Abstract
Transgenic tobacco plants engineered with bacterial merA and merB genes via the chloroplast genome were investigated to study the uptake, translocation of different forms of mercury (Hg) from roots to shoots, and their volatilization. Untransformed plants, regardless of the form of Hg supplied, reached a saturation point at 200 µM of phenylmercuric acetate (PMA) or HgCl2, accumulating Hg concentrations up to 500 µg g−1 with significant reduction in growth. In contrast, chloroplast transgenic lines continued to grow well with Hg concentrations in root tissues up to 2000 µg g−1. Chloroplast transgenic lines accumulated both the organic and inorganic Hg forms to levels surpassing the concentrations found in the soil. The organic-Hg form was absorbed and translocated more efficiently than the inorganic-Hg form in transgenic lines, whereas no such difference was observed in untransformed plants. Chloroplast-transgenic lines showed about 100-fold increase in the efficiency of Hg accumulation in shoots compared to untransformed plants. This is the first report of such high levels of Hg accumulation in green leaves or tissues. Transgenic plants attained a maximum rate of elemental-Hg volatilization in two days when supplied with PMA and in three days when supplied with inorganic-Hg, attaining complete volatilization within a week. The combined expression of merAB via the chloroplast genome enhanced conversion of Hg2+ into Hg,0 conferred tolerance by rapid volatilization and increased uptake of different forms of mercury, surpassing the concentrations found in the soil. These investigations provide novel insights for improvement of plant tolerance and detoxification of mercury.
Introduction
Mercury is a highly toxic heavy metal that has been introduced to the environment from natural sources and anthropogenic activities. Although mercury is usually released in its metal or ionic form, it is readily methylated by bacteria in the environment to methyl mercury, a highly toxic organomercurial compound (1). Methyl mercury toxicity is magnified in organisms at higher trophic levels due to its high accumulation in tissues (2, 3). Organomercurials are easily absorbed and known to act as neurotoxins; over 90% of methyl mercury is absorbed into the blood stream from the gastrointestinal tract, compared to less than 10% for mercury salts, and 0.1% for elemental mercury (4).
Because of the continuous buildup of mercury in the environment, there is an urgent need to develop appropriate technologies for mercury remediation. Power plants in the U.S. alone emit about 48 tons of mercury annually; it would cost $40000–70000 to remove each pound of mercury with currently available technologies (5). Traditional remediation strategies for mercury-contaminated environments are expensive, environmentally invasive, and oftentimes ineffective for large-scale cleanups (6). On the other hand, plants have the ability to adapt to diverse environmental conditions and can contain disrupted ecosystems; because of this, they may be used as a cost-effective and environmentally friendly approach for large-scale cleanups (7, 8). While plants have natural resistance against some toxic pollutants, they have limited applicability for the treatment of places with intermediate and high levels of contaminants, including mercury. Plant genetic engineering may be used to integrate new traits from organisms like bacteria to enhance their phytoremediation capabilities and resistance to toxic pollutants.
Bacterial genes merA and merB confer resistance to mercury compounds. MerB is a 638 bp gene that encodes a 24 kDa enzyme that undergoes the protonolysis of organomercurials by cleavage of the carbon-mercury bond (9), releasing a reduced organic moiety and the mercury ion. The merA gene codes for the mercuric ion reductase, which catalyzes the conversion ofHg2+ to elemental mercury (Hg0), a volatile, nonreactive, and less toxic form of mercury (10). Plants have been engineered via the nuclear genome with merA and merB genes to resist relatively low concentrations of toxic organomercurials (11, 12). Because the previous approach did not directly protect the chloroplast, high levels of resistance were difficult to achieve. The redox reaction catalyzed by the MerA requires the availability of nicotinamide adenine dinucleotide phosphate (NADPH) as an electron donor. NADPH is generated from the chloroplast reaction that occurs in photosystem I. Because of this, activity of the MerA enzyme depends on the integrity of the chloroplast (11). Organomercurial compounds strongly inhibit chloroplast functions, including electron transport; oxygen evolution (13); Hill reaction; photophosphorylation; chlorophyll fluorescence (14); and chlorophyll content (15). Therefore, phytoremediation approaches for mercury that genetically modify the chloroplast genome provide an ideal environment for optimal functionality of the mercury detoxifying enzymes encoded by merA and merB genes (16). In addition, mercury-induced root damage may have serious consequences for nutrient and water supply to above ground plant parts and should be taken into account when assessing the effect of mercury on the physiology of leaves (15). While HgCl2 affects the plasma membrane, methyl mercury, and other organomercurials, may primarily affect organelle metabolism in the cytoplasm.
The integration of foreign genes into plant nucleargenome has been shown to alter the mobilization of metal ions, including uptake into the root, sequestration, and detoxification (6, 14). However, a major limitation of all of these studies is their inability to show transport of organic or inorganic mercury to above ground tissues, which constitute more than 90% of the plant biomass. There is a considerable body of evidence suggesting that translocation of Hg2+ from root to shoot is negligible or nonexistent in wild type plants (18). Heaton et al. (19) compared the accumulation of Hg2+ by wild type and nuclear transgenic rice plants expressing the merA gene while growing in hydroponics media or in soil containing HgCl2. This study revealed that mercury was being accumulated in roots but was poorly translocated to shoots when grown in hydroponics and soil.
