SUMMARY
Elevated glucose levels in the presence of insulin are indicative of type 2 diabetes and the more inclusive metabolic syndrome. Alleles conferring susceptibility to these and other common conditions may be adaptations to past environments. It is possible that other mammals exhibiting environmental diversity harbor similar variants; therefore, we assessed glucose regulation in two species of deer mice (Peromyscus), a diverse endemic North American group. The prairie deer mouse, P. maniculatus bairdii (BW), and the Oldfield mouse, P. polionotus subgriseus (PO) differ in sexual dimorphism, behavior and habitat. PO animals exhibit better regulatory ability than BW animals, particularly among males, although both species display equivalent insulin levels/responses and non-fasted glucose levels. Hybrid males exhibit a PO glucose challenge response and subsequent analysis of consomic animals implicates Y chromosome variation as the genetic cause. Two pieces of evidence indicate that the male glucose regulatory differences are mediated by stress response: (1) fasting and handling alone account for most of the variation; (2) an inhibitor of glucocorticoid (GC) stress hormone synthesis eliminates these differences. PO males have GC levels that are twice those of BW males, indicating the presence of alleles that attenuate the GC response. We hypothesize that the interspecific physiological and behavioral differences are interrelated and that similar human variants exist.
INTRODUCTION
The complexity of many prevalent human diseases has led to the suggestion that moderate risk alleles at multiple loci may be a regular occurrence (Smith and Lusis, 2002). Termed the common disease/common variant (CD/CV) hypothesis, emerging data has generally supported this model (Peng and Kimmel, 2007; Spielman et al., 2007). Explanations for the CD/CV hypothesis (i.e. high frequency of disease susceptibility alleles) include a lack of negative selection (e.g. diseases which arise later than the average lifespan of pre-civilization humans) or adaptations to past environments. For example, multiple adaptive scenarios have been proposed to explain the rapid increase in type 2 diabetes mellitus (T2DM) and the more inclusive ‘metabolic syndrome’ (Freeman and Cox, 2006; Diamond, 2003; Speakman, 2007) – the latter also includes cardiovascular disease (CVD), stroke and nonalcoholic fatty liver disease. Several lines of evidence suggest the interrelated nature of these diseases (Zimmet et al., 2001). Although obesity-induced metabolic alterations are a cause of these diseases, symptoms may precede obesity and ∼20% of T2DM cases are not accompanied by obesity (www.niddk.gov). Given that there are multiple pathways involved in T2DM/metabolic syndrome, it seems likely that multiple relevant environmental factors have changed during the development of human civilization. Stress pathways, for example, are known to affect blood pressure and adipose deposition (Widgren et al., 1992), and to influence CVD (Alevizaki et al., 2007).
If a significant proportion of the human genetic variation involved in disease susceptibility was adaptive, then similar variation may exist in other mammalian species that are adapted to a variety of environments. Such models could be used to identify and study interactions among alleles conferring disease susceptibility in humans. Commonly used laboratory rodents and domesticated animals are not ideal for such studies because they do not represent naturally occurring populations (owing to both allelic combinations and homozygosity) (Beck et al., 2000; Smale et al., 2005).
Deer mice (Peromyscus) are the most common native North American mammals and inhabit nearly every terrestrial habitat on the continent (Dewey and Dawson, 2001). A study of five Peromyscus species suggested a correlation between metabolic rate and local caloric availability (environmental productivity) (Mueller and Diamond, 2001). For example, the California mouse (Peromyscus californicus) is found in chaparral, a relatively unproductive habitat, and has a tendency to develop fatty liver disease, pancreatic pathologies and other T2DM symptoms when given a high-fat diet (Krugner-Higby et al., 2000). These symptoms occur without significant obesity or increase in food intake. It may be relevant that P. californicus exhibits among the strictest monogamy of any mammalian species (Ribble, 1991). Monogamy is a rare adaptation with pleiotropic consequences (Clutton-Brock, 1989). Like many of the pathways involved in T2DM/metabolic syndrome, monogamy/pair bonding is at least partly regulated by the hypothalamic-pituitary-adrenal (HPA) axis (DeVries et al., 1995a; Good et al., 2005; Kramer et al., 2005; Taymans et al., 1997). Further, the social interactions required for the pair-bonding observed in monogamy induce stress (Chrousos and Kino, 2007; DeVries et al., 1995b).
Another Peromyscus species, P. polionotus (‘Oldfield mouse’), offers the potential for genetic analysis of monogamy-associated pathways and assessment of their influence on disease phenotypes. P. polionotus has also been documented as monogamous in the wild (Foltz, 1981) and is part of the larger P. maniculatus species complex – the best studied of this group is the prairie deer mouse, P.m. bairdii. Captive stocks derived from single wild populations are available for both P. polionotus and P. maniculatus (Fig. 1). These stocks retain ancestral behavioral differences (e.g. the degree of paternal care of offspring) (Dewey and Dawson, 2001; Kramer et al., 2005; Margulis, 1998).
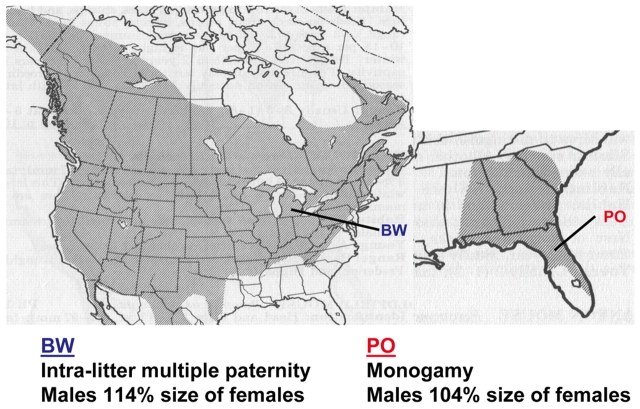
Fig. 1.
Summary of P. polionotus (PO)/P. maniculatus (BW) populations and behavior. A comparison of P. polionotus (PO) with P. maniculatus (BW) showing geographic ranges of the two species (maps), population origins of the captive stocks (lines), and selected differences (below) (Dawson et al., 1993; Dewey and Dawson, 2001). The BW stock is derived from P.m. bairdii trapped in Washtenaw Co, MI; the PO stock is derived from P. polionotus subgriseus in Ocala National Forest, FL.
Both captive and wild-trapped P. polionotus (captive stock=PO) animals display a calmer demeanor than their P. maniculatus (captive stock=BW) counterparts (Martin et al., 2007). Published data suggest that basal metabolic rates are approximately equal in both the more active, wider ranging P. maniculatus and the calmer, monogamous P. polionotus (Layne and Kirkland, 1989). Despite being mildly growth-retarded, the offspring of BW females crossed with PO males are healthy and fertile (Dawson et al., 1993). Therefore, these hybrids allow genetic analysis through backcrosses and/or intercrosses. In this system, analysis is facilitated by a nascent genetic map and immanent genome sequencing (Ramsdell et al., 2008).
