Scale bar = 50 μm.
Figure 2. Semi-quantitative analysis of FGF2 immunoreactivity.
Serial sections from the frontal cortex or hippocampus of saline and antidepressant treated-rats were prepared as described in Fig. 1. The number of FGF2/GFAP/neurofilament-positive cells was assessed in an area of 4000 μm2 (in each section) of the frontal cortex (A) or hippocampus (B) using MetaMorph® as described in Methods. Data are the mean ± SEM of six animals per group (20 sections per animal). #p<0.05, *p<0.01 vs control.
To examine the specificity of antidepressant's effect on neuronal FGF2, we analyzed FGF2 immunoreactivity in the hippocampus. The hippocampus is another FGF2-expressing area targeted by antidepressants (Mallei et al. 2002). However, unlike the cortex, both astrocytes and neurons of the CA2 region express FGF2 (Bland et al. 2007; Gomez-Pinilla et al. 1992; Woodward et al. 1992). Chronic DMI and FLU increased FGF2 immunoreactivity in astrocytes as well as in the CA2 region (ANOVA and Dunnett's test, DF = 2; p<0.05; Fig. 2B). These data suggest that antidepressant-mediated accumulation of FGF2 into neurons occurs only in the cerebral cortex.
To establish whether the effect of antidepressants on FGF2 accumulation requires a prolonged treatment, rats received saline, DMI or FLU acutely. Animals were then sacrificed 24 hr later. We did not observe a difference in FGF2 immunoreactivity between saline, DMI or FLU treatments in both the cerebral cortex (Kruskal-Wallis One Way ANOVA, H=0.801 with 2 DF, p=0.670 for Neurofilament+ cells; H=0.390 with 2 DF, p=0.823 for GFAP+ cells; Fig. 3A) and hippocampus (Kruskal-Wallis One Way ANOVA, H=0.789 with 2 DF, p=0.674 for Neurofilament+ cells; H=1.315 with 2 DF, p=0.518 for GFAP+ cells; Fig. 3B). Moreover, FGF2 immunoreactivity was mostly confined to GFAP positive cells in both cortex and hippocampus (Fig. 3). Thus, it appears that the accumulation of FGF2 in neurons by antidepressants occurs only after a chronic treatment.
Figure 3. Acute antidepressant treatment does not change FGF2 immunoreactivity.
Rats received a single injection of DMI (10 mg/kg) or FLU (5 mg/kg) and were sacrificed 24 hr later. FGF2 immunoreactivity was examined in serial sections from the cortex (A) and hippocampus (B) as described in the legend of Fig. 2. Data are the mean ± SEM of six animals per group.
DMI and FLU increase FGF2 isoforms in the cytoplasm
In control animals, FGF2 immunoreactivity was localized in both nuclei as well as in the cytosol. In the hippocampus of treated animals, accumulation of FGF2 was seen in the cytosol and the nucleus. Instead, antidepressants evoke an increase in FGF2 immunoreactivity in the cytosol of cortical neurons. Thus, the accumulation of FGF2 after antidepressants may represent a cell-specific event. However, histology cannot distinguish the multiple isoforms of FGF2 (18, 21 and 22 kDa) that are produced by alternative usage of translation start sites (reviewed in Sorensen et al., 2006). Moreover, the various isoforms of FGF2 exhibit different subcellular localizations and functions (Ma et al. 2007; Sorensen et al. 2006). Therefore, in an attempt to reveal whether antidepressants modulate intracellular distribution of FGF2, we analyzed FGF2 immunoreactive forms in the cerebral cortex.
