Abstract
Purpose
Report the results of using a permanently implantable dosimeter in radiation therapy: determine specific adverse events, degree of migration, and acquire dose measurements during treatment to determine difference between expected and measured dose.
Methods and Materials
DVS (Dose Verification System) is a wireless, permanently implantable MOSFET dosimeter using a bidirectional antenna for power and data transfer. The study cohort includes 36 breast (33 received 2 devices) and 29 prostate (21 received 2 devices) cancer patients. A total of 1783 and 1749 daily dose measurements were obtained on breast and prostate patients, respectively. The measurements were compared to the planned expected dose. Bi-weekly CT scans were obtained to evaluate migration and NCI CTCv3 used to evaluate adverse events.
Results
Only grade I/II adverse events of pain and bleeding were noted. There were only four instances of dosimeter migration of >5mm due to known factors. A deviation of ≥7% in cumulative dose was noted in 7 of 36 (19%) for breast cancer patients. In prostate cancer patients, a ≥7% deviation was noted in 6 of 29 (21%) and 8 of 19 (42%) during initial and boost irradiation, respectively. The two patterns of dose deviation were random and systematic. Some causes for these differences could involve organ movement, patient movement and/or treatment plan considerations.
Conclusions
The DVS was not associated with significant adverse events or migration. The dosimeter can measure dose in situ on a daily basis. The accuracy and utility of the DVS complements current IGRT and IMRT techniques.
Keywords: Implantable dosimeter, in-situ radiation, dosimetry prostate and breast cancer
Introduction
One of the most important tenets in oncology is to minimize toxicity and maximize malignant cell kill. The desire to hold to this tenet has been particularly important in radiation therapy because of the long term effects of radiation on normal tissue. Recent technological advances (including 3-D conformal therapy, intensity modulated radiation therapy [IMRT]) have allowed more complex and conformal treatment plans in order to deliver higher doses to smaller target volumes and avoid critical structures. In so doing, steep dose gradients are created making target coverage and dose delivery important. Several important factors including target motion and deformation, patient alignment and treatment delivery can alter the position of the target (1). Ultimately, the identification of an anatomical target and verification of the delivered dose become paramount to the physician as the nexus for improved therapeutic outcome.
Image guided radiation therapy techniques allow for more precise identification of the target volume. IGRT can reduce patient positioning errors due to daily setup variations and organ movement (2). Interfractional movement has been studied in bladder (3), rectum (4), gynecological (5) and prostate (6,7,8) while intrafractional movement has been investigated in lung (9), liver (10) pancreas (11) and prostate (12,13,14). Although there is general agreement on the importance of IGRT particularly when involving IMRT, there does appear to be uncertainty regarding the features and approaches of competing IGRT systems. In order to verify the validity of these techniques and be assured of achieving improved therapeutic ratio, knowing the dose delivered to the target would be beneficial. Errors in delivery of radiation and the efficacy of the IGRT positioning system can be known by performing direct dose measurements.
Reports of dose measurements are, except for a few cases, limited to work in phantoms or by measuring entrance and exit dose, the latter of which is not used extensively for IMRT because of its complexity. Regardless of the ex vivo technique used, the true in-situ dose delivered to the target volume in the patient has not been directly verified. The ability to monitor the delivered dose in situ at the target tissue(s) (15) introduces the next phase in radiation oncology that of dose guided radiation therapy (DGRT).
A previous report (16) introduced the first implantable dosimeter capable of measuring the radiation dose in-situ. The accuracy, safety and functionality of the device as well as the results of the pilot study have been reported (16).
This report contains the results of the clinical implementation of the DVS dosimeter from a pivotal study in breast and prostate patients and breast cancer patients from the initial pilot study. Based on the results of these studies, the implantable dosimeter has been cleared by the United States Food and Drug Administration (FDA) for use in breast and prostate cancer patients.
Methods and Materials
a) Study Design
The current analysis is based on an FDA approved pivotal and pilot study protocols, conducted at four institutions including Rex Cancer Center, Duke University School of Medicine, Wake Forest University School of Medicine and the Texas Cancer Clinic. The Institutional Review Board (IRB) of each institution approved the study. The nature of the procedure was explained and consent was obtained from each patient prior to insertion of the device. The primary objective of the study was to evaluate the safety (migration and frequency of adverse events) of the device after implantation and secondarily its ability to measure the delivered dose at the site of implantation. FDA study guidelines did not permit alterations of treatment parameters or methodology.
b) DVS Dosimeter
The Dose Verification System (DVS®, Sicel Technologies Inc., Morrisville, NC) is a wireless dosimeter designed to be permanently implanted in vivo to record the actual dose to the target on a daily basis. The structure and technical aspects of the dosimeter system have been previously described (16, 19). In summary, the DVS is a wireless MOSFET based dosimeter designed to be permanently implanted in situ at the tumor volume and provides a method for verifying the actual dose to the target on a daily basis. A portable telemetric readout system couples to the dosimeter antenna powering the dosimeter and permitting data transfer. Accuracy of the dosimeter used for the patient clinical trial was validated and found to be within the manufacturer specification of 5% (2σ) for these dosimeters (17). The active area of the MOSFET was 690μm × 15μm and can be considered a point detector. The DVS modulates the magnetic field generated by the reader to transmit the measured dose data.