Because P. polionotus and P. maniculatus differ in terms of their environment, and both social and non-social behaviors, we hypothesized that the two groups might differ in their susceptibility to T2DM/metabolic syndrome. First, we asked whether the two species were equally adept at glucose homeostasis, a primary diagnostic tool for these diseases.
RESULTS
Species and sex differences in fasting glucose regulatory ability
We performed glucose tolerance tests (GTTs) on at least 13 individuals of each sex from both the PO and BW stocks. Animals were fasted for 18 hours and then subjected to glucose administration (1.5 mg/g bodyweight) via intraperitoneal injection. We measured blood glucose concentration immediately prior to the glucose challenge (time 0) and every 30 minutes thereafter up until 180 minutes post-administration. All tests were carried out at the same time of day (2–4 p.m.) to avoid confounding effects of circadian rhythms. BW females exhibited significantly higher glucose concentrations (as assessed using the Student’s t-test) than PO females, both at time 0 and throughout most of the measuring period, and there was little overlap between the two groups (Fig. 2A; supplementary material Fig. S1A). Despite these differences, females of both species showed a return to pre-injection blood glucose levels by 90 minutes.
Fig. 2.
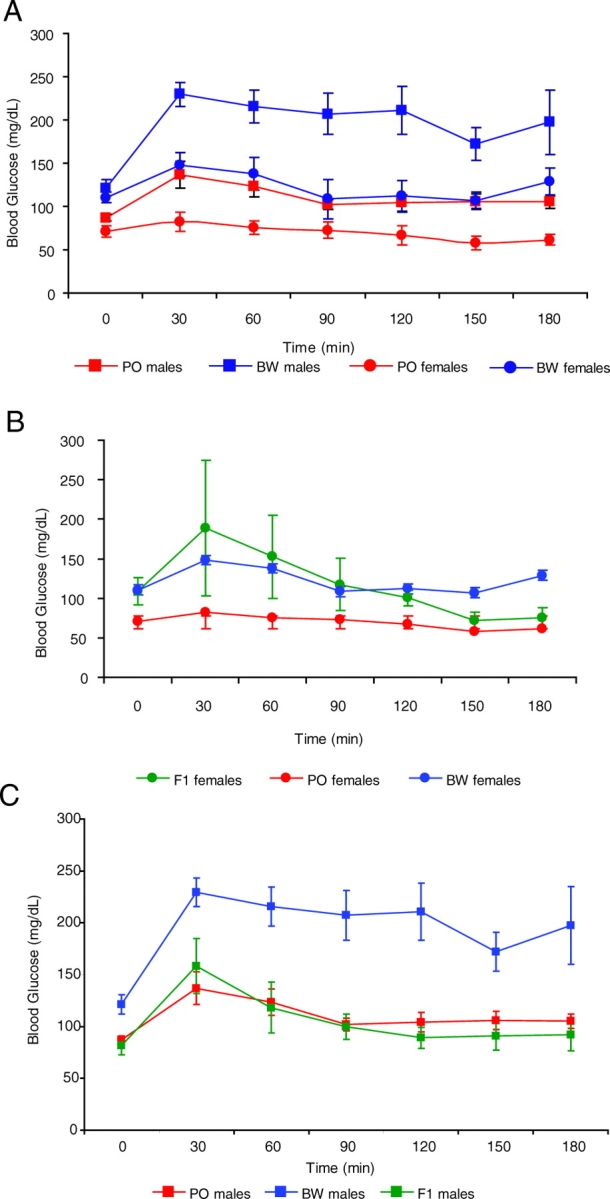
Results of Peromyscus GTTs performed after an 18-hour fast. (AC) Mean blood glucose values ± s.e. are shown for both sexes of both species (A), female hybrids compared with parental stocks (B), and males hybrids compared with parental stocks (C). Circles=females; squares=males. Red=PO, blue=BW.
A much larger difference was observed between males of the two species (Fig. 2A; supplementary material Fig. S1B). Initial blood glucose levels for BW males were also well above those of their PO counterparts. As expected, male blood glucose levels rose during the first 30 minutes post-injection and then declined over the next two 30-minute intervals. However, BW males showed only a minimal decline, whereas PO blood glucose concentrations returned to near baseline levels. As shown in Fig. 2A, male BW glucose values remained at almost double the pre-GTT levels, whereas the glucose levels of PO males remained static from 90 minutes after glucose administration. Therefore, the GTT data indicate that Peromyscus glucose regulation exhibits both sex- and species-specific differences.
In comparison with BW males, the male PO GTT response was more similar to the conspecific female response in both shape and absolute values (supplementary material Table S1; supplementary material Fig. S1C,D). For example, values for PO males differed from those of PO females by an average of only 39.6 mg/dL across all time points, whereas the BW sexes differed by an average of 71.5 mg/dL. Together, the pre-GTT data from both sexes suggest either a differential response to fasting or species-specific differences in baseline blood glucose concentrations.
Genetics of species differences in glucose regulation
The fertile offspring produced by mating BW females with PO males offer the opportunity for genetic analyses in this system. We performed GTTs on both male and female F1 hybrids to assess the genetics of the species differences in glucose regulation. Female hybrids were not significantly different from either parental strain, suggesting no species dominance (Fig. 2B). In contrast, hybrid males displayed a response that was indistinguishable from their PO fathers (Fig. 2C).