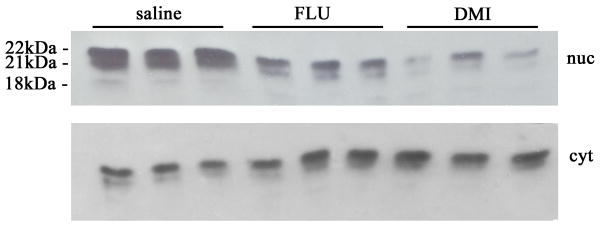
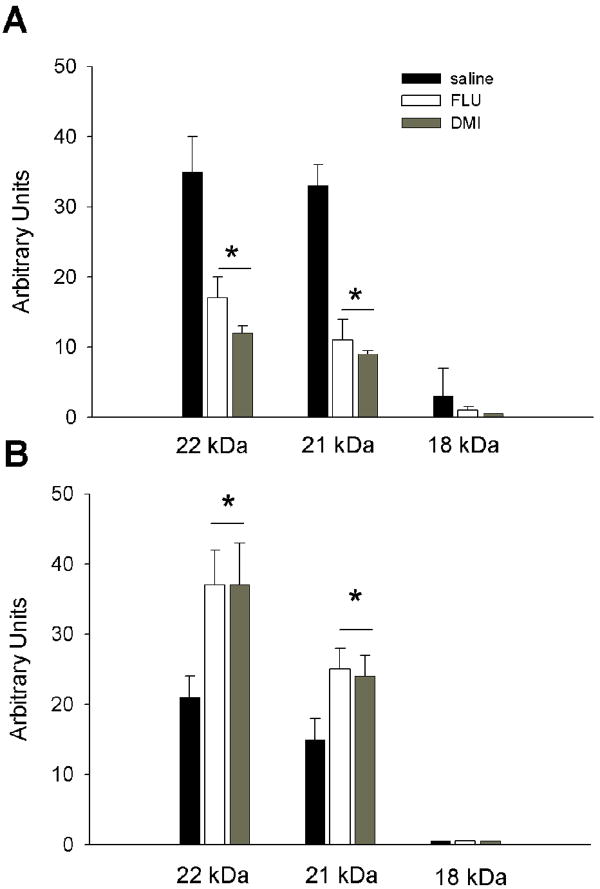
Lysates from the frontal cortex were prepared to separate nuclear and cytosol fractions and then processed for Western blot analysis of FGF2 isoforms. In the nuclear fraction, we were able to detect three isoforms that migrated with an apparent molecular weight of 18, 21 and 22 kDa (Fig. 4). The 22 kDa band was always more abundant than the other forms. The 18 kDa band was detected at very low levels. Densitometric analysis of the immunoreactive bands revealed that both DMI and FLU reduce all FGF2 isoforms in the nuclear extracts (Kruskal-Wallis One Way ANOVA, H=12.590 with 2 DF, p=0.002; Fig. 5A). Analyses of cytoplasmic extracts revealed a different profile. In fact, only the 22 and 21 kDa isoforms were readily visible (Fig. 4). In addition, both antidepressants increased these isoforms (ANOVA and Holm-Sidak test, 2 DF, p<0.05; Fig. 5B). Thus, it appears that antidepressants change the cellular distribution of FGF2 isoforms.
Figure 4. Antidepressants affect FGF2 isoform levels.
Rats received for 15 days a daily injection of saline, DMI or FLU. The frontal cortex was dissected and lysates prepared as described in Methods. Heparin binding proteins, enriched by affinity chromatography as described previously (Mallei et al. 2002), were loaded on to a 15% SDS-polyacrylamide gel and analyzed using a FGF2 monoclonal antibody (dilution 1:500). Examples of Western blot analysis of nuclear (nuc) and cytosolic (cyt) fractions from three separate samples.
Figure 5. Antidepressants regulate the cellular distribution of FGF2 isoforms.
Cortical lysates from saline, DMI and FLU-treated rats were prepared as described in Methods and analyzed by Western blot as described in Fig. 4. The arbitrary levels of the FGF2 isoforms were calculated by densitometric analysis of the FGF2 immunoreactive bands in the nuclear (A) and cytoplasmic (B) extracts. Data, expressed as arbitrary units, are the mean ± SEM of six independent samples. *p<0.002 vs saline in panel A, *p< 0.05 vs saline in panel B.