The device characteristics, in vitro calibration methodology, and test results have been previously reported (16-19). The dosimeters used in the clinical trials were individually calibrated to perform within 5% (2σ) up to 80Gy and had a capsule size of 27 mm × 3.1 mm. In vitro testing found minimal day to day variation with reproducibility of ±2% (17). Another in vitro study included a heterogeneous phantom and different positions of the dosimeter in the isodose configuration showing a performance within ±5% when compared to the predicted dose from the planning system (16). Currently the DVS is commercially available in a smaller capsule size (20 mm × 2.1 mm) with lot calibration with an accuracy of <5.5% (2σ) up to 20 Gy and < 6.5% (2σ) up to 74 Gy). Phantom testing has verified the accuracy and performance of the new dosimeter for different irradiation conditions (18)
c) Study Population
The study population included 36 breast (30 from pivotal and 6 from pilot studies) and 29 prostate cancer patients. All patients were required to have biopsy proven malignancy (resectable or unresectable) and to be candidates for definitive radiation therapy. It was recommended but not required that each patient be implanted with two dosimeters - one associated with the malignant tissue and a second associated with the normal tissue being irradiated. Additional criteria included a prothrombin time of 10.2 to 12.2s and a partial thromboplastin time of 24 to 36s.
d) Breast Implantation
Two DVS devices were implanted; one in the tumor bed and, if possible, one in the opposite side of the same breast, either at the time of lumpectomy or in the radiation therapy department using local anesthesia and CT guidance. When implanted at the time of lumpectomy, the device was placed in a polyethylene mesh and sutured in the tumor bed. A second dosimeter was then placed in the normal tissue of the opposite quadrant with a specially designed trochar. To provide appropriate build up and comfort, it was recommended that the DVS dosimeter be implanted ≥ 3cm from the skin surface in the breast cancer patients.
e) Prostate Implantation
DVS dosimeters were inserted in the prostate either under monitored anesthesia care (MAC) or with a local anesthetic, using a transperineal approach. One device was placed at or inside the prostate capsule and defined as the Gross Tumor Volume (GTV). The second device was placed on the opposite side of the prostate 0.5 to 2.0 cm lateral to the prostate capsule but within the treatment volume and designated as normal tissue.
f) Simulation and Dosimetry
After implantation, simulation was generally delayed for at least 3-5 days to allow the dosimeter to become anchored in the tissue. All patients underwent computed tomography (CT) simulation in the treatment position. Once the DVS implant was identified, 1- 3 mm slices were obtained through the device beginning 2 cm cephalad and continuing to 2 cm caudal to the dosimeter. Sensor migration could lead to a discrepancy between measured and expected dose values; therefore the specific CT protocol was repeated every 2 weeks during treatment to determine any degree of migration. Migration of the device was evaluated by an independent board certified radiologist. Migration was defined as a change in the device location of greater than 5 mm from the initial CT, not attributable to patient movement or tumor shrinkage. The protocol required re-planning which established a new predicted dose, but did not allow for the implementation of a new treatment plan.
Standard treatment planning procedures were followed. The three main planning characteristics – heterogeneity correction, type of planning system and daily localization were not stipulated in the protocol. However, the planning details for the prostate patients have been reported (30). The active area of the dosimeter was obtained by using CT scout views and digitally reconstructed radiographs. The position of the MOSFET dosimeter within a specific isodose line was identified and designated in the treatment plan via a point of interest, to obtain the expected radiation dose. The only requirements for the delivery of radiation were that the patients receive at least 4 weeks of treatment and daily fractionated photon irradiation in the range of 150-250 cGy per fraction. The DVS was interrogated before and after each fraction of radiation to obtain the dose delivered to the sensor. The measured results were recorded and compared with the expected dose of radiation.
g) Clinical endpoints
This report represents the results of 36 breast patients and 29 prostate cancer patients. Thirty-three breast patients had 2 dosimeters inserted and 21 prostate patients had 2 dosimeters inserted. In each case, daily measured dose values were compared with expected plan values. Patients were evaluated at the time of insertion of the DVS and each week for adverse events attributable to the implanted dosimeter. All data was collected and referred to the Duke Clinical Research Center for independent registration and evaluation.
h) Statistics
A data point is defined as a single reading from any one dosimeter. Accordingly, the results presented for breast patients represents 1783 data points (during photon beam only) and for prostate patients, 1749 data points.
Cumulative percent difference is the percent difference between cumulative planned and measured doses. The cumulative measured dose was an accrual of daily fractional radiation dose measurements to the tumor or normal tissue site as obtained by the DVS dosimeter while the cumulative planned dose was the total prescribed dose to the MOSFET position. The percent difference between cumulative measured and planned doses was calculated for each dosimeter at tumor or normal tissue locations for each patient in the study.
Fractional dose difference (or fractional percent difference) was calculated for each patient as the difference between the daily fractional planned dose and the daily dose measurement obtained by the DVS dosimeter. The daily fractional planned dose was obtained from the treatment plan based on the patient's prescribed treatment and the MOSFET location. The numbers of fractional percent differences greater than or equal to 5 and 7% were counted (not mutually exclusive) for each patient. Those values were then divided by the total number of fractions for each patient, giving the percent frequency at which fractional dose differences greater than or equal to 5 and 7% occurred per patient.
Results
a) Safety
The primary objective of this study was to determine the safety of the device following implantation. Safety is defined as the degree of migration of the device following implantation and any associated adverse events. The National Cancer Institute Common Toxicity Criteria v3 (20) were utilized to evaluate adverse events. There were a total of 119 dosimeters inserted.
The most common adverse events associated with implantation of the device in the prostate patients were pain and bleeding as noted in Table 1. They were reported to be mild to moderate by patients and surgeons. All events were considered within the range of expected discomfort associated with the surgical procedure. Six of the eight events resolved within 24 hours and two by 23 days. There was one instance of migration of 6 mm attributed to the simulation being done immediately following the implant and not allowing sufficient time for the normal process of retention to occur.