Species equivalence in male insulin levels and insulin response
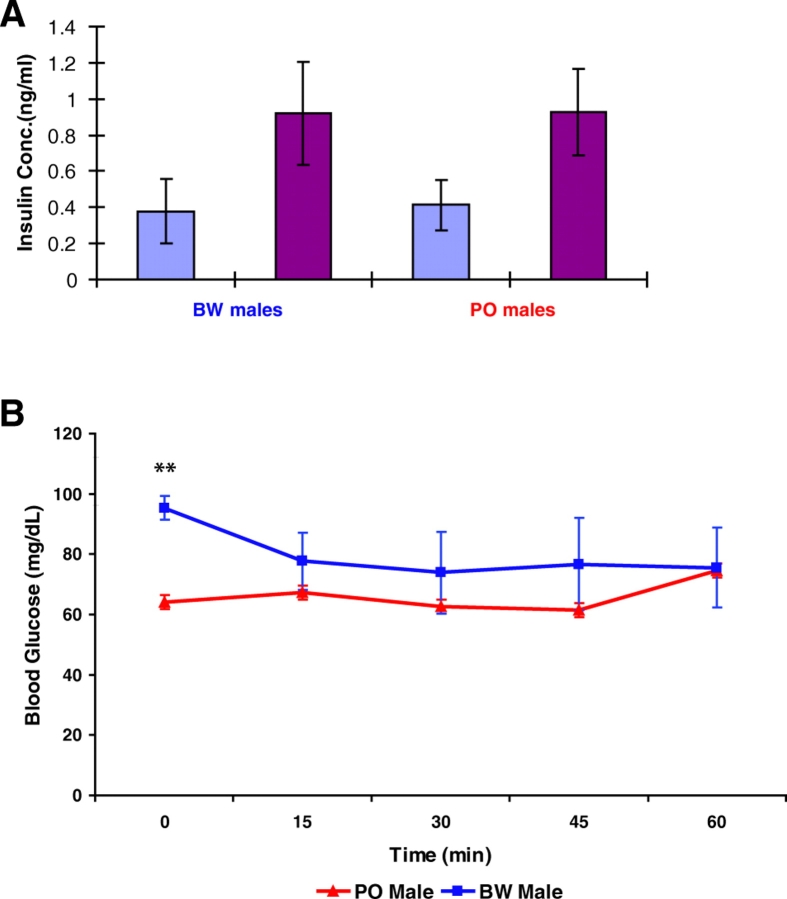
We focused on the differences in male glucose regulation owing to their greater magnitude. We postulated that the differences in male Peromyscus glucose regulation might be the result of different concentrations of circulating insulin. To test this hypothesis, we performed insulin ELISA assays on fasted males before and after glucose administration. These data revealed that the insulin levels in the two strains were equivalent at both time points (Fig. 3A). Computing insulin sensitivity indices based on fasted insulin and glucose values via the QUICKI formula (Katz et al., 2000) resulted in nearly identical values (BW=0.33, PO=0.34).
Fig. 3.
Insulin concentrations and tolerance in Peromyscus males. (A) Insulin ELISA data. Light blue columns indicate the insulin concentration at the time of glucose administration; burgundy columns indicate the values at 15 minutes post-administration. Mean values ± s.e. are shown. Genotype indicated beneath columns. (B) Insulin tolerance test (ITT) data for PO and BW males. Significance was determined by using the Student’s t-test; P values are indicated by asterisks: *P=0.05, **P≤0.005, ***P≤0.001.
We also considered that a differential insulin response might underlie the interspecific male variation. We therefore performed insulin tolerance tests (ITTs). The ITT procedure is similar to the GTT, except that insulin is administered rather than glucose. Therefore, blood glucose concentration is expected to initially drop and then return to the pre-test range. Insulin resistance, commonly associated with T2DM, is marked by a failure of blood glucose levels to fall after the administration of exogenous insulin. We assayed blood glucose concentrations at 15-minute intervals. The two species again displayed significantly different mean glucose concentrations after fasting (Fig. 3B). Females of the two species differed significantly only at time 0 (supplementary material Fig. S2A) and values for PO and BW males exhibited the same pattern. F1 males differed from both PO and BW at 15 minutes post-insulin administration (P<0.05) but were otherwise statistically equivalent (supplementary material Fig. S2B). The Peromyscus ITT curves are reminiscent of mild insulin resistance because the animals did not experience dramatic decreases in blood glucose levels. However, it has previously been shown that Peromyscus and some other rodents are not induced to feed by addition of physiological levels of insulin, or by glucose anti-metabolites (Rowland et al., 1985). These data suggest that neither differential insulin concentrations nor insulin sensitivity are the causes of the species differences in glucose homeostasis.
GTT and ITT responses of Y chromosome consomic animals
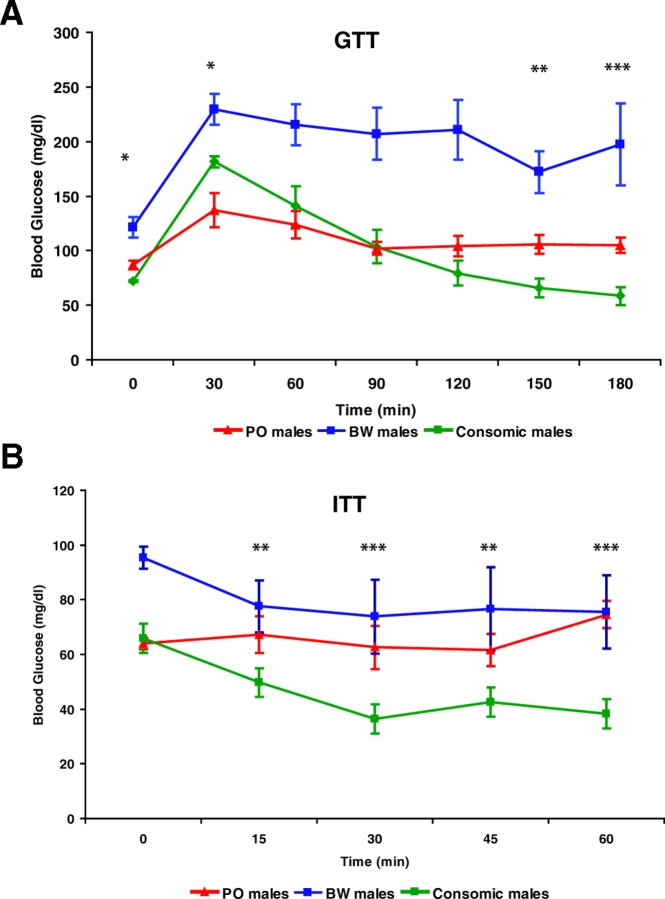
The apparent paternal inheritance of glucose homeostasis ability suggested several possibilities: (1) dominance of PO alleles at one or more loci; (2) one or more paternally expressed imprinted loci; or (3) Y chromosome-linked sequences underlie the species differences in glucose regulation. We were able to test the latter hypothesis by breeding consomic animals that had a PO Y chromosome on an otherwise BW genetic background (BW YPO).
The BW YPO animals had significantly different GTT values from BW males at every time point (Fig. 4A). Although more similar to PO males, the consomic animals also differed from them at several time points. The fasted BW YPO animals had lower glucose values than PO males (P=0.03), but at 30 minutes post-glucose administration they displayed values that were intermediate between PO and BW (with mean values significantly different from both). From 60 to 120 minutes the consomic pattern was not distinguishable from PO values; however, at 150 and 180 minutes post-glucose administration the consomic animals again displayed significantly lower values than PO males. Both PO and BW YPO values at 180 minutes were indistinguishable from their starting blood glucose levels, unlike BW males, whose values were higher than the fasting values.
Fig. 4.
Response of BW YPO consomic males to glucose and insulin challenges. (A) GTT data. (B) ITT data. In both (A) and (B), blood glucose levels are shown for BW YPO consomic males, and for PO and BW males after an 18-hour fast. Asterisks denote P values as in Fig. 3, but only the outcomes of Student’s t-tests between consomic males and PO males are indicated. Consomic males exhibited significant differences from BW males at all time points in both (A) and (B).