Antidepressants increase FGF-binding protein in the cerebral cortex
It has been suggested that FGF2 binds to a secreted FBP that mobilizes FGFs from extracellular matrix (ECM) heparin sulfate protoglycans [reviewed in (Abuharbeid et al. 2006)]. Thus, FBP functions as an extracellular chaperone for locally stored FGF2. These considerations prompted us to examine whether chronic antidepressants change the content of FBP.
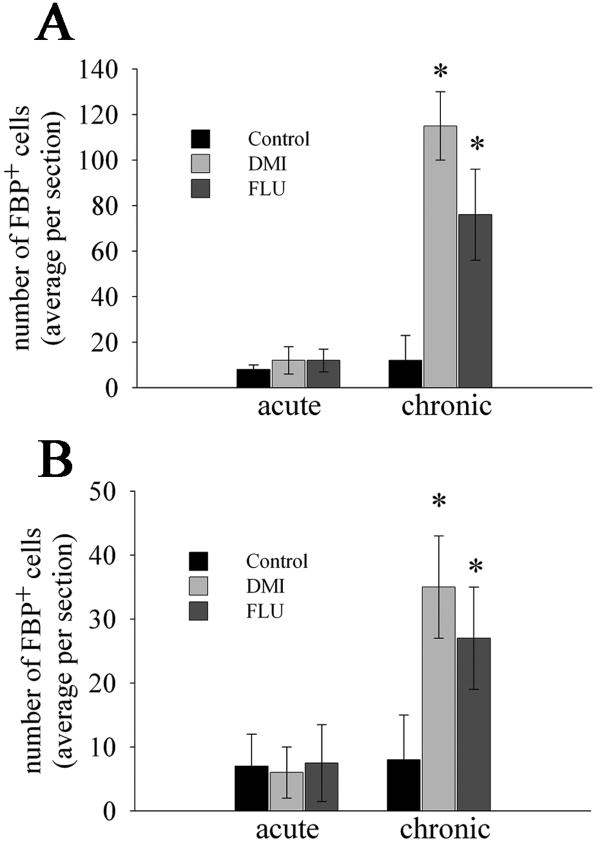
Rats received saline, DMI, or FLU for 15 days and were sacrificed 24 hr after the last injection. Serial coronal sections were then prepared throughout the telencephalon and stained for FBP and neurofilament, as well as FBP and GFAP, to visualize FGF immunoreactivity in neurons and glia, respectively. In chronically saline-treated rats, FBP immunoreactivity was undetectable or rarely seen in the cortex (Fig. 6A), striatum or corpus callosum (data not shown). In chronic DMI (Fig. 6B) or FLU-treated rats (Fig. 6C), FBP immunoreactivity was clearly observed in the frontal cortex in neurofilament positive cells, indicating neuronal expression. Moreover, FBP immunoreactivity was localized in the soma (Figs. 6B and C, arrows). Semiquantitative analysis of FBP immunoreactivity revealed that both antidepressants increase the number of neurons positive for the chaperone protein (ANOVA and Dunnett's test, p<0.01; Fig. 7A). No changes were observed after an acute injection (Kruskal-Wallis One Way ANOVA, H=0.108 with 2 DF, p=0.948; Fig. 7A).
Figure 6. Chronic antidepressant treatments increase FBP immunoreactivity.
Rats received saline, DMI or FLU for two weeks. Serial coronal sections throughout the cortex were prepared and stained with an antibody against FBP. (A) saline- (B) DMI- and (C) FLU-treated rats. Red=neurofilament, green=FBP. Saline-treated rats showed no FBP immunoreactivity. In DMI and FLU-treated rats, FBP immunoreactivity is mostly localized perinuclearly in neurons (arrows). Bar= 50 μm.
Figure 7. Semiquantitative analysis of FBP positive cells.