Table 1. Safety Analysis.
| Site | Adverse Events* | Migration > 5 mm | |||
|---|---|---|---|---|---|
| Pain | Bleeding | ||||
| # Patients | Grade | # Patients | Grade | ||
| Prostate | |||||
| Surgical | 2 | 1 | 3 | 1 | 1 |
| Non-surgical | 3 | 1 | 0 | ||
| Breast | |||||
| Surgical | 2 | 1 | 1 | 1 | 3 |
| Non-surgical | 2 | 2 | |||
NCI/RTOG CTCv3
Grade (Gr) 1 = minimal; 2 = moderate
Surgical – as reported by surgeon
Non-surgical – as reported by patient
The 5 adverse events associated with breast implantation were directly attributable to the device. All events resolved within 24 hours. There were two instances of post surgical adverse events. One event was related to improper placement of the device and the second to a retained suture. There were three episodes of migration. One case was attributed to the shallow placement of the device (noted above), a second due to soft tissue displacement associated with evolving post operative changes and a third, noted as movement of 8 mm by serial CT scans, indicated conformational differences led to an apparent shift of the dosimeter with respect to the appointed landmarks.
b) In vivo dose measurements
Breast Patients
All patients were treated with tangential fields to the entire breast. The data represents dose measurements during photon irradiation. No electron measurements are reported at this time. All patients had a device placed in the tumor bed; three did not have a device implanted in the normal tissue.
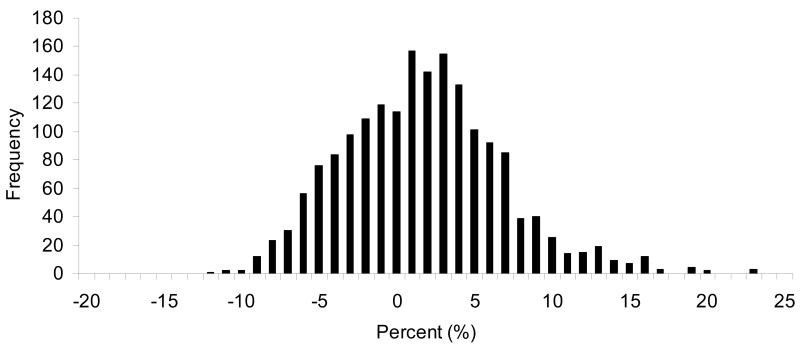
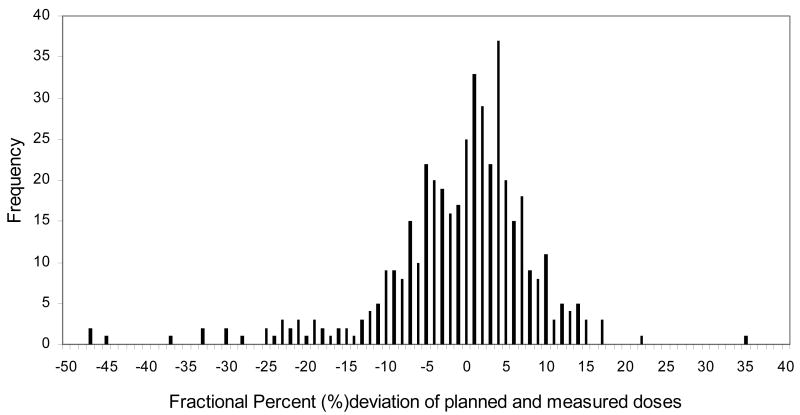
The distribution of observed differences between measured and predicted doses of all daily fractions are shown in Fig. 1. The majority of measurements (1111 or 62%) were within 5% of the expected dose, however, 38% of the fractions differed from the expected by ≥5% and 19% differed by ≥ 7%.
Fig.1.
The frequency of the percent difference between planned and measured daily dose in the breast cancer patients during whole breast irradiation.
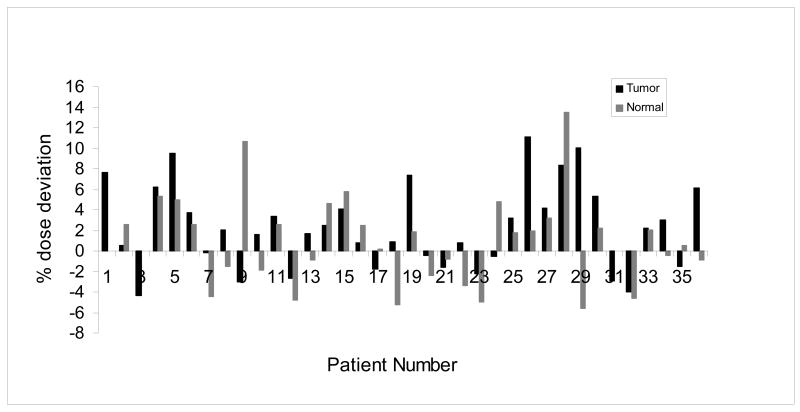
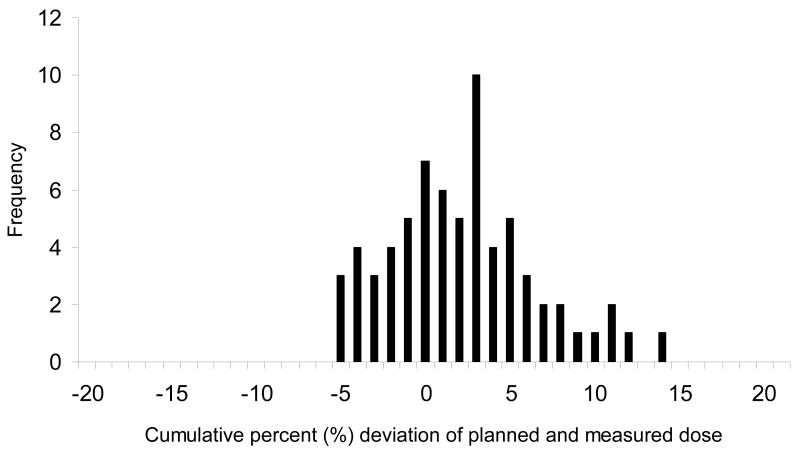
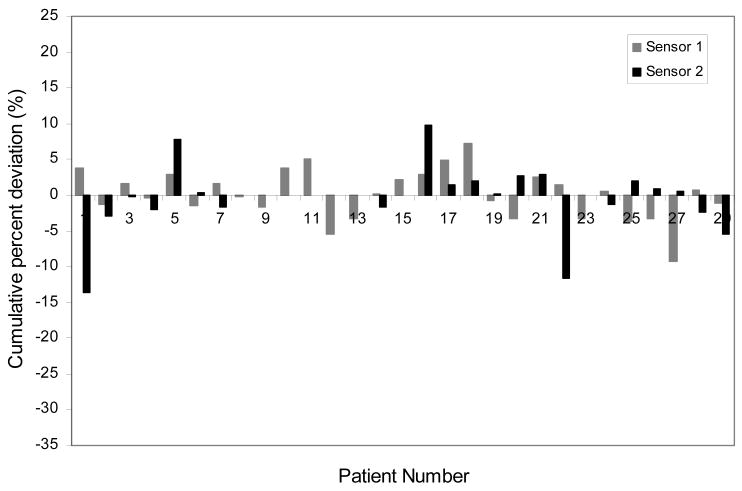
Figure 2 depicts the cumulative percent difference in each patient including the tumor and normal sites. There are 13 patients (36%) who had a ≥5% cumulative percent difference in at least one site. There was ≥7% difference in cumulative dose in 7 of the 36 (19%) patients and one patient with ≥14% difference. Fig.3 is a histogram of the cumulative percent error which graphically displays the tendency toward the delivery of higher doses than expected in the breast group as a whole.
