Abstract
Genistein is a major soy isoflavone with multiple properties. The impact of soy/genistein on breast cancer is controversial. One of the issues is whether soy/genistein has a genotoxic effect at physiological concentrations. To address this question using an in vitro model, we first established MCF-10A/G0 and MCF-10A/G1 cell lines, which were MCF-10A cells exposed to 0.01% DMSO (as vehicle control), i.e. MCF-10A/G0, or 1 µM of genistein for three months, MCF-10A/G1, respectively. Chromosomal changes were compared between the two cell lines by routine G-banded chromosome analyses, regular CGH and oligo array-based CGH. After three months of exposure to genistein, the cell line MCF-10A/G1 showed a loss of a normal chromosome 8, a gain of extra chromosome 20, plus a loss of a chromosomal segment on the short arm of chromosome 9, which leads to a homozygous deletion of the tumor suppressor genes, INK4/p16 and INK4/p15. Our results suggest that long-term/low concentration exposure to genistein may have the potential to induce chromosomal imbalances. These genotoxic effects may work in concert with other factors to induce genetic lesions that contribute to soy/genistein associated risk.
1. Introduction
Genistein is a major isoflavone derived from soy. Earlier studies suggest that soy/genistein may provide several health benefits, such as relief of menopausal symptoms and breast cancer prevention (1, 2). However, reports increasingly suggest that genistein might also be a risk factor under certain conditions (3, 4). As soy/genistein-associated risk is becoming a serious concern to soy/genistein consumers and breast cancer survivors (5, 6), it is important to investigate the impact and conditions associated with soy/genistein’s risk.
Genistein is a bioactive molecule with multiple properties. It may function as an estrogen receptor (ER) modulator, a tyrosine kinase inhibitor and a topoisomerase inhibitor (1). Genistein-associated risk was mainly attributed to its pro-estrogenic activity in a previous study. Recent reports suggest that genistein-mediated genotoxicity may also induce potential risk (7). As a strong topoisomerase II inhibitor, genistein induces DNA breakage and genetic lesions (8, 9). It has been shown that genistein induces genomic aberrations, such as chromatid exchange, micronuclei and chromosomal translocation in peripheral lymphocytes and Chinese hamster V79 cells (10–12). In particular, it was demonstrated that genistein induces mixed-lineage leukemia (MLL) gene translocation in primary human CD34+ cells (13, 14). As 75% of infant leukemia demonstrates a specific abnormality involving the MLL gene, this has led to the speculation that maternal exposure to soy/genistein might be a risk factor of infant leukemia (15, 16).
In contrast to the positive correlation between soy/genistein treatment and genetic aberrations, results from other groups show a lack of significant genotoxicity post-soy/genistein (17). The key issue in this controversy is the variation in treatment conditions among different studies. In most in vitro studies, genistein genotoxicity was observed in cells treated with genistein at higher concentrations (≥5 µM) for a shorter period (≥72 hr) (10, 18). This effect might not be evident if the cells were exposed to lower concentrations for a short period. Because in vivo exposure to soy/genistein is a low-concentration/long-term process, it is necessary to examine genistein’s genotoxic effects under conditions similar to in vivo exposure.
In this study, we aimed to investigate whether long-term (relative to a few days’ treatment) in vitro exposure to low-dose genistein induces chromosomal aberrations. We established MCF-10A breast epithelial cell lines with defined exposure conditions and examined the effect of genistein on chromosomal aberrations with chromosomal genomic hybridization (CGH) analysis. We found that long-term/low concentration exposure to genistein induced genetic aberrations in our cell line model.
2. Materials and Methods
2.1 Cell lines and treatment
MCF-10A breast epithelial cell line was purchased from ATCC. MCF-10A/G0 and MCF-10A/G1 cells were derived from MCF-10A cells by growing the cells in IMDM medium (supplemented with 10% FBS, 10 µg/ml insulin, 20 ng/ml EGF, 100 ng/ml cholera toxin and 0.5 µg/ml hydrocortisone) in the presence of 0.01% DMSO (as solvent control) and 1 µM of genistein, respectively. The cells were subcultured in the same medium with a split every three days. After three months’ exposure to different concentrations of genistein (0 or 1 µM), MCF-10A/G0 and MCF-10A/G1 cells were collected for further analysis. With MCF-10A/G0 cells as the control, MCF-10A/G1 cells represent breast epithelial cells with long term exposure to low doses of genistein.