We also performed ITTs on the consomic animals, which exhibited values within the ranges of both parental strain males (Fig. 4B). Again, the consomic animals displayed starting values that were equivalent to PO males, but diverged to lower values thereafter. These data suggest there may be interactions between the PO Y chromosome and BW autosomal alleles that result in increased insulin sensitivity and/or levels. Testing the effects of the reciprocal combination, a BW Y chromosome on a PO genetic background, is problematic. The difficulty in producing this genetic combination lies in the non-viability of offspring produced by PO females mated to BW males (Duselis and Vrana, 2007).
Fasting and procedural stress contribute to differences in glucose regulatory ability
To assess whether the relatively long duration of fasting (18 hours) was responsible for the differences in male response, we conducted GTTs after a 6-hour fast. Differences in the response were clearly present at this time, but were attenuated relative to the 18-hour fast data (supplementary material Fig. S3). However, pre-administration blood glucose levels for both PO and BW males were equivalent to the conspecific levels observed after 18-hour fasts. We also assessed glucose concentration in animals that were fed ad libitum. Without induced fasting or exogenous glucose administration, males of the two species did not have significantly different mean blood glucose values (∼80–100 mg/dL) (supplementary material Table S1). That is, both non-fasted PO and BW males had approximately the same blood glucose concentrations as seen in fasted PO males. Indeed, an entire GTT series performed on non-fasted males indicated no significant differences under these conditions (data not shown). These data suggested that fasting-induced stress might be responsible for a substantial portion of the interspecific differences in glucose regulation.
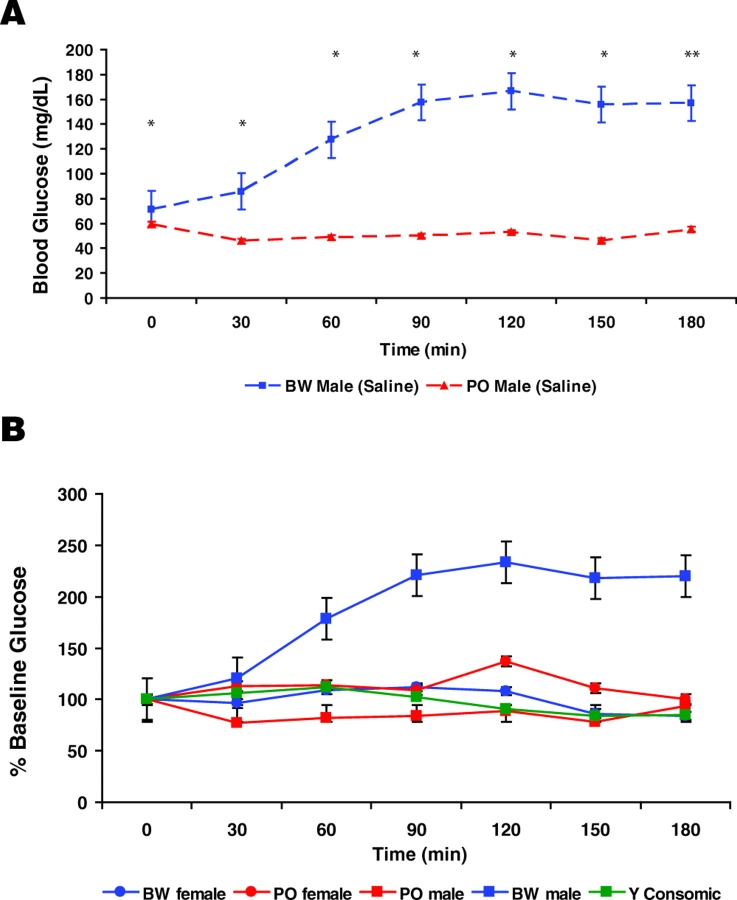
We therefore performed a mock GTT, in which animals were fasted and then injected with only saline solution. As no glucose is administered, this experiment is a control for stress arising from fasting and procedures. As expected, neither PO nor BW males showed a sharp rise in blood glucose levels during the first 30-minute interval after the saline injection (Fig. 5A). However, BW blood glucose levels rose slowly for approximately 90 minutes thereafter and remained at elevated levels. In contrast, the blood glucose levels did not rise in PO mice. We also tested P. polionotus males derived from a distinct and more recently captured population (Santa Rosa Island FL; LS stock) (Lacy and Ballou, 1998). The LS males exhibited the same stability in glucose levels as their PO conspecifics (supplementary material Fig. S2B and data not shown).
Fig. 5.
Involvement of the stress response in Peromyscus glucose homeostasis variation. (A) Mock GTT data for PO and BW males. This test was performed in an identical manner to the GTTs in Fig. 2, except that 1× PBS was injected instead of a glucose solution. Asterisks denote P values as in Fig. 3. (B) Mock GTT data depicted as a percentage of starting (fasted) values.
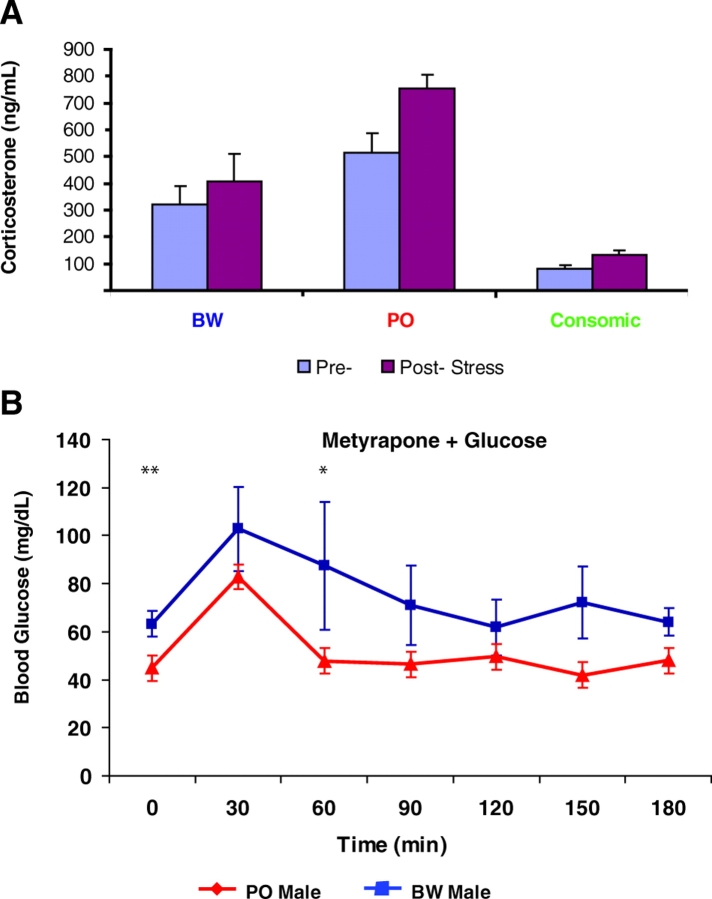
We performed the mock GTT on the consomic males (BW YPO), and on both PO and BW females. Plotting this saline response as a percentage of the starting glucose value for each group generated a relatively flat line (transient elevations of =140%) for consomic and PO males as well as females of both species. In contrast, BW males reach and remain at ∼250% of baseline glucose levels (Fig. 5B). We reanalyzed the GTT data to assess the extent to which the differential stress response underlies the differences in blood glucose concentration. We normalized these data using the values obtained with saline injection only, and compared them to consomic data treated in the same fashion. The normalized patterns are nearly identical, suggesting that the Y chromosome-mediated differences in glucose regulation are largely owing to a differential stress response (supplementary material Fig. S4). These data strongly suggest that there are species-specific aspects of glucose regulation that are influenced by a differential response to both fasting and procedural stress (i.e. handling, transport, injection).
