Abstract
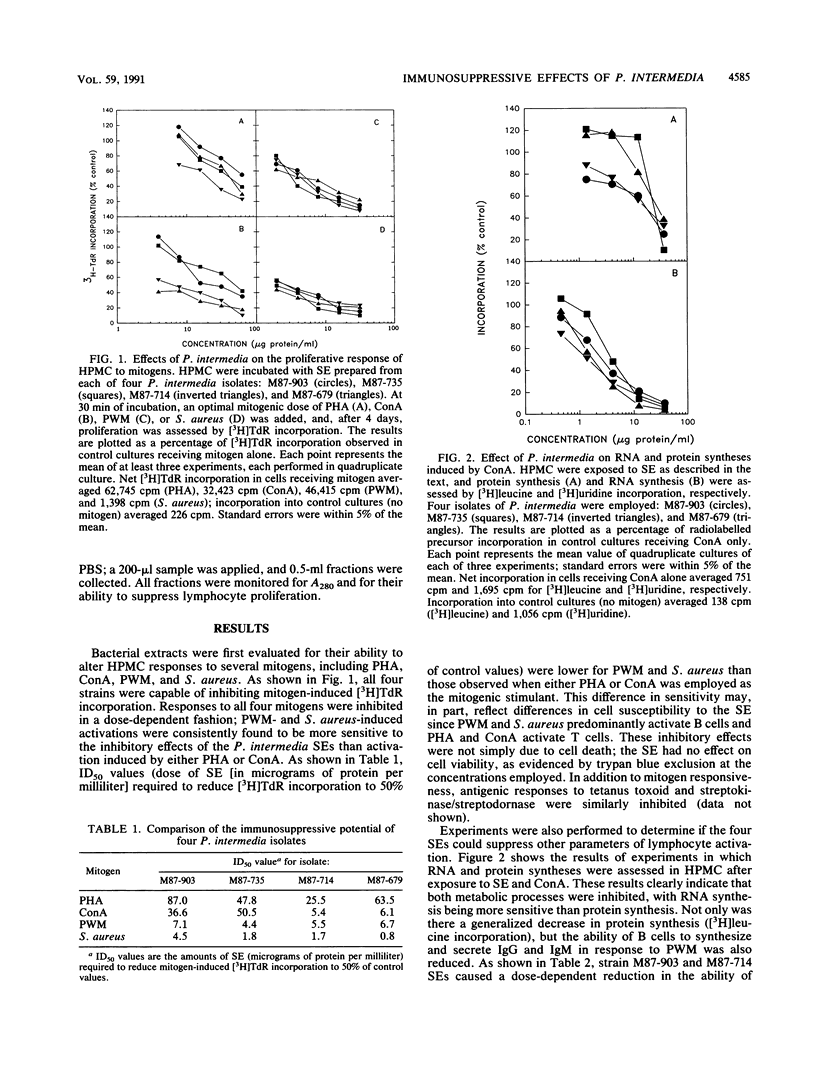
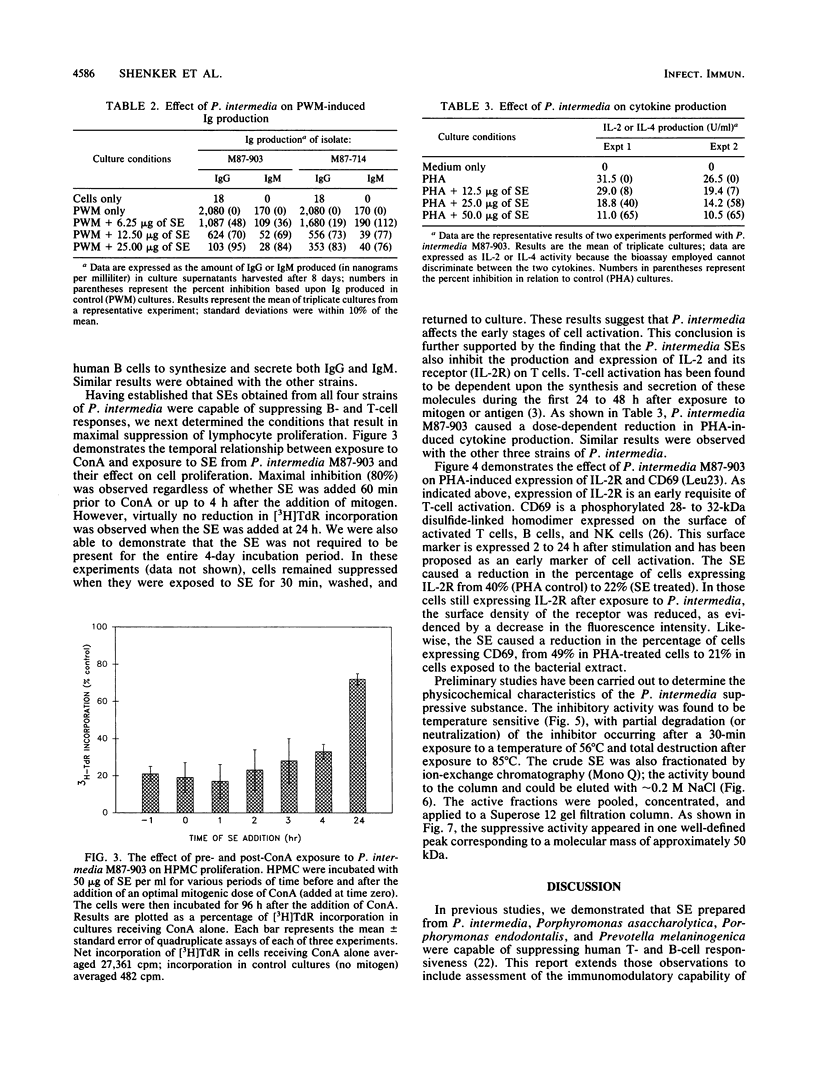
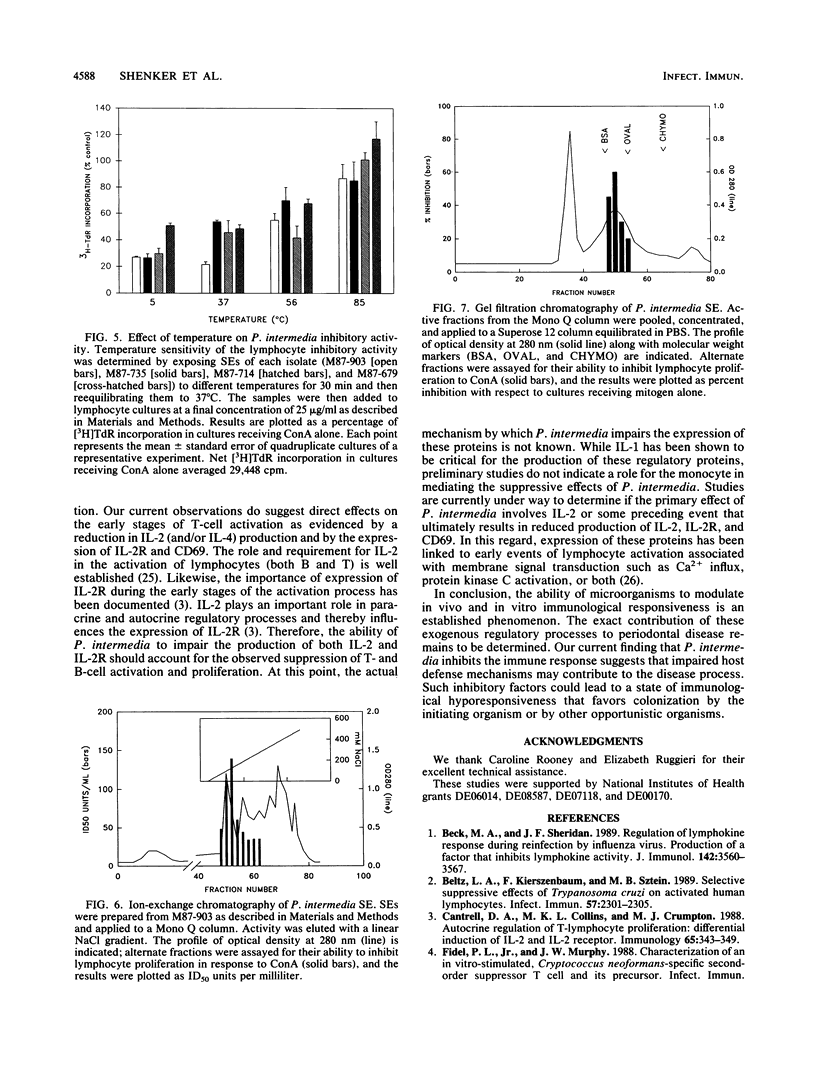
In this study, we have assessed four strains of Prevotella intermedia, isolated from periodontally involved lesions, for their ability to inhibit lymphocyte functions. All four strains were found to cause a dose-dependent inhibition of B- and T-cell proliferation in response to mitogens and antigens. This was reflected in altered DNA, RNA, and protein syntheses. Furthermore, P. intermedia appeared to affect the early stages of cell activation. This was ascertained by kinetic analysis in which it was determined that the extract had to be present during the first 24 h of incubation to cause suppression. Moreover, direct assessment of the early stages of cell activation indicated that release of cytokines and expression of the interleukin 2 receptor and CD69 on T cells were inhibited by P. intermedia sonic extracts. Finally, preliminary characterization of the immunosuppressive agent indicates that it has a molecular mass of approximately 50 kDa and is heat labile. It has been proposed that impaired host defense may play a pivotal role in the pathogenesis of many infections. The data presented in this paper suggest that microbially mediated immunosuppression may contribute to the pathogenesis of periodontal disease by altering the nature and consequences of host-parasite interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck M. A., Sheridan J. F. Regulation of lymphokine response during reinfection by influenza virus. Production of a factor that inhibits lymphokine activity. J Immunol. 1989 May 15;142(10):3560–3567. [PubMed] [Google Scholar]
- Beltz L. A., Kierszenbaum F., Sztein M. B. Selective suppressive effects of Trypanosoma cruzi on activated human lymphocytes. Infect Immun. 1989 Aug;57(8):2301–2305. doi: 10.1128/iai.57.8.2301-2305.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Collins M. K., Crumpton M. J. Autocrine regulation of T-lymphocyte proliferation: differential induction of IL-2 and IL-2 receptor. Immunology. 