2.2 Routine cytogenetics and CGH assay
MCF-10A/G0 and MCF-10A/G1 cells were harvested according to our laboratory standard protocols. Chromosomes were treated and stained by trypsin-Giemsa banding (GTG-banding). A total of 50 cells were analyzed and karyotyped from each cell line. DNA was extracted from cultured cells using a Qiagnen DNA isolation kit. CGH was performed according to the previously described protocol with minor modifications. Briefly, DNA extracted from MCF-10A G0 and MCF-10A G1 was directly labeled with spectrum-green-dUTP by a CGH nick translation kit (Vysis Inc, Downers Grove, IL). Digestion time and enzyme concentration were adjusted to obtain an average fragment size of 500–2000 base pairs. Next, 200–300 ng of spectrum-green-tumor DNA and 100–150 ng of spectrum-red-normal male reference DNA purchased from Vysis Inc, Downers Grove, IL were co-precipitated with 10 µg of cot-1 DNA (Gibco BRL, Gaithersburg, MD), and then redissolved in 10 µl of hybridization solution (2X SSC, 50% formamide, 10% dextran sulfate, pH 7.0). The mixture was denatured and hybridized with denatured normal male metaphase spreads for three days in a humid chamber. Post-hybridization washes were carried out at 70°C with 0.4X SSC/0.3% NP-40 (pH 7.0) and at room temperature with 2X SSC/1% NP-40 (pH 7.0) for one minute each, and the slides were counterstained with 4,6-diamidino-2-phenylindole-dihydrochloride (Sigma Chemical Co., St. Louis, MO).
2.3 Array CGH assay
The genomic DNA was extracted from the cell lines using a QIAamp DNA mini kit. Human genomic reference DNA (Cat# G1471) was purchased from Promega. The sonicated cell line and reference DNA were labeled with either Cyanine 3 (Cy-3) or Cyanine 5 (Cy-5) by random priming (Trilink Biotechnologies) and then hybridized to a NimbleGen high capacity 385K oligo microarray chip via incubation in the MAUI Hybridization System for 18 hours according to NimbleGen’s CGH protocols. The array was scanned at 532 nm and 635 nm using the GenePix scanner (Molecular Devices); NimbleScan and SignalMap were applied for data analysis (19).
3. Results