The main goal of this research is to investigate how uptake and translocation of Hg from roots to shoots may be altered in chloroplast transgenic tobacco plants expressing merA and merB genes compared to untransformed plants. Furthermore, investigations were performed to determine whether detoxification in the transgenic plants is accompanied by the conversion of mercury into the volatilizable elemental form when supplied in inorganic (mercuric chloride) or organic (phenyl mercuric acetate) forms.
Experimental Section
Construction of Chloroplast Vectors Containing merA and merB Genes
As described earlier (16), the bacterial native genes, merA (1.69 kb) and merB (638 bp), were cloned into the chloroplast transformation vector. This vector allows site-specific integration of the transgenes into the inverted repeat regions of the chloroplast genome between the trnI and trnA genes by homologous recombination. The constitutive promoter Prrn drives the transcription of the downstream genes that include the aadA (aminoglycoside 3′- adenylyl-transferase) gene, which confers resistance to spectinomycin, and the merAB operon. Two versions of the chloroplast transformation vectors were made, one with a 3′ untranslated region (3′UTR) from the chloroplast psbA gene inserted downstream of the merA,B operon, to confer transcript stability. The second vector did not contain the psbA 3′UTR. Chloroplast transgenic tobacco lines were obtained with each construct and were designated as pLDR-MerAB-3′UTR and pLDR-MerAB, respectively. A complete description regarding the chloroplast vector construction containing both the merA and merB genes, the development of chloroplast transgenic lines and the molecular characterization of the transgenic plants have been reported in our previous publication (16).
Plant Germination in Contaminated Soil
Tobacco seeds of both transgenic lines and wild type (untransformed) were surface-sterilized by shaking in 70% (v/v) ethanol for 30 s, followed by 10% (v/v) sodium hypochlorite for 30 min, and five 5-min washes with sterile double-distilled water. During the sterilization procedure, the seeds were kept in closed, sterile, plastic tubes, and shaken on a rocking platform to ensure that bleach and alcohol covered all of the seeds. As described in Ruiz et al. (16), sterilized seeds were transferred to plates containing half-strength MS medium (20) with 500 µg/mL spectinomycin and 0.3% (w/v) phytoagar (pH 5.7). Plates were incubated in the dark at 4 °C for three days, and then were maintained in a growth chamber with controlled temperature (22–24 °C), humidity (75/90%) and light (750 µE.m−2) with 16 h day length. Ten-day old seedlings were transferred to soil (50:50 sand and potting soil) in the greenhouse at 22 °C using 16 h of light. Each pot contained a single seedling, either untransformed or chloroplast transgenic plants (pLDR-MerAB or pLDR-MerAB-3′UTR). All pots were watered twice a week with half-strength Hoagland’s solution for 10 days.
Pots of five replicates representing the untransformed and two transgenic lines (of approximately the same age) were transferred to PVC plastic trays 3 in. high. Different concentrations (100, 200, and 300 µM) of phenyl mercuric acetate (PMA) and mercuric chloride (HgCl2) were prepared using a stock solution in half-strength Hoagland’s solution. For each treatment, a single tray maintained approximately 200 mL (to about half-of the pot’s height) of the Hg-Hoagland’s solution. This semihydroponic system ensures a common source of feeding solution and avoids variations in irrigation of individual pots. Moreover, all plants in the same treatment were exposed to exactly the same concentration of mercury. The control tray was filled with half-strength Hoagland’s solution without Hg.
After about 15 days, the plants were harvested, washed thoroughly in distilled water, shoots and roots were separated, and immersed directly in liquid nitrogen. The frozen plants were dried using a freeze-dryer, and the dry weight was determined. Samples were stored at −80 °C for chemical analysis.
Determination of Total Mercury Concentration in Plants
Freeze–dried samples were ground to a fine powder using liquid nitrogen and analyzed via automated CVAAS (cold vapor atomic absorption spectroscopy) with a continuous flow Vapor Generation Accessory, VGA-77 as described in Varian Operation Manual (21).
Plant samples (five replicates of each treatment) were acid-digested by stepwise additions of 70% (v/v) nitric acid, 30% (v/v) hydrogen peroxide, and concentrated HCl at 95 °C in a modification of EPA method 3010A (22). Potassium permanganate (2% w/v) and potassium persulfate (5% w/v) were added to the samples before addition of nitric acid to reduce organic mercury in the digestion solution (23). After digestion, the excess potassium permanganate was reduced with hydroxylamine hydrochloride. Blanks and standard reference materials of San Joaquin soil were run as external quality controls for analyses of Hg in soil and plant samples.
Measurement of Hg Volatilization
Volatilized Hg0 was measured by germinating seeds of untransformed and two transgenic lines (in three replicates) as mentioned above. PMA and HgCl2 were added separately at a concentration of 100 µM to each pot using a stock solution in half-strength Hoagland’s solution. Pots, each containing a single plant, were placed in 3 L volume gastight acrylic volatilization chambers in the greenhouse through which a continuous airflow (1.5 L min−1) was passed by applying suction at the outlet and by bubbling incoming air into the oxidizing trap solution (see Figure 4) The background Hg volatilization from the soil was obtained from chambers containing only pots with soil amended with the same concentration (100 µM) of PMAor HgCl2. The actual plant volatilization was determined by subtracting the total volatilization from the background values. Volatile Hg0 was quantitatively trapped in alkaline peroxide liquid traps composed of 0.1% (w/v) NaOH and 30% (v/v)H2O2 (1:1) as described previously (24, 25). Aliquots (10 mL) of trap solution were collected every 24 h, after which the solutions were replaced with new trap solution for 13 successive days. The trap solution samples were heated at 95 °C to remove the peroxide. The Hg concentration was measured by vapor-generation atomic absorption spectroscopy as described above.