Fig.2.
The cumulative percent difference between measured and planned dose from the dosimeters implanted in the tumor bed and normal tissue of each patient.
Fig.3.
Frequency of cumulative percent difference between measured and planned dose of all breast cancer patients.
Prostate patients
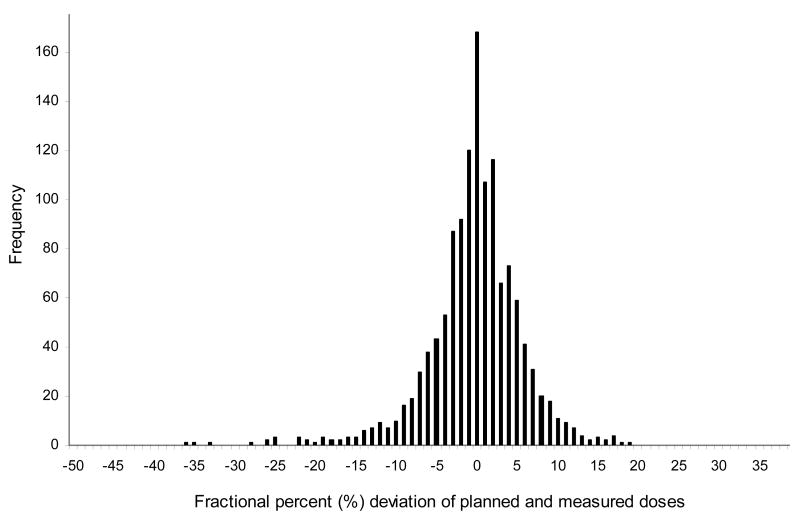
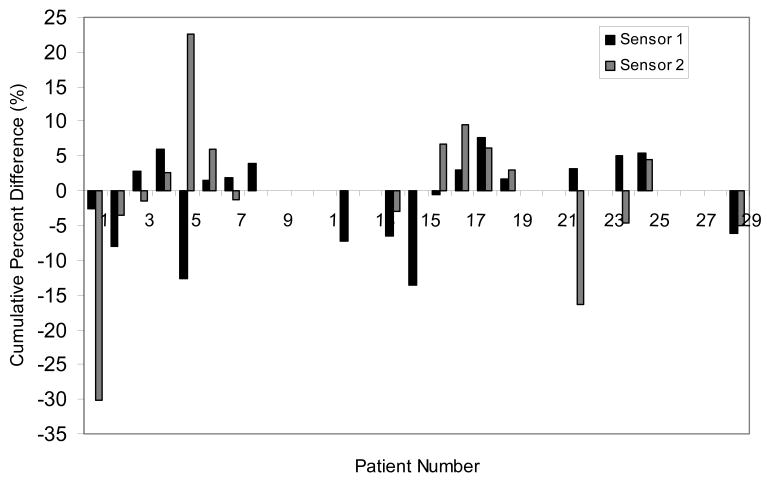
A total of 50 devices were implanted in 29 patients; 21 patients received 2 dosimeters and 8 patients received one. A total of 1749 data points were obtained - 1308 data points during treatment of the primary field and 441 data points during delivery of the boost or reduced field. The distribution of observed differences between measured and predicted doses of all daily fractions during large field are shown in Fig.4. A total of 33% of the fractions differed from expected dose by ≥5% and 19% by ≥7% and some ≥20%. The cumulative percent difference between the observed and measured doses for each patient is shown in Fig.5. Thirty-one percent (9/29 patients) had a ≥5% absolute difference and 21% (6/29 patients) demonstrated a ≥7% absolute difference during large field radiation.
Fig.4.
The frequency of the percent difference between planned and measured daily doses during large field irradiation in all prostate patients
Fig.5.
The cumulative percent difference between measured and planned dose during large field irradiation in all the prostate patients.
Figure 6 shows the frequency distribution during the boost field when field sizes were reduced and treatment margins of 1.0 to 1.5 cm were frequently employed. A total of 51% of fractions differed from expected dose by ≥5% and 35% differed by ≥7%. Figure 7 shows the cumulative percent difference for prostate boost treatment only. A total of 19 patients received reduced field (boost) irradiation; 15 patients or 79% had values that differed by ≥5% and 8 patients or 42% had ≥ 7% difference from expected. It is also important to note that in all but one case, the dosimeter was within the treatment isodose line. A summary of the percent variation of the data on all the patients is presented in Table 2.
Fig. 6.
The frequency of the percent difference between planned and measured daily doses during boost irradiation in all the prostate patients.
Fig. 7.
The cumulative percent difference between measured and planned dose during boost field irradiation in all prostate patients.