3.1. Cytogenetic characterization of MCF-10A/G0 and MCF-10A/G1 cells by G-banded karyotyping
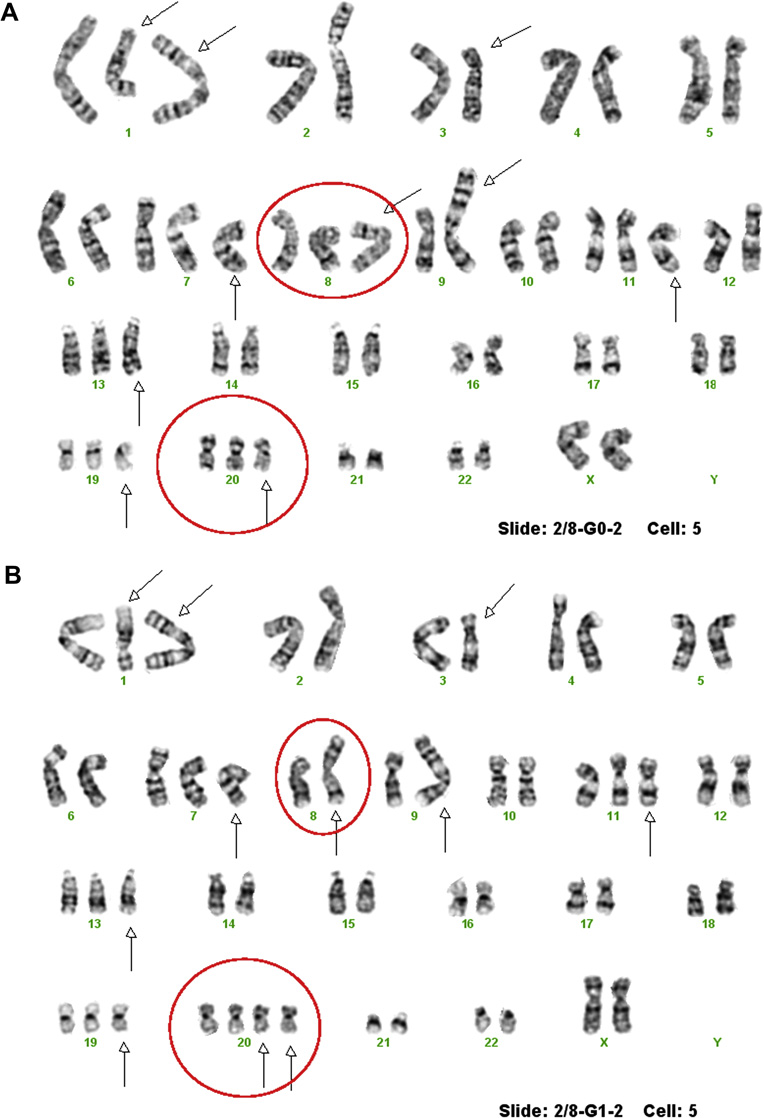
To define the cytogenetic patterns between MCF-10A/G0 and MCF-10A/G1 cells, we first performed G-banded karyotyping. The results show that the control cell line, MCF-10A/G0, has very complex, consistent chromosomal changes, including an isochromosome of the long arm of chromosome 1, an extra chromosome 1 with a deletion of the long arm, a derivative chromosome 3, trisomy 7, an extra derivative chromosome 8, a derivative chromosome 9, trisomy 11, trisomy 13, trisomy 19 and trisomy 20 (Fig. 1A), our findings were confirmed by M-FISH (Fig. 2). The karyotype was defined as 53,XX,i(1)(q10),+del(1)(q12q32), der(3)t(3;9)(p13;p22),+7,+der(8)t(8;8)(q22;p23),der(9)t(3;9)(p13;p22)t(3;5)(p26;q31),+11,+13, +19,+20. The MCF-10A/G1 cell lines, which were treated with 1 µM of genistein, had a different loss or gain of chromosomal changes, as compared to the MCF-10A/G0. This includes the loss of a normal chromosome 8 and gain of chromosome 20 (Fig. 1B). The differences in chromosomes 8 and 20 between the two cell lines suggest that long term exposure to low doses of genistein may induce cytogenetic changes, at least in cultured cells. The rest chromosome changes were the same. Since our genotyping results are based on 50 metaphase cells from each subline cultures, genistein-induced changes in chromosomes 8 and 20 are specific.
Figure 1.
A and B. G-banded karyotype of MCF-10A/G0 (A) showed complex chromosomal changes including an isochromosome of the long arm of chromosome 1, an extra derivative chromosome 1, a derivative chromosome 3, trisomy 7, an extra derivative chromosome 8, a derivative chromosome 9, trisomy 11, trisomy 13, trisomy 19 and trisomy 20, which they marked by arrows. Chromosomes 8 and 20 were highlighted with red circles. G-banded karyotype of MCF-10A/G1 showed the similar chromosome changes like MCF-10A/G0 except the loss of a normal chromosome 8 and a gain of chromosome 20, which were highlighted with red circles.
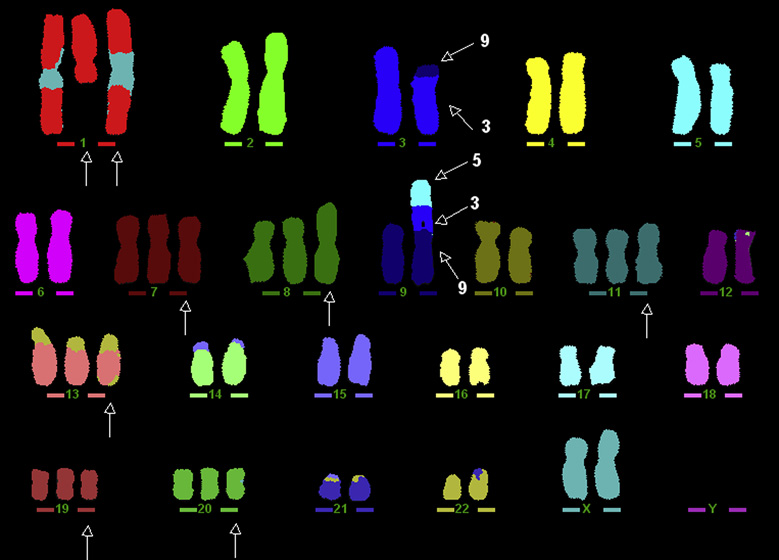
Figure 2.