FIGURE 4.
Gastight acrylic volatilization chambers used to collect the volatilized mercury from untransformed and chloroplast engineered pLDR-MerAB and pLDR-MerAB 3′UTR tobacco lines (in three replicates) grown over a 13-day period on soil amended with 100 µM of either PMA or HgCl2. Volatile Hg was quantitatively trapped in alkaline peroxide liquid traps solution (1:1 of 0.1% NaOH and 30% H2O2).
Results and Discussion
Accumulation of Mercury by Roots and Translocation to Shoots
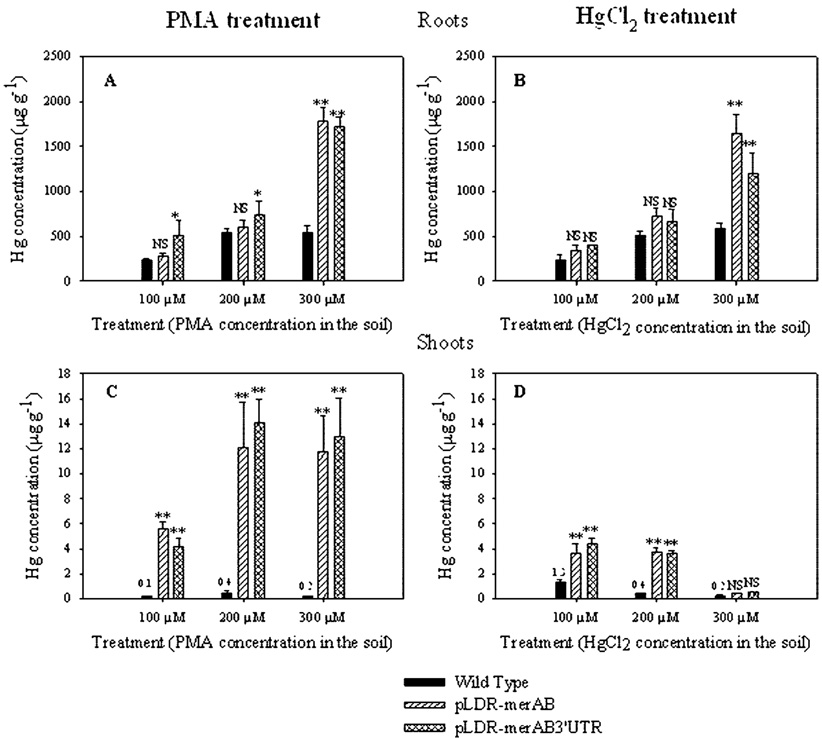
Mercury uptake and translocation was determined by measuring mercury content in tobacco root and shoot tissues of untransformed and merAB chloroplast transgenic lines. These results showed that substantial quantities of Hg were accumulated in roots. Mercury concentrations in roots ranged from 230 µg g−1 to 1780 µg g−1 depending on genotype and on the concentration of external Hg (Figure 1A,B). In general, Hg concentrations in roots increased with increasing external concentration of Hg but were not much affected by the form of Hg supplied (PMA or HgCl2). One of the most interesting aspects of these results was the substantial increase in Hg concentration in roots of transgenic lines supplied with Hg at the highest level, 300 µM; these concentrations were 2- to 3-fold higher in the transgenic lines than in untransformed plants. When supplied with external concentrations of 100 and 200 µM mercury, root Hg concentrations of transgenic plants were only slightly higher than those of wild type. Thus, the insertion of merA and merB genes into the tobacco chloroplast genome substantially improved the ability of plants to accumulate Hg in their roots and survive when the external Hg concentrations were high; this observation was consistent regardless of whether the Hg was supplied in the organic or inorganic form.
FIGURE 1.
Hg concentration (µg g−1) in roots and shoots of untransformed (black bars) and chloroplast transgenic lines (pLDR-MerAB and pLDR-MerAB-3-UTR) grown for 15 days in soil amended with 100, 200, and 300 µM of either PMA or HgCl2. Values shown are the average ± standard deviation of five replicates. (*, Significant difference at P < 0.05 from the untransformed plants of the same treatment. **, Significant difference at P < 0.001 from the untransformed plants of the same treatment. NS, Not significant from the untransformed plants of the same treatment).
As observed, the maximum level Hg accumulation obtained in untransformed plants was about 500 µg g−1 regardless of the form of mercury in the soil. This concentration did not vary by treating plants with 200 or 300 µM, suggesting that a saturation point was reached at 200 µM. Such a saturation point was not observed in transgenic lines, which at the higher concentrations accumulated up to 3-fold more than the untransformed plants; this saturation effect could be explained by the susceptibility of plant cells to Hg, which disrupts the cellular function and integrity. Cellular integrity and functionality are probably needed for processes that facilitate uptake of Hg and assimilation.