Table 2. Summary of Percent Difference Between Expected and Measured Dose.
| Site | Fractional Percent * | Cumulative Percent + | ||
|---|---|---|---|---|
| ≥ 5% | ≥ 7% | ≥ 5% | ≥ 7% | |
| Breast | 38 | 19 | 36 (13/36) | 19 (7/36) |
| Prostate | ||||
| Large Field | 33 | 19 | 31 (9/29) | 21 (6/29) |
| Boost | 51 | 35 | 79 (15/19) | 42 (8/19) |
Cumulative numbers are taken as percent of patients.
Fractional numbers are taken as percent of fractions.
Total number of fractions for breast patients was 1783.
Total number of fractions for prostate was 1749 (large field = 1308, boost = 441).
c) Patterns of Dose Differences
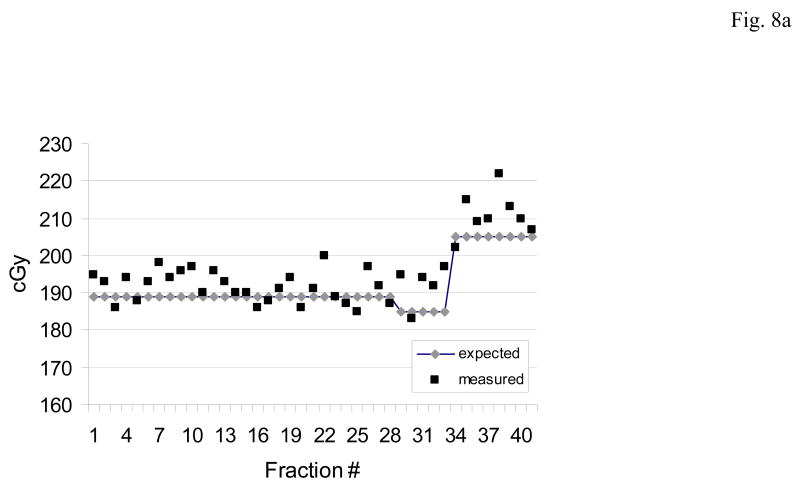
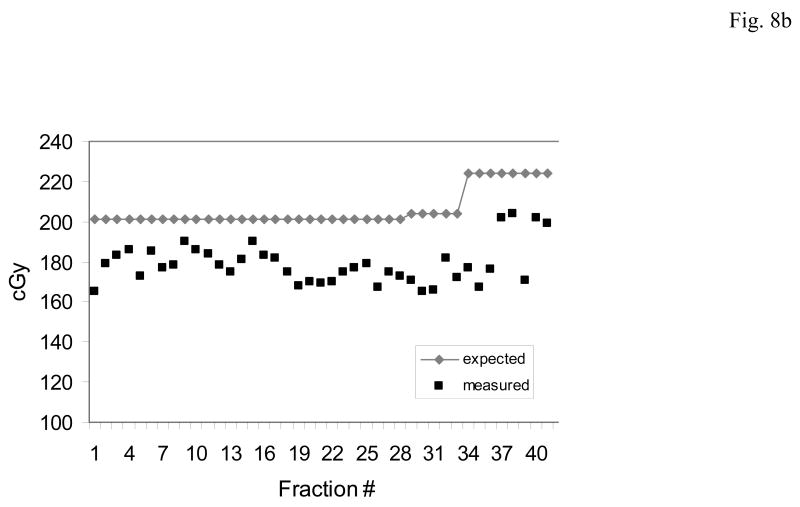
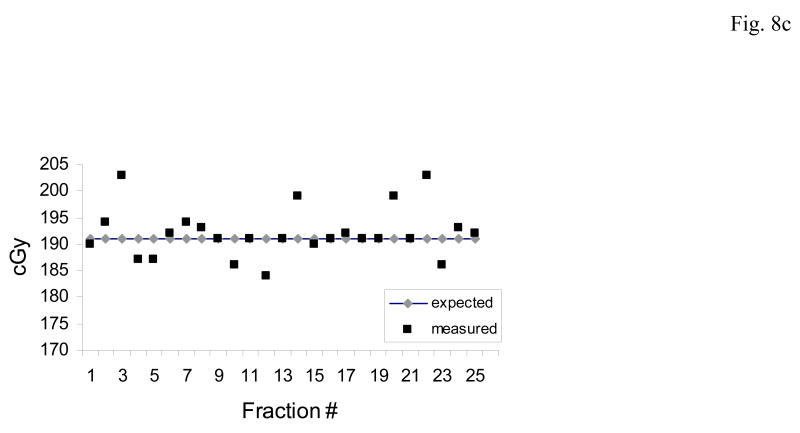
Figures 8a,b,c illustrate two patterns of dose measurements observed in several prostate patients. In each case the expected (blue) and measured (red) doses are shown for each day.
Fig. 8.
The predominant patterns of daily dose measurements observed with the implanted dosimeter.(a) An acceptable pattern where the measured dose differs < 5% from the planned dose. (b) Systematic pattern: where the measured dose is consistently higher or lower than the planned dose. The systematic pattern usually results in a cumulative dose difference greater than 7%. (c) Random pattern: when there is no definite pattern in measured dose, rather the measurements may vary from higher than expected (day 3) or lower (day 12). The random pattern usually will not result in a cumulative dose difference of greater than 5%.
Fig. 8a illustrates what would be considered an acceptable result (i.e. dose measurements within the 5% acceptable range). The expected dose in this patient was 187.0 cGy on the majority of days this was delivered. Of note is the change in expected dose on days 29 and 34 which corresponded to a change from large field to boost field and the recognition of the change by the dosimeter. The cumulative dose measurement in this patient was +1.40% of expected.
Fig. 8b is representative of a systematic pattern of dose deviations. In this case the expected dose is 200.0 cGy which increased due to field change on day 34. The measured dose is consistently or systematically low throughout the course of treatment and followed the change in expected dose during the boost field treatment. The cumulative dose delivered was -11.64% of expected.