M-FISH confirmed the findings of MCF-10A/G0 detected by G-banded chromosomal analysis. It is the M-FISH assay which helps to determine exactly how the derivative chromosome 9 was formed, der(9)t(9;5;3).
3.2. Examination of chromosomal imbalance between MCF-10A/G0 and MCF-10A/G1 cells by regular CGH
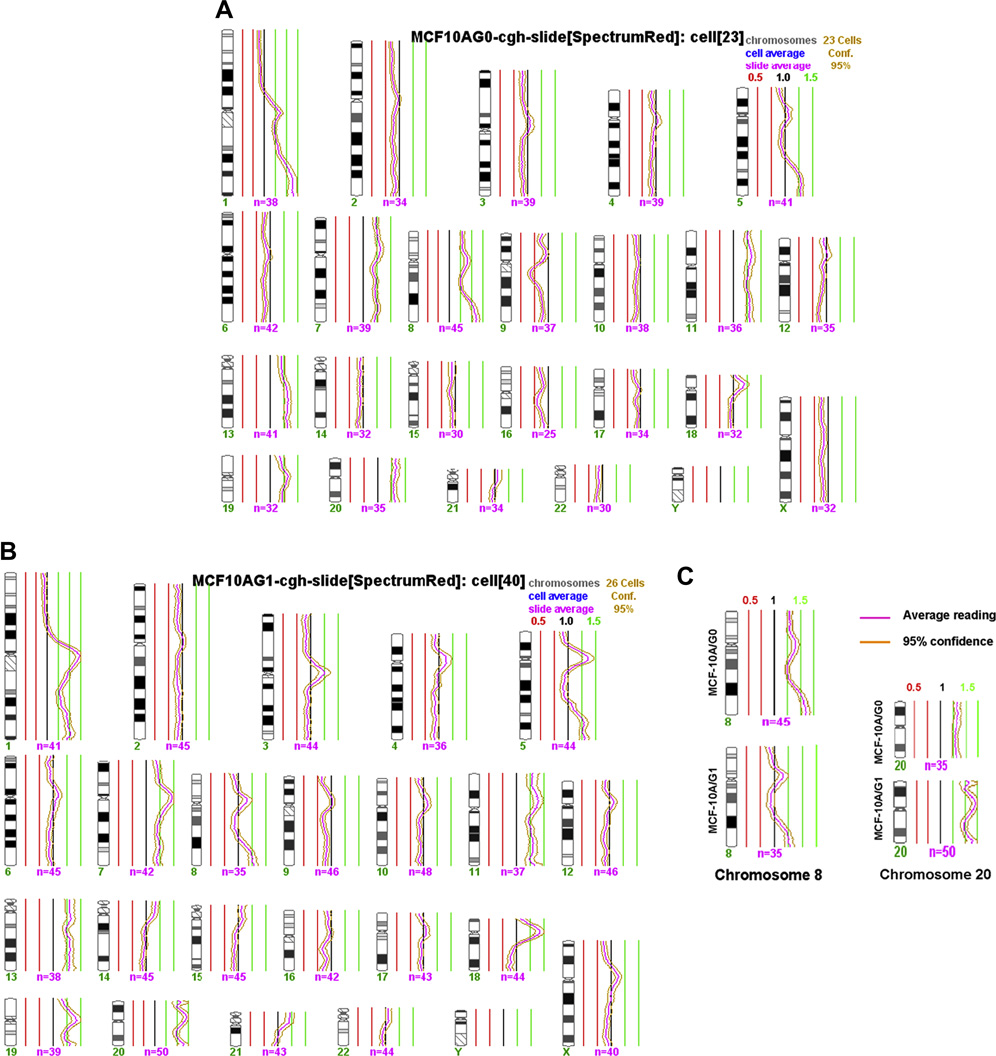
To confirm the results detected in G-banded karyotyping, we examined the chromosomal imbalances between MCF-10A/G0 and MCF-10A/G1 cells by regular CGH. CGH results confirmed the findings detected by G-banded in the two cell lines (Fig. 3A and 3B). The both cell lines showed a gain of the long arm of chromosome 1, a partial gain of the long arm of chromosome 5 at 5q31-5qter, the whole chromosomes 7, 11, 13, and 19. CGH also confirmed that the cell line MCF-10A/G1 had a loss of normal chromosome 8 and a gain of another extra chromosome 20. It showed there was a duplication of the short arm of chromosome 18 by CGH, however, it could not confirmed by high resolution oligo based array CHG (data not shown). It could be due to the variation of the alpha satellite sequences in the centromeric region of chromosome 18.