Evidence that differential glucocorticoid sensitivity results in the species-specific glucose homeostasis
The effects of stress are mediated by the HPA axis via glucocorticoids (GCs; e.g. cortisol in primates, corticosterone in rodents). These hormones are known to have major effects on glucose metabolism, including stimulation of glycogen synthesis, gluconeogenesis and inhibition of insulin secretion (Lambillotte et al., 1997; Tomlinson and Stewart, 2007). Although elevated GC levels are associated with diabetic or pre-diabetic phenotypes, it is not clear whether this is always the case. For example, another monogamous mammal, the vole Microtus ochrogaster, has higher GC levels and displays an attenuated GC response relative to a non-monogamous sister species (Taymans et al., 1997).
Data from previous studies suggested higher GC levels in the monogamous P. polionotus than in P. maniculatus (Good et al., 2003; Harper and Austad, 2000). In both cases, the captive PO and BW stocks have been shown to have similar responses/GC levels to their wild counterparts; however, the two species have not been compared in the same study. We therefore assayed plasma corticosterone levels from BW, PO and consomic males both prior to and after the stress regimen of fasting and saline injection. As expected, the stress caused GC levels in all three groups to rise (Fig. 6A). However, prior to stress, PO corticosterone levels were ∼1.5 times greater than those in BW animals. After fasting and saline injection, PO levels were closer to double the levels in BW animals. In contrast, the consomic males exhibit GC concentrations of only ∼25% of those seen in the BW males. The latter values were surprising in that hybrid males had levels equivalent to BW males (supplementary material Fig. S5).
Fig. 6.
Evidence for GC involvement in Peromyscus glucose homeostasis variation. (A) ELISA measuring corticosterone concentrations in PO, BW and consomic males. Genotypes are indicated beneath columns; light blue columns represent pre-stress values and burgundy columns represent post-stress values. Stress was induced by fasting, transport from the vivarium to the lab and through handling/saline injection. Data are shown as mean values of all individuals tested ± s.e. Analysis of variance (ANOVA) suggests that, for each genotype, pre- and post-stress values are significantly different (P≤0.001). (B) Effects of the GC synthesis inhibitor metyrapone on response to glucose challenge. Asterisks denote P values as in Fig. 3.
To functionally test the involvement of GC activity in the glucose regulation disparity, we treated animals with the drug metyrapone. Metyrapone acts as a dose-dependent inhibitor of 11-beta-hydroxylase, an essential enzyme in corticosterone/cortisol production. Treatment of fasted males with metyrapone did not affect blood glucose levels in PO animals but lowered the levels in BW males into the PO range at 60–90 minutes post-injection (Fig. 6B). The delay in this reaction is consistent with the time required for metyrapone to act.
DISCUSSION
These data indicate differential abilities between the recently diverged species P. polionotus and P. maniculatus in post-fasting glucose homeostasis. BW males display diabetic phenotypes such as elevated blood glucose, whereas PO males, and females of both species do not. Consistent with the greater variation in males, the genetic data suggest Y chromosome-linked sequences are primarily responsible for the effects. Both fasting and being subjected to sham procedures without a glucose challenge induced the differences in male glucose regulation, implying that these effects are mediated by an underlying differential stress response. We believe this is the first example of natural variation in stress responses resulting in immediate diabetic phenotypes.
Despite the greater stress sensitivity of BW males with regard to glucose regulation, corticosterone levels are much higher in PO males. However, the effects of metyrapone treatment strongly suggest that this stress pathway involves GC hormones. Together, these data imply that PO males are able to attenuate the effects of GC signaling on glucose homeostasis. As both pre- and post-stress insulin levels are similar between the two groups, we hypothesize that differences in glucagon release and/or gluconeogenesis are responsible for the differences. We therefore predict that variation in a Y-linked locus, whose product regulates these pathways, is primarily responsible for this relative GC resistance. This model does not obviously explain the reduced GC levels observed in the consomic animals; however, other studies have also noted that a better understanding of GC turnover is necessary to understand their role in glucose regulation/insulin sensitivity (Dewbury et al., 2007).
Unidentified Y chromosomal sequences are also involved in stress-induced hyperglycemia and subsequent hyperinsulinemia in C3H.SW/SnJ inbred male mice (Leiter, 1988). Unlike the BW males, these animals develop hyperglycemia only after months of repeated stress and then lose this symptom as insulin levels rise, so do not represent naturally-occurring genotypes (Beck et al., 2000; Ideraabdullah et al., 2004). Unidentified human and rat Y chromosomal sequences have been implicated in the stress response and hypertension, but are largely unexamined with respect to glucose homeostasis (Charchar et al., 2002; Dumas et al., 2000; Strahorn et al., 2005). In addition, several studies have shown an association between cortisol metabolism and insulin resistance that is independent of obesity effects (Reynolds et al., 2001). Thus, this system represents an opportunity to develop a novel CD/CV animal model of T2DM/metabolic syndrome, and is an example of natural variation in stress response resulting in an immediate diabetic phenotype.
The species differences in the GTT pattern are reminiscent of the observation that monogamy (e.g. as seen in P. polionotus) is associated with a reduction in sex-specific differences. There is a greater similarity in the GTT responses between male and female PO animals than between male and female BW animals. Our hypothesis is that the superior glucose homeostasis ability of the PO males is related to their monogamy.
The relative GC resistance exhibited by PO males strengthens a trend associated with monogamous mammals (Table 1). Within Peromyscus, the monogamous P. eremicus exhibit corticosterone levels that are between 2–4 times higher than those of non-monogamous white-footed mice P. leucopus (Martin et al., 2007). The monogamous prairie vole M. ochrogaster exhibits corticosterone levels that are 8–10 times higher than the recently diverged non-monogamous montane vole M. montanus (Taymans et al., 1997). Increased GC levels and/or GC resistance is also associated with monogamy and/or paternal care of offspring in several primate species (Bales et al., 2006; da Silva Mota et al., 2006).
Table 1.