Fig. 8c demonstrates a random or inconsistent pattern in dose deviations. Although random measurements ≥ 7% of expected are noted on days 3, 14, 20 and 22, there was little effect on the cumulative dose which was -0.54% of expected.
Discussion
As this report represents the initial presentation of complete data from the pivotal study, specific observations related to variability in measured versus expected dose and the relevance to current radiotherapeutic practice will be emphasized:
a) The pivotal study verified the safety of the implanted device
There were only Grade 1 (minimal) and moderate adverse events associated with the implanted device, and the Grade 1events occurred primarily at the time of the insertion of the device. The incidence of migration was low, occurring in only 4 of 119 implanted devices and could be explained.
b) Approximately 20% of all patients had ≥ 7% difference in cumulative dose delivered
The variability of daily readings occurred sporadically during treatment in most patients. The percent frequency of ≥7% variability was 19, 19 and 35% in the breast, prostate (large field) and prostate (boost) respectively. The degree of variability during daily treatment did not translate into as high a percent of patients with cumulative dose difference which was 19, 21 and 42%. Although the degree of variability was similar in both the breast and prostate patients (19 and 21% respectively) the cause(s) may be different since one would not expect the same degree of uncontrollable organ movement to occur in breast (due to breathing) as in prostate. In breast patients, set up and movement of the patient would predominate. The major variation from expected dose is seen in prostate patients during treatment of reduced fields (most treated with IMRT), where tight margins were employed. In combination with organ movement, this could result in significant variation in delivered dose. Additional measurements and comparisons with phantoms would help evaluate the main causes for the discrepancy and would be of additional interest. However, the goal of the study was to summarize the initial findings on a large patient population and indicate how the dosimeter can help alert that something in the treatment delivery is not going as planned. This information can help physicians review the treatment plan, patient setup and equipment functionality to evaluate and possibly find the cause of the discrepancy.
Previous reports of in-vivo dose measurements included dosimeters placed daily in the anal-rectal lumen which limited the number of measurements made. The results of those studies showed discrepancies of 7% with diodes (21) and 5.8% with thermoluminescent dosimeters (22) between the measured and predicted dose and 6.5%+/- 21.6 with an ion chamber (23). The latter increased to > 30% if the probe was related to posterior dose gradient. The current study noted a higher percent of dose differences than were reported by others (21, 22,23). The primary differences were that the previous studies utilized a small number of observations, rigid systems and placement of the measuring devices in lumens. In contrast, the DVS dosimeter, placed in-situ and more reflective of the daily clinical processes of a patient receiving radiation therapy and therefore subject to the same influences affecting patient preparation, set up and organ movement. The current show the importance of verifying the actual dose delivered to the target during irradiation of malignant and normal tissue.
c) The variability in readings can occur in a random or systematic fashion
Two types of discrepancies were encountered in this study: random and systematic. Random variability refers to an inconsistent pattern of higher or lower than expected dose measurements. Systematic variability refers to dose measurements that are consistently lower or higher than expected and generally observed in the first 3-5 fractions.
Potential cause(s) for systematic variation particularly during large field irradiation include patient set up, treatment planning error, and use of heterogeneity correction factors in the treatment plan and/or tight margins. During reduced field irradiation with IMRT, variation is most likely related to organ movement, deformity, patient set up or inconsistent preparation of bowel or bladder in the case of prostate patients. The systematic pattern was also noted by Kupelian et al (24) in their initial report on real – time monitoring of the prostate during external radiotherapy. Others also noted shifts (25) and organ deformity of the prostate (26) More recently Yue et al, (27) utilizing a 4DCT scanning system, noted the potential for intrafractional motion during irradiation of both whole and partial breast irradiation.
Organ movement and deformity are events that can be inconsistent and not easily controlled or anticipated during a course of treatment but can be suggested with evidence of the final dose delivered. One of the major advantages of DVS is early detection of any dose deviation. Therefore the ability to address and correct for the cause of the discrepancy is important. Accordingly, specific guidelines have been proposed as a means of determining potential causes for the discrepancies. The guidelines are divided into three broad categories including a) evaluate the treatment plan including dose value and scaling, heterogeneity correction factors, dose gradient, b) evaluate the patient setup including patient position, SSD and separation, patient preparation and treatment margins and c) verify equipment function including linac output and report and verify transfer data.
The DVS addresses the concept of individualized therapy that is aligned with IGRT/IMRT as well as dose guided and adaptive radiotherapy. The implantable dosimeter is designed to measure the dose delivered to the target(s), verify treatment plans, ensure the delivery of the dose necessary to maximize tumor control, and minimize side effects and alert the physician to the observed difference in dose.
d. Dose variation and IGRT techniques
The DVS compliments IGRT by acting as a fiducial marker as well as a dosimeter, thereby verifying the accuracy of IGRT. There were 18 patients in the prostate group reported by Beyer et al (30) in whom IGRT methods were incorporated (2U/S, 3 CBCT/13MV imaging and fiducial markers). No clear relationship was correlated between predicted and measured dose and IGRT method. The small number of patients precludes any definitive reason for the lack of correlation, although it may be related to IGRT techniques (28.29) organ or patient movement. However the consistency in results across diverse institutions with different techniques and treatment planning systems indicates that this may represent the norm.