The ability of untransformed plants to transport Hg to shoots was extremely limited; average Hg concentrations in shoots ranged from 0.1 to 1.3 µg g−1 (Figure 1C,D) compared to 230 to 580 µg g−1 in roots. At the highest level of external Hg supplied, 300 µM, the amount of Hg accumulated per shoot was 0.20 ± 0.04 µg in plants supplied with PMA and 0.23 ± 0.10 µg in plants supplied with HgCl2. These values represented <0.2% of the total Hg accumulated per plant in each case. In sharp contrast to the untransformed plants, transgenic lines transported Hg from roots to shoots with much greater efficiency. Transgenic lines supplied with PMA accumulated Hg in shoots 100-fold greater than wild type (Figure 1C). This effect was less striking in transgenic lines supplied with HgCl2; Hg concentrations in the transgenic shoots increased only at 100 and 200 µM Hg and not at higher level of Hg supplied (Figure 1 D). Chloroplast transgenic lines supplied with 300 µM PMA accumulated 4.3% (pLDR-MerAB) and 3.4% (pLDR-MerAB-3′UTR) of the total Hg accumulated, while plants supplied with HgCl2 accumulated <0.3%. Low levels of accumulation of Hg in shoots compared to roots have been observed in previous studies in different plant species. For example, Greger et al. (26) reported that Hg concentration in six different plant species increased mostly in roots, 150–1083 times, whereas the increase in shoots was only 2.2–16.7 times depending on plant species, confirming inefficient Hg translocation.
Effect of merA and merB Expression on Mercury Tolerance and Growth
Phytotoxic effects of mercury compounds on plant growth have been reported in several plants, including Triticum aestivum (27), Oryza sativa (28), and several other grain crops. In general, the degree of impact depends on the concentration, the formulation, the mode of application, and the cultivar (3). At the levels of Hg applied in this study, there were major differences in root and shoot growth of untransformed and merAB engineered plants.
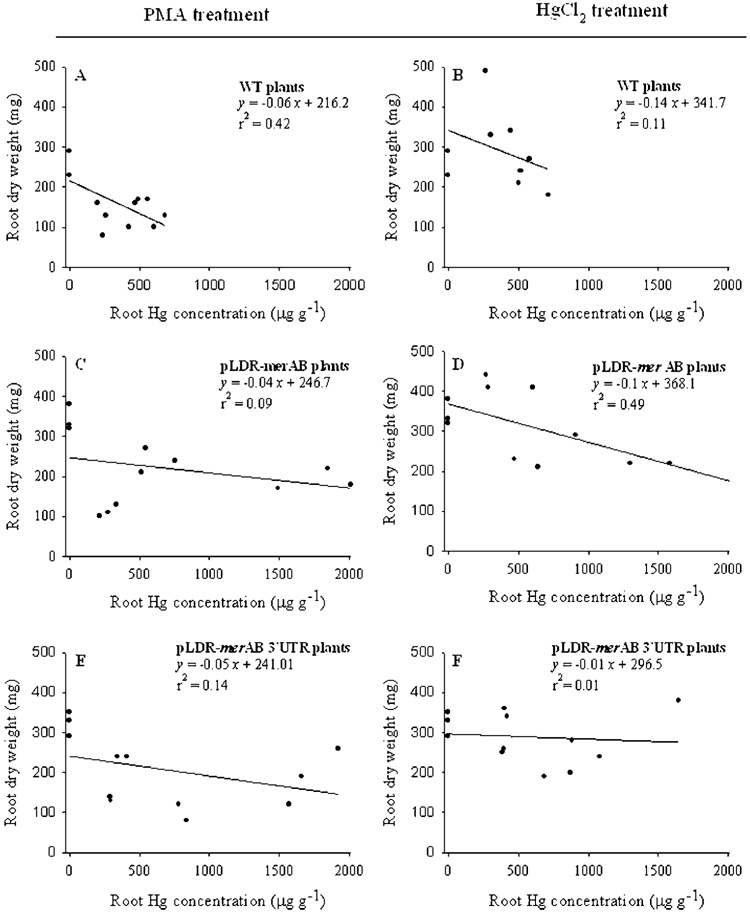
Untransformed plants, regardless of the form of Hg supplied, were only able to tolerate increases in root Hg concentrations up to 700 µg g−1 with significant reduction in growth as indicated by root dry weight (Figure 2A,B). Transgenic plants, on the other hand, continued to grow well with Hg concentration in root tissues up to 1500 µg g−1 for the plants supplied with inorganic Hg, and up to 2000 µg g−1 for plants supplied with PMA (Figure 2C,F).
FIGURE 2.
The relationship between root dry weight (mg/plant) and root Hg concentrations (µg g−1) of untransformed and chloroplast engineered pLDR-merAB and pLDR-merAB 3′UTR tobacco lines. The regression analysis between the dry weight and Hg concentration in tissues is derived from three different treatments with 100, 200, and 300 µM of either PMA or HgCl2.