Serial sections prepared as described in Fig. 6 from the frontal cortex (A) and hippocampus (B) were analyzed for FBP immunoreactivity using MetaMorph®. Data are the mean ± SEM of six animals per group (20 sections per animal). *p<0.01 vs control.
To determine the specificity of antidepressant treatments, we examined FBP in the hippocampus and striatum. DMI and FLU increased the number of FBP positive cells in the hippocampus (ANOVA and Dunnett's test, DF = 2, p<0.01; Fig. 7B) but not in the striatum (data not shown). In addition, we did not observe an effect on FBP immunoreactivity after an acute treatment (Kruskal-Wallis One Way ANOVA, H=0.0211 with 2 DF, p=0.990; Fig. 7B). Thus, similar to the cortex, a single injection of antidepressants does not change FBP levels.
Discussion
Neurotrophic factors are naturally occurring diffusible polypeptides that promote the survival of a variety of CNS cells and are equally essential for promoting synaptic plasticity. In the present study, we found that antidepressants regulate the distribution and expression of FGF2 and its binding protein in the brains of adult rats. FGF2 exhibits trophic activities that are significant for the therapeutic action of antidepressants, including neurite branching in cortical neurons (Korada et al. 2002; Szebenyi et al. 2001) and modification of excitatory synapses (Li et al. 2002). Thus, we propose that FGF2 could be added to the list of neurotrophic factors that are relevant to the therapeutic action of antidepressants.
In this study, we observed an area-specific increase in FGF2 immunoreactivity after antidepressant treatments. In the cortex, FGF2 was seen in the cytoplasm of neurons, whereas in the hippocampus it was seen in both astrocytes and neurons. Interestingly, in the cortex of antidepressant-treated rats, in addition to FGF2, we were able to detect an increase in FBP immunoreactivity in the cytosol of neurons. FBP (or HBp17) is a crucial chaperone for the biological activity of FGF2. Binding of FGF2 to FBP results in a marked reduction of the affinity of FGF2 to heparin, which culminates in release of stored FGF2 from ECM heparin sulfate protoglycans [reviewed in (Abuharbeid et al. 2006)]. This event is essential for the solubilization of FGF2 and activation of its receptors (Czubayko et al. 1997). Moreover, FBP protects FGFs from acid inactivation (Wu et al. 1991) and enhances FGF2-induced receptor signaling even at concentrations of FGF2 not sufficient to elicit maximal receptor activation (Tassi et al. 2001). Therefore, FBP is considered to be playing a pivotal role in FGF2 biological effects. Lastly, FBP expression is up-regulated following CNS injury (Tassi et al. 2007) with a time-course similar to that reported for FGF2 (Mocchetti et al. 1996a). Based on these considerations, we suggest including FBP in the variety of events that have been proposed to explain the therapeutic efficacy of antidepressants.
An interesting and novel finding reported here is the fact that antidepressants with a different pharmacological profile induce an accumulation of FGF2 isoforms in the cytoplasm. These isoforms originate from alterative splicing translation initiation sites within a single mRNA species. The significance of this finding is still under investigation. However, it is crucial to recall the fact that the smaller translocation product, which has a molecular weight of 18 kDa, can be secreted from cells to activate membrane associated FGF receptors. The other FGF2 products, which are amino terminal extended forms of FGF2 and migrate with an apparent molecular weight of 21 and 22 kDa (Sorensen et al. 2006), exhibit neurotrophic activity (Grothe et al. 2000) and appear to regulate cellular processes through an intracellular pathway independent of cell surface receptors (Ma et al. 2007). The 21 and 22 kDa products are consistently seen in nuclei of astrocytes and oligodendrocytes (Delrieu 2000; Woodward et al. 1992). Our results show that antidepressants reduce the nuclear accumulation of FGF2 isoforms while increasing them in the cytoplasm. Thus, it is appealing to speculate that a decrease in nuclear accumulation of FGF2 may represent a decreased synthesis in astrocytes, while an increase in cytosolic FGF2 is indicative of increased neuronal synthesis. This suggestion is consistent with the immunohistochemical data showing that antidepressants produce an accumulation of FGF2 immunoreactivity in the cytosol of neurofilament positive cells. Nuclear accumulation of FGF2 is essential for its mitogenic responses (Baguma-Nibasheka et al. 2007). Indeed, nuclear FGF2 is a negative prognosis for survival of patients with astrocytic tumors (Fukui et al. 2003). Thus, our data suggest that antidepressants reduce the potential proliferative properties of FGF2 while enhancing its neurotrophic activity.