1988 Nov;65(3):343–349. [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Khakpour F. R., Murphy J. W. Characterization of a third-order suppressor T cell (Ts3) induced by cryptococcal antigen(s). Infect Immun. 1987 Jul;55(7):1657–1662. doi: 10.1128/iai.55.7.1657-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi G., Vismara D., Piccolella E., Colizzi V., Asherson G. L. A non-specific inhibitor produced by Candida albicans activated T cells impairs cell proliferation by inhibiting interleukin-1 production. Clin Exp Immunol. 1985 May;60(2):303–310. [PMC free article] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Olson G. B., Dent P. B., Rawls W. E., South M. A., Montgomery J. R., Melnick J. L., Good R. A. Abnormalities of in vitro lymphocyte responses during rubella virus infections. J Exp Med. 1968 Jul 1;128(1):47–68. doi: 10.1084/jem.128.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra C., Montaño L. F., Huesca M., Rayón I., Willms K., Goodsaid F. Inhibition of mitogenesis induced by phytohemagglutinin and Lens culinaris lectin in adherent-cell supernatants treated with protein extract of Mycobacterium tuberculosis. Infect Immun. 1986 Apr;52(1):309–313. doi: 10.1128/iai.52.1.309-313.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie G., Lally E. T., Shenker B. J. Immunosuppressive properties of Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 1988 Jan;56(1):122–127. doi: 10.1128/iai.56.1.122-127.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner N. E. Parasite accessory cell interactions in murine leishmaniasis. I. Evasion and stimulus-dependent suppression of the macrophage interleukin 1 response by Leishmania donovani. J Immunol. 1987 Mar 15;138(6):1919–1925. [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B. J., Berthold P., Dougherty P., Porter K. K. Immunosuppressive effects of Centipeda periodontii: selective cytotoxicity for lymphocytes and monocytes. Infect Immun. 1987 Oct;55(10):2332–2340. doi: 10.1128/iai.55.10.2332-2340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B. J., DiRienzo J. M. Suppression of human peripheral blood lymphocytes by Fusobacterium nucleatum. J Immunol. 1984 May;132(5):2357–2362. [PubMed] [Google Scholar]