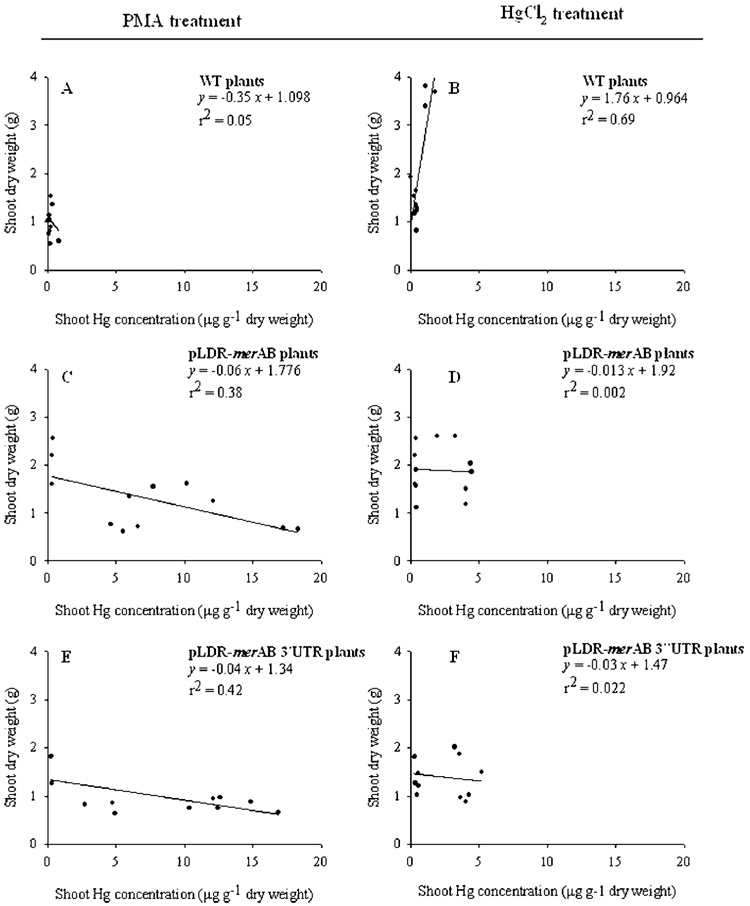
Although Hg concentration in shoots was substantially lower compared to roots, it was greatly affected by the form of Hg supplied. Wild type plants were able to accumulate Hg in shoot tissues up to 0.8 µg g−1 for plant supplied with organic Hg and up to 1.7 µg g−1 when supplied with inorganic Hg (Figure 3A,B). In contrast to roots, Hg concentration in shoots of transgenic plants was more affected by the form of Hg supplied. The transgenic plants were able to accumulate Hg in shoot tissues up to 18 µg g−1 when organic Hg was supplied (Figure 3C,E). When inorganic Hg was supplied, shoots of transgenic plants accumulated Hg up to 4 µg g−1 (Figure 3D,F). These results show that untransformed plants were affected equally by both inorganic and organic forms of Hg. In transgenic plants, Hg accumulation was 25% less in roots of plants supplied with inorganic Hg than the organic form. Similarly, the average concentration of Hg in shoots was 77% less in plants supplied with inorganic Hg than organic form. Thus, organic form is absorbed and translocated more efficiently than the inorganic form in transgenic lines while no such difference was observed in untransformed plants. The reduction in Hg accumulation in shoots of both untransformed and transgenic lines at higher concentrations of external inorganic mercury in soil might be explained by the mechanism of action of Hg[II] itself. An earlier study carried out by Godbold and Huttermann (29) showed that the inorganic mercury (HgCl2 in particular) affects the plasma membrane causing injury to cells. On the other hand, organic mercury is rapidly moved across the membranes and affects organelle metabolism in the cytoplasm, in particular the chloroplasts.
FIGURE 3.
The relationship between shoot dry weight (mg/plant) and shoot Hg concentrations (µg g−1) of untransformed and chloroplast engineered pLDR-merAB and pLDR-merAB 3′UTR tobacco lines. The regression analysis between the dry weight and Hg concentration in tissues is derived from three different treatments with 100, 200, and 300 µM of either PMA or HgCl2.
To date, there is no report of a naturally occurring plant with the ability to accumulate Hg. Plants growing in heavy metal polluted soil often have evolved mechanisms to exclude the toxic metals from the cell (30). Chloroplast transgenic plants are able to accumulate high levels of Hg in either metal or organic form to levels surpassing the concentrations found in the soil. The data reflects that the accumulation and translocation from roots to shoots of the transgenic plants is dependent on the chemical form of Hg and its concentration in the soil. However, this is not the case for wild-type plants that were affected by even low cellular concentrations of Hg. The highly efficient absorption and accumulation of Hg in roots of transgenic lines may be due to the integrity of root cell metabolism resulting from plastid detoxification. Efficient translocation from root to shoot in the transgenic lines may facilitate the conversion of toxic organo-Hg and Hg2+ into less toxic Hg0 by making toxic-Hg available in large amounts to the chloroplast expressing merA and merB genes. In addition, transgenic plants showed a great potential for mercury volatilization as a result of expression of merA gene as discussed below.
Mercury Volatilization
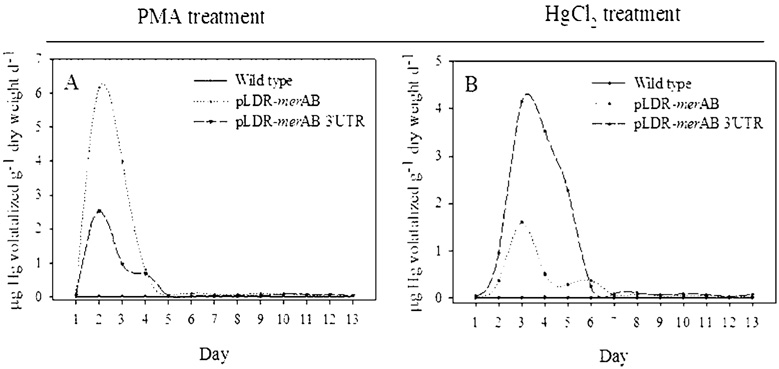
Volatilization of elemental mercury is the final step in the detoxification pathway of organic-Hg. Our results show that insertion of merA and merB genes into the tobacco plastid genome resulted in significant volatilization of Hg. Twenty-day-old tobacco plants grown in small pots were treated with 100 µM of Hg, either as PMA (organic form) or HgCl2 (inorganic form). After the addition of Hg, the entire plant–soil-pot was enclosed in a 3 L volume gastight acrylic volatilization chamber for the measurement of Hg volatilization (Figure 4). The rate of volatilization was measured for a period of 13 days. No volatile Hg was detected from untransformed plants supplied with PMA or HgCl2 (Figure 5 A, B). In contrast, the transgenic plants volatilized considerable amounts of Hg when supplied with PMA or HgCl2 (Figure 5A,B). Transgenic plants supplied with PMA attained a maximum rate of volatilization in two days; rates of volatilization decreased to the background levels by the fifth day (Figure 5A), probably by the complete absorption and detoxification of supplied PMA. Transgenic plants supplied with inorganic-Hg, on the other hand, attained a maximum rate of Hg volatilization in three days, the rate declining to background levels after six or seven days (Figure 5 B).