In this report, we have examined the association between antidepressants and FGF2. Antidepressants have been shown to increase the expression of BDNF (Nibuya et al. 1995). Thus, it would be naive to ignore the likelihood that antidepressants modify the levels of this neurotrophic factor as well. BDNF and FGF2 possess similar trophic activities, as these trophic factors are notoriously crucial for the plasticity of overlapping neuronal populations. In addition, both trophic factors exhibit properties that are relevant for the clinical efficacy of antidepressants, namely reduction of neuronal apoptosis and induction of neurogenesis (Ganat et al. 2002; Jin et al. 2005; Mohapel et al. 2005). On the other hand, BDNF and FGF2 affect distinct neuronal populations. For instance, BDNF is crucial for the maintenance of plasticity in serotonergic neurons (Lyons et al. 1999). FGF2, instead, appears to play a role in the developmental maturation of neurotransmitter transporters, which are critical components of synapses. These include dopamine uptake in mesencephalic cultures (Ferrari et al. 1990), the noradrenergic transport in the locus coeruleus (Sieber-Blum and Ren 2000), and glutamate transport in astrocytes (Figiel et al. 2003). Considering the vast number of synapses present in the brain with a crucial role in regulating brain function, the ability of antidepressants to increase multiple neurotrophic factors is functionally relevant. In fact, each neurotrophic factor may influence several forms of synaptic plasticity in different neuronal populations. Our data support the notion that antidepressants, by increasing the availability of these neurotrophic factors, may overcome a loss of synaptic connections seen in patients. Given the well established neurotrophic property of FGF2 in terms of neuroprotection and plasticity in the adult CNS, we suggest that FGF2 participates in the neurotrophic activity of antidepressants.
The molecular mechanism underlying the induction of FGF2 immunoreactivity is still under investigation. Previous studies have shown that antidepressants increase FGF2 mRNA levels, suggesting an increase in synthesis/expression (Mallei et al. 2002; Maragnoli et al. 2004). At least two phenomena can explain this effect. Antidepressants may increase FGF2 expression in neurons by activating cis-elements and trans-acting factors crucial for its cell-specific expression. Indeed, the FGF2 promoter contains a cAMP responsive region (Peng et al. 2001) that binds to the transcription factor cAMP-responsive element binding protein (CREB). Antidepressants increase the activity of adenylyl cyclase (Menkes et al. 1983), thus enabling the activation of protein kinase A, a prerequisite for CREB phosphorylation. Thus, the FGF2 promoter may be turned on when CREB is activated. However, activation of cAMP pathway in vivo, as in that obtained by beta-adrenergic agonists, increases FGF2 expression in glial cells (Hayes et al. 1995). A similar induction of FGF2 expression in glia has been obtained by glucocorticoid receptor agonists (Mocchetti et al. 1996b). Thus, a direct mechanism involving cAMP or other signaling pathways may not be sufficient to explain the effect of antidepressants on neuronal FGF2. A second and most likely mechanism might occur if we consider that FGF2 is internalized by neurons (Ferguson and Johnson 1991). In this scenario, antidepressants may evoke the release of FGF2 from glial cells, the primary sites of its synthesis. Antidepressants may also induce the synthesis of FBP in neurons. Released FBP binds to FGF2 and promotes its mobilization from the extracellular matrix (Czubayko et al. 1997) and is subsequently internalized as a FGF-FBP complex inside neurons. This event might explain why we observed an accumulation of FGF2 isoforms and FBP immunoreactivity in the cytoplasm of cortical neurons. Although still speculative, this hypothesis suggests that the ability of antidepressants to accumulate FGF2 in neurons may represent a unique and novel property of these pharmacologically active compounds.