- Shenker B. J. Immunologic dysfunction in the pathogenesis of periodontal diseases. J Clin Periodontol. 1987 Oct;14(9):489–498. doi: 10.1111/j.1600-051x.1987.tb00989.x. [DOI] [PubMed] [Google Scholar]
- Shenker B. J., Listgarten M. A., Taichman N. S. Suppression of human lymphocyte responses by oral spirochetes: a monocyte-dependent phenomenon. J Immunol. 1984 Apr;132(4):2039–2045. [PubMed] [Google Scholar]
- Shenker B. J., McArthur W. P., Tsai C. C. Immune suppression induced by Actinobacillus actinomycetemcomitans. I. Effects on human peripheral blood lymphocyte responses to mitogens and antigens. J Immunol. 1982 Jan;128(1):148–154. [PubMed] [Google Scholar]
- Shenker B. J., Slots J. Immunomodulatory effects of Bacteroides products on in vitro human lymphocyte functions. Oral Microbiol Immunol. 1989 Mar;4(1):24–29. doi: 10.1111/j.1399-302x.1989.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Shenker B. J., Vitale L. A., Welham D. A. Immune suppression induced by Actinobacillus actinomycetemcomitans: effects on immunoglobulin production by human B cells. Infect Immun. 1990 Dec;58(12):3856–3862. doi: 10.1128/iai.58.12.3856-3862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B. J., Willoughby W. F., Hollingdale M. R., Mann T. N. In vivo responses to inhaled proteins. III. Inhibition of experimental immune complex pneumonitis after suppression of peripheral blood lymphocytes. J Immunol. 1980 Apr;124(4):1763–1772. [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Testi R., Phillips J. H., Lanier L. L. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989 Mar 15;142(6):1854–1860. [PubMed] [Google Scholar]
- Tomai M. A., Elmquist B. J., Warmka S. M., Fitzgerald T. J. Macrophage-mediated suppression of con A-induced IL-2 production in spleen cells from syphilitic rabbits. J Immunol. 1989 Jul 1;143(1):309–314. [PubMed] [Google Scholar]
- Weir D. M., Blackwell C. C. Interaction of bacteria with the immune system. J Clin Lab Immunol. 1983 Jan;10(1):1–12. [PubMed] [Google Scholar]