FIGURE 5.
Rates of Hg[0] volatilization from untransformed (solid line) and chloroplast engineered pLDR-merAB (dotted line) and pLDR-merAB 3′UTR (dashed line) tobacco plants grown for 13 days in soil amended with 100 µM of either PMA (A) or HgCl2 (B). Values shown are the average of three replicates.
The primary goal of this study was to characterize the phytoremediation capabilities of chloroplast transgenic plants, including mercury absorption by the root system, accumulation, translocation to the shoot system, and conversion of toxic-Hg forms into the less toxic elemental Hg, with the purpose of cleaning up Hg contaminated soil. However, no specific attempt was made to distinguish between volatilization occurring from roots or shoots or both systems. Higher uptake of Hg by transgenic roots may be the result of enhanced Hg volatilization. This explanation seems to be supported by previous studies that showed volatilization of elemental mercury enhanced by the function of merA in plant roots (31). In this study, tobacco plants (var. xanthi) were genetically modified through the nuclear genome by expressing the merApe9 gene. Their results of mercury volatilization by isolated organs indicated that the root system of the transgenic plants volatilized most of the reduced mercury (up to 5 times more than in shoots or leaves). Rugh et al. (32) succeeded in transforming a bacterial merA gene into the nuclear genome of yellow poplar plantlets; they reported that Hg volatilization was 10-fold higher when compared to the wild type, most likely from the plant root. A study by Bizili et al. (33) indicated that when roots of the merA and merB nuclear transgenics were supplied with 25 µM organomercurials in solution, elemental mercury was volatilized at an estimated rate between 14.4 and 85.0 µg Hg0 g−1 fresh biomass day−1. Zayed and Terry (24) also support this concept for Selenium volatilization.
It has been known that the chloroplast thylakoid membranes and photosynthesis are the main sites of action of Hg toxicity in plants (13, 14). We observed the effect of Hg in untransformed plants by chlorosis of leaves, ultimately resulting in death. However, the high levels of PMA and HgCl2 resistance and elemental Hg volatilization showed that the transgenic chloroplasts expressing MerA and MerB were active and protected plants from toxic effects of Hg. Both transgenic lines (with or without 3′ UTR) performed similarly in bioassays with similar levels of resistance, accumulation, and translocation of mercury, confirming that both enzymes are expressed at similar levels. This is supported by recent studies (34) that provide evidence that policistronic mRNAs containing two or more genes have increased stability over monocistrons in transgenic chloroplasts, even when they do not contain 3′UTR. As shown in our previous publication (16), merA / merB transcripts in both transgenic lines (with or without 3′UTR) were polycistrons (dicistrons and tricistrons) and had similar abundance, correlating well with the observation that both enzymes are expressed at similar levels.
In untransformed plants, yellowing of leaves observed after treatment with Hg was due to lack of organomercurial lyase and mercuric ion reductase in the cells, which in turn caused higher accumulation of Hg in chloroplasts. In contract, transgenic plants expressing both merA and merB genes via the chloroplast genome were able to enhance their tolerance by rapid volatilization of Hg, rapidly compensating for greater Hg uptake. From these observations, we envision two possible scenarios for Hg tolerance. Scenario 1: Mercury is detoxified in the root system: detoxification of mercury occurs in roots, and Hg0 is volatilized directly from roots. Support for this scenario comes from the fact that Hg concentrations are approximately 100 times greater in roots than in shoots. Kumar et al. (35) have shown that root proplastids in transgenic plants express about 70% of foreign protein as leaf chloroplasts. This, combined with the fact that mercury concentrations are higher in roots, suggests that the expression of merA and merB in root plastids may play a vital role in mercury detoxification. Scenario 2: Mercury is detoxified in shoots: mercury is transported from roots to shoots in an as yet unknown form. MerA and merB are expressed in transgenic chloroplasts, where the conversion of HgCl2 to Hg0 occurs. In this case, the greater part of mercury volatilization would be from shoots rather than from roots. In support of this is the fact that expression of merA and merB genes are expected to be extremely high in the chloroplasts because of their maximum copy number (up to 10000 copies of transgenes per cell). Therefore, low concentrations of mercury observed in shoots of chloroplast transgenic lines could be due to the fact that mercury is converted to Hg0 and volatilized so efficiently from shoots that mercury does not accumulate to a significant quantity. It is quite possible that both scenarios coexist.
Chloroplast genetic engineering system has several advantages including transgene containment via maternal maternal inheritance (36, 37), high levels of foreign gene expression (38) and engineering multiple genes or pathways in a single transformation event (16, 34). The present study shows that chloroplast genetic engineering offers an advantageous system for phytoremediation of mercury by protecting the chloroplast, which is the main site of mercury toxicity in plants. Expressing the merA and merB genes in leaf chloroplasts and proplastids in roots protects normal cell functions to allow Hg to move from one cell to the next, either by crossing the cell membrane or via plasmodesmata, until it reaches shoots. This effect could create a net influx of Hg from soil to the root system and then to green tissues. In untransformed plants, on the other hand, the greater toxicity of mercury negatively impacts root metabolism, disrupting normal cell functions, impeding the movement of Hg between cells and root uptake. Therefore, plastid transformation with merA and merB genes significantly improved the ability of transgenic lines to survive higher levels of external Hg. Successful engineering of phytovolatilization of mercury in the transgenic plant shows that it is possible to introduce an entire microbial pathway into plants without apparent disturbances in plant physiology and biochemistry (39).