Acknowledgments
We would like to thank Eli-Lilly for fluoxetine and M Diane Leader for diligent editorial assistance. Supported by HHS grant NS 047977
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abuharbeid S, Czubayko F, Aigner A. The fibroblast growth factor-binding protein FGF-BP. Int J Biochem Cell Biol. 2006;38(9):1463–1468. doi: 10.1016/j.biocel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of Human Immunodeficiency Virus Type 1 envelope glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26(25):6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguma-Nibasheka M, Li AW, Murphy PR. The fibroblast growth factor-2 antisense gene inhibits nuclear accumulation of FGF-2 and delays cell cycle progression in C6 glioma cells. Mol Cell Endocrinol. 2007;267(12):127–136. doi: 10.1016/j.mce.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Bal-Klara A, Bird MM. The effects of various antidepressant drugs on the fine-structure of neurons of the cingulate cortex in culture. Neuroscience. 1990;37(3):685–692. doi: 10.1016/0306-4522(90)90099-p. [DOI] [PubMed] [Google Scholar]
- Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, Day HE, Maier SF. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 2007;144(4):1219–1228. doi: 10.1016/j.neuroscience.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo AM, Johnson PF, Mocchetti I. beta-adrenergic receptor-induced activation of nerve growth factor gene transcription in rat cerebral cortex involves CCAAT/enhancer-binding protein delta. Proc Natl Acad Sci U S A. 1998;95(18):10920–10925. doi: 10.1073/pnas.95.18.10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98(22):12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubayko F, Liaudet-Coopman ED, Aigner A, Tuveson AT, Berchem GJ, Wellstein A. A secreted FGF-binding protein can serve as the angiogenic switch in human cancer. Nat Med. 1997;3(10):1137–1140. doi: 10.1038/nm1097-1137. [DOI] [PubMed] [Google Scholar]
- Delrieu I. The high molecular weight isoforms of basic fibroblast growth factor (FGF-2): an insight into an intracrine mechanism. FEBS Lett. 2000;468(1):6–10. doi: 10.1016/s0014-5793(00)01189-3. [DOI] [PubMed] [Google Scholar]
- Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, Walsh DM, Myers RM, Bunney WE, Watson SJ, Jones EG, Akil H. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci USA. 2004;101(43):15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson IA, Johnson EMJ. Fibroblast growth factor receptor-bearing neurons in the CNS: identification by receptor-mediated retrograde transport. J Comp Neurol. 1991;22(313):693–706. doi: 10.1002/cne.903130412. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Minozzi MC, Toffano G, Leon A, Skaper SD. Basic fibroblast growth factor affects the survival and development of mesencephalic neurons in culture. Adv Exp Med Biol. 1990;265:93–99. doi: 10.1007/978-1-4757-5876-4_8. [DOI] [PubMed] [Google Scholar]
- Figiel M, Maucher T, Rozyczka J, Bayatti N, Engele J. Regulation of glial glutamate transporter expression by growth factors. Exp Neurol. 2003;183(1):124–135. doi: 10.1016/s0014-4886(03)00134-1. [DOI] [PubMed] [Google Scholar]
- Follesa P, Gale K, Mocchetti I. Regional and temporal pattern of expression of nerve growth factor and basic fibroblast growth factor mRNA in rat brain following electroconvulsive shock. Exp Neurol. 1994;127(1):37–44. doi: 10.1006/exnr.1994.1077. [DOI] [PubMed] [Google Scholar]
- Fukui S, Nawashiro H, Otani N, Ooigawa H, Nomura N, Yano A, Miyazawa T, Ohnuki A, Tsuzuki N, Katoh H, Ishihara S, Shima K. Nuclear accumulation of basic fibroblast growth factor in human astrocytic tumors. Cancer. 2003;97(12):3061–3067. doi: 10.1002/cncr.11450. [DOI] [PubMed] [Google Scholar]