Figure 3.
A, B and C. These figures showed the regular CGH profiles of the cell lines MCF-10A/G0 (A) and MCF-10A/G1 (B) respectively. The profiles of chromosomes 8 and 10 were highlighted (C). It is evident that the loss of chromosome 8 and gain of chromosome 10 in the cell line MCF-10A/G1.
3.3 Identification of a deletion on chromosome 9 in MCF-10A/G1 cells using array CGH
High density oligo array CGH is a powerful tool for the detection of DNA copy number changes and mapping of the associated breakpoints in unbalanced chromosome rearrangements. To further identify more specific cytogenetic differences between the two cell lines, we performed oligo array CGH analysis on MCF-10A/G0 and MCF-10A/G1 cells. Similar to the results from karyotyping and regular CGH, array CGH data also indicate a decrease of DNA copy numbers of chromosome 8 and an increase in chromosome 20 (data not shown). This further supports the differential cytogenetic patterns between the two cell lines.
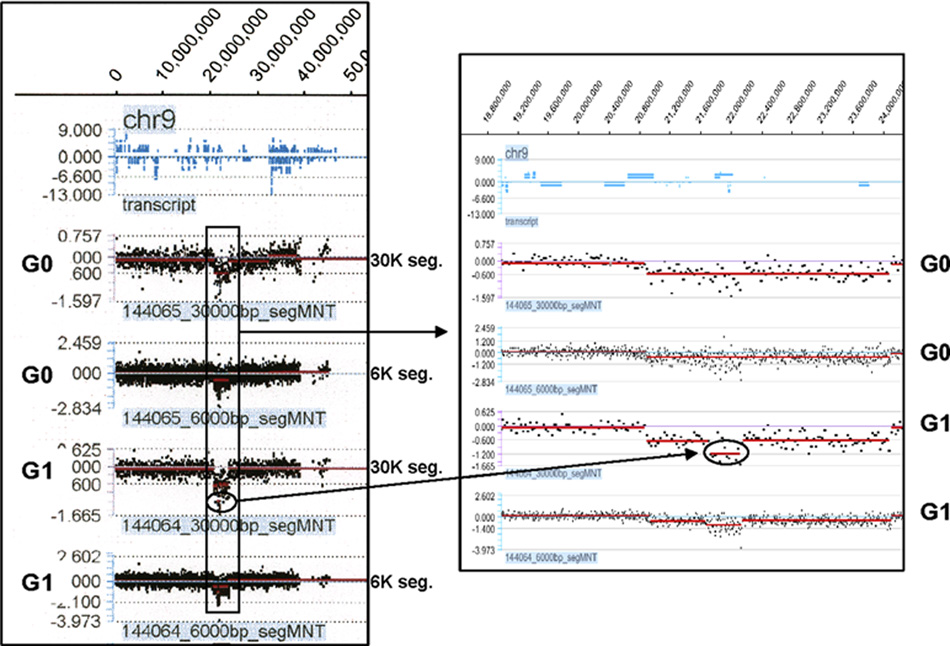
More interestingly, we found chromosome anomalies on the short arm of chromosome 9 in both cell lines not visible by routine karyotype. In MCF-10A/G0, the deletion is approximately 3.18 Mb (20895000 to 24075000) (Fig. 4A). In MCF-10A/G1, not only same 3.18 Mb deletion was present, but also a smaller segment, approximately 390 Kb (21735000 to 22125000) within the deleted region is further deleted (Fig. 4B). The evident difference between the two cell lines suggests that genistein treatment may result in this deletion. More interestingly, tracking of the corresponding genes indicates that this further deleted region contains tumor suppressor genes p15/Ink4b and p16/Ink4a (Table 1). Since p15/Ink4b and p16/Ink4a are well known tumor suppressor genes, genistein-induced deletion of these genes might increase the risk of cell transformation in the affected cells.