Same-study glucocorticoid comparisons of monogamous versus promiscuous species/populations
| Monogamy/paternal care | Promiscuous/lack of care | Relative GC levels | Reference |
|---|---|---|---|
| P. polionotus | P. maniculatus | Monog ∼2 higher | This study |
| P. eremicus | P. leucopus | Monog ∼2–4 higher | Glasper and DeVries, 2005 |
| M. ochrogaster | M. montanus | Monog ∼5–10 higher | DeVries et al., 1995a; Taymans et al., 1997a |
| Leontopithecus rosalia | Leontopithecus rosalia | Monog ∼2 higher | Bales et al., 2006 |
| Callithrix jacchus | Callithrix jacchus | Exp fathers ∼2–3 higher | da Silva Mota et al., 2006 |
Abbreviations: Monog=monogamous; Exp=experienced.
Monogamy is thought to be an adaptation to those environments that demand greater paternal involvement in offspring care and/or stricter guarding of females against potential rivals (Clutton-Brock, 1989; Marlowe, 2000). Compared with BW males, PO males exhibit both increased paternal care and greater aggressiveness towards unrelated males (Layne and Kirkland, 1989; Margulis, 1998; Martin et al., 2007). We suggest that P. polionotus has adapted to these stressors by increasing corticosterone levels, but attenuating the hormone’s effects on glucose homeostasis. This combination results in the calmer demeanor exhibited by the PO males and their ability to maintain normal blood glucose values. Supporting the former hypothesis, addition of exogenous GCs have been shown to aid humans in reducing fear (Soravia et al., 2006).
Despite their relative resistance, male PO corticosterone levels still rise in response to stress. Prior studies using P. polionotus have shown an association between males with elevated corticosterone levels and increased mortality rates of their first litters (Good et al., 2005). GC signaling is enormously complex; GCs are known to affect many pathways, most of which are subject to tissue-specific regulation (Buckingham, 2006; Chrousos and Kino, 2007). Even within the central nervous system, GC signaling appears to differ between the hippocampus and amygdala (Herbert et al., 2006). For example, memory formation and recall have been shown to be differentially affected by increased GC levels (Herbert et al., 2006; Roozendaal et al., 2006). Thus, we predict that increased GC levels in PO animals means that other pathways and organs that are affected by these hormones exhibit greater sensitivity. For example, we predict that like the similarly monogamous P. californicus, P. polionotus will be more susceptible to the effects of a higher fat diet. Major efforts in treating T2DM/metabolic syndrome symptoms are focused on the local effects of GC signaling (Tomlinson and Stewart, 2007). Elucidation of the mechanisms employed by P. polionotus to attenuate the effects of GC signaling on glucose homeostasis could significantly aid these efforts. We believe that studying the variation in the P. maniculatus species complex experimental system has a unique potential for understanding the relationship between environment-associated adaptations and mammalian disease susceptibility. In general, we suggest that further studies of mammals with natural allelic combinations will yield insights that are not possible with mixed and/or inbred lines (Smale et al., 2005).
It appears possible that variations in the human stress response may have been shaped by environmental and/or cultural differences. Social mores in much of the world are indicative of a general trend towards social/genetic monogamy in humans (Wellings et al., 2006). However, cultural variation still exists, and there is evidence that the shift towards monogamy is recent (Dupanloup et al., 2003). Ample evidence indicates roles for GC signaling in modulating sexual/social bonding and other interactions in humans (Curtis et al., 2006; Halpern et al., 2002; Storey et al., 2000; Wyart et al., 2007). Thus, we predict the existence of GC pathway-related alleles that were selected for in earlier non-monogamous cultures, which now result in increased disease susceptibility.
Detailing the molecular mechanisms by which this specificity is conferred will be a key component of deciphering the relationship between common alleles related to stress/GC influenced pathways and disease. However, we believe that understanding environmental and social influences on these pathways, including distinctions between varieties of social stress, will also be essential.
METHODS
Animals
Origins and husbandry
P. polionotus and P. maniculatus animals were purchased from the Peromyscus Genetic Stock Center (PGSC; http://stkctr.biol.sc.edu/). These animals were caught from single populations in the wild as noted in the text and on the PGSC website. Animals bred at the PGSC or descendents bred at UCI were used in the tests. Animals were housed with food and water ad libitum on a 16:8 hour light:dark cycle. All animals tested were at least 65 days old but not more than 20 months old (both species breed past 2 years of age and live for >4 years). Animals were fed a standard low-fat (4% by weight) rodent chow. We bred BW females with PO males to obtain hybrids. All experiments were approved by the University of California Irvine Institutional Animal Care and Use Committee.
BWYPO animals
The Y chromosome consomic line was bred by backcrossing hybrid males and their subsequent male progeny to BW females for eleven generations. The consomic animals tested were from tenth and eleventh generation backcrosses. The sex ratios produced from the offspring of BW females bred to BW YPO males were not significantly different from the PO and BW stocks (supplementary material Fig. S6). We genotyped BW YPO animals at eight unlinked autosomal and X-linked loci, all of which showed the expected BW genotype. The consomic animals were equivalent in size to BW males. Female animals produced by the consomic line were indistinguishable from other BW females in body weight and GTT response.
Assays
Glucose tolerance tests (GTTs)
As noted in the text, adult animals were fasted prior to testing. Animals were weighed and then given an intraperitoneal injection of 1.5 mg glucose per gram of body weight in a saline solution. Blood glucose concentration was measured from a small cut made at the end of the tail. The scab was gently scraped off at subsequent measurements. Approximately 5 μls of blood was then spotted onto commercially available glucose test strips and analyzed using the accompanying glucose meter (Ascensia Elite XL model).
Glucose readings were taken at the time of injection and at subsequent 30-minute intervals. We performed at least two tests per animal at each time point. A third test was often performed, and always performed in (rare) cases where the two readings had a difference of ≥25 mg/dL. The value for a given animal was then obtained by averaging the two or more values that did not differ by ≥25 mg/dL. Every time point/genotype combination was represented by ≥10 animals. Student’s t-tests were performed on the data as implemented in Microsoft Excel. Note that while BW fasting glucose values are always significantly higher than PO values from animals of the same sex, these values fluctuate over time, which is likely to be dependent on the time of year and/or other external cues.
Insulin tolerance tests (ITTs)
As with the GTT procedure described above, adult animals were fasted prior to testing. The fasting duration was reduced to 8 hours to reduce mortality during the monitoring period (i.e. owing to hypoglycemic shock). We tested insulin concentrations of both 1 and 0.5 units per kilogram of bodyweight. Insulin was administered and blood glucose measured as described for the GTT procedure.
Stress inhibitor assays
These assays were performed in a similar way to the GTT and ITT procedures described above, except that the indicated drug was administered in a saline solution. The dosages used were: metyrapone, 50 mg/kg bodyweight and propranolol hydrochloride, 10 mg/kg bodyweight.