The importance of consistency and accuracy in delivering dose is embodied in the concept of dose response curves (sigmoid) which describe the relationship between dose, tumor control and outcome as well as dose and complications from radiation. The literature is replete with good objective data on dose response to radiation of malignant tissue obtained from tissue culture and animal studies. Clinically, dose response curves have been suggested for tumors of the larynx (31,32), Hodgkin's lymphoma (33) and breast (34) which have relied on clinical response or local control rates and not objective measurement of dose delivered. The dose response curve for an individual site can be steep and a 7% or greater difference in cumulative dose delivered can be significant. Hanks et al (35) and more recently Eade et al (36) reported on dose response in prostate. Eade (36) described the dose groups as <70Gy, 70-74.9 Gy, 75-79.9 Gy and ≥80 Gy. The 5 year estimates for freedom from biochemical failure for the four dose groups were 70%, 81%, 83%, and 89% respectively. The results indicate a steep dose response curve and pertinent to the current study a difference in cumulative dose ≥7% could move the patient down on the dose response curve to a less favorable outcome, e.g. a patient assigned to receive 80.0Gy receiving >7% less dose or <75Gy, would have a 6% less chance for biochemical control.
Clinically, the dose response curves generated are by inference or the absence of in situ data on dose delivered. Variability between predicted and measured dose does occur and must be considered when interpreting dose response curves without associated in situ data. In addition, dose measurements made in situ should help to identify more definitive and effective dose limits to maximize tumor control and minimize normal tissue toxicity.
Conclusions
The implantation of the DVS dosimeter caused 8% of patient to experience GrI level of either pain, which lasted one day, or bleeding, which resolved with in 30 minutes post implantation. Dose measurements showed that when using large radiation treatment fields, the cumulative dose difference between delivered and planned was 20%, which increased to 42% with cone-down fields. The discrepancy is most likely due to tight field margins, organ movement and deformity. Specific patterns of variability can be recognized early and addressed in order to allow a consistent delivery of a tumoricidal dose to the malignant tissue while minimizing the dose to normal tissue. The variability between measured and predictive dose observed in this pivotal trial of DVS reflects the importance and need for true in vivo dosimetry.
The ability of DVS to be both a fiducial marker and in situ dosimeter allows it a central role in discerning and verifying the complex interactions of IMRT and IGRT. The future application for in situ dosimetry is in adaptive radiotherapy and dose guided radiotherapy where dose alterations can be identified and adjusted on line in real time.
Acknowledgments
Supported in part by NCI Grant R21CA 97859
We would like to acknowledge T. Dixon, T. Carrea, A. Lombardo, L. McCumber for their efforts.
Footnotes
Conflict of Interest Statement: The following participants are associated with Sicel Technologies, Inc., the manufacturer of the implantable dosimeter used in the study: Charles W. Scarantino, MD, PhD, is a co-founder of the company, medical director for the company, and member of the Board of Directors; Gloria P. Beyer, Ph.D., is an officer of the company; Robert D. Black, PhD, was an officer of the company during the study and a consultant for the company at the time of data analysis; Natasha Bolick is an employee of the company.
The study analyzes the data obtained from a FDA approved study protocol. The data used and presented in this research was collected, monitored, and managed in accordance with the FDA's adopted ICH Guideline for Good Clinical Practice and 21 CFR Parts 50, 54, 56, and 812 and 45 CFR Parts 160 and 164.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Byrne TE. A review of prostate motion with consideration for the treatment of prostate cancer. Med Dosim. 2005;30:155–161. doi: 10.1016/j.meddos.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Scarbrough TJ, Golden NM, Ting JY, et al. Comparison of ultrasound and implanted seed marker prostate localization methods: Implications for image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:378–387. 161. doi: 10.1016/j.ijrobp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Turner SL, Swindell R, Bowl N, et al. Bladder movement during radiation therapy for bladder cancer: Implications for treatment planning. Int J Radiat Oncol Biol Phys. 1997;39:355–360. doi: 10.1016/s0360-3016(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 4.Lebesque JV, Bruce AM, Kroes APG, et al. Variation in volumes, dose-volume histograms, and estimated normal tissue complication probabilities of rectum and bladder during conformal radiotherapy of T3 prostate cancer. Int J Radiat Oncol Biol Phys. 1995;33:1109–1119. doi: 10.1016/0360-3016(95)00253-7. [DOI] [PubMed] [Google Scholar]
- 5.Buchali A, Koswig S, Dinges S, et al. Impact of the filling status of the bladder and rectum on their integral dose distribution and the movement of the uterus in the treatment planning of gynecological cancer. Radiother Oncol. 1999;52:29–34. doi: 10.1016/s0167-8140(99)00068-7. [DOI] [PubMed] [Google Scholar]
- 6.Nederveen A, et al. Measurements and clinical consequences of prostate motion during a radiotherapy fraction. Int J Radiat Oncol Biol Phys. 2002;53:206–214. doi: 10.1016/s0360-3016(01)02823-1. [DOI] [PubMed] [Google Scholar]
- 7.Balter J, Sandler HM, Lam K, et al. Measurement of prostate movement over the course of routine radiotherapy using implanted markers. Int J Radiat Oncol Biol Phys. 1995;31:113–118. doi: 10.1016/0360-3016(94)00382-U. [DOI] [PubMed] [Google Scholar]
- 8.Britton KR, Takai Y, Mitsuya M, et al. Evaluation of inter and intrafraction organ motion during intensity modulated radiation therapy (IMRT) for localized prostate cancer measured by a newly developed on-board image-guided system. Radiat Med. 2005;23:14–24. [PubMed] [Google Scholar]