The large biomass of the tobacco plant yielding up to 170 t of biomass per hectare (40), and the extensive root system should facilitate high levels of phytoremediation. Tobacco is a rapid system for scale up because a single plant produces up to one million seeds, which is adequate to plant more than 100 acres (with 10000 plants per acre, (41)). In addition, it is easy to engineer the tobacco chloroplast genome and to regenerate transgenic lines within a few months. Tobacco is a nonfood and nonfeed crop and it is self-pollinated, minimizing transgene escape and contamination of the food chain. Most importantly, maternal inheritance of the tobacco chloroplast genome should minimize escape of mer genes via pollen (36, 37). Furthermore, a cytoplasmic male sterility system for tobacco plants has been developed that inhibits formation of pollen (42). Thus, phytoremediation using tobacco transgenic lines is an environmental friendly approach. Because the insertion of the bacterial merA and merB genes into the chloroplast genome greatly increases the ability of the tobacco plants to extract and volatilize high concentrations of Hg, while maintaining adequate growth rate, large biomass and extensive root system, this method is ideal for phytoremediation. However, phytoremediation of Hg polluted soil is complex and multifactorial (depth and degree of contamination, climate, etc). Therefore, further research is needed to explore advantages of this method in polluted soil in field conditions.
Acknowledgments
Investigations reported in this article were supported in part by grants from USDA 3611-21000-017-00D and NIH R01GM 63879 to H.D.
Literature Cited
- 1.Meagher RB. Phytoremediation of toxic elemental and organic pollutants. Curr. Opin. Plant Biol. 2000;3:153–162. doi: 10.1016/s1369-5266(99)00054-0. [DOI] [PubMed] [Google Scholar]
- 2.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 3.Patra M, Sharma A. Mercury Toxicity in Plants. Bot. Rev. 2000;66:379–422. [Google Scholar]
- 4.Goldman LR, Shannon MW. The Committee on Environmental Health. Technical Report. Mercury in the environment: Implications for pediatricians. PEDIATRICS. 2001;108(1):197–205. doi: 10.1542/peds.108.1.197. [DOI] [PubMed] [Google Scholar]
- 5.EPA. Clean Air Mercury Rule. Washington, DC: U.S. Environmental Protection Agency; 2005. Mar, [Google Scholar]
- 6.Kärenlampi S, Schat H, Vangronsveld J, Verkleij JAC, Lelie D, Mergeay M, Tervahauta AI. Genetic engineering in the improvement of plants for phytoremediation of metal polluted soils. Environ. Pollut. 2000;107:225–231. doi: 10.1016/s0269-7491(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 7.Meagher RB, Rugh CL, Kandasamy MK, Gragson G, Wang NJ. Engineered phytoremediation of mercury pollution in soil and water using bacterial genes. In: Terry N, Bañuelos G, editors. Phytoremediation of Contaminated Soil and Water. Berkeley CA: Ann Arbor Press Inc.; 2000. pp. 201–209. [Google Scholar]
- 8.Pilon-Smits E. Phytoremediation. Annu. Rev. Plant Biol. 2005;56:15–39. doi: 10.1146/annurev.arplant.56.032604.144214. [DOI] [PubMed] [Google Scholar]
- 9.Barrineau P, Summers AO. A second positive regulatory function in the mer (mercury resistance) operon. Gene. 1983;25:209–221. doi: 10.1016/0378-1119(83)90225-1. [DOI] [PubMed] [Google Scholar]
- 10.Jackson WJ, Summers AO. Biochemical characterization of HgCl2-inducible polypeptide encoded by mer operon R100. J. Bacteriol. 1982;151:962–970. doi: 10.1128/jb.151.2.962-970.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugh CL, Wilde HD, Stack NM, Thompson DM, Summers AO, Meagher RB. Mercuric ion reduction and resistance in transgenic Arabidopsis thaliana plants expressing a modified bacterial merA gene. Proc. Natl. Acad. Sci. U.S.A. 1996;93(8):3182–3187. doi: 10.1073/pnas.93.8.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard MB, Heaton ACP. Strategies for the engineered phytoremediation of toxic element pollution: mercury and arsenic. J. Ind. Microbiol. Biotechnol. 2005;32:502–213. doi: 10.1007/s10295-005-0255-9. [DOI] [PubMed] [Google Scholar]
- 13.Bernier M, Popovic R, Carpentier R. Mercury inhibition of photosystem II. FEBS Lett. 1993;32:19–23. doi: 10.1016/0014-5793(93)80612-x. [DOI] [PubMed] [Google Scholar]
- 14.Kupper H, Kupper F, Spiller M. Environmental relevance of heavy metal substituted chlorophylls using the example of water plants. J. Exp. Bot. 1996;47:259–266. [Google Scholar]
- 15.Sen AK, Mondal NG. Silvinia natans as the scavenger of Hg (II) Water Air Soil Pollut. 1987;34:439–446. [Google Scholar]
- 16.Ruiz ON, Hussein HS, Terry N, Daniell H. Phytoremediation of organomercurial compounds via chloroplast genetic engineering. Plant Physiol. 2003;132:1344–1352. doi: 10.1104/pp.103.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh OV, Labana S, Dey G, Hiraja R, Jain RK. Phytoremediation: an overview of metallic ion decontamination from soil. Appl. Microbiol. Biotechnol. 2003;61:405–412. doi: 10.1007/s00253-003-1244-4. [DOI] [PubMed] [Google Scholar]