ELISAs
A corticosterone ELISA kit (Assay Systems, Ann Arbor, MI) was used to measure plasma corticosterone levels as per the instructions of the manufacturer. The insulin ELISA was performed using a kit from Crystal Chem (Downers Grove, IL; Cat. #90060) as per manufacturer’s instructions. All assays were performed on blood from adult animals obtained from the tail. Six or more animals per genotype were analyzed for each assay.
Acknowledgments
We thank Lisa Krugner-Higby, Benno Roozendaal, Maike Sander, and Ping Wang for helpful discussions on experimental design. We thank Phillip Seymour and Jia-Ying Yang for aid in procedures. We thank the Dept of Biological Chemistry, University of California Irvine and NSF award MCB-0517754 for funding. Deposited in PMC for immediate release.
Footnotes
AUTHOR CONTRIBUTIONS
R.C.O., C.D.W., M.J.D. and P.B.V. conceived, designed and performed the experiments. P.B.V. wrote the paper.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/content/1/4-5/255/suppl/DC1
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Alevizaki M, Cimponeriu A., Lekakis J., Papamichael C, Chrousosa G. P. (2007)High anticipatory stress plasma cortisol levels and sensitivity to glucocorticoids predict severity of coronary artery disease in subjects undergoing coronary angiography. Metab. Clin. Exp. 56, 222–226 [DOI] [PubMed] [Google Scholar]
- Bales K. L., French J. A., McWilliams J., Lake R. A., Dietza J. M. (2006)Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia). Horm. Behav. 49, 88–95 [DOI] [PubMed] [Google Scholar]
- Beck J. A., Lloyd S., Hafezparast M., Lennon-Pierce M., Eppig J. T., Festing M. F., Fisher E. M. (2000)Genealogies of mouse inbred strains. Nat. Genet. 24, 23–25 [DOI] [PubMed] [Google Scholar]
- Buckingham J. C. (2006)Glucocorticoids: exemplars of multi-tasking. Br. J. Pharmacol. 147, S258–S268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charchar F. J., Tomaszewski M., Padmanabhan S., Lacka B., Upton M. N., Inglis G. C., Anderson N. H., McConnachie A., Zukowska-Szczechowska E., Grzeszczak W., et al. (2002)The Y chromosome effect on blood pressure in two European populations. Hypertension 39, 353–356 [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Kino T. (2007)Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress 10, 213–219 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H. (1989)Mammalian mating systems. Proc. R. Soc. Lond., B, Biol. Sci. 236, 339–372 [DOI] [PubMed] [Google Scholar]
- Curtis J. T., Liu Y., Aragona B. J., Wang Z. (2006)Dopamine and monogamy. Brain Res. 1126, 76–90 [DOI] [PubMed] [Google Scholar]
- da Silva Mota M. T., Franci C. R., Cordeiro de Sousa M. B. (2006)Hormonal changes related to paternal and alloparental care in common marmosets (Callithrix jacchus). Horm. Behav. 49, 293–302 [DOI] [PubMed] [Google Scholar]
- Dawson W. D., Sagedy M. N., Enyu L., Kass D. H., Crossland J. P. (1993)Growth regulation in Peromyscus species hybrids: a test for mitochondrial-nuclear genomic interaction. Growth Dev. Aging 57, 121–133 [PubMed] [Google Scholar]
- DeVries A. C., DeVries M. B., Taymans S., Carter C. S. (1995a). Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc. Natl. Acad. Sci. USA 92, 7744–7748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries A. C., DeVries M. B., Taymans S. E., Carter C. S. (1995b). The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc. Natl. Acad. Sci. USA 93, 11980–11984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewbury K., Wood P. J., Phillips D. I., Byrne C. D. (2007)Cortisol clearance and associations with insulin sensitivity, body fat and fatty liver in middle-aged men. Diabetologia 50,1024–1032 [DOI] [PubMed] [Google Scholar]
- Dewey M. J., Dawson W. D. (2001)Deer mice: “the Drosophila of North American mammalogy”. Genesis 29, 105–109 [DOI] [PubMed] [Google Scholar]
- Diamond J. (2003)The double puzzle of diabetes. Nature 423, 599–602 [DOI] [PubMed] [Google Scholar]
- Dumas P., Pausová Z., Kren V., Krenová D., Pravenec M., Dumont M., Ely D., Turner M., Sun Y., Tremblay J, et al. (2000)Contribution of autosomal loci and the Y chromosome to the stress response in rats. Hypertension 35, 568–573 [DOI] [PubMed] [Google Scholar]
- Dupanloup I., Pereira L., Bertorelle G., Calafell F., Prata M. J., Amorim A, Barbujani G. (2003)A recent shift from polygyny to monogamy in humans is suggested by the analysis of worldwide Y-chromosome diversity. J. Mol. Evol. 57, 85–97 [DOI] [PubMed] [Google Scholar]
- Duselis A. R., Vrana P. B. (2007)Assessment and disease comparisons of hybrid developmental defects. Hum. Mol. Genet. 16, 808–819 [DOI] [PubMed] [Google Scholar]
- Foltz D. W. (1981)Genetic evidence for long term monogamy in a small rodent, Peromyscus polionotus. Am. Nat. 117, 665–675 [Google Scholar]
- Freeman H., Cox R. D. (2006)Type-2 diabetes: a cocktail of genetic discovery. Hum. Mol. Genet. 15, R202–R209 [DOI] [PubMed] [Google Scholar]
- Glasper E. R., DeVries A. C. (2005)Social structure influences effects of pair-housing on wound healing. Brain Behav. Immun. 19, 61–68 [DOI] [PubMed] [Google Scholar]
- Good T., Khan M. Z., Lynch J. W. (2003)Biochemical and physiological validation of a corticosteroid radioimmunoassay for plasma and fecal samples in oldfield mice (Peromyscus polionotus). Physiol. Behav. 80, 405–411 [DOI] [PubMed] [Google Scholar]
- Good T. C., Harris K. K., Ihunnah C. A. (2005)Corticosteroids as potential mechanism regulating variability in reproductive success in monogamous oldfield mice (Peromyscus polionotus). Physiol. Behav. 86, 96–102 [DOI] [PubMed] [Google Scholar]
- Halpern C. T., Campbell B., Agnew C. R., Thompson V, Udry J. R. (2002)Associations between stress reactivity and sexual and nonsexual risk taking in young adult human males. Horm. Behav. 42, 387–398 [DOI] [PubMed] [Google Scholar]
- Harper J. M., Austad S. N. (2000)Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol. Biochem. Zool. 73, 12–22 [DOI] [PubMed] [Google Scholar]
- Herbert J., Goodyer I. M., Grossman A. B., Hastings M. H., de Kloet E. R., Lightman S. L., Lupien S. J., Roozendaal B, Seckl J. R. (2006)Do corticosteroids damage the brain? J. Neuroendocrinol. 18, 393–411 [DOI] [PubMed] [Google Scholar]