- 9.Ross CS, Hussey DH, Pennington EC, et al. Analysis of movement of intrathoracic neoplasms using ultrafast computerized tomography. Int J Radiat Oncol Biol Phys. 1990;18:671–677. doi: 10.1016/0360-3016(90)90076-v. [DOI] [PubMed] [Google Scholar]
- 10.Weiss PH, Baker JM, Potchen EJ. Assessment of hepatic respiratory excursion. J Nucl Med. 1972;13:758–759. [PubMed] [Google Scholar]
- 11.Suramo I, Päivänsalo M, Myllyla V. Cranio-caudal movements of the liver pancreas and kidneys in respiration. Acta Radiol Diag. 1984;25:129–131. doi: 10.1177/028418518402500208. [DOI] [PubMed] [Google Scholar]
- 12.Vigneault E, Pouliot J, Laverdière J, et al. Electronic portal imaging device detection of radioopaque markers for the evaluation of prostate position during megavoltage irradiation: A clinical study. Int J Radiat Oncol Biol Phys. 1997;37:205–212. doi: 10.1016/s0360-3016(96)00341-0. [DOI] [PubMed] [Google Scholar]
- 13.Padhani A, et al. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys. 1999;44:525–533. doi: 10.1016/s0360-3016(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 14.Ghilezan MJ, Jaffray DA, Siewerdsen JH, et al. Prostate gland motion assessed with cne-magnetic resonance imaging (cine-MRI) Int J Radiat Oncol Biol Phys. 2005;62:406–417. doi: 10.1016/j.ijrobp.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Scarantino CW, Rini CJ, Aquino M, et al. Initial clinical results of an in vivo dosimeter during external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:606–613. doi: 10.1016/j.ijrobp.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 16.Black RD, Scarantino CW, Mann GG, et al. An analysis of an implantable dosimeter system for external beam therapy. Int J Radiat Oncol Biol Phys. 2005;63:290–300. doi: 10.1016/j.ijrobp.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beddar AS, Salehpour M, Briere TM, et al. Preliminary evaluation of implantable MOSFET radiation dosimeters. Phys Med Biol. 2005;50:141–149. doi: 10.1088/0031-9155/50/1/011. [DOI] [PubMed] [Google Scholar]
- 18.Beyer GP, Mann G, Pursley J, et al. An implantable MOSFET dosimeter for the measurement of radiation dose in tissue during cancer therapy. IEEE J. 2008;8:38–51. [Google Scholar]
- 19.Scarantino CW, Ruslander DM, Rini CJ, et al. An implantable radiation dosimeter for use in external beam radiation therapy. Med Phys. 2004;31:2658–71. doi: 10.1118/1.1778809. [DOI] [PubMed] [Google Scholar]
- 20.Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: Version 2.0. An improved reference for grading the acute effects of cancer treatment: Impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:13–47. doi: 10.1016/s0360-3016(99)00559-3. [DOI] [PubMed] [Google Scholar]
- 21.Hayne D, Johnson U, D'Souza D, et al. Anorectal irradiation in pelvic radiotherapy: An assessment using in-vivo dosimetry. Clin Oncol (R Coll Radiol) 2001;13:126–129. doi: 10.1053/clon.2001.9235. [DOI] [PubMed] [Google Scholar]
- 22.Weber DC, Nouet P, Kurtz JM, et al. Assessment of target dose delivery in anal cancer using in vivo thermoluminescent dosimetry. Radiother Oncol. 2001;59:39–43. doi: 10.1016/s0167-8140(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 23.Wertz H, Boda-Heggemann J, Walter C, et al. Image guided in vivo dosimetry for quality assurance of IMRT treatment for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:288–295. doi: 10.1016/j.ijrobp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Kupelian P, Willoughby T, Mahadevan A, et al. Multi-institutional clinical experience with the Calypso system in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1088–1098. doi: 10.1016/j.ijrobp.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Litzenberg DW, Balter JM, Hadley SW, et al. Influence of intrafraction motion on margins for prostate radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:528–534. doi: 10.1016/j.ijrobp.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Langen KM, Zhang Y, Andrews RD, et al. Initial experience with megavoltage (MV) CT guidance for daily prostate alignments. Int J Radiat Oncol Biol Phys. 2005;62:1517–1524. doi: 10.1016/j.ijrobp.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Yue NJ, Li X, Beriwal S, et al. The intrafraction motion induced dosimetric impacts in breast 3D radiation treatment: A 4DCT based study. Med Phys. 2007;34:2789–2800. doi: 10.1118/1.2739815. [DOI] [PubMed] [Google Scholar]
- 28.Lattanzi J, Mc Neeley S, Pinover W, et al. A comparison of daily CT localization to a daily ultrasound-based system in prostate cancer. Int J Radiat Oncol Biol Phys. 1999;43:719–723. doi: 10.1016/s0360-3016(98)00496-9. [DOI] [PubMed] [Google Scholar]
- 29.Langen KM, Pouliot J, Anezinos C, et al. Evaluation of ultrasound-based prostate localization for image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:635–644. doi: 10.1016/s0360-3016(03)00633-3. [DOI] [PubMed] [Google Scholar]
- 30.Beyer GP, Scarantino CW, Prestidge BR, et al. Technical evaluation of radiation dose delivered in prostate patients as measured by an implantable MOSFET dosimeter. Int J Radiat Oncol Biol Phys. 2007;69:925–935. doi: 10.1016/j.ijrobp.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 31.Shukovsky LJ. Dose, time, volume relationships in squamous cell carcinoma of the supraglottic larynx. Am J Roent Rad Ther Nucl Med. 1970;108:27–29. doi: 10.2214/ajr.108.1.27. [DOI] [PubMed] [Google Scholar]
- 32.Stewart JF, Jackson AW. The steepness of the dose response curve for tumor and normal tissue injury. Laryngoscope. 1975;85:1107–1111. doi: 10.1288/00005537-197507000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan HS. Evidence for a tumoricidal dose level in the radiotherapy of Hodgkin's disease. Cancer Res. 1966;26:1221–1224. [PubMed] [Google Scholar]
- 34.Hellman S. improving the therapeutic index in breast cancer treatment. Cancer Res. 1980;40:4335–4342. [PubMed] [Google Scholar]
- 35.Hanks GE, Hanlon AL, Epstein B, Horwitz EM. Dose response in prostate cancer with 8-12 years' follow-up. In J Radiat Oncol Biol Phys. 2002;54:427–435. doi: 10.1016/s0360-3016(02)02954-1. [DOI] [PubMed] [Google Scholar]
- 36.Eade TN, Hanlon AL, Horwitz EM, et al. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys. 2007;68:682–689. doi: 10.1016/j.ijrobp.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]