Figure 4.
A and B showed the interesting high density oligo based array CGH results which showed that genistein could induces additional deletion in chromosome 9 in MCF-10A/G1 cells.
Table 1.
Genes located in the deleted region on Chr. 9 of MCF-10A/G1 cells (circled in Fig. 3)
| Position | Symbol | Location | Description |
|---|---|---|---|
| 21684998 to 21792352 | LOC402359 | 9p21.3 | similar to KH-type splicing regulatory protein (FUSE binding protein 2) |
| 21792635 to 21855970 | MTAP | 9p21 | methylthioadenosine phosphorylase |
| 21957751 to 21984490 | CDKN2A | 9p21 | cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) |
| 21992902 to 21999312 | CDKN2B | 9p21 | cyclin-dependent kinase inhibitor 2B (p15, inhibits CDK4) |
Discussion
Studies on genistein associated protection and risk is underscored by the popular use or exposure to soy products in daily life. Genistein is present as unconjugated aglycones in unfermented soy products. After ingestion, soybean isoflavones, including genistein, are hydrolyzed by intestinal glucosidases, which release the aglycones, daidzein, genistein and glycitein. These may be absorbed or further metabolized to many specific metabolites, including equol and p-ethylphenol by gut microflora (20, 21). As a major isoflavone, genistein has been extensively used in vitro studies.
It has been demonstrated that genistein induces genotoxic effects on cells at higher concentrations (>10 µM) (22). However, serum concentration of genistein is rarely above 5 µM in humans or animals that are fed a soy/genistein diet (23, 24). Whether soy/genistein-associated genotoxicity has any impact on genomic integrity of soy/genistein consumers remains unclear. In this study, we tested the effect of long-term (over three months) in vitro exposure to low concentrations (1 µM) of genistein on cytogenetic patterns of MCF-10A breast epithelial cells. These treatment conditions mimic in vivo exposure to soy/genistein and takes both concentration and exposure time into consideration. Results from these experiments may have more translational value.
We found that exposure to genistein may induce both chromosomal number change and regional deletion. Using G-banded chromosome analysis and regular CGH, we found that genistein induced loss in chromosome 8 and gain in chromosome 20 in MCF-10A/G1 cells, as compared to control MCF-10A/G0 cells. Data from array CGH not only confirm the above changes but also reveal more subtle genetic lesions in smaller fragments, such as the deletion on the short arm of chromosome 9. These results suggest that genistein may affect cytogenetic stability by inducing chromosomal number alterations and regional deletions. The numerical changes of chromosomes 8 and 20 may reflect the changes in immortalized cell lines, which had gained chromosomal imbalance before genistein treatment. More meaningful change is the regional deletion on chromosome 9. In particular, the further deleted region of chromosome 9 includes tumor suppressor genes p15/Ink4b and p16/Ink4a. This suggests that the locus encoding p15/Ink4b and p16/Ink4a is a potential target of genistein induced genetic aberrations, which could be a candidate marker for in vivo studies.
Findings in this report are consistent with our previous animal studies in which cell lines derived from mice fed a soy diet have more chromosomal aberrations than the control diet group (25). On top of these, several reports indicate that soy/genistein may induce the translocation of MLL genes, which has been linked to the risk of infant acute leukemia (14, 16). In a broader consideration, genistein-induced chromosomal changes are part of genistein’s general genotoxicity, which also includes nuclear condensation and nuclear fragmentation under extreme conditions (10, 26). Taken together, results from multiple systems suggest that soy/genistein may affect genomic stability and induce other genetic lesions under certain conditions.