- Ideraabdullah F. Y., de la Casa-Esperon E., Bell T. A., Detwiler D. A., Magnuson T., Sapienza C, de Villena F. P. (2004)Genetic and haplotype diversity among wild-derived mouse inbred strains. Genome Res. 14, 1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Nambi S. S., Mather K., Baron A. D., Follman D. A., Sullivan G, Quon M. J. (2000)Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 85, 2402–2410 [DOI] [PubMed] [Google Scholar]
- Kramer K. M., Yamamoto Y., Hoffman G. E., Cushing B. S. (2005)Estrogen receptor alpha and vasopressin in the paraventricular nucleus of the hypothalamus in Peromyscus. Brain Res. 1032, 154–161 [DOI] [PubMed] [Google Scholar]
- Krugner-Higby L., Shadoan M., Carlson C., Gendron A., Cofta P., Marler C, Wagner J. (2000)Type 2 diabetes mellitus, hyperlipidemia, and extremity lesions in California mice (Peromyscus californicus) fed commercial mouse diets. Comp. Med. 50, 412–418 [PubMed] [Google Scholar]
- Lacy R. C., Ballou J. D. (1998)Effectiveness of selection in reducing the genetic load in populations of Peromyscus polionotus during generations of inbreeding Evolution 52, 900–909 [DOI] [PubMed] [Google Scholar]
- Lambillotte C., Gilon P., Henquin J. C. (1997)Direct glucocorticoid inhibition of insulin secretion. J. Clin. Invest. 99, 414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne J. N., Kirkland G. L. (1989)Advances in the Study of Peromyscus. Lubbock, TX: Texas Tech University Press [Google Scholar]
- Leiter E. H. (1988)Control of spontaneous glucose intolerance, hyperinsulinemia, and islet hyperplasia in nonobese C3H.SW male mice by Y-linked locus and adrenal gland. Metab. Clin. Exp. 37, 689–696 [DOI] [PubMed] [Google Scholar]
- Margulis S. W. (1998)Relationships among parental inbreeding, parental behavior and offspring viability in oldfield mice. Anim. Behav. 55, 427–438 [DOI] [PubMed] [Google Scholar]
- Marlowe F. (2000)Paternal investment and the human mating system. Behavioural Processes 51, 45–61 [DOI] [PubMed] [Google Scholar]
- Martin L. B., Trainor B. C., Nelson R. J. (2007)HPA activity and neotic and anxiety-like behavior vary among Peromyscus species. Gen. Comp. Endocrinol. 151, 342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P., Diamond J. (2001)Metabolic rate and environmental productivity: well-provisioned animals evolved to run and idle fast. Proc. Natl. Acad. Sci. USA 98, 12550–12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B., Kimmel M. (2007)Simulations provide support for the common disease-common variant hypothesis. Genetics 175, 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell C. M., Lewandowski A. A., Glenn J. L. W, Vrana P. B., O’Neill R. J., Dewey M. J. (2008)Comparative genome mapping of the deer mouse (Peromyscus maniculatus) reveals greater similarity to rat (Rattus norvegicus) than to the lab mouse (Mus musculus ). BMC Evol. Biol. 8, 65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. M., Walker B. R., Syddall H. E., Andrew R., Wood P. J., Whorwood C. B., Phillips D. I. (2001)Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J. Clin. Endocrinol. Metab. 86, 245–250 [DOI] [PubMed] [Google Scholar]
- Ribble D. (1991)The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 29, 161–166 [Google Scholar]
- Roozendaal B., Okuda S., de Quervain D. J., McGaugh J. L. (2006)Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience 138, 901–910 [DOI] [PubMed] [Google Scholar]
- Rowland N., Watkins L., Carlton J. (1985)Failure of 2-Deoxy-D-Glucose to stimulate feeding in deermice. Physiol. Behav. 34, 155–157 [DOI] [PubMed] [Google Scholar]
- Smale L., Heideman P. D., French J. A. (2005)Behavioral neuroendocrinology in nontraditional species of mammals: things the ‘knockout’ mouse CAN’T tell us. Horm. Behav. 48, 474–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Lusis A. J. (2002)The allelic structure of common disease. Hum. Mol. Genet. 11, 2455–2461 [DOI] [PubMed] [Google Scholar]
- Soravia L. M., Heinrichs M., Aerni A., Maroni C., Schelling G., Ehlert U., Roozendaal B, de Quervain D. J. (2006)Glucocorticoids reduce phobic fear in humans. Proc. Natl. Acad. Sci. USA 103, 5585–5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J. R. (2007)A nonadaptive scenario explaining the genetic predisposition to obesity: the ‘‘Predation Release’’ hypothesis. Cell Metab. 6, 5–12 [DOI] [PubMed] [Google Scholar]
- Spielman R. S., Bastone L. A., Burdick J. T., Morley M., Ewens W. J., Cheung V. G. (2007)Common genetic variants account for differences in gene expression among ethnic groups. Nat. Genet. 39, 226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey A. E., Walsh C. J., Quinton R. L., Wynne-Edwards K. E. (2000)Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol. Hum. Behav. 21, 79–95 [DOI] [PubMed] [Google Scholar]
- Strahorn P., Graham D., Charchar F. J., Sattar N., McBride M. W., Dominiczak A. F. (2005)Genetic determinants of metabolic syndrome components in the stroke-prone spontaneously hypertensive rat. J. Hypertens. 23, 2179–2186 [DOI] [PubMed] [Google Scholar]
- Taymans S. E., DeVries A. C., DeVries M. B., Nelson R. J., Friedman T. C., Castro M., Detera-Wadleigh S., Carter C. S., Chrousos G. P. (1997)The hypothalamic-pituitary-adrenal axis of prairie voles (Microtus ochrogaster): evidence for target tissue glucocorticoid resistance. Gen. Comp. Endocrinol. 106, 48–61 [DOI] [PubMed] [Google Scholar]
- Tomlinson J. W., Stewart P. M. (2007)Modulation of glucocorticoid action and the treatment of type-2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 21, 607–619 [DOI] [PubMed] [Google Scholar]
- Wellings K., Collumbien M., Slaymaker E., Singh S., Hodges Z., Patel D, Bajos N. (2006)Sexual behaviour in context: a global perspective. Lancet 368, 1706–1728 [DOI] [PubMed] [Google Scholar]
- Widgren B. R., Wikstrand J., Berglund G, Andersson O. K. (1992)Increased response to physical and mental stress in men with hypertensive parents. Hypertension 20, 606–611 [DOI] [PubMed] [Google Scholar]
- Wyart C., Webster W. W., Chen J. H., Wilson S. R., McClary A., Khan R. M., Sobel N. (2007)Smelling a single component of male sweat alters levels of cortisol in women. J. Neurosci. 27, 1261–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet P., Alberti K. G. M. M., Shaw J. (2001)Global and societal implications of the diabetes epidemic. Nature 414, 782–787 [DOI] [PubMed] [Google Scholar]