- 18.Suszcynsky EM, Shann JR. Phytotoxicity and accumulation of mercury in tobacco subjected to different exposure routes. Environ. Toxicol. Chem. 1995;14(1):61–67. [Google Scholar]
- 19.Heaton ACP, Rugh CL, Kim T, Wang NJ, Meagher RB. Toward detoxifying mercury-polluted aquatic sediments with rice genetically engineered for mercury resistance. Environ. Toxicol. Chem. 2003;22:2940–2947. doi: 10.1897/02-442. [DOI] [PubMed] [Google Scholar]
- 20.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- 21.Hams GA. Determination of mercury in blood and urine by Cold Vapor AAS using the VGA-77.AAInstruments at work. Varian: Palo Alto, CA: Varian operation manual, AA-126; 1997. pp. 1–4. [Google Scholar]
- 22.Methods for Chemical Analysis of Water and Wastes. Washington, DC: U.S. Environmental Protection Agency; 1992. EPA Method 3010A. [Google Scholar]
- 23.Munns RK, Holland DC. Determination of mercury in fish by flamless atomic absorption. J. Assoc. Off. Anal. Chem. 1971;54:202–205. [PubMed] [Google Scholar]
- 24.Zayed AM, Terry N. Selenium volatilization in broccoli as influenced by sulfate supply. J. Plant. Physiol. 1992;140:646–652. [Google Scholar]
- 25.Zayed A, Lytle CM, Terry N. Accumulation and volatilization of different chemical species of selenium by plants. Planta. 1998;206:284–292. [Google Scholar]
- 26.Greger M, Wang Y, Neuschütz C. Absence of Hg transpiration by shoot after Hg uptake by roots of six terrestrial plant species. Environ. Pollut. 2005;134(2):201–208. doi: 10.1016/j.envpol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Mosso HJ. Phytotoxicity Test of Mercury Phenyl Acetate in Wheat Seeds (Triticum aestivum) Facultad de Agronomia: Buenos Aires: Universidad de Buenos Aires; 1979. [Google Scholar]
- 28.Shimizu TMM, Nakamura M. Effect of lead and mercury on the rice plant and some vegetables, Bull. Osaka. Agric. Res. Center. 1975;12:117–129. [Google Scholar]
- 29.Godbold DL, Huttermann A. The uptake and toxicity of mercury and lead to spruce seedlings. Water, Air, Soil Pollut. 1986;31:509–515. [Google Scholar]
- 30.Meharg AA, Macnair MR. Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J. Exp. Bot. 1992;43:519–524. [Google Scholar]
- 31.He HY, Sun JG, Feng XZ, Czako M, Marton L. Differential mercury volatilization by tobacco organs expressing a modified bacterial merA gene. Cell Res. 2001;11:231–236. doi: 10.1038/sj.cr.7290091. [DOI] [PubMed] [Google Scholar]
- 32.Rugh CL, Senecoff JF, Meagher RB, Merkle SA. Development of transgenic yellow poplar for mercury phytoremediation. Nat. Biotechnol. 1998;16(10):925–928. doi: 10.1038/nbt1098-925. [DOI] [PubMed] [Google Scholar]
- 33.Bizily S, Rugh CC, Meagher RB. Phytoremediation of hazardous organomercurials by genetically engineered plant. Nat. Biotechnol. 2000;18:213–217. doi: 10.1038/72678. [DOI] [PubMed] [Google Scholar]
- 34.Quesada-Vargas T, Ruiz ON, Daniell H. Characterization of Heterologous multigene operons in transgenic chloroplast: transcription, processing and translation. Plant Physiol. 2005;138:1746–1762. doi: 10.1104/pp.105.063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Dhingra A, Daniell H. Plastid expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots and leaves confers enhanced salt tolerance. Plant Physiol. 2004;136:2843–2854. doi: 10.1104/pp.104.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniell H. Transgene containment by maternal inheritance: Effective or elusive. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6879–6880. doi: 10.1073/pnas.0702219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniell H. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 2002;20:581–587. doi: 10.1038/nbt0602-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeCosa B, Moar W, Lee SB, Miller M, Daniell H. Hyper-expression of the Bt Cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat. Biotechnol. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persidis A. Agricultural biotechnology. Nat. Biotechnol. 1999;17:612–614. doi: 10.1038/9940. [DOI] [PubMed] [Google Scholar]
- 40.Cramer CL, Boothe JG, Oishi KK. Transgenic plants for therapeutic proteins: linking upstream and downstream strategies. Curr. Top. Microbiol. Immunol. 1999;240:95–118. doi: 10.1007/978-3-642-60234-4_5. [DOI] [PubMed] [Google Scholar]
- 41.Arlen PA, Falconer R, Cherukumilli S, Cole A, Cole AM, Oishi KK, Daniell H. Field production and functional evaluation of chloroplast-derived interferon-α2b. Plant Biotechnol. J. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz ON, Daniell H. Engineering cytoplasmic male sterility via the chloroplast genome. Plant Physiol. 2005;138:1232–1246. doi: 10.1104/pp.104.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]