- Ganat Y, Soni S, Chacon M, Schwartz ML, Vaccarino FM. Chronic hypoxia up-regulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor-responsive radial glial cells in the sub-ependymal zone. Neuroscience. 2002;112(4):977–991. doi: 10.1016/s0306-4522(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70(3):221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Lee JW, Cotman CW. Basic FGF in adult rat brain: cellular distribution and response to entorhinal lesion and fimbria-fornix transection. J Neurosci. 1992;12(1):345–355. doi: 10.1523/JNEUROSCI.12-01-00345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46(11):1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Grothe C, Schulze A, Semkova I, Muller-Ostermeyer F, Rege A, Wewetzer K. The high molecular weight fibroblast growth factor-2 isoforms (21,000 mol. wt and 23,000 mol. wt) mediate neurotrophic activity on rat embryonic mesencephalic dopaminergic neurons in vitro. Neuroscience. 2000;100(1):73–86. doi: 10.1016/s0306-4522(00)00247-5. [DOI] [PubMed] [Google Scholar]
- Grothe C, Timmer M. The physiological and pharmacological role of basic fibroblast growth factor in the dopaminergic nigrostriatal system. Brain Res Rev. 2007;54(1):80–91. doi: 10.1016/j.brainresrev.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40(11):1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes VY, Isackson PJ, Fabrazzo M, Follesa P, Mocchetti I. Induction of nerve growth factor and basic fibroblast growth factor mRNA following clenbuterol: contrasting anatomical and cellular localization. Exp Neurol. 1995;132(1):33–41. doi: 10.1016/0014-4886(95)90056-x. [DOI] [PubMed] [Google Scholar]
- Jin K, LaFevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, Logvinova A, Ross CA, Greenberg DA, Ellerby LM. FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2005;102(50):18189–18194. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korada S, Zheng W, Basilico C, Schwartz ML, Vaccarino FM. Fibroblast growth factor 2 is necessary for the growth of glutamate projection neurons in the anterior neocortex. J Neurosci. 2002;22(3):863–875. doi: 10.1523/JNEUROSCI.22-03-00863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbeater WE, Gonzalez AM, Logaras N, Berry M, Turnbull JE, Logan A. Intracellular trafficking in neurones and glia of fibroblast growth factor-2, fibroblast growth factor receptor 1 and heparan sulphate proteoglycans in the injured adult rat cerebral cortex. J Neurochem. 2006;96(4):1189–1200. doi: 10.1111/j.1471-4159.2005.03632.x. [DOI] [PubMed] [Google Scholar]
- Li AJ, Suzuki S, Suzuki M, Mizukoshi E, Imamura T. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur J Neurosci. 2002;16(7):1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96(26):15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Dang X, Claus P, Hirst C, Fandrich RR, Jin Y, Grothe C, Kirshenbaum LA, Cattini PA, Kardami E. Chromatin compaction and cell death by high molecular weight FGF-2 depend on its nuclear localization, intracrine ERK activation, and engagement of mitochondria. J Cell Physiol. 2007;213(3):690–698. doi: 10.1002/jcp.21139. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol Pharmacol. 2002;61(5):1017–1024. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- Maragnoli ME, Fumagalli F, Gennarelli M, Racagni G, Riva MA. Fluoxetine and olanzapine have synergistic effects in the modulation of fibroblast growth factor 2 expression within the rat brain. Biol Psychiatry. 2004;55(11):1095–1102. doi: 10.1016/j.biopsych.2004.02.003. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Menkes DB, Rasenick MM, Wheeler MA, Bitensky MW. Guanosine triphosphate activation of brain adenylate cyclase: enhancement by long-term antidepressant treatment. Science. 