Increasing reports on genistein-associated genotoxicity, especially at physiologically relevant concentration, underscore the involvement of genotoxicity in genistein-associated risk, which may work in context with genistein-mediated estrogenic effect. This may partially explain an interesting observation found in our previous study. In that study, we found that MMTV-erbB-2 transgenic mice fed a soy diet had a longer latency in tumor development than the control diet group. However, soy-fed mice developed tumors at a faster rate (a sharp drop of tumor free animal numbers) in later age once a tumor started to grow (27). It is possible that accumulation of genistein-induced genotoxicity in the mammary epithelial cells contributes to the faster tumor development at an older age.
Regarding the potential impact of genistein-induced genotoxicity on breast cancer development, it should be pointed out that our observation is based on an in vitro model using immortalized cell lines at one ending point. On the basis of this report, we will examine chromosomal changes at shorter intervals and multiple ending points in future studies to test the presence, stability and continuity of the unstable chromosomal aberrations. While our findings suggest a link between long-term, continuous exposure to genistein and the risk of chromosomal imbalance, further investigation of soy/genistein-mediated genotoxicity in vivo using animal models is required. Genistein associated genotoxicity in vivo may vary with cell type, developmental stage and/or presence of other mutagens. Appropriate evaluation of soy/genistein-associated risk will have significant impact on breast cancer prevention and risk control.
Acknowledgement
Authors express special thanks to Mallory Martin for her technical support. S Li and Young Mi Kim were supported in part by the grant CA115320 to the University of Oklahoma from the National Cancer Institute, National Institute of Health. X Yang and S Yang were supported in part by the Oklahoma Center for Advancement of Science and Technology Grant HR07-108 to XY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131:3095S–3108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- 2.Polkowski K, Mazurek AP. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol Pharm. 2000;57:135–155. [PubMed] [Google Scholar]
- 3.Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- 4.Ju YH, Doerge DR, Allred KF, Allred CD, Helferich WG. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–2477. [PubMed] [Google Scholar]
- 5.Bouker KB, Hilakivi-Clarke L. Genistein: does it prevent or promote breast cancer? Environ Health Perspect. 2000;108:701–708. doi: 10.1289/ehp.00108701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick LA. Soy isoflavones: hope or hype? Maturitas. 2003;44 Suppl 1:S21–S29. doi: 10.1016/s0378-5122(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 7.Morris SM, Chen JJ, Domon OE, McGarrity LJ, Bishop ME, Manjanatha MG, Casciano DA. p53, mutations, and apoptosis in genistein-exposed human lymphoblastoid cells. Mutat Res. 1998;405:41–56. doi: 10.1016/s0027-5107(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar FH, Li Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metastasis Rev. 2002;21:265–280. doi: 10.1023/a:1021210910821. [DOI] [PubMed] [Google Scholar]
- 9.Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Le Pecq JB, Larsen AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- 10.Kulling SE, Metzler M. Induction of micronuclei, DNA strand breaks and HPRT mutations in cultured Chinese hamster V79 cells by the phytoestrogen coumoestrol. Food Chem Toxicol. 1997;35:605–613. doi: 10.1016/s0278-6915(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 11.Di Virgilio AL, Iwami K, Watjen W, Kahl R, Degen GH. Genotoxicity of the isoflavones genistein, daidzein and equol in V79 cells. Toxicol Lett. 2004;151:151–162. doi: 10.1016/j.toxlet.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Kulling SE, Rosenberg B, Jacobs E, Metzler M. The phytoestrogens coumoestrol and genistein induce structural chromosomal aberrations in cultured human peripheral blood lymphocytes. Arch Toxicol. 1999;73:50–54. doi: 10.1007/s002040050585. [DOI] [PubMed] [Google Scholar]