1983;219(4580):65–67. doi: 10.1126/science.6849117. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Rabin SJ, Colangelo AM, Whittemore SR, Wrathall JR. Increased basic fibroblast growth factor expression following contusive spinal cord injury. Exp Neurol. 1996a;141(1):154–164. doi: 10.1006/exnr.1996.0149. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Spiga G, Hayes VY, Isackson PJ, Colangelo A. Glucocorticoids differentially increase nerve growth factor and basic fibroblast growth factor expression in the rat brain. J Neurosci. 1996b;16(6):2141–2148. doi: 10.1523/JNEUROSCI.16-06-02141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-Derived Growth Factor (PDGF-BB) and Brain-Derived Neurotrophic Factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132(3):767. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan CL, Moore GJ, Madden R, Farchione T, Bartoi M, Lorch E, Stewart CM, Rosenberg DR. Prefrontal cortical volume in childhood-onset major depression: preliminary findings. Arch Gen Psychiatry. 2002;59(2):173–179. doi: 10.1001/archpsyc.59.2.173. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20(11):2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Moffett J, Myers J, Fang X, Stachowiak EK, Maher P, Kratz E, Hines J, Fluharty SJ, Mizukoshi E, Bloom DC, Stachowiak MK. Novel nuclear signaling pathway mediates activation of fibroblast growth factor-2 gene by type 1 and type 2 angiotensin II receptors. Mol Biol Cell. 2001;12(2):449–462. doi: 10.1091/mbc.12.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27(18):4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93(9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber-Blum M, Ren Z. Norepinephrine transporter expression and function in noradrenergic cell differentiation. Mol Cell Biochem. 2000;212(12):61–70. [PubMed] [Google Scholar]
- Sorensen V, Nilsen T, Wiedlocha A. Functional diversity of FGF-2 isoforms by intracellular sorting. Bioessays. 2006;28(5):504–514. doi: 10.1002/bies.20405. [DOI] [PubMed] [Google Scholar]
- Szebenyi G, Dent EW, Callaway JL, Seys C, Lueth H, Kalil K. Fibroblast growth factor-2 promotes axon branching of cortical neurons by influencing morphology and behavior of the primary growth cone. J Neurosci. 2001;21(11):3932–3941. doi: 10.1523/JNEUROSCI.21-11-03932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassi E, Al-Attar A, Aigner A, Swift MR, McDonnell K, Karavanov A, Wellstein A. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J Biol Chem. 2001;276(43):40247–40253. doi: 10.1074/jbc.M104933200. [DOI] [PubMed] [Google Scholar]
- Tassi E, Henke RT, Bowden ET, Swift MR, Kodack DP, Kuo AH, Maitra A, Wellstein A. Expression of a fibroblast growth factor-binding protein during the development of adenocarcinoma of the pancreas and colon. Cancer Res. 2006;66(2):1191–1198. doi: 10.1158/0008-5472.CAN-05-2926. [DOI] [PubMed] [Google Scholar]
- Tassi E, Walter S, Aigner A, Cabal-Manzano RH, Ray R, Reier PJ, Wellstein A. Effects on neurite outgrowth and cell survival of a secreted fibroblast growth factor binding protein upregulated during spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R775–783. doi: 10.1152/ajpregu.00737.2006. [DOI] [PubMed] [Google Scholar]
- Woodward WR, Nishi R, Meshul CK, Williams TE, Coulombe M, Eckenstein FP. Nuclear and cytoplasmic localization of basic fibroblast growth factor in astrocytes and CA2 hippocampal neurons. J Neurosci. 1992;12(1):142–152. doi: 10.1523/JNEUROSCI.12-01-00142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DQ, Kan MK, Sato GH, Okamoto T, Sato JD. Characterization and molecular cloning of a putative binding protein for heparin-binding growth factors. J Biol Chem. 1991;266(25):16778–16785. [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]