- 13.Strick R, Strissel PL, Borgers S, Smith SL, Rowley JD. Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc Natl Acad Sci U S A. 2000;97:4790–4795. doi: 10.1073/pnas.070061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Waalwijk van Doorn-Khosrovani SB, Janssen J, Maas LM, Godschalk RW, Nijhuis JG, van Schooten FJ. Dietary flavonoids induce MLL translocations in primary human CD34+ cells. Carcinogenesis. 2007;28:1703–1709. doi: 10.1093/carcin/bgm102. [DOI] [PubMed] [Google Scholar]
- 15.Ross JA. Maternal diet and infant leukemia: a role for DNA topoisomerase II inhibitors? Int J Cancer Suppl. 1998;11:26–28. [PubMed] [Google Scholar]
- 16.Abe T. Infantile leukemia and soybeans--a hypothesis. Leukemia. 1999;13:317–320. doi: 10.1038/sj.leu.2401344. [DOI] [PubMed] [Google Scholar]
- 17.Miltyk W, Craciunescu CN, Fischer L, Jeffcoat RA, Koch MA, Lopaczynski W, Mahoney C, Crowell J, Paglieri J, Zeisel SH. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am J Clin Nutr. 2003;77:875–882. doi: 10.1093/ajcn/77.4.875. [DOI] [PubMed] [Google Scholar]
- 18.Miltyk W, Craciunescu CN, Fischer L, Jeffcoat RA, Koch MA, Lopaczynski W, Mahoney C, Jeffcoat RA, Crowell J, Paglieri J, Zeisel SH. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am J Clin Nutr. 2003;77:875–882. doi: 10.1093/ajcn/77.4.875. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZF, Ruivenkamp C, Staaf J, Zhu H, Barbaro M, Petillo D, Khoo SK, Borg A, Fan YS, Schoumans J. Detection of submicroscopic constitutional chromosome aberrations in clinical diagnostics: a validation of the practical performance of different array platforms. Eur J Hum Genet. 2008;16:786–792. doi: 10.1038/ejhg.2008.14. [DOI] [PubMed] [Google Scholar]
- 20.Axelson M, Sjovall J, Gustafsson BE, Setchell KD. Soya--a dietary source of the non-steroidal oestrogen equol in man and animals. J Endocrinol. 1984;102:49–56. doi: 10.1677/joe.0.1020049. [DOI] [PubMed] [Google Scholar]
- 21.Joannou GE, Kelly GE, Reeder AY, Waring M, Nelson C. A urinary profile study of dietary phytoestrogens. The identification and mode of metabolism of new isoflavonoids. J Steroid Biochem Mol Biol. 1995;54:167–184. doi: 10.1016/0960-0760(95)00131-i. [DOI] [PubMed] [Google Scholar]
- 22.Misra RR, Hursting SD, Perkins SN, Sathyamoorthy N, Mirsalis JC, Riccio ES, Crowell JA. Genotoxicity and carcinogenicity studies of soy isoflavones. Int J Toxicol. 2002;21:277–285. doi: 10.1080/10915810290096441. [DOI] [PubMed] [Google Scholar]
- 23.Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003;133:1027–1035. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 24.Doerge DR, Churchwell MI, Chang HC, Newbold RR, Delclos KB. Placental transfer of the soy isoflavone genistein following dietary and gavage administration to Sprague Dawley rats. Reprod Toxicol. 2001;15:105–110. doi: 10.1016/s0890-6238(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 25.Jeruss JS, Liu NX, Chung Y, Magrane G, Waldman F, Edgerton S, Yang X, Thor AD. Characterization and chromosomal instability of novel derived cell lines from a wt-erbB-2 transgenic mouse model. Carcinogenesis. 2003;24:659–664. doi: 10.1093/carcin/bgg001. [DOI] [PubMed] [Google Scholar]
- 26.Pagliacci MC, Smacchia M, Migliorati G, Grignani F, Riccardi C, Nicoletti I. Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur J Cancer. 1994;30A:1675–1682. doi: 10.1016/0959-8049(94)00262-4. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Edgerton SM, Kosanke SD, Mason TL, Alvarez KM, Liu N, Chatterton RT, Liu B, Wang Q, Kim A, Murthy S, Thor AD. Hormonal and dietary modulation of mammary carcinogenesis in mouse mammary tumor virus-c-erbB-2 transgenic mice. Cancer Res. 2003;63:2425–2433. [PubMed] [Google Scholar]